Tumor Treating Fields Concomitant with Sorafenib in Advanced Hepatocellular Cancer: Results of the HEPANOVA Phase II Study
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Ethics Oversight
2.2. Patients
2.3. Treatments
2.4. Assessments and Outcomes
2.5. Statistical Analysis
3. Results
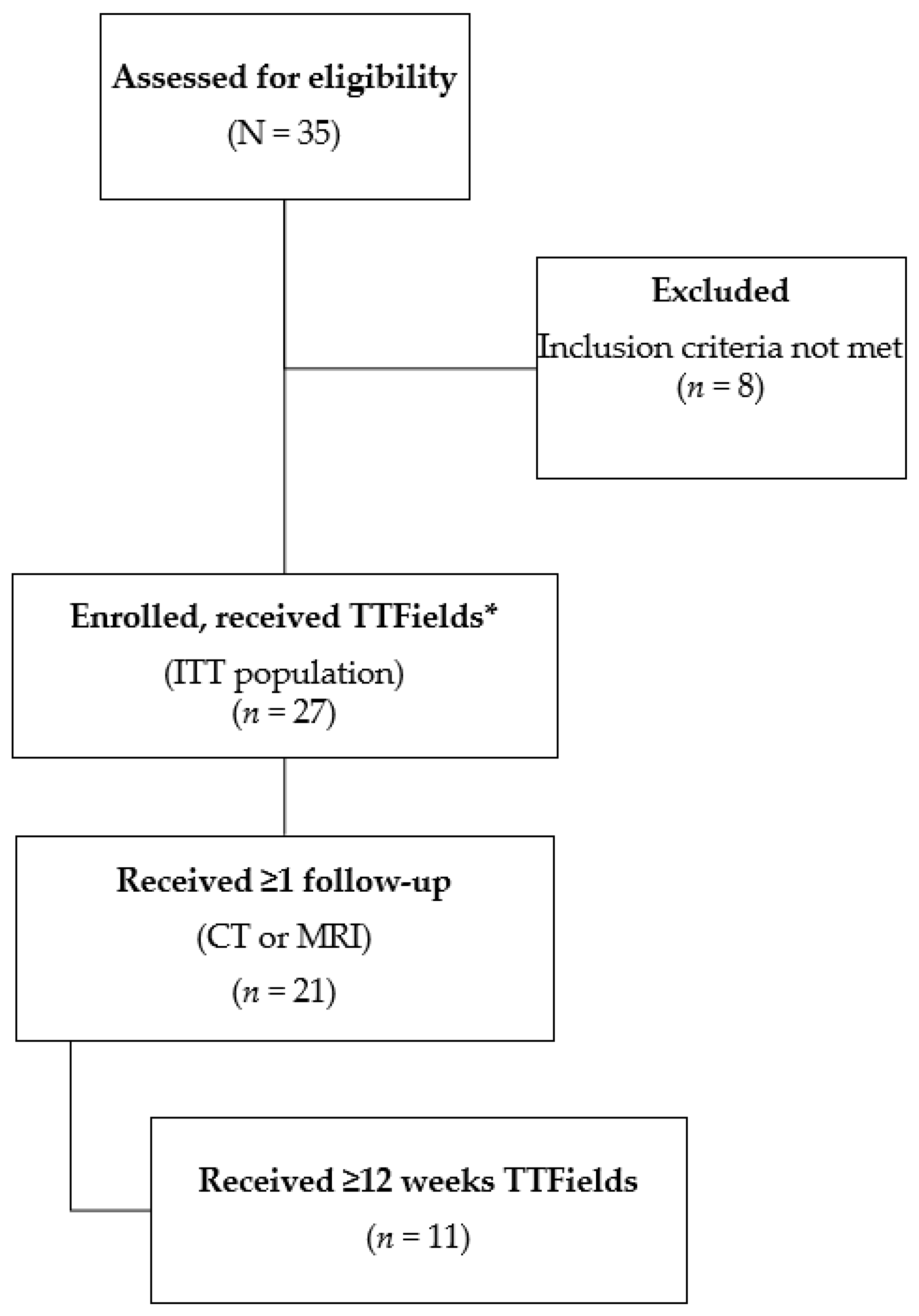
3.1. Patients
3.2. Treatments
3.3. Efficacy
3.4. Safety
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Llovet, J.M.; Kelley, R.K.; Villanueva, A.; Singal, A.G.; Pikarsky, E.; Roayaie, S.; Lencioni, R.; Koike, K.; Zucman-Rossi, J.; Finn, R.S. Hepatocellular carcinoma. Nat. Rev. Dis. Primers 2021, 7, 6. [Google Scholar] [CrossRef] [PubMed]
- Vogel, A.; Martinelli, E.; Vogel, A.; Cervantes, A.; Chau, I.; Daniele, B.; Llovet, J.M.; Meyer, T.; Nault, J.C.; Neumann, U.; et al. Updated treatment recommendations for hepatocellular carcinoma (HCC) from the ESMO Clinical Practice Guidelines. Ann. Oncol. 2021, 32, 801–805. [Google Scholar] [CrossRef]
- Kim, H.C. Radioembolization for the treatment of hepatocellular carcinoma. Clin. Mol. Hepatol. 2017, 23, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Finn, R.S.; Qin, S.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.Y.; Kudo, M.; Breder, V.; Merle, P.; Kaseb, A.O.; et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N. Engl. J. Med. 2020, 382, 1894–1905. [Google Scholar] [CrossRef] [PubMed]
- Kudo, M.; Finn, R.S.; Qin, S.; Han, K.H.; Ikeda, K.; Piscaglia, F.; Baron, A.; Park, J.W.; Han, G.; Jassem, J.; et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: A randomised phase 3 non-inferiority trial. Lancet 2018, 391, 1163–1173. [Google Scholar] [CrossRef]
- Llovet, J.M.; Ricci, S.; Mazzaferro, V.; Hilgard, P.; Gane, E.; Blanc, J.-F.; de Oliveira, A.C.; Santoro, A.; Raoul, J.-L.; Forner, A.; et al. Sorafenib in advanced hepatocellular carcinoma. N. Engl. J. Med. 2008, 359, 378–390. [Google Scholar] [CrossRef]
