The New Treatment Methods for Non-Hodgkin Lymphoma in Pediatric Patients
Abstract
:Simple Summary
Abstract
1. Introduction
2. New Approaches in Treatment
2.1. Monoclonal Antibodies (mAbs) Therapy
| mAbs name | Generation | Origin | Target | Antigen |
|---|---|---|---|---|
| rituximab | I | chimeric | B-cells | anti-CD20 |
| obinutzumab | II | humanized | ||
| ofatumumab | I | human | ||
| ublituximab | I | chimeric | ||
| tafasitamab | II | humanized | anti-CD19 | |
| inebilizumab | next generation | humanized | ||
| epratuzumab | next generation | humanized | anti-CD22 | |
| blinatumomab | next generation | mouse | T cells | anti-CD19 CD3 |
| mosunetuzumab | next generation | humanized | anti-CD20 CD3 | |
| glofitamab | next generation | humanized | ||
| odronextamab | next generation | humanized | ||
| epcoritamab | next generation | humanized |
2.2. Antibody–Drug Conjugates (ADCs)
| Antibody Type | Mechanism of Action | Approved Abs | Abs in Preclinical/Clinical Research |
|---|---|---|---|
| mAbs | activation of apoptosis binding of membrane receptors on the surface of the tumor cell causing inhibition of signal transduction pathway antibody-dependent cellular cytotoxicity complement-dependent cytotoxicities | rituximab (as single-agent therapy or conjugated with chemotherapy)—B-NHL, FL obinutuzumab + chemotherapy—FL | B43 + genistein—NHL epratuzumab galiximab tafasitamab—R/R NHL, FL, DLBCL, MCL MEDI-551—R/R FL, DLBCL |
| BiAbs | engaging the cells of the immune system to attack malignant cells by targeting the tumor-associated antigen binding to the CD3 antigen on T cells to induce T cell activation and proliferation in an MHC-independent manner | blinatumomab—R/R B-NHL | mosunetuzumab—DLBCL, FL, MCL odronextamab—DLBCL, FL, MCL epcoritamab—DLBCL, FL, MCL plamotamab—DLBCL glofitamab—DLBCL, FL, MCL |
| ADCs | preferential release of a potent cytotoxic agent at the tumor region, which is caused by proteases or alterations in pH bystander killing | Bv—R/R ALCL polatuzumab vedotin-piiq—DLBCL loncastuximab tesirine-lpyl—DLBCL | Bv—DLBCL Bv + chemotherapy—ALK+ ALCL IO JBH492 Pinatuzumab vedotin—R/R DLBCL, FL Vorsetuzumab mafodotin—R/R NHL Coltuximab Ravtansine (SAR3419) IMGN529 (CD37 ADC) |
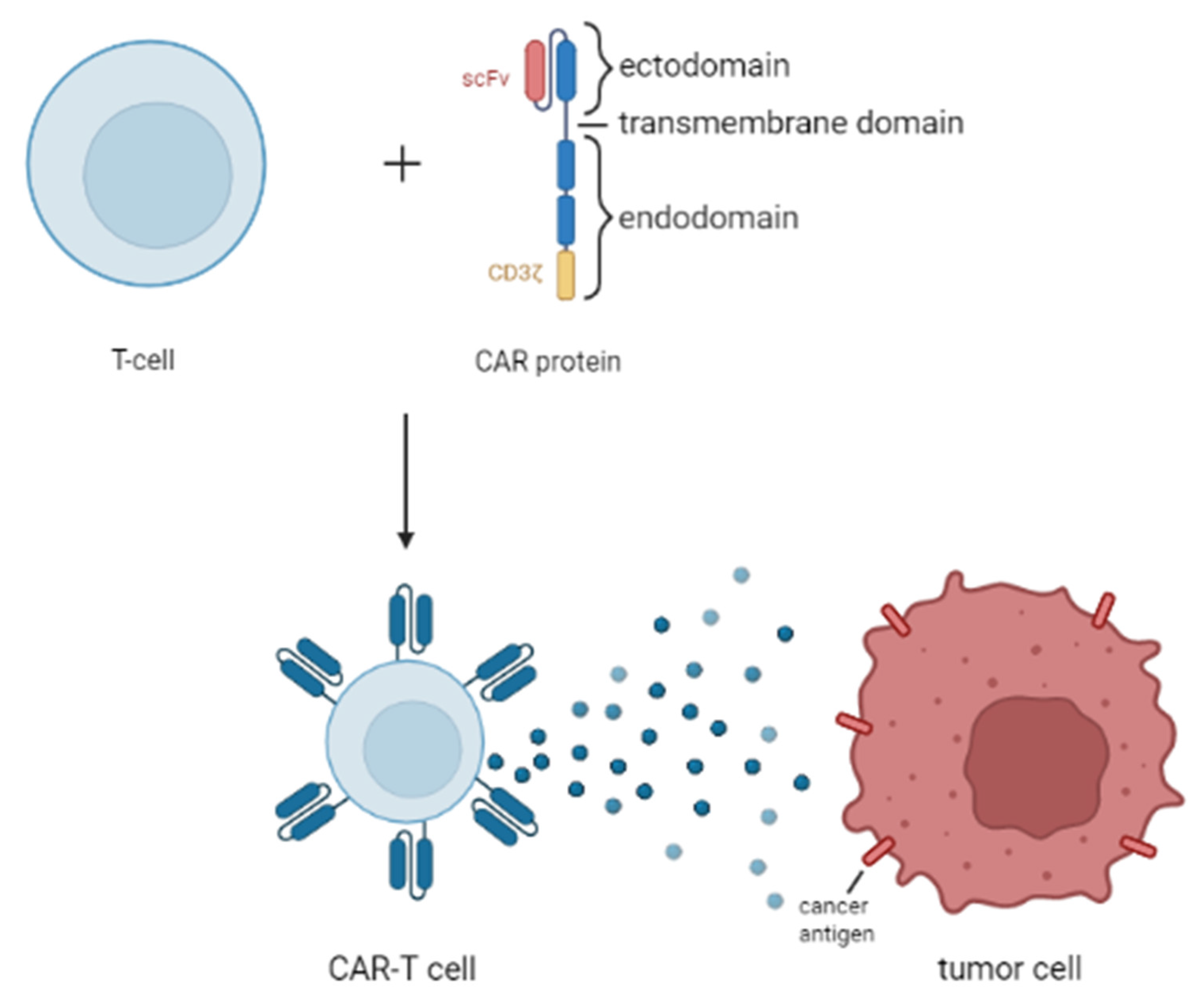
2.3. Chimeric Antigen Receptor T Cell (CAR-T Cell) Therapy
2.4. DNA Methyltransferase (DNMT) Inhibitors
2.5. Histone Deacetylase Inhibitors (HDACIs)
2.6. Immune Checkpoint Inhibitors (ICIs)
2.7. Enhancer of Zeste Homolog 2 (EZH2) Inhibitors
2.8. Isocitrate Dehydrogenase (IDH) Inhibitors
2.9. The B2 Cell Lymphoma Protein (BCL-2) Inhibitors
2.10. Anaplastic Lymphoma Kinase (ALK) Inhibitors
2.11. Ibrutinib/Bruton’s Tyrosine Kinase (BTK) Inhibitor
2.12. Bortezomib, Inhibitor of Proteasome
2.13. Temsirolimus
3. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| Abbreviations | Definitions |
| NHL | Non-Hodgkin lymphoma |
| DLBCL | Diffuse large B-cell lymphoma |
| BL | Burkitt lymphoma |
| PMBCL | Primary mediastinal B-cell lymphoma |
| FL | Follicular lymphoma |
| MZL | Marginal zone lymphoma |
| ALK+ | ALK-positive |
| ALCL | Anaplastic large cell lymphoma |
| LBL-T | T cell lymphoblastic lymphoma |
| LBL-B | B-cell lymphoblastic lymphoma |
| ID3 | Inhibitor of DNA binding 3 |
| BLL | Burkitt-like lymphoma |
| TCF3 | Transcription Factor 3 |
| ALK | Anaplastic lymphoma kinase gene |
| NPM | Nucleophosmin gene |
| ABDTRG | Absence of biallelic deletion of the TRG locusT cell receptor gamma |
| CNS | Central nervous system |
| RT | Radiotherapy |
| B-NHL | B-cell non-Hodgkin lymphoma |
| FDA | Food and Drug Administration |
| mAbs | Monoclonal antibodies |
| ICIs | Immune checkpoint inhibitors |
| ADCs | Antibody–drug conjugates |
| CAR | Chimeric antigen receptor |
| HDAC | Histone deacetylase |
| DNMT | DNA methyltransferase |
| IDH | Isocitrate dehydrogenase |
| Allo-HSCT | Allogenic hematopoietic stem-cell transplantation |
| Auto-ASCT | Autologous stem cell transplantation |
| CHOP | Cyclophosphamide, doxorubicin, vincristine, prednisone |
| allo-SCT | Allogenic stem cell transplantation |
| HDC/ASCT | High-dose chemotherapy supported by autologous stem cell transplantation |
| Bv | Brentuximab vedotin |
| BiAbs | Bispecific antibodies |
| scFv | Single-chain variable fragments |
| OS | Overall survival |
| R/R | Relapsed or refractory |
| MMAE | Monomethyl auristatin E |
| COG | Children Oncology Group |
| EFS | Event free survival |
| CR | Complete Response |
| PR | Partial response |
| IO | Inotuzumab ozogamicin |
| BCMA | B-cell maturation antigen |
| MCL | Mantle cell lymphoma |
| MRD | Minimal residual disease |
| HDACIs | Histone deacetylase inhibitors |
| GVHD | Graft-versus-host disease |
| EZH2 | Enhancer of Zeste Homolog 2 |
| GC | Germinal center |
| αKG | α-ketoglutarate |
| D-2HG | D-2-hydroxyglutarate |
| AML | Acute myeloid leukemia |
| BCL-2 | B2 cell lymphoma protein |
| ORRs | Overall responses rates |
| LL | Lymphoblastic lymphoma |
| ALK | Anaplastic lymphoma kinase |
| BTK | Bruton’s tyrosine kinase |
| BCR | B-cell antigen receptor |
| RICE | Rituximab plus ifosfamide, carboplatin, and etoposide |
| CRu | Complete response unconfirmed |
| IVB | Bortezomib with ifosfamide/vinorelbine |
| BICE | Bortezomib, ifosfamide, carboplatin, and etoposide |
| OR | Overall response |
| mTOR | Mammalian targets of the rapamycin |
| PI3K | Protein-3-phosphoinositide kinase B |
| PFS | Progression-free survival |
| BeRT | Bendamustine + rituximab + temsirolimus |
| DNA | Deoxyribonucleic acid |
References
- Moubadder, L.; McCullough, L.E.; Flowers, C.R.; Koff, J.L. Linking Environmental Exposures to Molecular Pathogenesis in Non-Hodgkin Lymphoma Subtypes. Cancer Epidemiol. Biomark. Prev. 2020, 29, 1844–1855. [Google Scholar] [CrossRef] [PubMed]
- Harker-Murray, P.D.; Pommert, L.; Barth, M.J. Novel Therapies Potentially Available for Pediatric B-Cell Non-Hodgkin Lymphoma. J. Natl. Compr. Cancer Netw. 2020, 18, 1125–1134. [Google Scholar] [CrossRef] [PubMed]
- Tsuyama, N.; Sakamoto, K.; Sakata, S.; Dobashi, A.; Takeuchi, K. Anaplastic Large Cell Lymphoma: Pathology, Genetics, and Clinical Aspects. J. Clin. Exp. Hematop. 2017, 57, 120–142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reedijk, A.M.J.; Beishuizen, A.; Coebergh, J.W.; Hoeben, B.A.W.; Kremer, L.C.M.; Hebeda, K.M.; Pieters, R.; Loeffen, J.L.C.; Karim-Kos, H.E. Progress against Non-Hodgkin′s Lymphoma in Children and Young Adolescents in the Netherlands since 1990: Stable Incidence, Improved Survival and Lower Mortality. Eur. J. Cancer 2022, 163, 140–151. [Google Scholar] [CrossRef]
