Regional Responses in Radiation-Induced Normal Tissue Damage
Abstract
:Simple Summary
Abstract
1. Introduction
2. Data by Organ
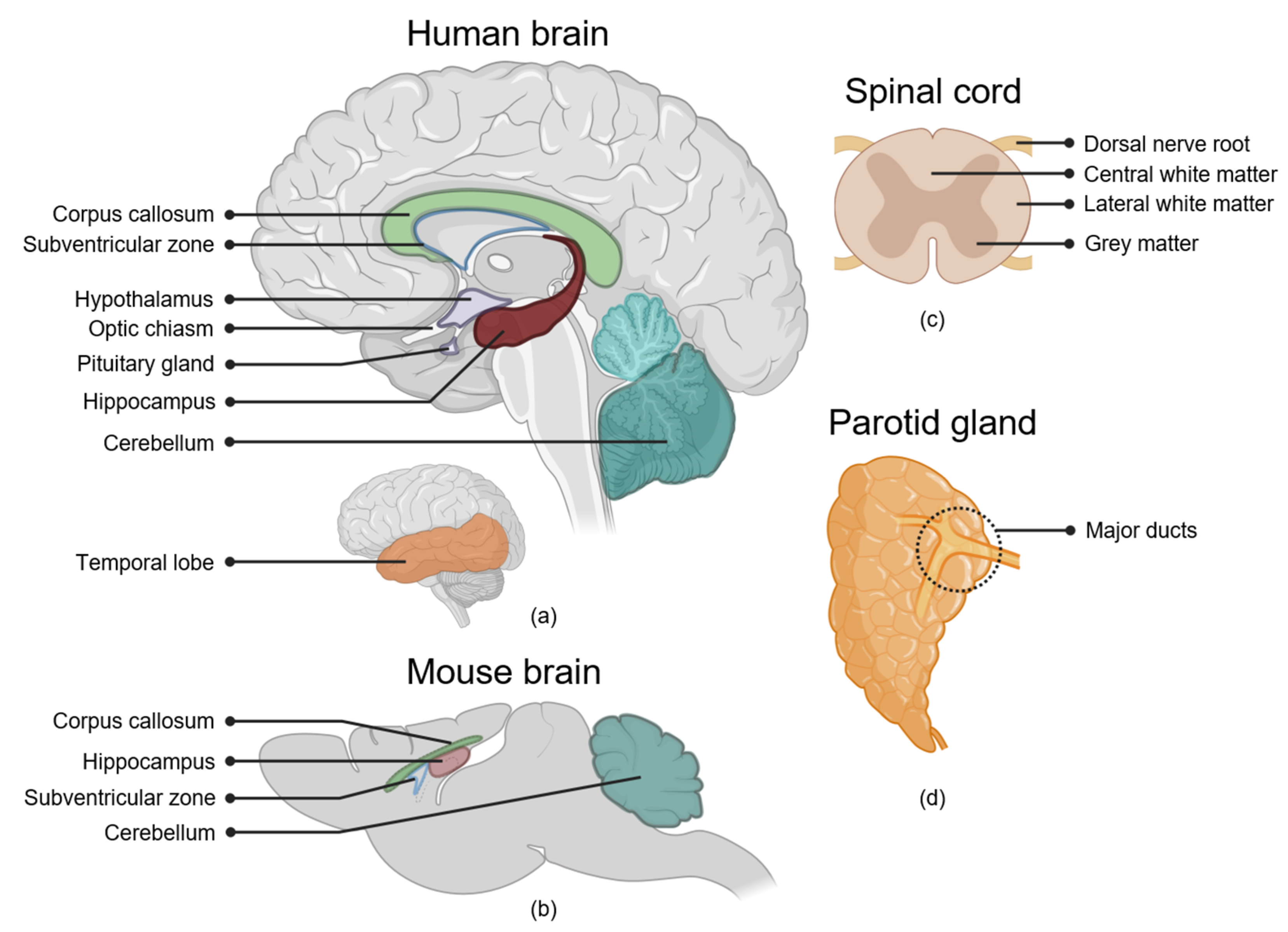
2.1. The Brain
2.1.1. Hippocampus
2.1.2. Subventricular Zone of the Lateral Ventricles
2.1.3. Cerebral Cortex
2.1.4. White Matter
2.1.5. Cerebellum
2.1.6. Hypothalamus and Pituitary Gland
2.1.7. Optic Nerve and Optic Chiasm
2.2. The Spinal Cord
White Matter
2.3. Salivary Glands
Parotid Gland Major Ducts
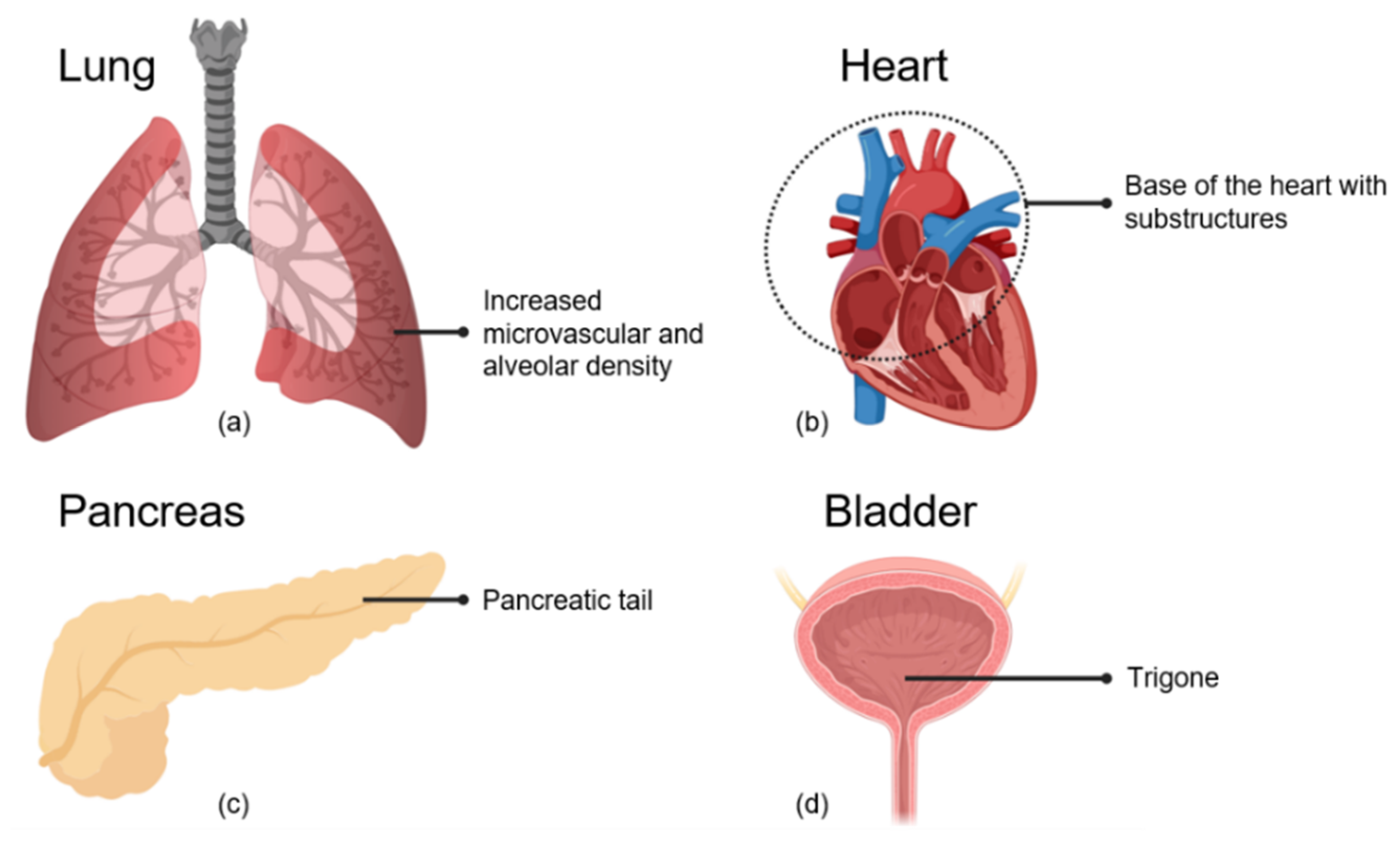
2.4. Cardiopulmonary System
2.4.1. Alveolar and Microvascular Dense Regions of the Lung
2.4.2. Basal Region of the Heart
2.4.3. Heart and Lung Interaction
2.5. The Pancreas
2.6. The Bladder
Bladder Trigone
3. Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Delaney, G.; Jacob, S.; Featherstone, C.; Barton, M. The Role of Radiotherapy in Cancer Treatment. Cancer 2005, 104, 1129–1137. [Google Scholar] [CrossRef]
- De Ruysscher, D.; Niedermann, G.; Burnet, N.G.; Siva, S.; Lee, A.W.M.; Hegi-Johnson, F. Radiotherapy Toxicity. Nat. Rev. Dis. Prim. 2019, 5, 13. [Google Scholar] [CrossRef]
- Borras, J.M.; Lievens, Y.; Barton, M.; Corral, J.; Ferlay, J.; Bray, F.; Grau, C. How Many New Cancer Patients in Europe Will Require Radiotherapy by 2025? An ESTRO-HERO Analysis. Radiother. Oncol. 2016, 119, 5–11. [Google Scholar] [CrossRef] [Green Version]
- Loeffler, J.S.; Durante, M. Charged Particle Therapy—Optimization, Challenges and Future Directions. Nat. Rev. Clin. Oncol. 2013, 10, 411–424. [Google Scholar] [CrossRef]
- Seibold, P.; Auvinen, A.; Averbeck, D.; Bourguignon, M.; Hartikainen, J.M.; Hoeschen, C.; Laurent, O.; Noël, G.; Sabatier, L.; Salomaa, S.; et al. Clinical and Epidemiological Observations on Individual Radiation Sensitivity and Susceptibility. Int. J. Radiat. Biol. 2020, 96, 324–339. [Google Scholar] [CrossRef]
- Britel, M.; Bourguignon, M.; Foray, N. The Use of the Term ‘Radiosensitivity’ through History of Radiation: From Clarity to Confusion. Int. J. Radiat. Biol. 2018, 94, 503–512. [Google Scholar] [CrossRef]
- Bentzen, S.M. Preventing or Reducing Late Side Effects of Radiation Therapy: Radiobiology Meets Molecular Pathology. Nat. Rev. Cancer 2006, 6, 702–713. [Google Scholar] [CrossRef]
- Dörr, W. Pathogenesis of Normal-Tissue Side-Effects. In Basic Clinical Radiobiology, 4th ed.; Joiner, M.C., van der Kogel, A.J., Eds.; Hodder Arnold: London, UK, 2009; pp. 169–189. [Google Scholar]
- Wittingen, J.; Frey, C.F. Islet Concentration in the Head, Body, Tail and Uncinate Process of the Pancreas. Ann. Surg. 1974, 179, 412–414. [Google Scholar] [CrossRef]
- Kocak, Z.; Borst, G.R.; Zeng, J.; Zhou, S.; Hollis, D.R.; Zhang, J.; Evans, E.S.; Folz, R.J.; Wong, T.; Kahn, D.; et al. Prospective Assessment of Dosimetric/Physiologic-Based Models for Predicting Radiation Pneumonitis. Int. J. Radiat. Oncol. Biol. Phys. 2007, 67, 178–186. [Google Scholar] [CrossRef] [Green Version]
- Mayo, C.; Martel, M.K.; Marks, L.B.; Flickinger, J.; Nam, J.; Kirkpatrick, J. Radiation Dose–Volume Effects of Optic Nerves and Chiasm. Int. J. Radiat. Oncol. Biol. Phys. 2010, 76, S28–S35. [Google Scholar] [CrossRef]
- Lawrence, Y.R.; Li, X.A.; el Naqa, I.; Hahn, C.A.; Marks, L.B.; Merchant, T.E.; Dicker, A.P. Radiation Dose–Volume Effects in the Brain. Int. J. Radiat. Oncol. Biol. Phys. 2010, 76, S20–S27. [Google Scholar] [CrossRef] [Green Version]
- Chemaitilly, W.; Li, Z.; Huang, S.; Ness, K.K.; Clark, K.L.; Green, D.M.; Barnes, N.; Armstrong, G.T.; Krasin, M.J.; Srivastava, D.K.; et al. Anterior Hypopituitarism in Adult Survivors of Childhood Cancers Treated with Cranial Radiotherapy: A Report from the St Jude Lifetime Cohort Study. J. Clin. Oncol. 2015, 33, 492–500. [Google Scholar] [CrossRef] [Green Version]
- Gonçalves, J.T.; Schafer, S.T.; Gage, F.H. Adult Neurogenesis in the Hippocampus: From Stem Cells to Behavior. Cell 2016, 167, 897–914. [Google Scholar] [CrossRef] [Green Version]
- Tobin, M.K.; Musaraca, K.; Disouky, A.; Shetti, A.; Bheri, A.; Honer, W.G.; Kim, N.; Dawe, R.J.; Bennett, D.A.; Arfanakis, K.; et al. Human Hippocampal Neurogenesis Persists in Aged Adults and Alzheimer’s Disease Patients. Cell Stem Cell 2019, 24, 974–982.e2. [Google Scholar] [CrossRef]
- Moreno-Jiménez, E.P.; Flor-García, M.; Terreros-Roncal, J.; Rábano, A.; Cafini, F.; Pallas-Bazarra, N.; Ávila, J.; Llorens-Martín, M. Adult Hippocampal Neurogenesis Is Abundant in Neurologically Healthy Subjects and Drops Sharply in Patients with Alzheimer’s Disease. Nat. Med. 2019, 25, 554–560. [Google Scholar] [CrossRef]
- Fike, J.R.; Rola, R.; Limoli, C.L. Radiation Response of Neural Precursor Cells. Neurosurg. Clin. N. Am. 2007, 18, 115–127. [Google Scholar] [CrossRef]
- Mizumatsu, S.; Monje, M.L.; Morhardt, D.R.; Rola, R.; Palmer, T.D.; Fike, J.R. Extreme Sensitivity of Adult Neurogenesis to Low Doses of X-Irradiation. Cancer Res. 2003, 63, 4021–4027. [Google Scholar]
- Raber, J.; Rola, R.; LeFevour, A.; Morhardt, D.; Curley, J.; Mizumatsu, S.; VandenBerg, S.R.; Fike, J.R. Radiation-Induced Cognitive Impairments Are Associated with Changes in Indicators of Hippocampal Neurogenesis. Radiat. Res. 2004, 162, 39–47. [Google Scholar] [CrossRef]
- Hellström, N.A.K.; Björk-Eriksson, T.; Blomgren, K.; Kuhn, H.G. Differential Recovery of Neural Stem Cells in the Subventricular Zone and Dentate Gyrus After Ionizing Radiation. Stem Cells 2009, 27, 634–641. [Google Scholar] [CrossRef]
- Saxe, M.D.; Battaglia, F.; Wang, J.-W.; Malleret, G.; David, D.J.; Monckton, J.E.; Garcia, A.D.R.; Sofroniew, M.V.; Kandel, E.R.; Santarelli, L.; et al. Ablation of Hippocampal Neurogenesis Impairs Contextual Fear Conditioning and Synaptic Plasticity in the Dentate Gyrus. Proc. Natl. Acad. Sci. USA 2006, 103, 17501–17506. [Google Scholar] [CrossRef] [Green Version]