- Cheng, A.L.; Kang, Y.K.; Chen, Z.; Tsao, C.J.; Qin, S.; Kim, J.S.; Luo, R.; Feng, J.; Ye, S.; Yang, T.S.; et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: A phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009, 10, 25–34. [Google Scholar] [CrossRef]
- Ren, Z.; Ma, X.; Duan, Z.; Chen, X. Diagnosis, therapy, and prognosis for hepatocellular carcinoma. Anal. Cell. Pathol. 2020, 2020, 8157406. [Google Scholar] [CrossRef]
- Kirson, E.D.; Dbalý, V.; Tovaryš, F.; Vymazal, J.; Soustiel, J.F.; Itzhaki, A.; Mordechovich, D.; Steinberg-Shapira, S.; Gurvich, Z.; Schneiderman, R.; et al. Alternating electric fields arrest cell proliferation in animal tumor models and human brain tumors. Proc. Natl. Acad. Sci. USA 2007, 104, 10152–10157. [Google Scholar] [CrossRef]
- Kirson, E.D.; Gurvich, Z.; Schneiderman, R.; Dekel, E.; Itzhaki, A.; Wasserman, Y.; Schatzberger, R.; Palti, Y. Disruption of cancer cell replication by alternating electric fields. Cancer Res. 2004, 64, 3288–3295. [Google Scholar] [CrossRef]
- Giladi, M.; Schneiderman, R.S.; Voloshin, T.; Porat, Y.; Munster, M.; Blat, R.; Sherbo, S.; Bomzon, Z.; Urman, N.; Itzhaki, A.; et al. Mitotic spindle disruption by alternating electric fields leads to improper chromosome segregation and mitotic catastrophe in cancer cells. Sci. Rep. 2015, 5, 18046. [Google Scholar] [CrossRef] [PubMed]
- Rominiyi, O.; Vanderlinden, A.; Clenton, S.J.; Bridgewater, C.; Al-Tamimi, Y.; Collis, S.J. Tumour treating fields therapy for glioblastoma: Current advances and future directions. Br. J. Cancer 2021, 124, 697–709. [Google Scholar] [CrossRef] [PubMed]