- Sandlund, J.T.; Martin, M.G. Non-Hodgkin lymphoma across the pediatric and adolescent and Young Adult Age Spectrum. Hematol. Am. Soc. Hematol. Educ. Progr. 2016, 2016, 589–597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burkhardt, B.; Oschlies, I.; Klapper, W.; Zimmermann, M.; Woessmann, W.; Meinhardt, A.; Landmann, E.; Attarbaschi, A.; Niggli, F.; Schrappe, M.; et al. Non-Hodgkin′s lymphoma in adolescents: Experiences in 378 adolescent NHL patients treated according to pediatric NHL-BFM Protocols. Leukemia 2010, 25, 153–160. [Google Scholar] [CrossRef]
- Chybicka, A.; Sawicz-Birkowska, K.; Kazanowska, B.; Adamkiewicz-Drożyńska, E.M. Onkologia I Hematologia Dziecięca; PZWL Wydawnictwo Lekarskie: Warszaw, Poland, 2021. [Google Scholar]
- Teachey, D.T.; O’Connor, D. How I treat newly diagnosed T-cell acute lymphoblastic leukemia and T-cell lymphoblastic lymphoma in children. Blood 2020, 135, 159–166. [Google Scholar] [CrossRef]
- Wei, J.; Liu, Y.; Wang, C.; Zhang, Y.; Tong, C.; Dai, G.; Wang, W.; Rasko, J.E.; Melenhorst, J.J.; Qian, W.; et al. The model of cytokine release syndrome in car T-cell treatment for B-cell non-Hodgkin Lymphoma. Signal Transduct. Target. Ther. 2020, 5, 134. [Google Scholar] [CrossRef] [PubMed]
- Eyre, T.A.; Khan, D.; Hall, G.W.; Collins, G.P. Anaplastic lymphoma kinase-positive anaplastic large cell lymphoma: Current and future perspectives in Adult and Paediatric Disease. Eur. J. Haematol. 2014, 93, 455–468. [Google Scholar] [CrossRef] [PubMed]
- Yunis, J.J.; Frizzera, G.; Oken, M.M.; McKenna, J.; Theologides, A.; Arnesen, M. Multiple recurrent genomic defects in follicular lymphoma. N. Engl. J. Med. 1987, 316, 79–84. [Google Scholar] [CrossRef]
- Barrans, S.L.; O′Connor, S.J.; Evans, P.A.; Davies, F.E.; Owen, R.G.; Haynes, A.P.; Morgan, G.J.; Jack, A.S. Rearrangement of the BCL6 locus at 3q27 is an independent poor prognostic factor in nodal diffuse large B-cell lymphoma. Br. J. Haematol. 2002, 117, 322–332. [Google Scholar] [CrossRef] [PubMed]
- Ramis-Zaldivar, J.E.; Gonzalez-Farré, B.; Balagué, O.; Celis, V.; Nadeu, F.; Salmerón-Villalobos, J.; Andrés, M.; Martin-Guerrero, I.; Garrido-Pontnou, M.; Gaafar, A.; et al. Distinct molecular profile of IRF4-rearranged large B-cell lymphoma. Blood 2020, 135, 274–286. [Google Scholar] [CrossRef] [PubMed]
- Zimonjic, D.B.; Keck-Waggoner, C.; Popescu, N.C. Novel genomic imbalances and chromosome translocations involving c-myc gene in Burkitt′s lymphoma. Leukemia 2001, 15, 1582–1588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Angi, M.; Kamath, V.; Yuvarani, S.; Meena, J.; Sitaram, U.; Manipadam, M.T.; Nair, S.; Ganapule, A.; Fouzia, N.A.; Abraham, A.; et al. The T(8;14)(q24.1;Q32) and its variant translocations: A study of 34 cases. Hematol. Oncol. Stem Cell Ther. 2017, 10, 126–134. [Google Scholar] [CrossRef]
- Nelson, M.; Perkins, S.L.; Dave, B.J.; Coccia, P.F.; Bridge, J.A.; Lyden, E.R.; Heerema, N.A.; Lones, M.A.; Harrison, L.; Cairo, M.S.; et al. An increased frequency of 13Q deletions detected by Fluorescencein situhybridization and its impact on survival in children and adolescents with Burkitt lymphoma: Results from the Children’s Oncology Group Study CCG-5961. Br. J. Haematol. 2010, 148, 600–610. [Google Scholar] [CrossRef] [Green Version]
- Rohde, M.; Bonn, B.R.; Zimmermann, M.; Lange, J.; Möricke, A.; Klapper, W.; Oschlies, I.; Szczepanowski, M.; Nagel, I.; Schrappe, M.; et al. Relevance of id3-TCF3-CCND3 pathway mutations in pediatric aggressive B-cell lymphoma treated according to the non-hodgkin lymphoma Berlin-frankfurt-münster protocols. Haematologica 2017, 102, 1091–1098. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez-Farre, B.; Ramis-Zaldivar, J.E.; Salmeron-Villalobos, J.; Balagué, O.; Celis, V.; Verdu-Amoros, J.; Nadeu, F.; Sábado, C.; Ferrández, A.; Garrido, M.; et al. Burkitt-like lymphoma with 11Q aberration: A germinal center-derived lymphoma genetically unrelated to Burkitt lymphoma. Haematologica 2019, 104, 1822–1829. [Google Scholar] [CrossRef]
- Abla, O.; Attarbaschi, A. Non-Hodgkin’s Lymphoma in Childhood and Adolescence; Springer Nature: Cham, Switzerland, 2019; pp. 52–53. [Google Scholar]
- Cairo, M.S.; Beishuizen, A. Childhood, adolescent and young adult non-Hodgkin lymphoma: Current perspectives. Br. J. Haematol. 2019, 185, 1021–1042. [Google Scholar] [CrossRef]
- Gravina, G.L.; Festuccia, C.; Marampon, F.; Popov, V.M.; Pestell, R.G.; Zani, B.M.; Tombolini, V. Biological rationale for the use of DNA methyltransferase inhibitors as new strategy for modulation of tumor response to chemotherapy and radiation. Mol. Cancer 2010, 9, 305. [Google Scholar] [CrossRef]
- Shiramizu, B.; Mussolin, L.; Woessmann, W.; Klapper, W. Paediatric non-Hodgkin Lymphoma-Perspectives in translational biology. Br. J. Haematol. 2016, 173, 617–624. [Google Scholar] [CrossRef] [Green Version]
- Neelapu, S.S.; Adkins, S.; Ansell, S.M.; Brody, J.; Cairo, M.S.; Friedberg, J.W.; Kline, J.P.; Levy, R.; Porter, D.L.; van Besien, K.; et al. Society for immunotherapy of cancer (SITC) clinical practice guideline on immunotherapy for the treatment of lymphoma. J. Immunother. Cancer 2020, 8, e001235. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, M.L.; Reyes-Garau, D.; Armengol, M.; Fernández-Serrano, M.; Roué, G. Recent Advances in the Targeting of Epigenetic Regulators in B-Cell Non-Hodgkin Lymphoma. Front. Genet. 2019, 10, 986. [Google Scholar] [CrossRef] [PubMed]
- van Bruggen, J.A.; Martens, A.W.; Tonino, S.H.; Kater, A.P. Overcoming the hurdles of autologous T-cell-based therapies in B-cell Non-Hodgkin Lymphoma. Cancers 2020, 12, 3837. [Google Scholar] [CrossRef] [PubMed]
- Brugieres, L.; Afify, Z.; Lowe, E. ALK inhibitors for Alk-altered paediatric malignancies. Lancet Oncol. 2021, 22, 1646–1648. [Google Scholar] [CrossRef]
- Brentuximab Vedotin or Crizotinib and Combination Chemotherapy in Treating Patients with Newly Diagnosed Stage II–IV Anaplastic Large Cell Lymphoma. Available online: https://clinicaltrials.gov/ct2/show/NCT01979536 (accessed on 12 March 2021).