- Hernández-Rabaza, V.; Llorens-Martín, M.; Velázquez-Sánchez, C.; Ferragud, A.; Arcusa, A.; Gumus, H.G.; Gómez-Pinedo, U.; Pérez-Villalba, A.; Roselló, J.; Trejo, J.L.; et al. Inhibition of Adult Hippocampal Neurogenesis Disrupts Contextual Learning but Spares Spatial Working Memory, Long-Term Conditional Rule Retention and Spatial Reversal. Neuroscience 2009, 159, 59–68. [Google Scholar] [CrossRef] [Green Version]
- Tomé, W.A.; Gökhan, Ş.; Brodin, N.P.; Gulinello, M.E.; Heard, J.; Mehler, M.F.; Guha, C. A Mouse Model Replicating Hippocampal Sparing Cranial Irradiation in Humans: A Tool for Identifying New Strategies to Limit Neurocognitive Decline. Sci. Rep. 2015, 5, 14384. [Google Scholar] [CrossRef] [Green Version]
- Takeshita, Y.; Watanabe, K.; Kakeda, S.; Hamamura, T.; Sugimoto, K.; Masaki, H.; Ueda, I.; Igata, N.; Ohguri, T.; Korogi, Y. Early Volume Reduction of the Hippocampus after Whole-Brain Radiation Therapy: An Automated Brain Structure Segmentation Study. Jpn. J. Radiol. 2020, 38, 118–125. [Google Scholar] [CrossRef]
- Seibert, T.M.; Karunamuni, R.; Bartsch, H.; Kaifi, S.; Krishnan, A.P.; Dalia, Y.; Burkeen, J.; Murzin, V.; Moiseenko, V.; Kuperman, J.; et al. Radiation Dose–Dependent Hippocampal Atrophy Detected with Longitudinal Volumetric Magnetic Resonance Imaging. Int. J. Radiat. Oncol. Biol. Phys. 2017, 97, 263–269. [Google Scholar] [CrossRef] [Green Version]
- Nieman, B.J.; de Guzman, A.E.; Gazdzinski, L.M.; Lerch, J.P.; Chakravarty, M.M.; Pipitone, J.; Strother, D.; Fryer, C.; Bouffet, E.; Laughlin, S.; et al. White and Gray Matter Abnormalities After Cranial Radiation in Children and Mice. Int. J. Radiat. Oncol. Biol. Phys. 2015, 93, 882–891. [Google Scholar] [CrossRef] [Green Version]
- Monje, M.L.; Vogel, H.; Masek, M.; Ligon, K.L.; Fisher, P.G.; Palmer, T.D. Impaired Human Hippocampal Neurogenesis after Treatment for Central Nervous System Malignancies. Ann. Neurol. 2007, 62, 515–520. [Google Scholar] [CrossRef]
- Acharya, S.; Wu, S.; Ashford, J.M.; Tinkle, C.L.; Lucas, J.T.; Qaddoumi, I.; Gajjar, A.; Krasin, M.J.; Conklin, H.M.; Merchant, T.E. Association between Hippocampal Dose and Memory in Survivors of Childhood or Adolescent Low-Grade Glioma: A 10-Year Neurocognitive Longitudinal Study. Neuro-Oncology 2019, 21, 1175–1183. [Google Scholar] [CrossRef]
- Zureick, A.H.; Evans, C.L.; Niemierko, A.; Grieco, J.A.; Nichols, A.J.; Fullerton, B.C.; Hess, C.B.; Goebel, C.P.; Gallotto, S.L.; Weyman, E.A.; et al. Left Hippocampal Dosimetry Correlates with Visual and Verbal Memory Outcomes in Survivors of Pediatric Brain Tumors. Cancer 2018, 124, 2238–2245. [Google Scholar] [CrossRef] [Green Version]
- Goda, J.S.; Dutta, D.; Krishna, U.; Goswami, S.; Kothavade, V.; Kannan, S.; Maitre, M.; Bano, N.; Gupta, T.; Jalali, R. Hippocampal Radiotherapy Dose Constraints for Predicting Long-Term Neurocognitive Outcomes: Mature Data from a Prospective Trial in Young Patients with Brain Tumors. Neuro-Oncology 2020, 22, 1677–1685. [Google Scholar] [CrossRef]
- Greenberger, B.A.; Pulsifer, M.B.; Ebb, D.H.; MacDonald, S.M.; Jones, R.M.; Butler, W.E.; Huang, M.S.; Marcus, K.J.; Oberg, J.A.; Tarbell, N.J.; et al. Clinical Outcomes and Late Endocrine, Neurocognitive, and Visual Profiles of Proton Radiation for Pediatric Low-Grade Gliomas. Int. J. Radiat. Oncol. Biol. Phys. 2014, 89, 1060–1068. [Google Scholar] [CrossRef]
- Redmond, K.J.; Mahone, E.M.; Terezakis, S.; Ishaq, O.; Ford, E.; McNutt, T.; Kleinberg, L.; Cohen, K.J.; Wharam, M.; Horska, A. Association between Radiation Dose to Neuronal Progenitor Cell Niches and Temporal Lobes and Performance on Neuropsychological Testing in Children: A Prospective Study. Neuro-Oncology 2013, 15, 360–369. [Google Scholar] [CrossRef] [Green Version]
- Gondi, V.; Hermann, B.P.; Mehta, M.P.; Tomé, W.A. Hippocampal Dosimetry Predicts Neurocognitive Function Impairment After Fractionated Stereotactic Radiotherapy for Benign or Low-Grade Adult Brain Tumors. Int. J. Radiat. Oncol. Biol. Phys. 2012, 83, e487–e493. [Google Scholar] [CrossRef] [Green Version]
- Ma, T.M.; Grimm, J.; McIntyre, R.; Anderson-Keightly, H.; Kleinberg, L.R.; Hales, R.K.; Moore, J.; Vannorsdall, T.; Redmond, K.J. A Prospective Evaluation of Hippocampal Radiation Dose Volume Effects and Memory Deficits Following Cranial Irradiation. Radiother. Oncol. 2017, 125, 234–240. [Google Scholar] [CrossRef]
- Okoukoni, C.; McTyre, E.R.; Ayala Peacock, D.N.; Peiffer, A.M.; Strowd, R.; Cramer, C.; Hinson, W.H.; Rapp, S.; Metheny-Barlow, L.; Shaw, E.G.; et al. Hippocampal Dose Volume Histogram Predicts Hopkins Verbal Learning Test Scores after Brain Irradiation. Adv. Radiat. Oncol. 2017, 2, 624–629. [Google Scholar] [CrossRef] [Green Version]
- Brown, P.D.; Gondi, V.; Pugh, S.; Tome, W.A.; Wefel, J.S.; Armstrong, T.S.; Bovi, J.A.; Robinson, C.; Konski, A.; Khuntia, D.; et al. Hippocampal Avoidance During Whole-Brain Radiotherapy Plus Memantine for Patients with Brain Metastases: Phase III Trial NRG Oncology CC001. J. Clin. Oncol. 2020, 38, 1019–1029. [Google Scholar] [CrossRef]
- Gondi, V.; Pugh, S.L.; Tome, W.A.; Caine, C.; Corn, B.; Kanner, A.; Rowley, H.; Kundapur, V.; DeNittis, A.; Greenspoon, J.N.; et al. Preservation of Memory with Conformal Avoidance of the Hippocampal Neural Stem-Cell Compartment During Whole-Brain Radiotherapy for Brain Metastases (RTOG 0933): A Phase II Multi-Institutional Trial. J. Clin. Oncol. 2014, 32, 3810–3816. [Google Scholar] [CrossRef]