- Chang, E.; Patel, C.B.; Pohling, C.; Young, C.; Song, J.; Flores, T.A.; Zeng, Y.; Joubert, L.-M.; Arami, H.; Natarajan, A.; et al. Tumor treating fields increases membrane permeability in glioblastoma cells. Cell Death Discov. 2018, 4, 113. [Google Scholar] [CrossRef] [PubMed]
- Voloshin, T.; Kaynan, N.; Davidi, S.; Porat, Y.; Shteingauz, A.; Schneiderman, R.S.; Zeevi, E.; Munster, M.; Blat, R.; Tempel Brami, C.; et al. Tumor-treating fields (TTFields) induce immunogenic cell death resulting in enhanced antitumor efficacy when combined with anti-PD-1 therapy. Cancer Immunol. Immunother. 2020, 69, 1191–1204. [Google Scholar] [CrossRef] [PubMed]
- Voloshin, T.; Schneiderman, R.S.; Volodin, A.; Shamir, R.R.; Kaynan, N.; Zeevi, E.; Koren, L.; Klein-Goldberg, A.; Paz, R.; Giladi, M.; et al. Tumor Treating Fields (TTFields) hinder cancer cell motility through regulation of microtubule and acting dynamics. Cancers 2020, 12, 3016. [Google Scholar] [CrossRef]
- Shteingauz, A.; Porat, Y.; Voloshin, T.; Schneiderman, R.S.; Munster, M.; Zeevi, E.; Kaynan, N.; Gotlib, K.; Giladi, M.; Kirson, E.D.; et al. AMPK-dependent autophagy upregulation serves as a survival mechanism in response to Tumor Treating Fields (TTFields). Cell Death Dis. 2018, 9, 1074. [Google Scholar] [CrossRef]
- Giladi, M.; Munster, M.; Schneiderman, R.S.; Voloshin, T.; Porat, Y.; Blat, R.; Zielinska-Chomej, K.; Haag, P.; Bomzon, Z.; Kirson, E.D.; et al. Tumor treating fields (TTFields) delay DNA damage repair following radiation treatment of glioma cells. Radiat. Oncol. 2017, 12, 206. [Google Scholar] [CrossRef]
- Health Technology Assessment Program. Tumor Treating Fields, (Optune®)—Final Evidence Report. 2018; Washington State Health Care Authority. Available online: https://www.hca.wa.gov/assets/program/tumor-treating-fields-final-report-20181016.pdf (accessed on 31 January 2022).