- Hare, L.; Burke, G.A.; Turner, S.D. Resistance to targeted agents used to treat paediatric Alk-positive ALCL. Cancers 2021, 13, 6003. [Google Scholar] [CrossRef] [PubMed]
- Phase I Study of LDK378 in Pediatric, Malignancies with a Genetic Alteration in Anaplastic Lymphoma Kinase (ALK). Available online: https://clinicaltrials.gov/ct2/show/NCT01742286 (accessed on 12 March 2021).
- Ferreri, A.J.M.; Govi, S.; Pileri, S.A.; Savage, K.J. Anaplastic large cell lymphoma, Alk-positive. Crit. Rev. Oncol. Hematol. 2012, 83, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Willard, P.; McKay, J.; Yazbeck, V. Role of antibody-based therapy in indolent non-Hodgkin′s lymphoma. Leuk. Res. Rep. 2021, 16, 100275. [Google Scholar]
- Baldo, B.A. Monoclonal antibodies approved for cancer therapy. Saf. Biol. Ther. 2016, 57–140. [Google Scholar]
- Minard-Colin, V.; Aupérin, A.; Pillon, M.; Burke, G.A.A.; Barkauskas, D.A.; Wheatley, K.; Delgado, R.F.; Alexander, S.; Uyttebroeck, A.; Bollard, C.M.; et al. Rituximab for high-risk, mature B-cell Non-Hodgkin’s lymphoma in children. N. Engl. J. Med. 2020, 382, 2207–2219. [Google Scholar] [CrossRef]
- Pytlik, R.; Polgarova, K.; Karolova, J.; Klener, P. Current immunotherapy approaches in Non-Hodgkin lymphomas. Vaccines 2020, 8, 708. [Google Scholar] [CrossRef]
- Kyriakidis, I.; Vasileiou, E.; Rossig, C.; Roilides, E.; Groll, A.H.; Tragiannidis, A. Invasive fungal diseases in children with hematological malignancies treated with therapies that target cell surface antigens: Monoclonal antibodies, immune checkpoint inhibitors and car T-cell therapies. J. Fungi 2021, 7, 186. [Google Scholar] [CrossRef] [PubMed]
- Salvaris, R.; Ong, J.; Gregory, G.P. Bispecific antibodies: A review of development, clinical efficacy and toxicity in B-cell lymphomas. J. Pers. Med. 2021, 11, 355. [Google Scholar] [CrossRef] [PubMed]
- Castaneda-Puglianini, O.; Chavez, J.C. Bispecific antibodies for non-Hodgkin’s lymphomas and multiple myeloma. Drugs Context 2021, 10, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Clements, J.D.; Zhu, M.; Kuchimanchi, M.; Terminello, B.; Doshi, S. Population pharmacokinetics of Blinatumomab in pediatric and adult patients with hematological malignancies. Clin. Pharmacokinet. 2019, 59, 463–474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dufner, V.; Sayehli, C.M.; Chatterjee, M.; Hummel, H.D.; Gelbrich, G.; Bargou, R.C.; Goebeler, M.-E. Long-term outcome of patients with relapsed/refractory B-cell non-hodgkin lymphoma treated with blinatumomab. Blood Adv. 2019, 3, 2491–2498. [Google Scholar] [CrossRef] [Green Version]
- Chu, Y.; Zhou, X.; Wang, X. Antibody-drug conjugates for the treatment of lymphoma: Clinical advances and latest progress. J. Hematol. Oncol. 2021, 14, 88. [Google Scholar] [CrossRef]
- Pro, B.; Advani, R.; Brice, P.; Bartlett, N.L.; Rosenblatt, J.D.; Illidge, T.; Matous, J.; Ramchandren, R.; Fanale, M.; Connors, J.M.; et al. Five-year results of brentuximab vedotin in patients with relapsed or refractory systemic anaplastic large cell lymphoma. Blood 2017, 130, 2709–2717. [Google Scholar] [CrossRef] [Green Version]
- van der Weyden, C.A.; Pileri, S.A.; Feldman, A.L.; Whisstock, J.; Prince, H.M. Understanding CD30 biology and therapeutic targeting: A historical perspective providing insight into future directions. Blood Cancer J. 2017, 7, e603. [Google Scholar] [CrossRef] [Green Version]
- Okeley, N.M.; Miyamoto, J.B.; Zhang, X.; Sanderson, R.J.; Benjamin, D.R.; Sievers, E.L.; Senter, P.D.; Alley, S.C. Intracellular activation of SGN-35, a potent anti-cd30 antibody-drug conjugate. Clin. Cancer Res. 2010, 16, 888–897. [Google Scholar] [CrossRef] [Green Version]
- Sekimizu, M.; Iguchi, A.; Mori, T.; Koga, Y.; Kada, A.; Saito, A.M.; Horibe, K. Phase I clinical study of Brentuximab Vedotin (SGN-35) involving children with recurrent or refractory CD30-positive hodgkin’s lymphoma or systemic anaplastic large cell lymphoma: Rationale, design and methods of BV-HLALCL study: Study protocol. BMC Cancer 2018, 18, 122. [Google Scholar] [CrossRef] [Green Version]
- Lowe, E.J.; Reilly, A.F.; Lim, M.S.; Gross, T.G.; Saguilig, L.; Barkauskas, D.A.; Wu, R.; Alexander, S.; Bollard, C.M. Brentuximab Vedotin in combination with chemotherapy for pediatric patients with ALK+ ALCL: Results of cog trial ANHL12P1. Blood 2021, 137, 3595–3603. [Google Scholar] [CrossRef] [PubMed]
- Locatelli, F.; Mauz-Koerholz, C.; Neville, K.; Llort, A.; Beishuizen, A.; Daw, S.; Pillon, M.; Aladjidi, N.; Klingebiel, T.; Landman-Parker, J.; et al. Brentuximab Vedotin for paediatric relapsed or refractory Hodgkin’s lymphoma and anaplastic large-cell lymphoma: A Multicentre, open-label, phase 1/2 study. Lancet Haematol. 2018, 5, 450–461. [Google Scholar] [CrossRef]
- Solimando, A.G.; Ribatti, D.; Vacca, A.; Einsele, H. Targeting B-cell non Hodgkin Lymphoma: New and old tricks. Leuk. Res. 2016, 42, 93–104. [Google Scholar] [CrossRef] [PubMed]
- Yurkiewicz, I.R.; Muffly, L.; Liedtke, M. Inotuzumab Ozogamicin: A CD22 mab–drug conjugate for adult relapsed or refractory B-cell precursor Acute lymphoblastic leukemia. Drug Des. Devel. Ther. 2018, 12, 2293–2300. [Google Scholar] [CrossRef] [Green Version]
- Inotuzumab Ozogamicin in Treating Younger Patients with B-Lymphoblastic Lymphoma or Relapsed or Refractory CD22 Positive B Acute Lymphoblastic Leukemia. Available online: https://clinicaltrials.gov/ct2/show/NCT02981628 (accessed on 13 January 2022).
- Sehn, L.H.; Herrera, A.F.; Flowers, C.R.; Kamdar, M.K.; McMillan, A.; Hertzberg, M.; Assouline, S.; Kim, T.M.; Kim, W.S.; Ozcan, M.; et al. Polatuzumab vedotin in relapsed or refractory diffuse large B-cell lymphoma. J. Clin. Oncol. 2020, 38, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Autologous Stem Cell Transplant Followed by Polatuzumab Vedotin in Patients with B-Cell Non-Hodgkin and Hodgkin Lymphoma. Available online: https://clinicaltrials.gov/ct2/show/NCT04491370 (accessed on 13 January 2022).
- Safety and Preliminary Efficacy of JBH492 Monotherapy in Patients with Chronic Lymphocytic Leukemia (CLL) and Non-Hodgkin’s Lymphoma (NHL). Available online: https://clinicaltrials.gov/ct2/show/NCT04240704 (accessed on 13 January 2022).
- Marofi, F.; Rahman, H.S.; Achmad, M.H.; Sergeevna, K.N.; Suksatan, W.; Abdelbasset, W.K.; Mikhailova, M.V.; Shomali, N.; Yazdanifar, M.; Hassanzadeh, A.; et al. A deep insight into CAR-T cell therapy in Non-Hodgkin Lymphoma: Application, opportunities, and future directions. Front. Immunol. 2021, 12, 681984. [Google Scholar] [CrossRef] [PubMed]
- Wysocki, P.J. Monoclonal antibodies in medical oncology—Mechanism of action. Oncol. Clin. Pract. 2014, 10, 175–183. [Google Scholar]
- Tong, J.T.; Harris, P.W.; Brimble, M.A.; Kavianinia, I. An insight into FDA approved antibody-drug conjugates for cancer therapy. Molecules 2021, 26, 5847. [Google Scholar] [CrossRef]
- Zhao, Z.; Chen, Y.; Francisco, N.M.; Zhang, Y.; Wu, M. The application of CAR-T cell therapy in hematological malignancies: Advantages and challenges. Acta Pharm. Sin. B 2018, 8, 539–551. [Google Scholar] [CrossRef]
- Du, J.; Zhang, Y. Sequential anti-cd19, 22, and 20 autologous chimeric antigen receptor T-cell (CAR-T) treatments of a child with relapsed refractory Burkitt lymphoma: A case report and literature review. J. Cancer Res. Clin. Oncol. 2020, 146, 1575–1582. [Google Scholar] [CrossRef]
- Chu, Y.; Gardenswartz, A.; Termuhlen, A.M.; Cairo, M.S. Advances in cellular and humoral immunotherapy—Implications for the treatment of poor risk childhood, adolescent, and Young Adult B-cell non-hodgkin lymphoma. Br. J. Haematol. 2019, 185, 1055–1070. [Google Scholar] [CrossRef]
- Poh, W.J.; Wee, C.P.; Gao, Z. DNA methyltransferase activity assays: Advances and challenges. Theranostics 2016, 6, 369–391. [Google Scholar] [CrossRef] [PubMed]
- Nepali, K.; Liou, J.-P. Recent developments in Epigenetic cancer therapeutics: Clinical advancement and emerging trends. J. Biomed. Sci. 2021, 28, 27. [Google Scholar] [CrossRef] [PubMed]
- Decitabine-Primed Tandem CD19/CD20 CAR T Cells Plus Epigenetic Agents in Aggressive r/r B-NHL with Huge Tumor Burden. Available online: https://clinicaltrials.gov/ct2/show/NCT04553393 (accessed on 22 January 2022).