- Barazzuol, L.; Ju, L.; Jeggo, P.A. A Coordinated DNA Damage Response Promotes Adult Quiescent Neural Stem Cell Activation. PLoS Biol. 2017, 15, e2001264. [Google Scholar] [CrossRef] [Green Version]
- Nait-Oumesmar, B.; Picard-Riéra, N.; Kerninon, C.; Baron-Van Evercooren, A. The Role of SVZ-Derived Neural Precursors in Demyelinating Diseases: From Animal Models to Multiple Sclerosis. J. Neurol. Sci. 2008, 265, 26–31. [Google Scholar] [CrossRef]
- Lindvall, O.; Kokaia, Z. Neurogenesis Following Stroke Affecting the Adult Brain. Cold Spring Harb. Perspect. Biol. 2015, 7, a019034. [Google Scholar] [CrossRef] [Green Version]
- Capilla-Gonzalez, V.; Guerrero-Cazares, H.; Bonsu, J.M.; Gonzalez-Perez, O.; Achanta, P.; Wong, J.; Garcia-Verdugo, J.M.; Quiñones-Hinojosa, A. The Subventricular Zone Is Able to Respond to a Demyelinating Lesion After Localized Radiation. Stem Cells 2014, 32, 59–69. [Google Scholar] [CrossRef] [Green Version]
- van West, S.E.; de Bruin, H.G.; van de Langerijt, B.; Swaak-Kragten, A.T.; van den Bent, M.J.; Taal, W. Incidence of Pseudoprogression in Low-Grade Gliomas Treated with Radiotherapy. Neuro-Oncology 2016, 19, now194. [Google Scholar] [CrossRef] [Green Version]
- Bahn, E.; Bauer, J.; Harrabi, S.; Herfarth, K.; Debus, J.; Alber, M. Late Contrast Enhancing Brain Lesions in Proton-Treated Patients with Low-Grade Glioma: Clinical Evidence for Increased Periventricular Sensitivity and Variable RBE. Int. J. Radiat. Oncol. Biol. Phys. 2020, 107, 571–578. [Google Scholar] [CrossRef]
- Khatua, S.; Dhall, G.; O’Neil, S.; Jubran, R.; Villablanca, J.G.; Marachelian, A.; Nastia, A.; Lavey, R.; Olch, A.J.; Gonzalez, I.; et al. Treatment of Primary CNS Germinomatous Germ Cell Tumors with Chemotherapy Prior to Reduced Dose Whole Ventricular and Local Boost Irradiation. Pediatr. Blood Cancer 2010, 55, 42–46. [Google Scholar] [CrossRef]
- Peiffer, A.M.; Leyrer, C.M.; Greene-Schloesser, D.M.; Shing, E.; Kearns, W.T.; Hinson, W.H.; Tatter, S.B.; Ip, E.H.; Rapp, S.R.; Robbins, M.E.; et al. Neuroanatomical Target Theory as a Predictive Model for Radiation-Induced Cognitive Decline. Neurology 2013, 80, 747–753. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gui, C.; Vannorsdall, T.D.; Kleinberg, L.R.; Assadi, R.; Moore, J.A.; Hu, C.; Quiñones-Hinojosa, A.; Redmond, K.J. A Prospective Cohort Study of Neural Progenitor Cell-Sparing Radiation Therapy Plus Temozolomide for Newly Diagnosed Patients with Glioblastoma. Neurosurgery 2020, 87, E31–E40. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Lee, J.E.; Kahng, J.Y.; Kim, S.H.; Park, J.S.; Yoon, S.J.; Um, J.-Y.; Kim, W.K.; Lee, J.-K.; Park, J.; et al. Human Glioblastoma Arises from Subventricular Zone Cells with Low-Level Driver Mutations. Nature 2018, 560, 243–247. [Google Scholar] [CrossRef] [PubMed]
- Altmann, C.; Keller, S.; Schmidt, M.H.H. The Role of SVZ Stem Cells in Glioblastoma. Cancers 2019, 11, 448. [Google Scholar] [CrossRef] [Green Version]
- Nagtegaal, S.H.J.; David, S.; van der Boog, A.T.J.; Leemans, A.; Verhoeff, J.J.C. Changes in Cortical Thickness and Volume after Cranial Radiation Treatment: A Systematic Review. Radiother. Oncol. 2019, 135, 33–42. [Google Scholar] [CrossRef]
- Seibert, T.M.; Karunamuni, R.; Kaifi, S.; Burkeen, J.; Connor, M.; Krishnan, A.P.; White, N.S.; Farid, N.; Bartsch, H.; Murzin, V.; et al. Cerebral Cortex Regions Selectively Vulnerable to Radiation Dose-Dependent Atrophy. Int. J. Radiat. Oncol. Biol. Phys. 2017, 97, 910–918. [Google Scholar] [CrossRef] [Green Version]
- Nagtegaal, S.H.J.; David, S.; Snijders, T.J.; Philippens, M.E.P.; Leemans, A.; Verhoeff, J.J.C. Effect of Radiation Therapy on Cerebral Cortical Thickness in Glioma Patients: Treatment-Induced Thinning of the Healthy Cortex. Neuro-Oncol. Adv. 2020, 2, 1–8. [Google Scholar] [CrossRef]
- Merchant, T.E.; Kiehna, E.N.; Li, C.; Xiong, X.; Mulhern, R.K. Radiation Dosimetry Predicts IQ after Conformal Radiation Therapy in Pediatric Patients with Localized Ependymoma. Int. J. Radiat. Oncol. Biol. Phys. 2005, 63, 1546–1554. [Google Scholar] [CrossRef] [PubMed]
- Doger de Speville, E.; Robert, C.; Perez-Guevara, M.; Grigis, A.; Bolle, S.; Pinaud, C.; Dufour, C.; Beaudré, A.; Kieffer, V.; Longaud, A.; et al. Relationships between Regional Radiation Doses and Cognitive Decline in Children Treated with Cranio-Spinal Irradiation for Posterior Fossa Tumors. Front. Oncol. 2017, 7, 166. [Google Scholar] [CrossRef] [PubMed]
- Jalali, R.; Mallick, I.; Dutta, D.; Goswami, S.; Gupta, T.; Munshi, A.; Deshpande, D.; Sarin, R. Factors Influencing Neurocognitive Outcomes in Young Patients with Benign and Low-Grade Brain Tumors Treated with Stereotactic Conformal Radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2010, 77, 974–979. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, K.-Y.; Yeh, S.-A.; Chang, C.-C.; Tsai, P.-C.; Wu, J.-M.; Gau, J.-S. Cognitive Function Before and After Intensity-Modulated Radiation Therapy in Patients with Nasopharyngeal Carcinoma: A Prospective Study. Int. J. Radiat. Oncol. Biol. Phys. 2010, 77, 722–726. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, G.T.; Jain, N.; Liu, W.; Merchant, T.E.; Stovall, M.; Srivastava, D.K.; Gurney, J.G.; Packer, R.J.; Robison, L.L.; Krull, K.R. Region-Specific Radiotherapy and Neuropsychological Outcomes in Adult Survivors of Childhood CNS Malignancies. Neuro-Oncology 2010, 12, 1173–1186. [Google Scholar] [CrossRef] [PubMed]
- Panagiotakos, G.; Alshamy, G.; Chan, B.; Abrams, R.; Greenberg, E.; Saxena, A.; Bradbury, M.; Edgar, M.; Gutin, P.; Tabar, V. Long-Term Impact of Radiation on the Stem Cell and Oligodendrocyte Precursors in the Brain. PLoS ONE 2007, 2, e588. [Google Scholar] [CrossRef] [Green Version]