- Centanni, M.; Moes, D.; Troconiz, I.F.; Ciccolini, J.; van Hasselt, J.G.C. Clinical Pharmacokinetics and Pharmacodynamics of Immune Checkpoint Inhibitors. Clin. Pharm. 2019, 58, 835–857. [Google Scholar] [CrossRef]
- Di Francia, R.; De Monaco, A.; Saggese, M.; Iaccarino, G.; Crisci, S.; Frigeri, F.; De Filippi, R.; Berretta, M.; Pinto, A. Pharmacological Profile and Pharmacogenomics of Anti-Cancer Drugs Used for Targeted Therapy. Curr. Cancer Drug. Targets 2018, 18, 499–511. [Google Scholar] [CrossRef]
- Masson, E.; Zamboni, W.C. Pharmacokinetic optimisation of cancer chemotherapy. Effect on outcomes. Clin. Pharm. 1997, 32, 324–343. [Google Scholar] [CrossRef]
- Ceresoli, G.L.; Aerts, J.G.; Dziadziuszko, R.; Ramlau, R.; Cedres, S.; van Meerbeeck, J.P.; Mencoboni, M.; Planchard, D.; Chella, A.; Crino, L.; et al. Tumour Treating Fields in combination with pemetrexed and cisplatin or carboplatin as first-line treatment for unresectable malignant pleural mesothelioma (STELLAR): A multicentre, single-arm phase 2 trial. Lancet Oncol. 2019, 20, 1702–1709. [Google Scholar] [CrossRef]
- Stupp, R.; Taillibert, S.; Kanner, A.A.; Kesari, S.; Steinberg, D.M.; Toms, S.A.; Taylor, L.P.; Lieberman, F.; Silvani, A.; Fink, K.L.; et al. Maintenance therapy with tumor-treating fields plus temozolomide vs temozolomide alone for glioblastoma: A randomized clinical trial. JAMA 2015, 314, 2535–2543. [Google Scholar] [CrossRef] [PubMed]
- Novocure. Optune®: Instructions for Use for Unrescetable Malignant Pleural Mesothelioma. Available online: https://www.optunelua.com/pdfs/Optune-Lua-MPM-IFU.pdf?uh=18f20e383178129b5d6cd118075549592bae498860854e0293f947072990624c&administrationurl=https%3A%2F%2Foptunelua-admin.novocure.intouch-cit.com%2F (accessed on 14 September 2021).
- Novocure. Optune®: Instructions for Use. Available online: https://www.optune.com/Content/pdfs/Optune_IFU_8.5x11.pdf (accessed on 14 September 2021).
- Shi, W.; Blumenthal, D.T.; Oberheim Bush, N.A.; Kebir, S.; Lukas, R.V.; Muragaki, Y.; Zhu, J.J.; Glas, M. Global post-marketing safety surveillance of Tumor Treating Fields (TTFields) in patients with high-grade glioma in clinical practice. J. Neurooncol. 2020, 148, 489–500. [Google Scholar] [CrossRef] [PubMed]
- Stupp, R.; Taillibert, S.; Kanner, A.; Read, W.; Steinberg, D.; Lhermitte, B.; Toms, S.; Idbaih, A.; Ahluwalia, M.S.; Fink, K.; et al. Effect of Tumor-Treating Fields plus maintenance temozolomide vs maintenance temozolomide alone on survival in patients with glioblastoma: A randomized clinical trial. JAMA 2017, 318, 2306–2316. [Google Scholar] [CrossRef] [PubMed]
- Stupp, R.; Wong, E.T.; Kanner, A.A.; Steinberg, D.; Engelhard, H.; Heidecke, V.; Kirson, E.D.; Taillibert, S.; Liebermann, F.; Dbalý, V.; et al. NovoTTF-100A versus physician’s choice chemotherapy in recurrent glioblastoma: A randomised phase III trial of a novel treatment modality. Eur. J. Cancer 2012, 48, 2192–2202. [Google Scholar] [CrossRef] [PubMed]
- Mrugala, M.M.; Engelhard, H.H.; Dinh Tran, D.; Kew, Y.; Cavaliere, R.; Villano, J.L.; Annenelie Bota, D.; Rudnick, J.; Love Sumrall, A.; Zhu, J.-J.; et al. Clinical practice experience with NovoTTF-100A™ system for glioblastoma: The Patient Registry Dataset (PRiDe). Semin. Oncol. 2014, 41, S4–S13. [Google Scholar] [CrossRef]
