Defining Tumor Microenvironment as a Possible Target for Effective GEP-NENs Immunotherapy—A Systematic Review
Abstract
:Simple Summary
Abstract
1. Introduction
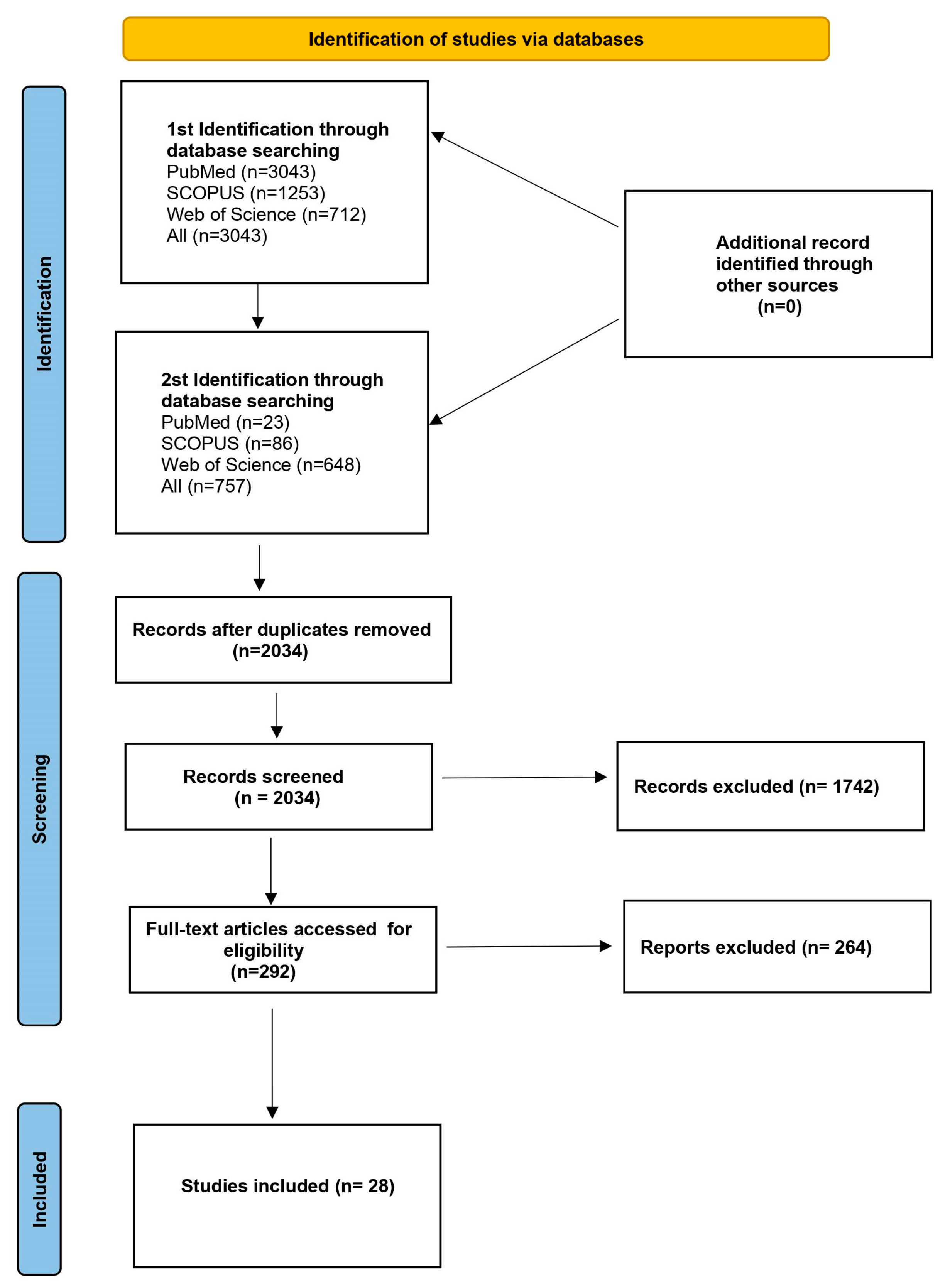
2. Materials and Methods
3. Results
3.1. Immune Checkpoints
3.2. Immune Cells Infiltrates
3.3. Cytokines
3.4. Cancer-Associated Fibroblasts
3.5. Neoangiogenesis
3.6. Microbiome
4. Conclusions and Future Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| NEN | neuroendocrine neoplasm |
| NET | neuroendocrine tumor |
| NEC | neuroendocrine carcinoma |
| GEP-NET | gastrointestinal neuroendocrine tumor |
| panNET | pancreatic neuroendocrine tumor |
| SI-NETs | small intestine neuroendocrine tumor |
| TME | tumor microenvironment |
| CT | computed tomography |
| MRI | magnetic resonance imaging |
| EUS | endoscopic ultrasound |
| SSA | somatostatin analog |
| PET | positron emission tomography |
| mTOR | mammalian target of rapamycin |
| TKI | tyrosine kinase inhibitor |
| IHC | immunohistochemistry |
| IF | immunofluorescence |
| FFPE | formalin-fixed paraffin-embedded |
| PD-1 | programmed cell death-1 |
| PD-L1 | programmed cell death-1 ligand |
| SHP-2 | homology region 2 domain-containing phosphatase (SHP-2) |
| TAM | tumor-associated macrophages |
| TIL | tumor-infiltrating lymphocyte |
| RT-PCR | reverse transcription polymerase chain reaction |
| HHLA2 | human endogenous retrovirus H long terminal repeat-associating 2 |
| B7x | B7 homolog x |
| HIF-1α | hypoxia-inducible factor 1 alpha |
| FOXM1 | Forkhead box protein M1 |
| IGF1R | type 1 insulin-like growth factor receptor |
| HLA | human leukocyte antigen |
| TNF-α | tumor necrosis factor alpha |
| VEGF | vascular endothelial growth factor |
| IDO | indoleamine 2,3-dioxygenase |
| TDO | tryptophan 2,3-dioxygenase |
| B7-H3 | B7 homolog 3 protein |
| OS | overall survival |
| PFS | progression-free survival. |
| CTLA-4 | cytotoxic T-lymphocyte-associated protein-4 |
| CAF | cancer-associated fibroblast |
| VASH-1 | vasohibin-1 |
| MDSC | myeloid-derived suppressor cells |
| FCM | flow cytometry |
| irAEs | immune-related adverse events |
References
- Cives, M.; Strosberg, J. An update on gastroenteropancreatic neuroendocrine tumors. Oncology 2014, 28, 749–756, 758. [Google Scholar] [PubMed]
- Alexandraki, K.I.; Tsoli, M.; Kyriakopoulos, G.; Angelousi, A.; Nikolopoulos, G.; Kolomodi, D.; Kaltsas, G.A. Current concepts in the diagnosis and management of neuroendocrine neoplasms of unknown primary origin. Minerva Endocrinol. 2020, 44, 378–386. [Google Scholar] [CrossRef] [PubMed]
- Rindi, G.; Mete, O.; Uccella, S.; Basturk, O.; La Rosa, S.; Brosens, L.A.A.; Ezzat, S.; de Herder, W.W.; Klimstra, D.S.; Papotti, M.; et al. Overview of the 2022 WHO Classification of Neuroendocrine Neoplasms. Endocr. Pathol. 2022, 33, 115–154. [Google Scholar] [CrossRef] [PubMed]