- Piao, J.; Major, T.; Auyeung, G.; Policarpio, E.; Menon, J.; Droms, L.; Gutin, P.; Uryu, K.; Tchieu, J.; Soulet, D.; et al. Human Embryonic Stem Cell-Derived Oligodendrocyte Progenitors Remyelinate the Brain and Rescue Behavioral Deficits Following Radiation. Cell Stem Cell 2015, 16, 198–210. [Google Scholar] [CrossRef] [Green Version]
- Connor, M.; Karunamuni, R.; McDonald, C.; White, N.; Pettersson, N.; Moiseenko, V.; Seibert, T.; Marshall, D.; Cervino, L.; Bartsch, H.; et al. Dose-Dependent White Matter Damage after Brain Radiotherapy. Radiother. Oncol. 2016, 121, 209–216. [Google Scholar] [CrossRef] [Green Version]
- Ajithkumar, T.; Price, S.; Horan, G.; Burke, A.; Jefferies, S. Prevention of Radiotherapy-Induced Neurocognitive Dysfunction in Survivors of Paediatric Brain Tumours: The Potential Role of Modern Imaging and Radiotherapy Techniques. Lancet Oncol. 2017, 18, e91–e100. [Google Scholar] [CrossRef] [Green Version]
- Jacola, L.M.; Ashford, J.M.; Reddick, W.E.; Glass, J.O.; Ogg, R.J.; Merchant, T.E.; Conklin, H.M. The Relationship between Working Memory and Cerebral White Matter Volume in Survivors of Childhood Brain Tumors Treated with Conformal Radiation Therapy. J. Neurooncol. 2014, 119, 197–205. [Google Scholar] [CrossRef]
- Reddick, W.E.; Taghipour, D.J.; Glass, J.O.; Ashford, J.; Xiong, X.; Wu, S.; Bonner, M.; Khan, R.B.; Conklin, H.M. Prognostic Factors That Increase the Risk for Reduced White Matter Volumes and Deficits in Attention and Learning for Survivors of Childhood Cancers. Pediatr. Blood Cancer 2014, 61, 1074–1079. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Partanen, M.; Bouffet, E.; Laughlin, S.; Strother, D.; Hukin, J.; Skocic, J.; Szulc-Lerch, K.; Mabbott, D.J. Early Changes in White Matter Predict Intellectual Outcome in Children Treated for Posterior Fossa Tumors. Neuroimage Clin. 2018, 20, 697–704. [Google Scholar] [CrossRef]
- Carey, M.E.; Haut, M.W.; Reminger, S.L.; Hutter, J.J.; Theilmann, R.; Kaemingk, K.L. Reduced Frontal White Matter Volume in Long-Term Childhood Leukemia Survivors: A Voxel-Based Morphometry Study. Am. J. Neuroradiol. 2008, 29, 792–797. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rueckriegel, S.M.; Bruhn, H.; Thomale, U.W.; Hernáiz Driever, P. Cerebral White Matter Fractional Anisotropy and Tract Volume as Measured by MR Imaging Are Associated with Impaired Cognitive and Motor Function in Pediatric Posterior Fossa Tumor Survivors. Pediatr. Blood Cancer 2015, 62, 1252–1258. [Google Scholar] [CrossRef] [PubMed]
- Mulhern, R.K.; Palmer, S.L.; Reddick, W.E.; Glass, J.O.; Kun, L.E.; Taylor, J.; Langston, J.; Gajjar, A. Risks of Young Age for Selected Neurocognitive Deficits in Medulloblastoma Are Associated with White Matter Loss. J. Clin. Oncol. 2001, 19, 472–479. [Google Scholar] [CrossRef] [PubMed]
- Edelmann, M.N.; Krull, K.R.; Liu, W.; Glass, J.O.; Ji, Q.; Ogg, R.J.; Sabin, N.D.; Srivastava, D.K.; Robison, L.L.; Hudson, M.M.; et al. Diffusion Tensor Imaging and Neurocognition in Survivors of Childhood Acute Lymphoblastic Leukaemia. Brain 2014, 137, 2973–2983. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bovi, J.A.; Pugh, S.L.; Sabsevitz, D.; Robinson, C.G.; Paulson, E.; Mehta, M.P.; Gondi, V.; Kundapur, V.; Shahin, M.S.; Chao, S.T.; et al. Pretreatment Volume of MRI-Determined White Matter Injury Predicts Neurocognitive Decline After Hippocampal Avoidant Whole-Brain Radiation Therapy for Brain Metastases: Secondary Analysis of NRG Oncology Radiation Therapy Oncology Group 0933. Adv. Radiat. Oncol. 2019, 4, 579–586. [Google Scholar] [CrossRef] [Green Version]
- Qiu, D.; Kwong, D.L.W.; Chan, G.C.F.; Leung, L.H.T.; Khong, P.-L. Diffusion Tensor Magnetic Resonance Imaging Finding of Discrepant Fractional Anisotropy between the Frontal and Parietal Lobes after Whole-Brain Irradiation in Childhood Medulloblastoma Survivors: Reflection of Regional White Matter Radiosensitivity? Int. J. Radiat. Oncol. Biol. Phys. 2007, 69, 846–851. [Google Scholar] [CrossRef]
- Schneider, J.F.L.; Il’yasov, K.A.; Hennig, J.; Martin, E. Fast Quantitative Diffusion-Tensor Imaging of Cerebral White Matter from the Neonatal Period to Adolescence. Neuroradiology 2004, 46, 258–266. [Google Scholar] [CrossRef] [Green Version]
- Redmond, K.J.; Hildreth, M.; Sair, H.I.; Terezakis, S.; McNutt, T.; Kleinberg, L.; Cohen, K.J.; Wharam, M.; Horska, A.; Mahone, E.M. Association of Neuronal Injury in the Genu and Body of Corpus Callosum After Cranial Irradiation in Children with Impaired Cognitive Control: A Prospective Study. Int. J. Radiat. Oncol. Biol. Phys. 2018, 101, 1234–1242. [Google Scholar] [CrossRef]
- Rashid, A.; Ram, A.N.; Kates, W.R.; Redmond, K.J.; Wharam, M.; Mark Mahone, E.; Horska, A.; Terezakis, S. A Prospective Study of Corpus Callosum Regional Volumes and Neurocognitive Outcomes Following Cranial Radiation for Pediatric Brain Tumors. Child’s Nerv. Syst. 2017, 33, 965–972. [Google Scholar] [CrossRef] [PubMed]
- Palmer, S.L.; Reddick, W.E.; Glass, J.O.; Gajjar, A.; Goloubeva, O.; Mulhern, R.K. Decline in Corpus Callosum Volume among Pediatric Patients with Medulloblastoma: Longitudinal MR Imaging Study. Am. J. Neuroradiol. 2002, 23, 1088–1094. [Google Scholar] [PubMed]
- Makola, M.; Douglas Ris, M.; Mahone, E.M.; Yeates, K.O.; Cecil, K.M. Long-Term Effects of Radiation Therapy on White Matter of the Corpus Callosum: A Diffusion Tensor Imaging Study in Children. Pediatr. Radiol. 2017, 47, 1809–1816. [Google Scholar] [CrossRef] [PubMed]
- Redmond, K. Neurocognitive Functioning with Genu-Sparing Whole Brain Radiation Therapy for Brain Metastases. Available online: https://clinicaltrials.gov/ct2/show/NCT03223922 (accessed on 30 November 2020).