- Rivera, F.; Benavides, M.; Gallego, J.; Guillen-Ponce, C.; Lopez-Martin, J.; Küng, M. Tumor treating fields in combination with gemcitabine or gemcitabine plus nab-paclitaxel in pancreatic cancer: Results of the PANOVA phase 2 study. Pancreatology 2019, 19, 64–72. [Google Scholar] [CrossRef]
- Benavides, M.; Guillen, C.; Rivera, F.; Gallego, J.; Lopez-Martin, J.A.; Küng, M. PANOVA: A phase II study of TTFields (150 kHz) concomitant with standard chemotherapy for front-line therapy of advanced pancreatic adenocarcinoma—Updated efficacy results. J. Clin. Oncol. 2017, 35, e15790. [Google Scholar] [CrossRef]
- Pless, M.; Droege, C.; von Moos, R.; Salzberg, M.; Betticher, D. A phase I/II trial of Tumor Treating Fields (TTFields) therapy in combination with pemetrexed for advanced non-small cell lung cancer. Lung Cancer 2013, 81, 445–450. [Google Scholar] [CrossRef]
- Vergote, I.; von Moos, R.; Manso, L.; Van Nieuwenhuysen, E.; Concin, N.; Sessa, C. Tumor Treating Fields in combination with paclitaxel in recurrent ovarian carcinoma: Results of the INNOVATE pilot study. Gynecol. Oncol. 2018, 150, 471–477. [Google Scholar] [CrossRef]
- Baas, P.; Scherpereel, A.; Nowak, A.K.; Fujimoto, N.; Peters, S.; Tsao, A.S.; Mansfield, A.S.; Popat, S.; Jahan, T.; Antonia, S.; et al. First-line nivolumab plus ipilimumab in unresectable malignant pleural mesothelioma (CheckMate 743): A multicentre, randomised, open-label, phase 3 trial. Lancet 2021, 397, 375–386. [Google Scholar] [CrossRef]
- Ceresoli, G.; Pless, M.; Benavides, M.; Vergote, I. P2.06-05 TTFields Applied to the Torso and Upper Abdomen: Safety Meta-Analysis of 176 Patients from four Phase I-II Trials. J. Thorac. Oncol. 2018, 13, S744. [Google Scholar] [CrossRef]
- Novocure. Optune®: Instructions for Use (EU). Available online: https://www.optune.de/wp-content/uploads/2020/11/Optune_User_Manual_ver2.0.pdf (accessed on 14 September 2021).
- Grosu, A.; Strouthos, I.; Brunner, T.B.; Weinberg, U. HEPANOVA: A phase II trial of tumor treating fields concomitant with sorafenib for advanced hepatocellular carcinoma. Cancer Res. 2018, 78, Abstract. [Google Scholar] [CrossRef]
- Davidi, S.; Brami, C.T.; Munster, M.; Gotlib, K.; Zeevi, E.; Schneiderman, R.S.; Voloshin, T.; Giladi, M.; Kinzel, A.; Kirson, E.; et al. Tumor Treating Fields (TTFields) Plus Sorafenib Is Safe and Effective in Hepatocellular Carcinoma Tested in Vitro and in an Animal Model. Int. J. Radiat. Oncol. Biol. Phys. 2019, 105, E675–E676. [Google Scholar] [CrossRef]
- Davidi, S.; Tempel-Brami, C.; Munster, M.; Gotlib, K.; Zeevi, E.; Schneiderman, R.S.; Voloshin, T.; Giladi, M.; Kinzel, A.; Kirson, E.D.; et al. In vitro and in vivo efficacy and safety of tumor treating fields (TTFields) and sorafenib combination in hepatocellular carcinoma. J. Clin. Oncol. 2020, 38, 551. [Google Scholar] [CrossRef]
- Blatt, R.; Davidi, S.; Munster, M.; Shteingauz, A.; Cahal, S.; Zeidan, A.; Marciano, T.; Bomzon, Z.; Haber, A.; Giladi, M.; et al. In Vivo Safety of Tumor Treating Fields (TTFields) Applied to the Torso. Front. Oncol. 2021, 11, 670809. [Google Scholar] [CrossRef]