- A Clinical Trial of Decitabine in Relapsed or Refractory T-Lymphoblastic Lymphoma. Available online: https://clinicaltrials.gov/ct2/show/NCT03558412 (accessed on 22 January 2022).
- Prince, H.M.; Bishton, M.J.; Harrison, S.J. Clinical studies of histone deacetylase inhibitors. Clin. Cancer Res. 2009, 15, 3958–3969. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drummond, D.C.; Noble, C.O.; Kirpotin, D.B.; Guo, Z.; Scott, G.K.; Benz, C.C. Clinical development of histone deacetylase inhibitors as anticancer agents. Annu. Rev. Pharmacol. Toxicol. 2005, 45, 495–528. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.-J.; Seto, E. HATs and hdacs: From structure, function and regulation to novel strategies for therapy and prevention. Oncogene 2007, 26, 5310–5318. [Google Scholar] [CrossRef] [PubMed]
- Chen, I.-C.; Sethy, B.; Liou, J.-P. Recent update of HDAC inhibitors in lymphoma. Front. Cell Dev. Biol. 2020, 8, 906. [Google Scholar] [CrossRef]
- Vorinostat and Combination Chemotherapy before Donor Stem Cell Transplantation for the Treatment of Relapsed Aggressive B-Cell or T-Cell Non-Hodgkin Lymphoma. Available online: https://clinicaltrials.gov/ct2/show/NCT04220008 (accessed on 22 January 2022).
- Vorinostat for Graft, vs. Host Disease Prevention in Children, Adolescents and Young Adults Undergoing Allogeneic Blood and Marrow Transplantation. Available online: https://clinicaltrials.gov/ct2/show/NCT03842696 (accessed on 22 January 2022).
- van Tilburg, C.M.; Milde, T.; Witt, R.; Ecker, J.; Hielscher, T.; Seitz, A.; Schenk, J.-P.; Buhl, J.L.; Riehl, D.; Frühwald, M.C.; et al. Phase I/II intra-patient dose escalation study of Vorinostat in children with relapsed solid tumor, lymphoma, or leukemia. Clin. Epigenetics 2019, 11, 188. [Google Scholar] [CrossRef] [Green Version]
- Goldberg, J.; Sulis, M.L.; Bender, J.; Jeha, S.; Gardner, R.; Pollard, J.; Aquino, V.; Laetsch, T.; Winick, N.; Fu, C.; et al. A phase I study of Panobinostat in children with relapsed and refractory hematologic malignancies. Pediatr. Hematol. Oncol. J. 2020, 37, 465–474. [Google Scholar] [CrossRef]
- Shi, Y.; Jia, B.; Xu, W.; Li, W.; Liu, T.; Liu, P.; Zhao, W.; Zhang, H.; Sun, X.; Yang, H.; et al. Chidamide in relapsed or refractory peripheral T cell lymphoma: A multicenter real-world study in China. J. Hematol. Oncol. 2017, 10, 69. [Google Scholar] [CrossRef] [Green Version]
- Ji, J.; Liu, Z.; Kuang, P.; Dong, T.; Chen, X.; Li, J.; Zhang, C.; Liu, J.; Zhang, L.; Shen, K.; et al. A new conditioning regimen with chidamide, cladribine, Gemcitabine and busulfan significantly improve the outcome of high-risk or relapsed/refractory non-Hodgkin′s lymphomas. Int. J. Cancer 2021, 149, 2075–2082. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Jiang, Y.; Zhu, Q.; Duan, Y.; Chen, X.; Xu, T.; Jin, Z.; Li, C.; Wu, D.; Huang, H. Clinical features and treatment outcomes of 14 patients with hepatosplenic γ δ T-cell lymphoma. J. Cancer Res. Clin. Oncol. 2021, 147, 3441–3445. [Google Scholar] [CrossRef]
- Makena, M.R.; Nguyen, T.H.; Koneru, B.; Hindle, A.; Chen, W.-H.; Verlekar, D.U.; Kang, M.H.; Reynolds, C.P. Vorinostat and FENRETINIDE synergize in preclinical models of T-cell lymphoid malignancies. Anti Cancer. Drugs 2020, 32, 34–43. [Google Scholar] [CrossRef]
- Panuciak, K.; Margas, M.; Makowska, K.; Lejman, M. Insights into modern therapeutic approaches in pediatric acute leukemias. Cells 2022, 11, 139. [Google Scholar] [CrossRef] [PubMed]
- Kabir, T.F.; Chauhan, A.; Anthony, L.; Hildebrandt, G.C. Immune checkpoint inhibitors in pediatric solid tumors: Status in 2018. Ochsner J. 2018, 18, 370–376. [Google Scholar] [CrossRef] [Green Version]
- Topalian, S.L.; Hodi, F.S.; Brahmer, J.R.; Gettinger, S.N.; Smith, D.C.; McDermott, D.F.; Powderly, J.D.; Carvajal, R.D.; Sosman, J.A.; Atkins, M.B.; et al. Safety, activity, and immune correlates of anti–PD-1 antibody in cancer. N. Engl. J. Med. 2012, 366, 2443–2454. [Google Scholar] [CrossRef] [PubMed]
- Nivolumab with or without Ipilimumab in Treating Younger Patients with Recurrent or Refractory Solid Tumors or Sarcomas. Available online: https://clinicaltrials.gov/ct2/show/NCT02304458 (accessed on 22 January 2022).
- Davis, K.L.; Fox, E.; Merchant, M.S.; Reid, J.M.; Kudgus, R.A.; Liu, X.; Minard, C.G.; Voss, S.; Berg, S.L.; Weigel, B.J.; et al. Nivolumab in children and young adults with relapsed or refractory solid tumours or lymphoma (ADVL1412): A Multicentre, open-label, single-arm, phase 1–2 trial. Lancet Oncol. 2020, 21, 541–550. [Google Scholar] [CrossRef]
- Rigaud, C.; Abbou, S.; Minard-Colin, V.; Geoerger, B.; Scoazec, J.Y.; Vassal, G.; Jaff, N.; Heuberger, L.; Valteau-Couanet, D.; Brugieres, L. Efficacy of nivolumab in a patient with systemic refractory ALK+ anaplastic large cell lymphoma. Pediatr. Blood Cancer 2017, 65, e26902. [Google Scholar] [CrossRef] [PubMed]
- Lesokhin, A.M.; Ansell, S.M.; Armand, P.; Scott, E.C.; Halwani, A.; Gutierrez, M.; Millenson, M.M.; Cohen, A.D.; Schuster, S.J.; Lebovic, D.; et al. Nivolumab in patients with relapsed or refractory hematologic malignancy: Preliminary results of a phase IB study. J. Clin. Oncol. 2016, 34, 2698–2704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, J.; Li, S.; Medeiros, L.J.; Lin, P.; Wang, S.A.; Tang, G.; Yin, C.C.; You, M.J.; Khoury, J.D.; Iyer, S.P.; et al. PD-L1 expression is associated with ALK positivity and STAT3 activation, but not outcome in patients with systemic anaplastic large cell lymphoma. Mod. Pathol. 2019, 33, 324–333. [Google Scholar] [CrossRef]
- Herbst, R.S.; Soria, J.-C.; Kowanetz, M.; Fine, G.D.; Hamid, O.; Gordon, M.S.; Sosman, J.A.; McDermott, D.F.; Powderly, J.D.; Gettinger, S.N.; et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature 2014, 515, 563–567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geoerger, B.; Zwaan, C.M.; Marshall, L.V.; Michon, J.; Bourdeaut, F.; Casanova, M.; Corradini, N.; Rossato, G.; Farid-Kapadia, M.; Shemesh, C.S.; et al. Atezolizumab for children and young adults with previously treated solid tumours, non-hodgkin lymphoma, and Hodgkin Lymphoma (imatrix): A multicentre phase 1–2 study. Lancet Oncol. 2020, 21, 134–144. [Google Scholar] [CrossRef]
- Pasini, D.; Di Croce, L. Emerging roles for Polycomb proteins in cancer. Curr. Opin. Genet. Dev. 2016, 36, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Simon, J.A.; Lange, C.A. Roles of the EZH2 histone methyltransferase in cancer epigenetics. Mutat. Res. Mol. Mech. Mutagen. 2008, 647, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Garapaty-Rao, S.; Nasveschuk, C.; Gagnon, A.; Chan, E.Y.; Sandy, P.; Busby, J.; Balasubramanian, S.; Campbell, R.; Zhao, F.; Bergeron, L.; et al. Identification of EZH2 and EZH1 small molecule inhibitors with selective impact on diffuse large B cell lymphoma cell growth. Chem. Biol. 2013, 20, 1329–1339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morin, R.D.; Johnson, N.A.; Severson, T.M.; Mungall, A.J.; An, J.; Goya, R.; Paul, J.E.; Boyle, M.; Woolcock, B.W.; Kuchenbauer, F.; et al. Somatic mutations altering EZH2 (TYR641) in follicular and diffuse large B-cell lymphomas of germinal-center origin. Nat. Genet. 2010, 42, 181–185. [Google Scholar] [CrossRef] [PubMed]
- Su, I.; Basavaraj, A.; Krutchinsky, A.N.; Hobert, O.; Ullrich, A.; Chait, B.T.; Tarakhovsky, A. EZH2 controls B cell development through histone H3 methylation and igh rearrangement. Nat. Immunol. 2002, 4, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Béguelin, W.; Popovic, R.; Teater, M.; Jiang, Y.; Bunting, K.L.; Rosen, M.; Shen, H.; Yang, S.N.; Wang, L.; Ezponda, T.; et al. EZH2 is required for germinal center formation and somatic EZH2 mutations promote lymphoid transformation. Cancer Cell 2013, 23, 677–692. [Google Scholar] [CrossRef] [Green Version]
- Lunning, M.A.; Green, M.R. Mutation of chromatin modifiers; an emerging hallmark of germinal center B-cell lymphomas. Blood Cancer J. 2015, 5, 361. [Google Scholar] [CrossRef] [Green Version]