- Modlin, I.M.; Oberg, K.; Chung, D.C.; Jensen, R.T.; de Herder, W.W.; Thakker, R.V.; Caplin, M.; Delle Fave, G.; Kaltsas, G.A.; Krenning, E.P.; et al. Gastroenteropancreatic neuroendocrine tumours. Lancet Oncol. 2008, 9, 61–72. [Google Scholar] [CrossRef] [PubMed]
- Nagtegaal, I.D.; Odze, R.D.; Klimstra, D.; Paradis, V.; Rugge, M.; Schirmacher, P.; Washington, K.M.; Carneiro, F.; Cree, I.A. The 2019 WHO classification of tumours of the digestive system. Histopathology 2020, 76, 182–188. [Google Scholar] [CrossRef]
- Dasari, A.; Shen, C.; Halperin, D.; Zhao, B.; Zhou, S.; Xu, Y.; Shih, T.; Yao, J.C. Trends in the Incidence, Prevalence, and Survival Outcomes in Patients With Neuroendocrine Tumors in the United States. JAMA Oncol. 2017, 3, 1335. [Google Scholar] [CrossRef]
- Yao, J.C.; Hassan, M.; Phan, A.; Dagohoy, C.; Leary, C.; Mares, J.E.; Abdalla, E.K.; Fleming, J.B.; Vauthey, J.-N.; Rashid, A.; et al. One Hundred Years After “Carcinoid”: Epidemiology of and Prognostic Factors for Neuroendocrine Tumors in 35,825 Cases in the United States. J. Clin. Oncol. 2008, 26, 3063–3072. [Google Scholar] [CrossRef]
- Kaltsas, G.A.; Besser, G.M.; Grossman, A.B. The Diagnosis and Medical Management of Advanced Neuroendocrine Tumors. Endocr. Rev. 2004, 25, 458–511. [Google Scholar] [CrossRef]
- Oberg, K.; Couvelard, A.; Delle Fave, G.; Gross, D.; Grossman, A.; Jensen, R.T.; Pape, U.-F.; Perren, A.; Rindi, G.; Ruszniewski, P.; et al. ENETS Consensus Guidelines for the Standards of Care in Neuroendocrine Tumors: Biochemical Markers. Neuroendocrinology 2017, 105, 201–211. [Google Scholar] [CrossRef]
- Plöckinger, U.; Rindi, G.; Arnold, R.; Eriksson, B.; Krenning, E.P.; de Herder, W.W.; Goede, A.; Caplin, M.; Öberg, K.; Reubi, J.C.; et al. Guidelines for the Diagnosis and Treatment of Neuroendocrine Gastrointestinal Tumours. Neuroendocrinology 2004, 80, 394–424. [Google Scholar] [CrossRef]
- Sundin, A.; Arnold, R.; Baudin, E.; Cwikla, J.B.; Eriksson, B.; Fanti, S.; Fazio, N.; Giammarile, F.; Hicks, R.J.; Kjaer, A.; et al. ENETS Consensus Guidelines for the Standards of Care in Neuroendocrine Tumors: Radiological, Nuclear Medicine and Hybrid Imaging. Neuroendocrinology 2017, 105, 212–244. [Google Scholar] [CrossRef] [PubMed]
- Putzer, D.; Gabriel, M.; Henninger, B.; Kendler, D.; Uprimny, C.; Dobrozemsky, G.; Decristoforo, C.; Bale, R.J.; Jaschke, W.; Virgolini, I.J. Bone Metastases in Patients with Neuroendocrine Tumor: 68 Ga-DOTA-Tyr 3 -Octreotide PET in Comparison to CT and Bone Scintigraphy. J. Nucl. Med. 2009, 50, 1214–1221. [Google Scholar] [CrossRef] [PubMed]
- Schmid-Tannwald, C.; Schmid-Tannwald, C.M.; Morelli, J.N.; Neumann, R.; Haug, A.R.; Jansen, N.; Nikolaou, K.; Schramm, N.; Reiser, M.F.; Rist, C. Comparison of abdominal MRI with diffusion-weighted imaging to 68Ga-DOTATATE PET/CT in detection of neuroendocrine tumors of the pancreas. Eur. J. Nucl. Med. Mol. Imaging 2013, 40, 897–907. [Google Scholar] [CrossRef]