- Weinberg, U.; Bomzon, Z.; Naveh, A.; Yesharim, O.; Faber, O.; Kirson, E. Computational simulations to determine the effectiveness and thermal safety of tumor treating fields with delivery to the abdomen. Ann. Oncol. 2019, 30, iv70–iv71. [Google Scholar] [CrossRef]
- Naveh, A.; Hershkovich, H.S.; Urman, N.; Bomzon, Z. Tumor Treating Fields therapy to the abdomen is unlikely to cause thermal tissue damage: Results of an extensive computational. Int. J. Radiat. Oncol. Biol. Phys. 2019, 105, S243. [Google Scholar] [CrossRef]
- Therasse, P.; Arbuck, S.G.; Eisenhauer, E.A.; Wanders, J.; Kaplan, R.S.; Rubinstein, L.; Verweij, J.; Van Glabbeke, M.; van Oosterom, A.T.; Christian, M.C.; et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J. Natl. Cancer Inst. 2000, 92, 205–216. [Google Scholar] [CrossRef]
- Abou-Alfa, G.K.; Schwartz, L.; Ricci, S.; Amadori, D.; Santoro, A.; Figer, A.; Greve, J.D.; Douillard, J.-Y.; Lathia, C.; Schwartz, B.; et al. Phase II study of sorafenib in patients with advanced hepatocellular carcinoma. J. Clin. Oncol. 2006, 24, 4293–4300. [Google Scholar] [CrossRef]
- Cainap, C.; Qin, S.; Huang, W.-T.; Chung, I.J.; Pan, H.; Cheng, Y.; Kudo, M.; Kang, Y.-K.; Chen, P.-J.; Toh, H.-C.; et al. Linifanib versus sorafenib in patients with advanced hepatocellular carcinoma: Results of a randomized phase III trial. J. Clin. Oncol. 2015, 33, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Zhu, A.X.; Rosmorduc, O.; Evans, T.R.J.; Ross, P.J.; Santoro, A.; Carrilho, F.J.; Bruix, J.; Qin, S.; Thuluvath, P.J.; Llovet, J.M.; et al. SEARCH: A phase III, randomized, double-blind, placebo-controlled trial of sorafenib plus erlotinib in patients with advanced hepatocellular carcinoma. J. Clin. Oncol. 2015, 33, 559–566. [Google Scholar] [CrossRef] [PubMed]
- Shingina, A.; Hashim, A.M.; Haque, M.; Suen, M.; Yoshida, E.M.; Gill, S.; Donnellan, F.; Weiss, A.A. In a ‘Real-World’, clinic-based community setting, sorafenib dose of 400 mg/day is as effective as standard dose of 800 mg/day in patients with advanced hepatocellular carcimona, with better tolerance and similar survival. Can. J. Gastroenterol. 2013, 27, 393–396. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nakano, M.; Tanaka, M.; Kuromatsu, R.; Nagamatsu, H.; Tajiri, N.; Satani, M.; Niizeki, T.; Aino, H.; Okamura, S.; Iwamoto, H.; et al. Sorafenib for the treatment of advanced hepatocellular carcinoma with extrahepatic metastasis: A prospective multicenter cohort study. Cancer Med. 2015, 4, 1836–1843. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Sung, P.S.; Yang, H.; Lee, S.K.; Nam, H.C.; Yoo, S.H.; Lee, H.L.; Kim, H.Y.; Lee, S.W.; Kwon, J.H.; et al. A real-world comparative analysis of lenvatinib and sorafenib as a salvage therapy for fransarterial treatments in unresectable HCC. J. Clin. Med. 2020, 9, 4121. [Google Scholar] [CrossRef] [PubMed]
- Toms, S.A.; Kim, C.Y.; Nicholas, G.; Ram, Z. Increased compliance with tumor treating fields therapy is prognostic for improved survival in the treatment of glioblastoma: A subgroup analysis of the EF-14 phase III trial. J. Neurooncol. 2019, 141, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Bayer HealthCare Pharmaceuticals Inc. NEXAVAR (Sorafenib) Tablets for Pral Use—Prescribing Information. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/021923s020lbl.pdf (accessed on 31 January 2022).