- Beera, K.G.; Li, Y.-Q.; Dazai, J.; Stewart, J.; Egan, S.; Ahmed, M.; Wong, C.S.; Jaffray, D.A.; Nieman, B.J. Altered Brain Morphology after Focal Radiation Reveals Impact of Off-Target Effects: Implications for White Matter Development and Neurogenesis. Neuro-Oncology 2018, 20, 788–798. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cantelmi, D.; Schweizer, T.A.; Cusimano, M.D. Role of the Cerebellum in the Neurocognitive Sequelae of Treatment of Tumours of the Posterior Fossa: An Update. Lancet Oncol. 2008, 9, 569–576. [Google Scholar] [CrossRef]
- Sándor, N.; Walter, F.R.; Bocsik, A.; Sántha, P.; Schilling-Tóth, B.; Léner, V.; Varga, Z.; Kahán, Z.; Deli, M.A.; Sáfrány, G.; et al. Low Dose Cranial Irradiation-Induced Cerebrovascular Damage Is Reversible in Mice. PLoS ONE 2014, 9, e112397. [Google Scholar] [CrossRef] [Green Version]
- Zhou, K.; Boström, M.; Ek, C.J.; Li, T.; Xie, C.; Xu, Y.; Sun, Y.; Blomgren, K.; Zhu, C. Radiation Induces Progenitor Cell Death, Microglia Activation, and Blood-Brain Barrier Damage in the Juvenile Rat Cerebellum. Sci. Rep. 2017, 7, 46181. [Google Scholar] [CrossRef]
- Moore, D.M.; D’Mello, A.M.; McGrath, L.M.; Stoodley, C.J. The Developmental Relationship between Specific Cognitive Domains and Grey Matter in the Cerebellum. Dev. Cogn. Neurosci. 2017, 24, 1–11. [Google Scholar] [CrossRef]
- Merchant, T.E.; Sharma, S.; Xiong, X.; Wu, S.; Conklin, H. Effect of Cerebellum Radiation Dosimetry on Cognitive Outcomes in Children with Infratentorial Ependymoma. Int. J. Radiat. Oncol. Biol. Phys. 2014, 90, 547–553. [Google Scholar] [CrossRef] [Green Version]
- Crowne, E.; Gleeson, H.; Benghiat, H.; Sanghera, P.; Toogood, A. Effect of Cancer Treatment on Hypothalamic–Pituitary Function. Lancet Diabetes Endocrinol. 2015, 3, 568–576. [Google Scholar] [CrossRef]
- Rose, S.R.; Horne, V.E.; Howell, J.; Lawson, S.A.; Rutter, M.M.; Trotman, G.E.; Corathers, S.D. Late Endocrine Effects of Childhood Cancer. Nat. Rev. Endocrinol. 2016, 12, 319–336. [Google Scholar] [CrossRef] [PubMed]
- Schmiegelow, M.; Lassen, S.; Poulsen, H.S.; Feldt-Rasmussen, U.; Schmiegelow, K.; Hertz, H.; Müller, J. Growth Hormone Response to a Growth Hormone-Releasing Hormone Stimulation Test in a Population-Based Study Following Cranial Irradiation of Childhood Brain Tumors. Horm. Res. Paediatr. 2000, 54, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Darzy, K.H.; Pezzoli, S.S.; Thorner, M.O.; Shalet, S.M. The Dynamics of Growth Hormone (GH) Secretion in Adult Cancer Survivors with Severe GH Deficiency Acquired after Brain Irradiation in Childhood for Nonpituitary Brain Tumors: Evidence for Preserved Pulsatility and Diurnal Variation with Increased Secretor. J. Clin. Endocrinol. Metab. 2005, 90, 2794–2803. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kountouri, M.; Pica, A.; Walser, M.; Albertini, F.; Bolsi, A.; Kliebsch, U.; Bachtiary, B.; Combescure, C.; Lomax, A.J.; Schneider, R.; et al. Radiation-Induced Optic Neuropathy after Pencil Beam Scanning Proton Therapy for Skull-Base and Head and Neck Tumours. Br. J. Radiol. 2020, 93, 20190028. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, I.S.; Hoskin, P.J. Stereotactic Body Radiotherapy for Spinal and Bone Metastases. Clin. Oncol. 2015, 27, 298–306. [Google Scholar] [CrossRef]
- Van der Kogel, A.J. Late Effects of Radiation on the Spinal Cord; Dose-Effect Relationships and Pathogenesis. Ph.D. Thesis, University of Amsterdam, Amsterdam, The Netherlands, 1979. [Google Scholar]
- Bijl, H.P.; van Luijk, P.; Coppes, R.P.; Schippers, J.M.; Konings, A.W.T.; van der Kogel, A.J. Regional Differences in Radiosensitivity across the Rat Cervical Spinal Cord. Int. J. Radiat. Oncol. Biol. Phys. 2005, 61, 543–551. [Google Scholar] [CrossRef]
- Medin, P.M.; Foster, R.D.; van der Kogel, A.J.; Sayre, J.W.; McBride, W.H.; Solberg, T.D. Spinal Cord Tolerance to Single-Fraction Partial-Volume Irradiation: A Swine Model. Int. J. Radiat. Oncol. Biol. Phys. 2011, 79, 226–232. [Google Scholar] [CrossRef] [Green Version]
- Medin, P.M.; Foster, R.D.; van der Kogel, A.J.; Sayre, J.W.; McBride, W.H.; Solberg, T.D. Spinal Cord Tolerance to Single-Session Uniform Irradiation in Pigs: Implications for a Dose-Volume Effect. Radiother. Oncol. 2013, 106, 101–105. [Google Scholar] [CrossRef] [Green Version]
- Philippens, M.E.P.; Pop, L.A.M.; Visser, A.G.; van der Kogel, A.J. Dose-Volume Effects in Rat Thoracolumbar Spinal Cord: The Effects of Nonuniform Dose Distribution. Int. J. Radiat. Oncol. Biol. Phys. 2007, 69, 204–213. [Google Scholar] [CrossRef]
- Franklin, R.J.M.; Gilson, J.M.; Blakemore, W.F. Local Recruitment of Remyelinating Cells in the Repair of Demyelination in the Central Nervous System. J. Neurosci. Res. 1997, 50, 337–344. [Google Scholar] [CrossRef]
- van Luijk, P.; Bijl, H.P.; Konings, A.W.T.; van der Kogel, A.J.; Schippers, J.M. Data on Dose-Volume Effects in the Rat Spinal Cord Do Not Support Existing NTCP Models. Int. J. Radiat. Oncol. Biol. Phys. 2005, 61, 892–900. [Google Scholar] [CrossRef] [PubMed]
- Vissink, A.; Jansma, J.; Spijkervet, F.K.L.; Burlage, F.R.; Coppes, R.P. Oral Sequelae of Head and Neck Radiotherapy. Crit. Rev. Oral Biol. Med. 2003, 14, 199–212. [Google Scholar] [CrossRef] [PubMed]
- Langendijk, J.A.; Doornaert, P.; Verdonck-de Leeuw, I.M.; Leemans, C.R.; Aaronson, N.K.; Slotman, B.J. Impact of Late Treatment-Related Toxicity on Quality of Life among Patients with Head and Neck Cancer Treated with Radiotherapy. J. Clin. Oncol. 2008, 26, 3770–3776. [Google Scholar] [CrossRef] [PubMed]
- Sciubba, J.J.; Goldenberg, D. Oral Complications of Radiotherapy. Lancet Oncol. 2006, 7, 175–183. [Google Scholar] [CrossRef]
- Little, M.; Schipper, M.; Feng, F.Y.; Vineberg, K.; Cornwall, C.; Murdoch-Kinch, C.-A.; Eisbruch, A. Reducing Xerostomia After Chemo-IMRT for Head-and-Neck Cancer: Beyond Sparing the Parotid Glands. Int. J. Radiat. Oncol. Biol. Phys. 2012, 83, 1007–1014. [Google Scholar] [CrossRef] [Green Version]
- Eisbruch, A.; Kim, H.M.; Terrell, J.E.; Marsh, L.H.; Dawson, L.A.; Ship, J.A. Xerostomia and Its Predictors Following Parotid-Sparing Irradiation of Head-and-Neck Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2001, 50, 695–704. [Google Scholar] [CrossRef]
- Deasy, J.O.; Moiseenko, V.; Marks, L.; Chao, K.S.C.; Nam, J.; Eisbruch, A. Radiotherapy Dose–Volume Effects on Salivary Gland Function. Int. J. Radiat. Oncol. Biol. Phys. 2010, 76, S58–S63. [Google Scholar] [CrossRef] [Green Version]
- Nutting, C.M.; Morden, J.P.; Harrington, K.J.; Urbano, T.G.; Bhide, S.A.; Clark, C.; Miles, E.A.; Miah, A.B.; Newbold, K.; Tanay, M.A.; et al. Parotid-Sparing Intensity Modulated versus Conventional Radiotherapy in Head and Neck Cancer (PARSPORT): A Phase 3 Multicentre Randomised Controlled Trial. Lancet Oncol. 2011, 12, 127–136. [Google Scholar] [CrossRef] [Green Version]
- Martinez, J.R. Ion Transport and Water Movement. J. Dent. Res. 1987, 66, 638–647. [Google Scholar] [CrossRef]
- Konings, A.W.T.; Coppes, R.P.; Vissink, A. On the Mechanism of Salivary Gland Radiosensitivity. Int. J. Radiat. Oncol. Biol. Phys. 2005, 62, 1187–1194. [Google Scholar] [CrossRef]