- Brenner, R.; Metens, T.; Bali, M.; Demetter, P.; Matos, C. Pancreatic neuroendocrine tumor: Added value of fusion of T2-weighted imaging and high b-value diffusion-weighted imaging for tumor detection. Eur. J. Radiol. 2012, 81, e746–e749. [Google Scholar] [CrossRef] [PubMed]
- Anderson, M. Endoscopic ultrasound is highly accurate and directs management in patients with neuroendocrine tumors of the pancreas. Am. J. Gastroenterol. 2000, 95, 2271–2277. [Google Scholar] [CrossRef]
- Partelli, S.; Maurizi, A.; Tamburrino, D.; Baldoni, A.; Polenta, V.; Crippa, S.; Falconi, M. GEP–NETS UPDATE: A review on surgery of gastro-entero-pancreatic neuroendocrine tumors. Eur. J. Endocrinol. 2014, 171, R153–R162. [Google Scholar] [CrossRef]
- Pavel, M.; Öberg, K.; Falconi, M.; Krenning, E.P.; Sundin, A.; Perren, A.; Berruti, A. Gastroenteropancreatic neuroendocrine neoplasms: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2020, 31, 844–860. [Google Scholar] [CrossRef]
- Uri, I.; Grozinsky-Glasberg, S. Current treatment strategies for patients with advanced gastroenteropancreatic neuroendocrine tumors (GEP-NETs). Clin. Diabetes Endocrinol. 2018, 4, 16. [Google Scholar] [CrossRef]
- Rinke, A.; Müller, H.-H.; Schade-Brittinger, C.; Klose, K.-J.; Barth, P.; Wied, M.; Mayer, C.; Aminossadati, B.; Pape, U.-F.; Bläker, M.; et al. Placebo-Controlled, Double-Blind, Prospective, Randomized Study on the Effect of Octreotide LAR in the Control of Tumor Growth in Patients With Metastatic Neuroendocrine Midgut Tumors: A Report From the PROMID Study Group. J. Clin. Oncol. 2009, 27, 4656–4663. [Google Scholar] [CrossRef]
- Caplin, M.E.; Pavel, M.; Ćwikła, J.B.; Phan, A.T.; Raderer, M.; Sedláčková, E.; Cadiot, G.; Wolin, E.M.; Capdevila, J.; Wall, L.; et al. Lanreotide in Metastatic Enteropancreatic Neuroendocrine Tumors. N. Engl. J. Med. 2014, 371, 224–233. [Google Scholar] [CrossRef]
- Yao, J.C.; Pavel, M.; Lombard-Bohas, C.; Van Cutsem, E.; Voi, M.; Brandt, U.; He, W.; Chen, D.; Capdevila, J.; de Vries, E.G.E.; et al. Everolimus for the Treatment of Advanced Pancreatic Neuroendocrine Tumors: Overall Survival and Circulating Biomarkers From the Randomized, Phase III RADIANT-3 Study. J. Clin. Oncol. 2016, 34, 3906–3913. [Google Scholar] [CrossRef] [PubMed]
- Faivre, S.; Niccoli, P.; Castellano, D.; Valle, J.W.; Hammel, P.; Raoul, J.-L.; Vinik, A.; Van Cutsem, E.; Bang, Y.-J.; Lee, S.-H.; et al. Sunitinib in pancreatic neuroendocrine tumors: Updated progression-free survival and final overall survival from a phase III randomized study. Ann. Oncol. 2017, 28, 339–343. [Google Scholar] [CrossRef]