- Bouattour, M.; Mehta, N.; He, A.R.; Cohen, E.I.; Nault, J.C. Systemic treatment for advanced gepatocellular carcinoma. Liver Cancer 2019, 8, 341–358. [Google Scholar] [CrossRef]
- Lacouture, M.; Davis, M.E.; Elzinga, G.; Butowski, N.; Tran, D.; Villano, J.L.; DiMeglio, L.; Davies, A.M.; Wong, E.T. Characterization and management of dermatologic adverse events with the NovoTTF-100A System, a novel anti-mitotic electric field device for the treatment of recurrent glioblastoma. Semin. Oncol. 2014, 41, S1–S14. [Google Scholar] [CrossRef]
- Lacouture, M.; Anadkat, M.J.; Ballo, M.T.; Iwamoto, F.; Jeyapalan, S.A.; La Rocca, R.V.; Schwartz, M.; Serventi, J.N.; Glas, M. Prevention and management of dermatologic adverse events associated with Tumor Treating Fields in patients with glioblastoma. Front. Oncol. 2020, 10, 1045. [Google Scholar] [CrossRef]


| Characteristics | TTFields + Sorafenib (n = 27) |
|---|---|
| Age, years, median (range) | 65 (28–85) |
| Sex, n (%) | |
| Female | 1 (3.7) |
| Male | 26 (96.3) |
| ECOG performance status, n (%) | |
| 0 | 12 (44.4) |
| 1 | 9 (33.3) |
| 2 | 6 (22.2) |
| CTP score | |
| 5 | 9 (33.3) |
| 6 | 4 (14.8) |
| 7 | 10 (37.0) |
| 8 | 4 (14.8) |
| Number of prior treatments, median (range) | 1 (0–6) |
| BCLC stage, n (%) | |
| 0 | 1 (3.7) |
| B | 5 (18.5) |
| C | 21 (77.8) |
| Etiology, n (%) | |
| HBV | 2 (7.4) |
| HCV | 6 (22.2) |
| Alcoholic liver disease | 9 (33.3) |
| Non-alcoholic fatty liver | 3 (11.1) |
| Alcohol and dysmetabolism | 1 (3.7) |
| Alcoholic liver disease and non-alcoholic fatty liver | 1 (3.7) |
| NASH | 1 (3.7) |
| Cirrhosis | 1 (3.7) |
| Other | 1 (3.7) |
| Missing | 2 (7.4) |
| Extrahepatic spread, n (%) | 14 (51.9) |
| Time from diagnosis to enrollment, median (range) weeks | 25.6 (1.9–345.9) |
| Alpha-fetoprotein, median (range) ng/mL * | 80.6 (1.0–4.7 × 106) |
| Outcome | TTFields + Sorafenib (n = 21) | TTFields ≥12 Weeks Usage + Sorafenib (n = 11) | Historical Control † |
|---|---|---|---|
| Overall response rate, % | 9.5 | 18 | 4.5 (p = 0.24) * |
| Level of response rate, % | |||
| Complete | 0 | 0 | - |
| Partial | 9.5 | 18 | - |
| Stable disease | 66.5 | 73 | - |
| Disease control rate, % | 76 | 91 | - |
| In-field control rate at 1 year, % | 9.5 | 9.1 | – |
| Outcome | TTFields + Sorafenib (n = 27) | TTFields ≥12 Weeks + Sorafenib (n = 11) |
|---|---|---|
| OS rate at 1 year, % (95% CI) | 30 (11–52) | 64 (30–85) |
| PFS rate at 12 months, % (95% CI) | 23 (7–45) | 28 (5–58) |
| Distant metastases-free survival rate at 1 year, % (95% CI) | 26 (8–49) | 30.5 (5–62) |