- Lue, J.K.; Amengual, J.E. Emerging EZH2 inhibitors and their application in lymphoma. Curr. Hematol. Malig. Rep. 2018, 13, 369–382. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Chng, W.-J. EZH2 abnormalities in lymphoid malignancies: Underlying mechanisms and therapeutic implications. J. Hematol. Oncol. 2019, 12, 118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qi, W.; Chan, H.; Teng, L.; Li, L.; Chuai, S.; Zhang, R.; Zeng, J.; Li, M.; Fan, H.; Lin, Y.; et al. Selective inhibition of EZH2 by a small molecule inhibitor blocks tumor cells proliferation. Proc. Natl. Acad. Sci. USA 2012, 109, 21360–21365. [Google Scholar] [CrossRef] [Green Version]
- Konze, K.D.; Ma, A.; Li, F.; Barsyte-Lovejoy, D.; Parton, T.; MacNevin, C.J.; Liu, F.; Gao, C.; Huang, X.-P.; Kuznetsova, E.; et al. An orally bioavailable chemical probe of the lysine methyltransferases EZH2 and EZH1. ACS Chem. Biol. 2013, 8, 1324–1334. [Google Scholar] [CrossRef] [PubMed]
- Honma, D.; Kanno, O.; Watanabe, J.; Kinoshita, J.; Hirasawa, M.; Nosaka, E.; Shiroishi, M.; Takizawa, T.; Yasumatsu, I.; Horiuchi, T.; et al. Novel orally bioavailable ezh1/2 dual inhibitors with greater antitumor efficacy than an EZH2 selective inhibitor. Cancer Sci. 2017, 108, 2069–2078. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vaswani, R.G.; Gehling, V.S.; Dakin, L.A.; Cook, A.S.; Nasveschuk, C.G.; Duplessis, M.; Iyer, P.; Balasubramanian, S.; Zhao, F.; Good, A.C.; et al. Identification of (R)-N-((4-methoxy-6-methyl-2-oxo-1,2-dihydropyridin-3-yl)methyl)-2-METHYL-1-(1-(1-(2,2,2-trifluoroethyl)piperidin-4-yl)ethyl)-1h-indole-3-carboxamide (CPI-1205), a potent and selective inhibitor of histone methyltransferase EZH2, suitable for phase I clinical trials for B-cell lymphomas. J. Med. Chem. 2016, 59, 9928–9941. [Google Scholar] [PubMed] [Green Version]
- Maruyama, D.; Tobinai, K.; Makita, S.; Ishida, T.; Kusumoto, S.; Ishitsuka, K.; Yoshimitsu, M.; Imaizumi, Y.; Sawayama, Y.; Takeuchi, S.; et al. First-in-Human Study of the EZH1/2 Dual Inhibitor DS-3201b in Patients with Relapsed or Refractory Non-Hodgkin Lymphomas—Preliminary Results. Blood 2017, 130, 4070. [Google Scholar]
- Tazemetostat in Treating Patients with Relapsed or Refractory Advanced Solid Tumors, Non-Hodgkin Lymphoma, or Histiocytic Disorders with EZH2, SMARCB1, or SMARCA4 Gene Mutations (a Pediatric MATCH Treatment Trial). Available online: https://clinicaltrials.gov/ct2/show/NCT03213665 (accessed on 6 January 2022).
- Gulati, N.; Béguelin, W.; Giulino-Roth, L. Enhancer of zeste homolog 2 (EZH2) inhibitors. Leuk. Lymphoma 2018, 59, 1574–1585. [Google Scholar] [CrossRef] [PubMed]
- Open-Label, Multicenter, Phase 1/2 Study of Tazemetostat (EZH2 Histone Methyl Transferase [HMT] Inhibitor) as a Single Agent in Subjects with Adv. Solid Tumors or with B-Cell Lymphomas and Tazemetostat in Combination with Prednisolone in Subjects with DLBC. Available online: https://clinicaltrials.gov/ct2/show/NCT01897571 (accessed on 6 January 2022).
- Targeted Therapy Directed by Genetic Testing in Treating Pediatric Patients with Relapsed or Refractory Advanced Solid Tumors, Non-Hodgkin Lymphomas, or Histiocytic Disorders (The Pediatric MATCH Screening Trial). Available online: https://clinicaltrials.gov/ct2/show/NCT03155620 (accessed on 6 January 2022).
- Lee, S.; Urman, A.; Desai, P. Emerging drug profile: Krebs Cycle and cancer: IDH mutations and therapeutic implications. Leuk. Lymphoma 2019, 60, 2635–2645. [Google Scholar] [CrossRef]
- Churchill, H.; Naina, H.; Boriack, R.; Rakheja, D.; Chen, W. Discordant intracellular and plasma D-2-hydroxyglutarate levels in a patient with IDH2 mutated angioimmunoblastic T-cell lymphoma. Int. J. Clin. Exp. Pathol. 2015, 8, 11753–11759. [Google Scholar]
- Lin, A.-P.; Abbas, S.; Kim, S.-W.; Ortega, M.; Bouamar, H.; Escobedo, Y.; Varadarajan, P.; Qin, Y.; Sudderth, J.; Schulz, E.; et al. D2HGDH regulates alpha-ketoglutarate levels and dioxygenase function by modulating IDH2. Nat. Commun. 2015, 6, 7768. [Google Scholar] [CrossRef]
- FDA Granted Regular Approval to Enasidenib for the Treatment of Relapsed or Refractory AML. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-granted-regular-approval-enasidenib-treatment-relapsed-or-refractory-aml (accessed on 9 January 2022).
- FDA Approves Ivosidenib for Relapsed or Refractory Acute Myeloid Leukemia. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-ivosidenib-relapsed-or-refractory-acute-myeloid-leukemia (accessed on 9 January 2022).
- FDA Approves Ivosidenib as First-Line Treatment for AML with IDH1 Mutation. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-ivosidenib-first-line-treatment-aml-idh1-mutation (accessed on 9 January 2022).
- Ivosidenib in Treating Patients with Advanced Solid Tumors, Lymphoma, or Histiocytic Disorders with IDH1 Mutations (a Pediatric MATCH Treatment Trial). Available online: https://clinicaltrials.gov/ct2/show/NCT04195555 (accessed on 9 January 2022).
- Hata, A.N.; Engelman, J.A.; Faber, A.C. The BCL2 family: Key mediators of the apoptotic response to targeted anticancer therapeutics. Cancer Discov. 2015, 5, 475–487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nann, D.; Ramis-Zaldivar, J.E.; Müller, I.; Gonzalez-Farre, B.; Schmidt, J.; Egan, C.; Salmeron-Villalobos, J.; Clot, G.; Mattern, S.; Otto, F.; et al. Follicular lymphoma t(14;18)-negative is genetically a heterogeneous disease. Blood Adv. 2020, 4, 5652–5665. [Google Scholar] [CrossRef] [PubMed]
- Galteland, E.; Sivertsen, E.A.; Svendsrud, D.H.; Smedshammer, L.; Kresse, S.H.; Meza-Zepeda, L.A.; Myklebost, O.; Suo, Z.; Mu, D.; DeAngelis, P.M.; et al. Translocation T(14;18) and gain of chromosome 18/BCL2: Effects on BCL2 expression and apoptosis in B-cell non-Hodgkin′s lymphomas. Leukemia 2005, 19, 2313–2323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iqbal, J.; Sanger, W.G.; Horsman, D.E.; Rosenwald, A.; Pickering, D.L.; Dave, B.; Dave, S.; Xiao, L.; Cao, K.; Zhu, Q.; et al. BCL2 translocation defines a unique tumor subset within the Germinal Center B-cell-like diffuse large B-cell lymphoma. Am. J. Pathol. 2004, 165, 159–166. [Google Scholar] [CrossRef] [Green Version]
- Monni, O.; Joensuu, H.; Franssila, K.; Klefstrom, J.; Alitalo, K.; Knuutila, S. BCL2 overexpression associated with chromosomal amplification in diffuse large B-cell lymphoma. Blood 1997, 90, 1168–1174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Vos, S.; Leonard, J.P.; Friedberg, J.W.; Zain, J.; Dunleavy, K.; Humerickhouse, R.; Hayslip, J.; Pesko, J.; Wilson, W.H. Safety and efficacy of navitoclax, a bcl-2 and Bcl-XL inhibitor, in patients with relapsed or refractory lymphoid malignancies: Results from a phase 2A study. Leuk. Lymphoma 2020, 62, 810–818. [Google Scholar] [CrossRef] [PubMed]
- Souers, A.J.; Leverson, J.D.; Boghaert, E.R.; Ackler, S.L.; Catron, N.D.; Chen, J.; Dayton, B.D.; Ding, H.; Enschede, S.H.; Fairbrother, W.J.; et al. ABT-199, a potent and selective bcl-2 inhibitor, achieves antitumor activity while sparing platelets. Nat. Med. 2013, 19, 202–208. [Google Scholar] [CrossRef]
- Knight, T.; Luedtke, D.; Edwards, H.; Taub, J.W.; Ge, Y. A delicate balance-the bcl-2 family and its role in apoptosis, oncogenesis, and cancer therapeutics. Biochem. Pharmacol. 2019, 162, 250–261. [Google Scholar] [CrossRef]
- Davids, M.S.; Roberts, A.W.; Seymour, J.F.; Pagel, J.M.; Kahl, B.S.; Wierda, W.G.; Puvvada, S.; Kipps, T.J.; Anderson, M.A.; Salem, A.H.; et al. Phase I first-in-human study of Venetoclax in patients with relapsed or refractory Non-Hodgkin lymphoma. J. Clin. Oncol. 2017, 35, 826–833. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davids, M.S.; Roberts, A.W.; Kenkre, V.P.; Wierda, W.G.; Kumar, A.; Kipps, T.J.; Boyer, M.; Salem, A.H.; Pesko, J.C.; Arzt, J.A.; et al. Long-term follow-up of patients with relapsed or refractory non–hodgkin lymphoma treated with Venetoclax in a phase I, first-in-human study. Clin. Cancer Res. 2021, 27, 4690–4695. [Google Scholar] [CrossRef]
- An Extension Study of Venetoclax for Subjects Who Have Completed a Prior Venetoclax Clinical Trial. Available online: https://clinicaltrials.gov/ct2/show/NCT03844048 (accessed on 9 January 2022).