- Fazio, N.; Abdel-Rahman, O. Immunotherapy in Neuroendocrine Neoplasms: Where Are We Now? Curr. Treat. Options Oncol. 2021, 22, 19. [Google Scholar] [CrossRef] [PubMed]
- Anderson, N.M.; Simon, M.C. The tumor microenvironment. Curr. Biol. 2020, 30, R921–R925. [Google Scholar] [CrossRef] [PubMed]
- Duan, Q.; Zhang, H.; Zheng, J.; Zhang, L. Turning Cold into Hot: Firing up the Tumor Microenvironment. Trends Cancer 2020, 6, 605–618. [Google Scholar] [CrossRef]
- Fridman, W.H.; Pagès, F.; Sautès-Fridman, C.; Galon, J. The immune contexture in human tumours: Impact on clinical outcome. Nat. Rev. Cancer 2012, 12, 298–306. [Google Scholar] [CrossRef]
- Bindea, G.; Mlecnik, B.; Tosolini, M.; Kirilovsky, A.; Waldner, M.; Obenauf, A.C.; Angell, H.; Fredriksen, T.; Lafontaine, L.; Berger, A.; et al. Spatiotemporal Dynamics of Intratumoral Immune Cells Reveal the Immune Landscape in Human Cancer. Immunity 2013, 39, 782–795. [Google Scholar] [CrossRef]
- Vitale, I.; Manic, G.; Coussens, L.M.; Kroemer, G.; Galluzzi, L. Macrophages and Metabolism in the Tumor Microenvironment. Cell Metab. 2019, 30, 36–50. [Google Scholar] [CrossRef]
- Hui, L.; Chen, Y. Tumor microenvironment: Sanctuary of the devil. Cancer Lett. 2015, 368, 7–13. [Google Scholar] [CrossRef]
- Binnewies, M.; Roberts, E.W.; Kersten, K.; Chan, V.; Fearon, D.F.; Merad, M.; Coussens, L.M.; Gabrilovich, D.I.; Ostrand-Rosenberg, S.; Hedrick, C.C.; et al. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat. Med. 2018, 24, 541–550. [Google Scholar] [CrossRef]
- Gajewski, T.F.; Schreiber, H.; Fu, Y.-X. Innate and adaptive immune cells in the tumor microenvironment. Nat. Immunol. 2013, 14, 1014–1022. [Google Scholar] [CrossRef] [PubMed]
- Pitt, J.M.; Marabelle, A.; Eggermont, A.; Soria, J.-C.; Kroemer, G.; Zitvogel, L. Targeting the tumor microenvironment: Removing obstruction to anticancer immune responses and immunotherapy. Ann. Oncol. 2016, 27, 1482–1492. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Chan, H.L.; Chen, P. Immune Checkpoint Inhibitors: Basics and Challenges. Curr. Med. Chem. 2019, 26, 3009–3025. [Google Scholar] [CrossRef] [PubMed]
- Topalian, S.L.; Taube, J.M.; Anders, R.A.; Pardoll, D.M. Mechanism-driven biomarkers to guide immune checkpoint blockade in cancer therapy. Nat. Rev. Cancer 2016, 16, 275–287. [Google Scholar] [CrossRef] [PubMed]
- Galluzzi, L.; Humeau, J.; Buqué, A.; Zitvogel, L.; Kroemer, G. Immunostimulation with chemotherapy in the era of immune checkpoint inhibitors. Nat. Rev. Clin. Oncol. 2020, 17, 725–741. [Google Scholar] [CrossRef]
- Farhood, B.; Najafi, M.; Mortezaee, K. CD8+ cytotoxic T lymphocytes in cancer immunotherapy: A review. J. Cell. Physiol. 2019, 234, 8509–8521. [Google Scholar] [CrossRef]
- Guillerey, C.; Huntington, N.D.; Smyth, M.J. Targeting natural killer cells in cancer immunotherapy. Nat. Immunol. 2016, 17, 1025–1036. [Google Scholar] [CrossRef]