- Coppes, R.P.; Meter, A.; Latumalea, S.P.; Roffel, A.F.; Kampinga, H.H. Defects in Muscarinic Receptor-Coupled Signal Transduction in Isolated Parotid Gland Cells after in Vivo Irradiation: Evidence for a Non-DNA Target of Radiation. Br. J. Cancer 2005, 92, 539–546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weng, P.L.; Aure, M.H.; Maruyama, T.; Ovitt, C.E. Limited Regeneration of Adult Salivary Glands after Severe Injury Involves Cellular Plasticity. Cell Rep. 2018, 24, 1464–1470.e3. [Google Scholar] [CrossRef] [Green Version]
- Aure, M.H.; Konieczny, S.F.; Ovitt, C.E. Salivary Gland Homeostasis Is Maintained through Acinar Cell Self-Duplication. Dev. Cell 2015, 33, 231–237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maimets, M.; Rocchi, C.; Bron, R.; Pringle, S.; Kuipers, J.; Giepmans, B.N.G.; Vries, R.G.J.; Clevers, H.; de Haan, G.; van Os, R.; et al. Long-Term in Vitro Expansion of Salivary Gland Stem Cells Driven by Wnt Signals. Stem Cell Rep. 2016, 6, 150–162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Konings, A.W.T.; Cotteleer, F.; Faber, H.; van Luijk, P.; Meertens, H.; Coppes, R.P. Volume Effects and Region-Dependent Radiosensitivity of the Parotid Gland. Int. J. Radiat. Oncol. Biol. Phys. 2005, 62, 1090–1095. [Google Scholar] [CrossRef] [PubMed]
- Konings, A.W.T.; Faber, H.; Cotteleer, F.; Vissink, A.; Coppes, R.P. Secondary Radiation Damage as the Main Cause for Unexpected Volume Effects: A Histopathologic Study of the Parotid Gland. Int. J. Radiat. Oncol. Biol. Phys. 2006, 64, 98–105. [Google Scholar] [CrossRef]
- van Luijk, P.; Pringle, S.; Deasy, J.O.; Moiseenko, V.V.; Faber, H.; Hovan, A.; Baanstra, M.; van der Laan, H.P.; Kierkels, R.G.J.; van der Schaaf, A.; et al. Sparing the Region of the Salivary Gland Containing Stem Cells Preserves Saliva Production after Radiotherapy for Head and Neck Cancer. Sci. Transl. Med. 2015, 7, 305ra147. [Google Scholar] [CrossRef] [Green Version]
- Buettner, F.; Miah, A.B.; Gulliford, S.L.; Hall, E.; Harrington, K.J.; Webb, S.; Partridge, M.; Nutting, C.M. Novel Approaches to Improve the Therapeutic Index of Head and Neck Radiotherapy: An Analysis of Data from the PARSPORT Randomised Phase III Trial. Radiother. Oncol. 2012, 103, 82–87. [Google Scholar] [CrossRef]
- Jiang, W.; Lakshminarayanan, P.; Hui, X.; Han, P.; Cheng, Z.; Bowers, M.; Shpitser, I.; Siddiqui, S.; Taylor, R.H.; Quon, H.; et al. Machine Learning Methods Uncover Radiomorphologic Dose Patterns in Salivary Glands That Predict Xerostomia in Patients with Head and Neck Cancer. Adv. Radiat. Oncol. 2019, 4, 401–412. [Google Scholar] [CrossRef] [Green Version]
- Miah, A.B.; Gulliford, S.L.; Morden, J.; Newbold, K.L.; Bhide, S.A.; Zaidi, S.H.; Hall, E.; Harrington, K.J.; Nutting, C.M. Recovery of Salivary Function: Contralateral Parotid-Sparing Intensity-Modulated Radiotherapy versus Bilateral Superficial Lobe Parotid-Sparing Intensity-Modulated Radiotherapy. Clin. Oncol. 2016, 28, e69–e76. [Google Scholar] [CrossRef] [Green Version]
- Steenbakkers, R.J.H.M. Parotid-Gland Stem-Cell Sparing Intensity-Modulated Radiotherapy (SCS-IMRT). Available online: https://clinicaltrials.gov/ct2/show/NCT01955239 (accessed on 30 November 2020).
- Sari, S.Y.; Yilmaz, M.T.; Elmali, A.; Yedekci, F.Y.; Yuce, D.; Ozyigit, G.; Cengiz, M.; Yazici, G. Parotid Gland Stem Cells: Mini yet Mighty. Head Neck 2020. [Google Scholar] [CrossRef]
- Robertson, S.P.; Quon, H.; Kiess, A.P.; Moore, J.A.; Yang, W.; Cheng, Z.; Afonso, S.; Allen, M.; Richardson, M.; Choflet, A.; et al. A Data-Mining Framework for Large Scale Analysis of Dose-Outcome Relationships in a Database of Irradiated Head and Neck Cancer Patients. Med. Phys. 2015, 42, 4329–4337. [Google Scholar] [CrossRef]
- van Luijk, P.; Faber, H.; Schippers, J.M.; Brandenburg, S.; Langendijk, J.A.; Meertens, H.; Coppes, R.P. Bath and Shower Effects in the Rat Parotid Gland Explain Increased Relative Risk of Parotid Gland Dysfunction After Intensity-Modulated Radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2009, 74, 1002–1005. [Google Scholar] [CrossRef] [PubMed]
- Nagle, P.W.; Hosper, N.A.; Barazzuol, L.; Jellema, A.L.; Baanstra, M.; van Goethem, M.J.; Brandenburg, S.; Giesen, U.; Langendijk, J.A.; van Luijk, P.; et al. Lack of DNA Damage Response at Low Radiation Doses in Adult Stem Cells Contributes to Organ Dysfunction. Clin. Cancer Res. 2018, 24, 6583–6593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Luijk, P.; Langendijk, J.A.; Coppes, R.P. Understanding Mechanisms Yields Novel Approaches to Reduce Radiotherapy-Related Xerostomia. Ann. Transl. Med. 2017, 5, 63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aps, J.K.M.; Martens, L.C. Review: The Physiology of Saliva and Transfer of Drugs into Saliva. Forensic Sci. Int. 2005, 150, 119–131. [Google Scholar] [CrossRef] [PubMed]
- Dawes, C.; Ong, B.Y. Circadian Rhythms in the Concentrations of Protein and the Main Electrolytes in Human Unstimulated Parotid Saliva. Arch. Oral Biol. 1973, 18, 1233–1242. [Google Scholar] [CrossRef]
- Dawes, C. Circadian Rhythms in the Flow Rate and Composition of Unstimulated and Stimulated Human Submandibular Saliva. J. Physiol. 1975, 244, 535–548. [Google Scholar] [CrossRef]
- Beetz, I.; Burlage, F.R.; Bijl, H.P.; Hoegen-Chouvalova, O.; Christianen, M.E.M.C.; Vissink, A.; van der Laan, B.F.A.M.; de Bock, G.H.; Langendijk, J.A. The Groningen Radiotherapy-Induced Xerostomia Questionnaire: Development and Validation of a New Questionnaire. Radiother. Oncol. 2010, 97, 127–131. [Google Scholar] [CrossRef]
- Dijkema, T.; Raaijmakers, C.P.J.; Braam, P.M.; Roesink, J.M.; Monninkhof, E.M.; Terhaard, C.H.J. Xerostomia: A Day and Night Difference. Radiother. Oncol. 2012, 104, 219–223. [Google Scholar] [CrossRef]
- Beetz, I.; Schilstra, C.; Visink, A.; van der Schaaf, A.; Bijl, H.P.; van der Laan, B.F.A.M.; Steenbakkers, R.J.H.M.; Langendijk, J.A. Role of Minor Salivary Glands in Developing Patient-Rated Xerostomia and Sticky Saliva during Day and Night. Radiother. Oncol. 2013, 109, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Marks, L.B.; Bentzen, S.M.; Deasy, J.O.; Kong, F.-M.; Bradley, J.D.; Vogelius, I.S.; El Naqa, I.; Hubbs, J.L.; Lebesque, J.V.; Timmerman, R.D.; et al. Radiation Dose Volume Effects in the Lung. Int. J. Radiat. Oncol. Biol. Phys. 2010, 76, 70–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Graham, M.V.; Purdy, J.A.; Emami, B.; Harms, W.; Bosch, W.; Lockett, M.A.; Perez, C.A. Clinical Dose-Volume Histogram Analysis for Pneumonitis after 3D Treatment for Non-Small Cell Lung Cancer (NSCLC). Int. J. Radiat. Oncol. Biol. Phys. 1999, 45, 323–329. [Google Scholar] [CrossRef]
- Yorke, E.D.; Jackson, A.; Rosenzweig, K.E.; Merrick, S.A.; Gabrys, D.; Venkatraman, E.S.; Burman, C.M.; Leibel, S.A.; Ling, C.C. Dose-Volume Factors Contributing to the Incidence of Radiation Pneumonitis in Non-Small-Cell Lung Cancer Patients Treated with Three-Dimensional Conformal Radiation Therapy. Int. J. Radiat. Oncol. Biol. Phys. 2002, 54, 329–339. [Google Scholar] [CrossRef]
- Liao, Z.X.; Travis, E.L.; Tucker, S.L. Damage and Morbidity from Pneumonitis after Irradiation of Partial Volumes of Mouse Lung. Int. J. Radiat. Oncol. Biol. Phys. 1995, 32, 1359–1370. [Google Scholar] [CrossRef]
- Travis, E.L.; Liao, Z.-X.; Tucker, S.L. Spatial Heterogeneity of the Volume Effect for Radiation Pneumonitis in Mouse Lung. Int. J. Radiat. Oncol. Biol. Phys. 1997, 38, 1045–1054. [Google Scholar] [CrossRef]