- Expanded Access to Venetoclax. Available online: https://clinicaltrials.gov/ct2/show/NCT03123029 (accessed on 9 January 2022).
- Fenske, T.S.; Shah, N.M.; Kim, K.M.; Saha, S.; Zhang, C.; Baim, A.E.; Farnen, J.P.; Onitilo, A.A.; Blank, J.H.; Ahuja, H.; et al. A phase 2 study of weekly temsirolimus and bortezomib for relapsed or refractory B-cell non-Hodgkin Lymphoma: A wisconsin oncology network study. Cancer 2015, 121, 3465–3471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pullarkat, V.A.; Lacayo, N.J.; Jabbour, E.; Rubnitz, J.E.; Bajel, A.; Laetsch, T.W.; Leonard, J.; Colace, S.I.; Khaw, S.L.; Fleming, S.A.; et al. Venetoclax and Navitoclax in combination with chemotherapy in patients with relapsed or refractory acute lymphoblastic leukemia and lymphoblastic lymphoma. Cancer Discov. 2021, 11, 1440–1453. [Google Scholar] [CrossRef] [PubMed]
- Tiran, A.L.; Claperon, A.; Davidson, J.; Starck, J.-B.; Diguarher, T.L.; Chanrion, M.; Mistry, P.; Wang, Y.; Monceau, E.; Bernhardt, F.; et al. Abstract 1276: Identification of S65487/VOB560 as a potent and selective intravenous 2nd-generation BCL-2 inhibitor active in wild-type and clinical mutants resistant to Venetoclax. Cancer Res. 2021, 81, 1276. [Google Scholar]
- Armand, P.; Chen, Y.-B.; Redd, R.A.; Joyce, R.M.; Bsat, J.; Jeter, E.; Merryman, R.W.; Coleman, K.C.; Dahi, P.B.; Nieto, Y.; et al. PD-1 blockade with pembrolizumab for classical hodgkin lymphoma after Autologous Stem Cell Transplantation. Blood 2019, 134, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Hughes, M.E.; Landsburg, D.J.; Rubin, D.J.; Schuster, S.J.; Svoboda, J.; Gerson, J.N.; Namoglu, E.; Nasta, S.D. Treatment of patients with relapsed/refractory Non-Hodgkin lymphoma with Venetoclax: A single-center evaluation of off-label use. Clin. Lymphoma Myeloma Leuk. 2019, 19, 791–798. [Google Scholar] [CrossRef]
- Roberts, A.W.; Davids, M.S.; Pagel, J.M.; Kahl, B.S.; Puvvada, S.D.; Gerecitano, J.F.; Kipps, T.J.; Anderson, M.A.; Brown, J.R.; Gressick, L.; et al. Targeting BCL2 with Venetoclax in relapsed chronic lymphocytic leukemia. N. Engl. J. Med. 2016, 374, 311–322. [Google Scholar] [CrossRef] [PubMed]
- Vogler, M.; Butterworth, M.; Majid, A.; Walewska, R.J.; Sun, X.-M.; Dyer, M.J.; Cohen, G.M. Concurrent up-regulation of Bcl-XL and BCL2A1 induces approximately 1000-fold resistance to ABT-737 in chronic lymphocytic leukemia. Blood 2009, 113, 4403–4413. [Google Scholar] [CrossRef] [Green Version]
- Tahir, S.K.; Smith, M.L.; Hessler, P.; Rapp, L.R.; Idler, K.B.; Park, C.H.; Leverson, J.D.; Lam, L.T. Potential mechanisms of resistance to Venetoclax and strategies to circumvent it. BMC Cancer 2017, 17, 399. [Google Scholar] [CrossRef]
- Kerkhofs, M.; Vervloessem, T.; Stopa, K.B.; Smith, V.M.; Vogler, M.; Bultynck, G. DLBCL cells with acquired resistance to Venetoclax are not sensitized to bird-2 but can be resensitized to Venetoclax through bcl-XL inhibition. Biomolecules 2020, 10, 1081. [Google Scholar] [CrossRef]
- Espinos, E.; Lai, R.; Giuriato, S. The dual role of autophagy in crizotinib-treated Alk+ ALCL: From the lymphoma cells drug resistance to their demise. Cells 2021, 10, 2517. [Google Scholar] [CrossRef]
- Sharma, G.; Mota, I.; Mologni, L.; Patrucco, E.; Gambacorti-Passerini, C.; Chiarle, R. Tumor resistance against ALK targeted therapy-where it comes from and where it goes. Cancers 2018, 10, 62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Subbiah, V.; Kuravi, S.; Ganguly, S.; Welch, D.R.; Vivian, C.J.; Mushtaq, M.U.; Hegde, A.; Iyer, S.; Behrang, A.; Ali, S.M.; et al. Precision therapy with anaplastic lymphoma kinase inhibitor ceritinib in Alk-rearranged anaplastic large cell lymphoma. ESMO Open 2021, 6, 100172. [Google Scholar] [CrossRef] [PubMed]
- Greengard, E.; Mosse, Y.P.; Liu, X.; Minard, C.G.; Reid, J.M.; Voss, S.; Wilner, K.; Fox, E.; Balis, F.; Blaney, S.M.; et al. Safety, tolerability and pharmacokinetics of crizotinib in combination with cytotoxic chemotherapy for pediatric patients with refractory solid tumors or anaplastic large cell lymphoma (ALCL): A Children’s Oncology Group Phase 1 Consortium Study (ADVL1212). Cancer Chemother. Pharmacol. 2020, 86, 829–840. [Google Scholar] [CrossRef] [PubMed]
- Prokoph, N.; Probst, N.A.; Lee, L.C.; Monahan, J.M.; Matthews, J.D.; Liang, H.-C.; Bahnsen, K.; Montes-Mojarro, I.A.; Karaca Atabay, E.; Sharma, G.G.; et al. IL10RA modulates crizotinib sensitivity in NPM1-alk-positive anaplastic large cell lymphoma. Blood 2020, 136, 1657–1669. [Google Scholar] [CrossRef] [PubMed]
- Crizotinib in Treating Younger Patients with Relapsed or Refractory Solid Tumors or Anaplastic Large Cell Lymphoma. Available online: https://clinicaltrials.gov/ct2/show/NCT00939770 (accessed on 12 March 2021).
- Prokoph, N.; Larose, H.; Lim, M.; Burke, G.; Turner, S. Treatment options for paediatric anaplastic large cell lymphoma (ALCL): Current standard and beyond. Cancers 2018, 10, 99. [Google Scholar] [CrossRef] [Green Version]
- Lee, D.H.; Kim, G.W.; Kwon, S.H. The hdac6-selective inhibitor is effective against non-hodgkin lymphoma and synergizes with ibrutinib in follicular lymphoma. Mol. Carcinog. 2019, 58, 944–956. [Google Scholar] [CrossRef] [PubMed]
- Bradshaw, J.M. The SRC, Syk, and Tec family kinases: Distinct types of molecular switches. Cell. Signal. 2010, 22, 1175–1184. [Google Scholar] [CrossRef] [PubMed]
- Barf, T.; Covey, T.; Izumi, R.; van de Kar, B.; Gulrajani, M.; van Lith, B.; van Hoek, M.; de Zwart, E.; Mittag, D.; Demont, D.; et al. Acalabrutinib (ACP-196): A covalent Bruton tyrosine kinase inhibitor with a differentiated selectivity and in vivo potency profile. J. Pharmacol. Exp. Ther. 2017, 363, 240–252. [Google Scholar] [CrossRef]
- Owen, C.; Berinstein, N.L.; Christofides, A.; Sehn, L.H. Review of Bruton tyrosine kinase inhibitors for the treatment of relapsed or refractory mantle cell lymphoma. Curr. Oncol. 2019, 26, 233–240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stephens, D.M.; Spurgeon, S.E. Ibrutinib in mantle cell lymphoma patients: Glass Half Full? Evidence and opinion. Ther. Adv. Hematol. 2015, 6, 242–252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herman, S.E.; Mustafa, R.Z.; Gyamfi, J.A.; Pittaluga, S.; Chang, S.; Chang, B.; Farooqui, M.; Wiestner, A. Ibrutinib inhibits BCR and NF-ΚB signaling and reduces tumor proliferation in tissue-resident cells of patients with CLL. Blood 2014, 123, 3286–3295. [Google Scholar] [CrossRef] [PubMed]
- Dreyling, M.; Jurczak, W.; Jerkeman, M.; Silva, R.S.; Rusconi, C.; Trneny, M.; Offner, F.; Caballero, D.; Joao, C.; Witzens-Harig, M.; et al. Ibrutinib versus Temsirolimus in patients with relapsed or refractory mantle-cell lymphoma: An international, randomised, open-label, phase 3 study. Lancet 2016, 387, 770–778. [Google Scholar] [CrossRef] [Green Version]
- Advani, R.H.; Buggy, J.J.; Sharman, J.P.; Smith, S.M.; Boyd, T.E.; Grant, B.; Kolibaba, K.S.; Furman, R.R.; Rodriguez, S.; Chang, B.Y.; et al. Bruton tyrosine kinase inhibitor Ibrutinib (PCI-32765) has significant activity in patients with relapsed/refractory B-cell malignancies. J. Clin. Oncol. 2013, 31, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Hou, K.; Yu, Z.; Jia, Y.; Fang, H.; Shao, S.; Huang, L.; Feng, Y. Efficacy and safety of ibrutinib in diffuse large B-cell lymphoma: A single-arm meta-analysis. Crit. Rev. Oncol. Hematol. 2020, 152, 103010. [Google Scholar] [CrossRef] [PubMed]
- Chu, Y.; Lee, S.; Shah, T.; Yin, C.; Barth, M.; Miles, R.R.; Ayello, J.; Morris, E.; Harrison, L.; Van de Ven, C.; et al. Ibrutinib significantly inhibited Bruton’s tyrosine kinase (BTK) phosphorylation, in-vitroproliferation and enhanced overall survival in a preclinical Burkitt lymphoma (BL) model. Oncoimmunology 2018, 8, 1512455. [Google Scholar] [CrossRef]
- A Safety and Efficacy Study of Ibrutinib in Pediatric and Young Adult Participants with Relapsed or Refractory Mature B-Cell Non-Hodgkin Lymphoma. Available online: https://clinicaltrials.gov/ct2/show/NCT02703272 (accessed on 15 January 2022).