- Voskoboinik, I.; Smyth, M.J.; Trapani, J.A. Perforin-mediated target-cell death and immune homeostasis. Nat. Rev. Immunol. 2006, 6, 940–952. [Google Scholar] [CrossRef]
- Mehla, K.; Singh, P.K. Metabolic Regulation of Macrophage Polarization in Cancer. Trends Cancer 2019, 5, 822–834. [Google Scholar] [CrossRef]
- Aras, S.; Zaidi, M.R. TAMeless traitors: Macrophages in cancer progression and metastasis. Br. J. Cancer 2017, 117, 1583–1591. [Google Scholar] [CrossRef]
- Sakaguchi, S.; Yamaguchi, T.; Nomura, T.; Ono, M. Regulatory T Cells and Immune Tolerance. Cell 2008, 133, 775–787. [Google Scholar] [CrossRef]
- Shang, B.; Liu, Y.; Jiang, S.; Liu, Y. Prognostic value of tumor-infiltrating FoxP3+ regulatory T cells in cancers: A systematic review and meta-analysis. Sci. Rep. 2015, 5, 15179. [Google Scholar] [CrossRef]
- Huppert, L.A.; Green, M.D.; Kim, L.; Chow, C.; Leyfman, Y.; Daud, A.I.; Lee, J.C. Tissue-specific Tregs in cancer metastasis: Opportunities for precision immunotherapy. Cell. Mol. Immunol. 2022, 19, 33–45. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Sampedro-Núñez, M.; Serrano-Somavilla, A.; Adrados, M.; Cameselle-Teijeiro, J.M.; Blanco-Carrera, C.; Cabezas-Agricola, J.M.; Martínez-Hernández, R.; Martín-Pérez, E.; Muñoz de Nova, J.L.; Díaz, J.Á.; et al. Analysis of expression of the PD-1/PD-L1 immune checkpoint system and its prognostic impact in gastroenteropancreatic neuroendocrine tumors. Sci. Rep. 2018, 8, 17812. [Google Scholar] [CrossRef] [PubMed]
- Cives, M.; Strosberg, J.; Al Diffalha, S.; Coppola, D. Analysis of the immune landscape of small bowel neuroendocrine tumors. Endocr. Relat. Cancer 2019, 26, 119–130. [Google Scholar] [CrossRef]
- Rosery, V.; Reis, H.; Savvatakis, K.; Kowall, B.; Stuschke, M.; Paul, A.; Dechêne, A.; Yang, J.; Zhao, B.; Borgers, A.; et al. Antitumor immune response is associated with favorable survival in GEP-NEN G3. Endocr. Relat. Cancer 2021, 28, 683–693. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.-G.; Cho, S.I.; Choi, S.; Jung, W.; Shin, J.; Park, G.; Moon, J.; Ma, M.; Song, H.; Mostafavi, M.; et al. Artificial Intelligence-Powered Whole-Slide Image Analyzer Reveals a Distinctive Distribution of Tumor-Infiltrating Lymphocytes in Neuroendocrine Neoplasms. Diagnostics 2022, 12, 2340. [Google Scholar] [CrossRef]
- Yuan, Z.; Gardiner, J.C.; Maggi, E.C.; Huang, S.; Adem, A.; Bagdasarov, S.; Li, G.; Lee, S.; Slegowski, D.; Exarchakis, A.; et al. B7 immune-checkpoints as targets for the treatment of neuroendocrine tumors. Endocr. Relat. Cancer 2021, 28, 135–149. [Google Scholar] [CrossRef]
- Hiltunen, N.; Väyrynen, J.P.; Böhm, J.; Helminen, O. CD3+, CD8+, CD4+ and FOXP3+ T Cells in the Immune Microenvironment of Small Bowel Neuroendocrine Tumors. Diseases 2021, 9, 42. [Google Scholar] [CrossRef]