- Khan, M.A.; Hill, R.P.; van Dyk, J. Partial Volume Rat Lung Irradiation: An Evaluation of Early DNA Damage. Int. J. Radiat. Oncol. Biol. Phys. 1998, 40, 467–476. [Google Scholar] [CrossRef]
- Khan, M.A.; van Dykc, J.; Yeunge, I.W.T.; Hilla, R.P. Partial Volume Rat Lung Irradiation; Assessment of Early DNA Damage in Different Lung Regions and Effect of Radical Scavengers. Radiother. Oncol. 2003, 66, 95–102. [Google Scholar] [CrossRef]
- Boersma, L.; Theuws, J.; Kwa, S.; Damen, E.; JV, L. Regional Variation in Functional Subunit Density of the Lung: Regarding Liao et Al IJROBP 32(5):1359–1370; 1995. Int. J. Radiat. Oncol. Biol. Phys. 1995, 34, 1187–1188. [Google Scholar] [CrossRef]
- Novakova-Jiresova, A.; van Luijk, P.; Van Goor, H.; Kampinga, H.H.; Coppes, R.P. Pulmonary Radiation Injury: Identification of Risk Factors Associated with Regional Hypersensitivity. Cancer Res. 2005, 65, 3568–3576. [Google Scholar] [CrossRef] [Green Version]
- Ghobadi, G.; Bartelds, B.; van der Veen, S.J.; Dickinson, M.G.; Brandenburg, S.; Berger, R.M.F.; Langendijk, J.A.; Coppes, R.P.; van Luijk, P. Lung Irradiation Induces Pulmonary Vascular Remodelling Resembling Pulmonary Arterial Hypertension. Thorax 2012, 67, 334–341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coppes, R.P.; Muijs, C.T.; Faber, H.; Gross, S.; Schippers, J.M.; Brandenburg, S.; Langendijk, J.A.; van Luijk, P. Volume-Dependent Expression of In-Field and Out-of-Field Effects in the Proton-Irradiated Rat Lung. Int. J. Radiat. Oncol. Biol. Phys. 2011, 81, 262–269. [Google Scholar] [CrossRef] [PubMed]
- Kandathil, A.; Chamarthy, M. Pulmonary Vascular Anatomy & Anatomical Variants. Cardiovasc. Diagn. Ther. 2018, 8, 201–207. [Google Scholar] [CrossRef]
- Defraene, G.; van Elmpt, W.; Crijns, W.; de Ruysscher, D. Regional Variability in Radiation-Induced Lung Damage Can Be Predicted by Baseline CT Numbers. Radiother. Oncol. 2017, 122, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Stam, B.; Kwint, M.; Guckenberger, M.; Mantel, F.; Hope, A.; Giuliani, M.; Werner-Wasik, M.; Grills, I.; Sonke, J.-J.; Belderbos, J. Subgroup Survival Analysis in Stage I-II NSCLC Patients with a Central Tumor Partly Treated with Risk-Adapted SBRT. Int. J. Radiat. Oncol. Biol. Phys. 2019, 103, 132–141. [Google Scholar] [CrossRef]
- Schlaak, R.A.; Senthilkumar, G.; Boerma, M.; Bergom, C. Advances in Preclinical Research Models of Radiation-Induced Cardiac Toxicity. Cancers 2020, 12, 415. [Google Scholar] [CrossRef] [Green Version]
- Ghita, M.; Gill, E.K.; Walls, G.M.; Edgar, K.S.; McMahon, S.J.; Osorio, E.V.; Bergom, C.; Grieve, D.J.; Watson, C.J.; McWilliam, A.; et al. Cardiac Sub-Volume Targeting Demonstrates Regional Radiosensitivity in the Mouse Heart. Radiother. Oncol. 2020. [Google Scholar] [CrossRef]
- Stam, B.; Peulen, H.; Guckenberger, M.; Mantel, F.; Hope, A.; Werner-Wasik, M.; Belderbos, J.; Grills, I.; O’Connell, N.; Sonke, J.J. Dose to Heart Substructures Is Associated with Non-Cancer Death after SBRT in Stage I–II NSCLC Patients. Radiother. Oncol. 2017, 123, 370–375. [Google Scholar] [CrossRef]
- McWilliam, A.; Kennedy, J.; Hodgson, C.; Vasquez Osorio, E.; Faivre-Finn, C.; van Herk, M. Radiation Dose to Heart Base Linked with Poorer Survival in Lung Cancer Patients. Eur. J. Cancer 2017, 85, 106–113. [Google Scholar] [CrossRef]
- McWilliam, A.; Khalifa, J.; Vasquez Osorio, E.; Banfill, K.; Abravan, A.; Faivre-Finn, C.; van Herk, M. Novel Methodology to Investigate the Effect of Radiation Dose to Heart Substructures on Overall Survival. Int. J. Radiat. Oncol. Biol. Phys. 2020, 108, 1073–1081. [Google Scholar] [CrossRef]
- Hotca, A.; Thor, M.; Deasy, J.O.; Rimner, A. Dose to the Cardio-Pulmonary System and Treatment-Induced Electrocardiogram Abnormalities in Locally Advanced Non-Small Cell Lung Cancer. Clin. Transl. Radiat. Oncol. 2019, 19, 96–102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Luijk, P.; Novakova-Jiresova, A.; Faber, H.; Schippers, J.M.; Kampinga, H.H.; Meertens, H.; Coppes, R.P. Radiation Damage to the Heart Enhances Early Radiation-Induced Lung Function Loss. Cancer Res. 2005, 65, 6509–6511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghobadi, G.; van der Veen, S.; Bartelds, B.; de Boer, R.A.; Dickinson, M.G.; de Jong, J.R.; Faber, H.; Niemantsverdriet, M.; Brandenburg, S.; Berger, R.M.F.; et al. Physiological Interaction of Heart and Lung in Thoracic Irradiation. Int. J. Radiat. Oncol. Biol. Phys. 2012, 84, e639–e646. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tucker, S.L.; Liao, Z.; Dinh, J.; Bian, S.X.; Mohan, R.; Martel, M.K.; Grosshans, D.R. Is There an Impact of Heart Exposure on the Incidence of Radiation Pneumonitis? Analysis of Data from a Large Clinical Cohort. Acta Oncol. 2014, 53, 590–596. [Google Scholar] [CrossRef]
- Huang, E.X.; Hope, A.J.; Lindsay, P.E.; Trovo, M.; El Naqa, I.; Deasy, J.O.; Bradley, J.D. Heart Irradiation as a Risk Factor for Radiation Pneumonitis. Acta Oncol. 2011, 50, 51–60. [Google Scholar] [CrossRef] [Green Version]
- Teinturier, C.; Tournade, M.-F.; Caillat-Zucman, S.; Boitard, C.; Amoura, Z.; Bougneres, P.-F.; Timsit, J. Diabetes Mellitus after Abdominal Radiation Therapy. Lancet 1995, 346, 633–634. [Google Scholar] [CrossRef]
- de Vathaire, F.; El-Fayech, C.; Ben Ayed, F.F.; Haddy, N.; Guibout, C.; Winter, D.; Thomas-Teinturier, C.; Veres, C.; Jackson, A.; Pacquement, H.; et al. Radiation Dose to the Pancreas and Risk of Diabetes Mellitus in Childhood Cancer Survivors: A Retrospective Cohort Study. Lancet Oncol. 2012, 13, 1002–1010. [Google Scholar] [CrossRef]
- Friedman, D.N.; Moskowitz, C.S.; Hilden, P.; Howell, R.M.; Weathers, R.E.; Smith, S.A.; Wolden, S.L.; Tonorezos, E.S.; Mostoufi-Moab, S.; Chow, E.J.; et al. Radiation Dose and Volume to the Pancreas and Subsequent Risk of Diabetes Mellitus: A Report from the Childhood Cancer Survivor Study. JNCI J. Natl. Cancer Inst. 2020, 112, 525–532. [Google Scholar] [CrossRef]
- van Nimwegen, F.A.; Schaapveld, M.; Janus, C.P.M.; Krol, A.D.G.; Raemaekers, J.M.M.; Kremer, L.C.M.; Stovall, M.; Aleman, B.M.P.; van Leeuwen, F.E. Risk of Diabetes Mellitus in Long-Term Survivors of Hodgkin Lymphoma. J. Clin. Oncol. 2014, 32, 3257–3263. [Google Scholar] [CrossRef]
- Lobo, N.; Kulkarni, M.; Hughes, S.; Nair, R.; Khan, M.S.; Thurairaja, R. Urologic Complications Following Pelvic Radiotherapy. Urology 2018, 122, 1–9. [Google Scholar] [CrossRef]
- Zuppone, S.; Bresolin, A.; Spinelli, A.E.; Fallara, G.; Lucianò, R.; Scarfò, F.; Benigni, F.; Di Muzio, N.; Fiorino, C.; Briganti, A.; et al. Pre-Clinical Research on Bladder Toxicity After Radiotherapy for Pelvic Cancers: State-of-the Art and Challenges. Front. Oncol. 2020, 10, 527121. [Google Scholar] [CrossRef] [PubMed]
- Mylona, E.; Ebert, M.; Kennedy, A.; Joseph, D.; Denham, J.; Steigler, A.; Supiot, S.; Acosta, O.; de Crevoisier, R. Rectal and Urethro-Vesical Subregions for Toxicity Prediction After Prostate Cancer Radiation Therapy: Validation of Voxel-Based Models in an Independent Population. Int. J. Radiat. Oncol. Biol. Phys. 2020, 108, 1189–1195. [Google Scholar] [CrossRef] [PubMed]