- Pal Singh, S.; Dammeijer, F.; Hendriks, R.W. Role of Bruton’s tyrosine kinase in B cells and malignancies. Mol. Cancer 2018, 17, 57. [Google Scholar] [CrossRef]
- Wang, M.; Rule, S.; Zinzani, P.L.; Goy, A.; Casasnovas, O.; Smith, S.D.; Damaj, G.; Doorduijn, J.; Lamy, T.; Morschhauser, F.; et al. Acalabrutinib in relapsed or refractory mantle cell lymphoma (ace-ly-004): A single-arm, multicentre, phase 2 trial. Lancet 2018, 391, 659–667. [Google Scholar] [CrossRef]
- Tam, C.; Grigg, A.P.; Opat, S.; Ku, M.; Gilbertson, M.; Anderson, M.A.; Seymour, J.F.; Ritchie, D.S.; Dicorleto, C.; Dimovski, B.; et al. The BTK inhibitor, BGB-3111, is safe, tolerable, and highly active in patients with relapsed/ refractory B-cell malignancies: Initial report of a phase 1 first-in-human trial. Blood 2015, 126, 832. [Google Scholar] [CrossRef]
- Walter, H.S.; Rule, S.A.; Dyer, M.J.; Karlin, L.; Jones, C.; Cazin, B.; Quittet, P.; Shah, N.; Hutchinson, C.V.; Honda, H.; et al. A phase 1 clinical trial of the selective BTK inhibitor Ono/GS-4059 in relapsed and refractory mature B-cell malignancies. Blood 2016, 127, 411–419. [Google Scholar] [CrossRef] [Green Version]
- Burger, J.A. Bruton tyrosine kinase inhibitors. Cancer J. 2019, 25, 386–393. [Google Scholar] [CrossRef] [PubMed]
- Wen, T.; Wang, J.; Shi, Y.; Qian, H.; Liu, P. Inhibitors targeting Bruton’s tyrosine kinase in cancers: Drug development advances. Leukemia 2020, 35, 312–332. [Google Scholar] [CrossRef] [PubMed]
- FDA DISCO. Burst Edition: FDA approvals of Brukinsa (Zanubrutinib), for Adult Patients with Relapsed or Refractory Marginal Zone Lymphoma, and Exkivity (Mobocertinib) for Adult Patients with Locally Advanced or Metastatic Non-Small Cell Lung Cancer with Epidermal Growth Factor Receptor Exon 20 Insertion Mutations. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-disco-burst-edition-fda-approvals-brukinsa-zanubrutinib-adult-patients-relapsed-or-refractory (accessed on 15 January 2022).
- FDA Approves Zanubrutinib for Waldenström’s Macroglobulinemia. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-zanubrutinib-waldenstroms-macroglobulinemia (accessed on 15 January 2022).
- Project Orbis: FDA Approves Acalabrutinib for CLL and SLL. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/project-orbis-fda-approves-acalabrutinib-cll-and-sll (accessed on 15 January 2022).
- Paydas, S. Management of adverse effects/toxicity of ibrutinib. Crit. Rev. Oncol. Hematol. 2019, 136, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Tam, C.S.; Anderson, M.A.; Pott, C.; Agarwal, R.; Handunnetti, S.; Hicks, R.J.; Burbury, K.; Turner, G.; Di Iulio, J.; Bressel, M.; et al. Ibrutinib plus venetoclax for the treatment of mantle-cell lymphoma. N. Engl. J. Med. 2018, 378, 1211–1223. [Google Scholar] [CrossRef] [PubMed]
- Burke, G.A.; Beishuizen, A.; Bhojwani, D.; Burkhardt, B.; Minard-Colin, V.; Norris, R.E.; Kabickova, E.; Pinarli, F.G.; Tacyildiz, N.; Howes, A.; et al. Ibrutinib plus CIT for R/R mature B-NHL in children (sparkle trial): Initial safety, pharmacokinetics, and efficacy. Leukemia 2020, 34, 2271–2275. [Google Scholar] [CrossRef] [Green Version]
- Tan, C.R.; Abdul-Majeed, S.; Cael, B.; Barta, S.K. Clinical pharmacokinetics and pharmacodynamics of Bortezomib. Clin. Pharmacokinet. 2018, 58, 157–168. [Google Scholar] [CrossRef] [PubMed]
- O′Connor, O.A.; Czuczman, M.S. Novel approaches for the treatment of NHL: Proteasome inhibition and immune modulation. Leuk. Lymphoma 2008, 49, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Kunami, N.; Katsuya, H.; Nogami, R.; Ishitsuka, K.; Tamura, K. Promise of combining a Bcl-2 family inhibitor with bortezomib or SAHA for adult T-cell leukemia/lymphoma. Anticancer Res. 2014, 34, 5287–5294. [Google Scholar] [PubMed]
- Sánchez-Serrano, I. Success in translational research: Lessons from the development of Bortezomib. Nat. Rev. Drug Discov. 2006, 5, 107–114. [Google Scholar] [CrossRef]
- Adams, J.; Palombella, V.J.; Sausville, E.A.; Johnson, J.; Destree, A.; Lazarus, D.D.; Maas, J.; Pien, C.S.; Prakash, S.; Elliott, P.J. Proteasome inhibitors: A novel class of potent and effective antitumor agents. Cancer Res. 1999, 59, 2615–2622. [Google Scholar]
- Raedler, L. Velcade (Bortezomib) Receives 2 New FDA Indications: For Retreatment of Patients with Multiple Myeloma and for First-Line Treatment of Patients with Mantle-Cell Lymphoma. Am. Heal. Drug Benefits 2015, 8, 135–140. [Google Scholar]
- FDA Approves VELCADE® (Bortezomib) for Injection for Previously Untreated Patients with Mantle Cell Lymphoma. Available online: https://www.takeda.com/newsroom/newsreleases/2014/fda-approves-velcade-bortezomib-for-injection-for-previously-untreated-patients-with-mantle-cell-lymphoma (accessed on 19 January 2022).
- Ruan, J.; Martin, P.; Furman, R.R.; Lee, S.M.; Cheung, K.; Vose, J.M.; LaCasce, A.; Morrison, J.; Elstrom, R.; Ely, S.; et al. Bortezomib plus chop-rituximab for previously untreated diffuse large B-cell lymphoma and mantle cell lymphoma. J. Clin. Oncol. 2011, 29, 690–697. [Google Scholar] [CrossRef] [PubMed]
- Robak, P.; Robak, T. Bortezomib for the treatment of hematologic malignancies: 15 years later. Drugs R. D. 2019, 19, 73–92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bose, P.; Batalo, M.S.; Holkova, B.; Grant, S. Bortezomib for the treatment of non-Hodgkin’s lymphoma. Expert Opin. Pharmacother. 2014, 15, 2443–2459. [Google Scholar] [CrossRef] [PubMed]
- Gerecitano, J.; Goy, A.; Wright, J.; MacGregor-Cortelli, B.; Neylon, E.; Gonen, M.; Esseltine, D.; Boral, A.; Schenkein, D.; Busam, K.; et al. Drug-induced cutaneous vasculitis in patients with non-Hodgkin lymphoma treated with the novel proteasome inhibitor Bortezomib: A possible surrogate marker of response? Br. J. Haematol. 2006, 134, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Horton, T.M.; Drachtman, R.A.; Chen, L.; Cole, P.D.; McCarten, K.; Voss, S.; Guillerman, R.P.; Buxton, A.; Howard, S.C.; Hogan, S.M.; et al. A phase 2 study of bortezomib in combination with ifosfamide/vinorelbine in paediatric patients and young adults with refractory/recurrent Hodgkin Lymphoma: A children’s oncology group study. Br. J. Haematol. 2015, 170, 118–122. [Google Scholar] [CrossRef] [Green Version]
- Bortezomib, Ifosfamide, and Vinorelbine Tartrate in Treating Young Patients with Hodgkin’s Lymphoma That Is Recurrent or Did Not Respond to Previous Therapy. Available online: https://clinicaltrials.gov/ct2/show/study/NCT00381940 (accessed on 19 January 2022).
- Combination Chemotherapy with or without Bortezomib in Treating Patients with Classical Hodgkin Lymphoma That Has Returned or Does Not Respond to Prior Treatment. Available online: https://clinicaltrials.gov/ct2/show/study/NCT00967369 (accessed on 19 January 2022).