- Imam, R.; Chang, Q.; Black, M.; Yu, C.; Cao, W. CD47 expression and CD163+ macrophages correlated with prognosis of pancreatic neuroendocrine tumor. BMC Cancer 2021, 21, 320. [Google Scholar] [CrossRef] [PubMed]
- da Silva, A.; Bowden, M.; Zhang, S.; Masugi, Y.; Thorner, A.R.; Herbert, Z.T.; Zhou, C.W.; Brais, L.; Chan, J.A.; Hodi, F.S.; et al. Characterization of the Neuroendocrine Tumor Immune Microenvironment. Pancreas 2018, 47, 1123–1129. [Google Scholar] [CrossRef] [PubMed]
- Rösner, E.; Kaemmerer, D.; Sänger, J.; Lupp, A. Evaluation of PD-L1 expression in a large set of gastroenteropancreatic neuroendocrine tumours and correlation with clinicopathological data. Transl. Oncol. 2022, 25, 101526. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.A.; Gonzalez, R.S.; Das, S.; Berlin, J.; Shi, C. Expression of PD-1 and PD-L1 in poorly differentiated neuroendocrine carcinomas of the digestive system: A potential target for anti–PD-1/PD-L1 therapy. Hum. Pathol. 2017, 70, 49–54. [Google Scholar] [CrossRef]
- Wei, M.; Xu, J.; Hua, J.; Meng, Q.; Liang, C.; Liu, J.; Zhang, B.; Wang, W.; Yu, X.; Shi, S. From the Immune Profile to the Immunoscore: Signatures for Improving Postsurgical Prognostic Prediction of Pancreatic Neuroendocrine Tumors. Front. Immunol. 2021, 12, 654660. [Google Scholar] [CrossRef]
- Mo, S.; Zong, L.; Chen, X.; Chang, X.; Lu, Z.; Yu, S.; Chen, J. High Mast Cell Density Predicts a Favorable Prognosis in Patients with Pancreatic Neuroendocrine Neoplasms. Neuroendocrinology 2022, 112, 845–855. [Google Scholar] [CrossRef]
- Pereira, S.S.; Pereira, R.; Santos, A.P.; Costa, M.M.; Morais, T.; Sampaio, P.; Machado, B.; Afonso, L.P.; Henrique, R.; Monteiro, M.P. Higher IL-6 peri-tumoural expression is associated with gastro-intestinal neuroendocrine tumour progression. Pathology 2019, 51, 593–599. [Google Scholar] [CrossRef]
- Bösch, F.; Brüwer, K.; Altendorf-Hofmann, A.; Auernhammer, C.J.; Spitzweg, C.; Westphalen, C.B.; Boeck, S.; Schubert-Fritschle, G.; Werner, J.; Heinemann, V.; et al. Immune checkpoint markers in gastroenteropancreatic neuroendocrine neoplasia. Endocr. Relat. Cancer 2019, 26, 293–301. [Google Scholar] [CrossRef]
- Young, K.; Lawlor, R.T.; Ragulan, C.; Patil, Y.; Mafficini, A.; Bersani, S.; Antonello, D.; Mansfield, D.; Cingarlini, S.; Landoni, L.; et al. Immune landscape, evolution, hypoxia-mediated viral mimicry pathways and therapeutic potential in molecular subtypes of pancreatic neuroendocrine tumours. Gut 2021, 70, 1904–1913. [Google Scholar] [CrossRef]
- Busse, A.; Mochmann, L.H.; Spenke, C.; Arsenic, R.; Briest, F.; Jöhrens, K.; Lammert, H.; Sipos, B.; Kühl, A.A.; Wirtz, R.; et al. Immunoprofiling in Neuroendocrine Neoplasms Unveil Immunosuppressive Microenvironment. Cancers 2020, 12, 3448. [Google Scholar] [CrossRef] [PubMed]