- Viana, R.; Batourina, E.; Huang, H.; Dressler, G.R.; Kobayashi, A.; Behringer, R.R.; Shapiro, E.; Hensle, T.; Lambert, S.; Mendelsohn, C. The Development of the Bladder Trigone, the Center of the Anti-Reflux Mechanism. Development 2007, 134, 3763–3769. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Improta, I.; Palorini, F.; Cozzarini, C.; Rancati, T.; Avuzzi, B.; Franco, P.; Degli Esposti, C.; Del Mastro, E.; Girelli, G.; Iotti, C.; et al. Bladder Spatial-Dose Descriptors Correlate with Acute Urinary Toxicity after Radiation Therapy for Prostate Cancer. Phys. Med. 2016, 32, 1681–1689. [Google Scholar] [CrossRef]
- Heemsbergen, W.D.; Al-Mamgani, A.; Witte, M.G.; van Herk, M.; Pos, F.J.; Lebesque, J.V. Urinary Obstruction in Prostate Cancer Patients from the Dutch Trial (68 Gy vs. 78 Gy): Relationships with Local Dose, Acute Effects, and Baseline Characteristics. Int. J. Radiat. Oncol. Biol. Phys. 2010, 78, 19–25. [Google Scholar] [CrossRef]
- Ghadjar, P.; Zelefsky, M.J.; Spratt, D.E.; Munck af Rosenschöld, P.; Oh, J.H.; Hunt, M.; Kollmeier, M.; Happersett, L.; Yorke, E.; Deasy, J.O.; et al. Impact of Dose to the Bladder Trigone on Long-Term Urinary Function After High-Dose Intensity Modulated Radiation Therapy for Localized Prostate Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2014, 88, 339–344. [Google Scholar] [CrossRef] [Green Version]
- Schaake, W.; van der Schaaf, A.; van Dijk, L.V.; van den Bergh, A.C.M.; Langendijk, J.A. Development of a Prediction Model for Late Urinary Incontinence, Hematuria, Pain and Voiding Frequency among Irradiated Prostate Cancer Patients. PLoS ONE 2018, 13, e0197757. [Google Scholar] [CrossRef]
- Colaco, R.J.; Martin, P.; Kluger, H.M.; Yu, J.B.; Chiang, V.L. Does Immunotherapy Increase the Rate of Radiation Necrosis after Radiosurgical Treatment of Brain Metastases? J. Neurosurg. 2016, 125, 17–23. [Google Scholar] [CrossRef] [Green Version]
- Tran, T.T.; Jilaveanu, L.B.; Omuro, A.; Chiang, V.L.; Huttner, A.; Kluger, H.M. Complications Associated with Immunotherapy for Brain Metastases. Curr. Opin. Neurol. 2019, 32, 907–916. [Google Scholar] [CrossRef]
- Koper, P.C.M.; Heemsbergen, W.D.; Hoogeman, M.S.; Jansen, P.P.; Hart, G.A.M.; Wijnmaalen, A.J.; van Os, M.; Boersma, L.J.; Lebesque, J.V.; Levendag, P. Impact of Volume and Location of Irradiated Rectum Wall on Rectal Blood Loss after Radiotherapy of Prostate Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2004, 58, 1072–1082. [Google Scholar] [CrossRef]
- Maruyama, C.; Monroe, M.; Hunt, J.; Buchmann, L.; Baker, O. Comparing Human and Mouse Salivary Glands: A Practice Guide for Salivary Researchers. Oral Dis. 2019, 25, 403–415. [Google Scholar] [CrossRef] [PubMed]
- Jackson, I.L.; Vujaskovic, Z.; Down, J.D. A Further Comparison of Pathologies after Thoracic Irradiation among Different Mouse Strains: Finding the Best Preclinical Model for Evaluating Therapies Directed Against Radiation-Induced Lung Damage. Radiat. Res. 2011, 175, 510–518. [Google Scholar] [CrossRef] [PubMed]
- Groves, A.M.; Johnston, C.J.; Misra, R.S.; Williams, J.P.; Finkelstein, J.N. Whole-Lung Irradiation Results in Pulmonary Macrophage Alterations That Are Subpopulation and Strain Specific. Radiat. Res. 2015, 184, 639–649. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Widder, J. Evaluation of Radiation Induced Pulmonary Hypertension Using MRI in Stage III NSCLC Patients Treated with Chemoradiotherapy. A Pilot Study (MRI-HART). Available online: https://clinicaltrials.gov/ct2/show/NCT02377934 (accessed on 30 November 2020).
- Qiu, Y.; Guo, Z.; Han, L.; Yang, Y.; Li, J.; Liu, S.; Lv, X. Network-Level Dysconnectivity in Patients with Nasopharyngeal Carcinoma (NPC) Early Post-Radiotherapy: Longitudinal Resting State FMRI Study. Brain Imaging Behav. 2018, 12, 1279–1289. [Google Scholar] [CrossRef] [PubMed]
- Suckert, T.; Müller, J.; Beyreuther, E.; Azadegan, B.; Brüggemann, A.; Bütof, R.; Dietrich, A.; Gotz, M.; Haase, R.; Schürer, M.; et al. High-Precision Image-Guided Proton Irradiation of Mouse Brain Sub-Volumes. Radiother. Oncol. 2020, 146, 205–212. [Google Scholar] [CrossRef]
- Andrianova, N.V.; Buyan, M.I.; Zorova, L.D.; Pevzner, I.B.; Popkov, V.A.; Babenko, V.A.; Silachev, D.N.; Plotnikov, E.Y.; Zorov, D.B. Kidney Cells Regeneration: Dedifferentiation of Tubular Epithelium, Resident Stem Cells and Possible Niches for Renal Progenitors. Int. J. Mol. Sci. 2019, 20, 6326. [Google Scholar] [CrossRef] [Green Version]
- Dawson, L.A.; Kavanagh, B.D.; Paulino, A.C.; Das, S.K.; Miften, M.; Li, X.A.; Pan, C.; Ten Haken, R.K.; Schultheiss, T.E. Radiation-Associated Kidney Injury. Int. J. Radiat. Oncol. Biol. Phys. 2010, 76, S108–S115. [Google Scholar] [CrossRef]
- Lopez-Gaitan, J.; Ebert, M.A.; Robins, P.; Boucek, J.; Leong, T.; Willis, D.; Bydder, S.; Podias, P.; Waters, G.; O’Mara, B.; et al. Radiotherapy of Abdomen with Precise Renal Assessment with SPECT/CT Imaging (RAPRASI): Design and Methodology of a Prospective Trial to Improve the Understanding of Kidney Radiation Dose Response. BMC Cancer 2013, 13, 381. [Google Scholar] [CrossRef] [Green Version]
- Hawkins, M.M.; Smith, R.A. Pregnancy Outcomes in Childhood Cancer Survivors: Probable Effects of Abdominal Irradiation. Int. J. Cancer 1989, 43, 399–402. [Google Scholar] [CrossRef]
- Signorello, L.B.; Mulvihill, J.J.; Green, D.M.; Munro, H.M.; Stovall, M.; Weathers, R.E.; Mertens, A.C.; Whitton, J.A.; Robison, L.L.; Boice, J.D. Stillbirth and Neonatal Death in Relation to Radiation Exposure before Conception: A Retrospective Cohort Study. Lancet 2010, 376, 624–630. [Google Scholar] [CrossRef] [Green Version]
- Sudour, H.; Chastagner, P.; Claude, L.; Desandes, E.; Klein, M.; Carrie, C.; Bernier, V. Fertility and Pregnancy Outcome After Abdominal Irradiation That Included or Excluded the Pelvis in Childhood Tumor Survivors. Int. J. Radiat. Oncol. Biol. Phys. 2010, 76, 867–873. [Google Scholar] [CrossRef] [PubMed]
- Jadon, R.; Higgins, E.; Hanna, L.; Evans, M.; Coles, B.; Staffurth, J. A Systematic Review of Dose-Volume Predictors and Constraints for Late Bowel Toxicity Following Pelvic Radiotherapy. Radiat. Oncol. 2019, 14, 57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maeda, Y.; Høyer, M.; Lundby, L.; Norton, C. Faecal Incontinence Following Radiotherapy for Prostate Cancer: A Systematic Review. Radiother. Oncol. 2011, 98, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.; Facer, P.; Davis, J.; Smith, G.; Egerton, J.; Bountra, C.; Williams, N.; Anand, P. Sensory Fibres Expressing Capsaicin Receptor TRPV1 in Patients with Rectal Hypersensitivity and Faecal Urgency. Lancet 2003, 361, 385–391. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Voshart, D.C.; Wiedemann, J.; van Luijk, P.; Barazzuol, L. Regional Responses in Radiation-Induced Normal Tissue Damage. Cancers 2021, 13, 367. https://doi.org/10.3390/cancers13030367
Voshart DC, Wiedemann J, van Luijk P, Barazzuol L. Regional Responses in Radiation-Induced Normal Tissue Damage. Cancers. 2021; 13(3):367. https://doi.org/10.3390/cancers13030367
Chicago/Turabian StyleVoshart, Daniëlle C., Julia Wiedemann, Peter van Luijk, and Lara Barazzuol. 2021. "Regional Responses in Radiation-Induced Normal Tissue Damage" Cancers 13, no. 3: 367. https://doi.org/10.3390/cancers13030367
APA StyleVoshart, D. C., Wiedemann, J., van Luijk, P., & Barazzuol, L. (2021). Regional Responses in Radiation-Induced Normal Tissue Damage. Cancers, 13(3), 367. https://doi.org/10.3390/cancers13030367