- Shokati, T.; Hartmann, M.; Davari, B.; Klawitter, J.; Klawitter, J.; Christians, U. Temsirolimus Metabolic Pathways Revisited. Xenobiotica 2019, 50, 640–653. [Google Scholar] [CrossRef] [PubMed]
- Beevers, C.S.; Li, F.; Liu, L.; Huang, S. Curcumin inhibits the mammalian target of rapamycin-mediated signaling pathways in cancer cells. Int. J. Cancer 2006, 119, 757–764. [Google Scholar] [CrossRef]
- Hay, N.; Sonenberg, N. Upstream and downstream of mtor. Genes Dev. 2004, 18, 1926–1945. [Google Scholar] [CrossRef] [Green Version]
- Robak, T.; Smolewski, P.; Robak, P.; Dreyling, M. Mantle cell lymphoma: Therapeutic options in transplant-ineligible patients. Leuk. Lymphoma 2019, 60, 2622–2634. [Google Scholar] [CrossRef]
- Jurczak, W.; Ramanathan, S.; Giri, P.; Romano, A.; Mocikova, H.; Clancy, J.; Lechuga, M.; Casey, M.; Boni, J.; Giza, A.; et al. Comparison of two doses of intravenous temsirolimus in patients with relapsed/refractory mantle cell lymphoma. Leuk. Lymphoma 2017, 59, 670–678. [Google Scholar] [CrossRef] [Green Version]
- Schatz, J.H. Targeting the PI3K/AKT/mtor pathway in Non-Hodgkin’s lymphoma: Results, biology, and development strategies. Curr. Oncol. Reports 2011, 13, 398–406. [Google Scholar] [CrossRef] [PubMed]
- Hess, G.; Herbrecht, R.; Romaguera, J.; Verhoef, G.; Crump, M.; Gisselbrecht, C.; Laurell, A.; Offner, F.; Strahs, A.; Berkenblit, A.; et al. Phase III study to evaluate Temsirolimus compared with investigator’s choice therapy for the treatment of relapsed or refractory mantle cell lymphoma. J. Clin. Oncol. 2009, 27, 3822–3829. [Google Scholar] [CrossRef]
- Fakhri, B.; Kahl, B. Current and emerging treatment options for mantle cell lymphoma. Ther. Adv. Hematol. 2017, 8, 223–234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hess, G.; Wagner, K.; Keller, U.; La Rosee, P.; Atta, J.; Hübel, K.; Lerchenmueller, C.; Schoendube, D.; Witzens-Harig, M.; Ruckes, C.; et al. Final results of a phase I/II trial of the combination bendamustine and Rituximab with Temsirolimus (BERT) in relapsed mantle cell lymphoma and follicular lymphoma. HemaSphere 2020, 4, 398. [Google Scholar] [CrossRef]
- Witzens-Harig, M.; Viardot, A.; Keller, U.; Wosniok, J.; Deuster, O.; Klemmer, J.; Geueke, A.-M.; Meißner, J.; Ho, A.D.; Atta, J.; et al. The mtor inhibitor temsirolimus added to rituximab combined with dexamethasone, Cytarabine, and CISPLATINUM (R-DHAP) for the treatment of patients with relapsed or refractory DLBCL—results from the phase-II storm trial. HemaSphere 2021, 5, 636. [Google Scholar] [CrossRef] [PubMed]
- Temsirolimus, Dexamethasone, Mitoxantrone Hydrochloride, Vincristine Sulfate, and Pegaspargase in Treating Young Patients with Relapsed Acute Lymphoblastic Leukemia or Non-Hodgkin Lymphoma. Available online: https://clinicaltrials.gov/ct2/show/study/NCT01403415 (accessed on 22 January 2022).
- A Trial of Temsirolimus with Etoposide and Cyclophosphamide in Children with Relapsed Acute Lymphoblastic Leukemia and Non-Hodgkins Lymphoma. Available online: https://clinicaltrials.gov/ct2/show/study/NCT01614197 (accessed on 22 January 2022).





| NHL Type | Frequency of Occurrence in Pediatric NHLs | NHL Subtypes | Clinical Features | Genetic Rearrangements and Prognosis | |
|---|---|---|---|---|---|
| B-NHL | 86% | DLBCL BL BLL PMBCL pediatric type FLpediatric nodal MZL | generally localized lesionsabdominal tumor nasopharyngeal tumor jaw bone tumor solid tumor syndrome | DLBCL | |
| MYC—8q25 rearrangements | poor prognosis | ||||
| t(14;18)(q32;q21) IGH::BCL2 | poor prognosis | ||||
| BCL6—3q27 rearrangements | poor prognosis | ||||
| t(6;14)(p25;q32) IGH::IRF4 | favorable outcomes | ||||
| translocations MYC/BCL2, MYC/BCL6, MYC/BCL2/BCL6 | poor prognosis | ||||
| BL | |||||
| c-MYC translocations: t(8;14)(q24;q32) IGH::MYC, t(8;22)(q24.1;q11.2) IGL::MYC, t(2;8)(p12;q24.1) IGK::MYC | poor prognosis | ||||
| del(13q14.3) or del(13q34) | poor prognosis | ||||
| ID3-TCF3-CCND3 pathway mutations | no correlation to the outcome | ||||
| BLL | |||||
| 11q aberration with proximal gains and telomeric losses | favorable outcomes | ||||
| FL | |||||
| In pediatric FL, there rarely occurs t(14;18), which is typical for FL; lack of this translocation correlates with excellent outcomes in pediatric FL | |||||
| LBL-T/B | 1–4 years old 40% 15–19 years old 20% | LBL-T (75%) LBL-B | mediastinal tumor pleural effusion respiratory failure not likely to involve the CNS at diagnosis or relapse relapse into the marrow | LBL-T | |
| Chromosomal abnormalities including TCR genes, e.g., translocations in TAL1, LMO2, LYL1, HOXA9, TLX1, TLX3 | unknown | ||||
| t(7;14)(p15;q32) HOXA::TCL1A | unknown | ||||
| Notch1 mutations +/− FBXW7 mutations | favorable outcomes | ||||
| LOH6q16 | poor prognosis | ||||
| ABD | poor prognosis | ||||
| PTEN mutations | poor prognosis, unless presence of notch1 or absence of LOH6q | ||||
| PHF6 mutations | favorable outcomes | ||||
| NRAS/KRAS mutations | no correlation to the outcome | ||||
| ALCL | Median around 16 years −10% | ALCL extra-nodal NK/T cell lymphoma T cell hepatosplenic lymphoma subcutaneous panniculitis like T cell lymphoma | mediastinal tumor | t(2;5)(p23;q35) NPM1::ALK | unknown; although t(2;5) is found in aggressive high grade tumors, a 80% 5-yr survival seems to be associated with this anomaly |
| tumors in the digestive tract | |||||
| peripheral, mediastinal, or abdominal lymphadenopathy | |||||
| hepatosplenomegaly | |||||
| skin changes | |||||
| changes in the lung parenchyma | |||||
| extra-nodal lesions (brain, marrow, bones, liver, spleen) | |||||
| associated hemophagocytic lymphohistocytosis | |||||
| NHL Type | Classical Treatment | Treatment after Lack of Response to Classical Treatment or Relapse | Novel Treatment Options |
|---|---|---|---|
| B-NHL | rituximab prednisone vincristine methotrexate doxorubicin arabinoside cyclophosphamide etoposide | ibrutinib mega chemotherapy + allo-HSCT | mAbs (obinutuzumab) ADCs (inotuzumab) CAR-T cell therapy ICIs (pembrolizumab) pathway inhibitors (buparlisib, ibrutinib) |
| LBL-T/B | multidrug chemotherapy | chemotherapy with nelarabine, cyclophosphamide and etoposide mega chemotherapy + auto/allo-HSCT | ruxolitinib tyrosine-serotonin kinase inhibitors gamma secretase inhibitors |
| ALCL | methotrexate combination of cyclophosphamide, doxorubicin, vincristine, corticosteroids, ifosfamide and etoposide tumor removal surgery | allo-HSCT vinblastine re-induction salvage chemotherapy + auto-SCT re-induction salvage chemotherapy + alloSCT | mAbs Bv kinase inhibitors (ceretynib)ICIs (nivolumab) signaling pathway inhibitors (ruxolitinib) anaplastic lymphoma kinase inhibitors (crizotinib, alectinib, ceritinib) |
| ALK+ALCL | doxorubicin-containing polychemotherapy, typically CHOP 3-week induction therapy (vincristine, prednisone, cyclophosphamide, daunomycin, asparaginase) followed by a 3-week consolidation period (vincristine, prednisone, etoposide, 6-thioguanine, cytarabine, asparaginase, methotrexate), subsequently 6 courses of maintenance chemotherapy (cyclophosphamide, 6-thioguanine, vincristine, prednisone, asparaginase, methotrexate, etoposide, cytarabine) at 7-week intervals HDC/ASCT | HDC/ASCT allo-SCT | crizotinib crizotinib + multiagent chemotherapy ceritinib Bv |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Derebas, J.; Panuciak, K.; Margas, M.; Zawitkowska, J.; Lejman, M. The New Treatment Methods for Non-Hodgkin Lymphoma in Pediatric Patients. Cancers 2022, 14, 1569. https://doi.org/10.3390/cancers14061569
Derebas J, Panuciak K, Margas M, Zawitkowska J, Lejman M. The New Treatment Methods for Non-Hodgkin Lymphoma in Pediatric Patients. Cancers. 2022; 14(6):1569. https://doi.org/10.3390/cancers14061569
Chicago/Turabian StyleDerebas, Justyna, Kinga Panuciak, Mikołaj Margas, Joanna Zawitkowska, and Monika Lejman. 2022. "The New Treatment Methods for Non-Hodgkin Lymphoma in Pediatric Patients" Cancers 14, no. 6: 1569. https://doi.org/10.3390/cancers14061569
APA StyleDerebas, J., Panuciak, K., Margas, M., Zawitkowska, J., & Lejman, M. (2022). The New Treatment Methods for Non-Hodgkin Lymphoma in Pediatric Patients. Cancers, 14(6), 1569. https://doi.org/10.3390/cancers14061569