| Median time to progression, months (95% CI) | 8.9 (3.1–not reached) | 8.9 (5.8–not reached) |
| Preferred Term, n (%) | TTFields + Sorafenib (n = 27) |
|---|---|
| Patients with any ≥1 AE | 26 (96) |
| Diarrhea | 15 (56) |
| Asthenia | 11 (41) |
| Decreased appetite | 8 (30) |
| Ascites | 6 (22) |
| Dermatitis | 5 (19) |
| Dyspnea | 5 (19) |
| Edema peripheral | 5 (19) |
| Alanine aminotransferase increased | 4 (15) |
| Palmar-plantar erythrodysesthesia syndrome | 4 (15) |
| Skin erosion | 4 (15) |
| Anemia | 3 (11) |
| Aspartate aminotransferase increased | 3 (11) |
| Constipation | 3 (11) |
| Dry mouth | 3 (11) |
| Hypertension | 3 (11) |
| Nausea | 3 (11) |
| Transaminases increased | 3 (11) |
| MedDRA Version 21.0 Preferred Term, n (%) | TTFields + Sorafenib (n = 27) |
|---|---|
| Severe (Grade 3–4) | |
| Patients with ≥1 any AE | 16 (59) |
| Decreased appetite | 3 (11) |
| Ascites | 2 (7) |
| Diarrhea | 2 (7) |
| Asthenia | 2 (7) |
| Edema peripheral | 2 (7) |
| Pathological fracture | 2 (7) |
| Dyspnea | 2 (7) |
| Palmar-plantar erythrodysesthesia syndrome | 2 (7) |
| Hypertension | 2 (7) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gkika, E.; Grosu, A.-L.; Macarulla Mercade, T.; Cubillo Gracián, A.; Brunner, T.B.; Schultheiß, M.; Pazgan-Simon, M.; Seufferlein, T.; Touchefeu, Y. Tumor Treating Fields Concomitant with Sorafenib in Advanced Hepatocellular Cancer: Results of the HEPANOVA Phase II Study. Cancers 2022, 14, 1568. https://doi.org/10.3390/cancers14061568
Gkika E, Grosu A-L, Macarulla Mercade T, Cubillo Gracián A, Brunner TB, Schultheiß M, Pazgan-Simon M, Seufferlein T, Touchefeu Y. Tumor Treating Fields Concomitant with Sorafenib in Advanced Hepatocellular Cancer: Results of the HEPANOVA Phase II Study. Cancers. 2022; 14(6):1568. https://doi.org/10.3390/cancers14061568
Chicago/Turabian StyleGkika, Eleni, Anca-Ligia Grosu, Teresa Macarulla Mercade, Antonio Cubillo Gracián, Thomas B. Brunner, Michael Schultheiß, Monika Pazgan-Simon, Thomas Seufferlein, and Yann Touchefeu. 2022. "Tumor Treating Fields Concomitant with Sorafenib in Advanced Hepatocellular Cancer: Results of the HEPANOVA Phase II Study" Cancers 14, no. 6: 1568. https://doi.org/10.3390/cancers14061568
APA StyleGkika, E., Grosu, A.-L., Macarulla Mercade, T., Cubillo Gracián, A., Brunner, T. B., Schultheiß, M., Pazgan-Simon, M., Seufferlein, T., & Touchefeu, Y. (2022). Tumor Treating Fields Concomitant with Sorafenib in Advanced Hepatocellular Cancer: Results of the HEPANOVA Phase II Study. Cancers, 14(6), 1568. https://doi.org/10.3390/cancers14061568