- Sato, S.; Tsuchikawa, T.; Nakamura, T.; Sato, N.; Tamoto, E.; Okamura, K.; Shichinohe, T.; Hirano, S. Impact of the tumor microenvironment in predicting postoperative hepatic recurrence of pancreatic neuroendocrine tumors. Oncol. Rep. 2014, 32, 2753–2759. [Google Scholar] [CrossRef] [PubMed]
- Herman Mahečić, D.; Cigrovski Berković, M.; Zjačić-Rotkvić, V.; Čačev, T.; Kapitanović, S.; Ulamec, M. Inflammation-related cytokines and their roles in gastroenteropancreatic neuroendocrine neoplasms. Bosn. J. Basic Med. Sci. 2020, 20, 445–450. [Google Scholar] [CrossRef] [PubMed]
- Milione, M.; Miceli, R.; Barretta, F.; Pellegrinelli, A.; Spaggiari, P.; Tagliabue, G.; Centonze, G.; Paolino, C.; Mangogna, A.; Kankava, K.; et al. Microenvironment and tumor inflammatory features improve prognostic prediction in gastro-entero-pancreatic neuroendocrine neoplasms. J. Pathol. Clin. Res. 2019, 5, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Centonze, G.; Lagano, V.; Sabella, G.; Mangogna, A.; Garzone, G.; Filugelli, M.; Belmonte, B.; Cattaneo, L.; Crisafulli, V.; Pellegrinelli, A.; et al. Myeloid and T-Cell Microenvironment Immune Features Identify Two Prognostic Sub-Groups in High-Grade Gastroenteropancreatic Neuroendocrine Neoplasms. J. Clin. Med. 2021, 10, 1741. [Google Scholar] [CrossRef]
- de Hosson, L.D.; Takkenkamp, T.J.; Kats-Ugurlu, G.; Bouma, G.; Bulthuis, M.; de Vries, E.G.E.; van Faassen, M.; Kema, I.P.; Walenkamp, A.M.E. Neuroendocrine tumours and their microenvironment. Cancer Immunol. Immunother. 2020, 69, 1449–1459. [Google Scholar] [CrossRef]
- Ali, A.S.; Langer, S.W.; Federspiel, B.; Hjortland, G.O.; Grønbæk, H.; Ladekarl, M.; Welin, S.; Weber Vestermark, L.; Arola, J.; Osterlund, P.; et al. PD-L1 expression in gastroenteropancreatic neuroendocrine neoplasms grade 3. PLoS ONE 2020, 15, e0243900. [Google Scholar] [CrossRef]
- Takahashi, D.; Kojima, M.; Suzuki, T.; Sugimoto, M.; Kobayashi, S.; Takahashi, S.; Konishi, M.; Gotohda, N.; Ikeda, M.; Nakatsura, T.; et al. Profiling the Tumour Immune Microenvironment in Pancreatic Neuroendocrine Neoplasms with Multispectral Imaging Indicates Distinct Subpopulation Characteristics Concordant with WHO 2017 Classification. Sci. Rep. 2018, 8, 13166. [Google Scholar] [CrossRef]
- Baretti, M.; Zhu, Q.; Zahurak, M.; Bhaijee, F.; Xu, H.; Engle, E.L.; Kotte, A.; Pawlik, T.M.; Anders, R.A.; De Jesus-Acosta, A. Prognostic Implications of the Immune Tumor Microenvironment in Patients With Pancreatic and Gastrointestinal Neuroendocrine Tumors. Pancreas 2021, 50, 719–726. [Google Scholar] [CrossRef]
- Cai, L.; Michelakos, T.; Deshpande, V.; Arora, K.S.; Yamada, T.; Ting, D.T.; Taylor, M.S.; Castillo, C.F.; Warshaw, A.L.; Lillemoe, K.D.; et al. Role of Tumor-Associated Macrophages in the Clinical Course of Pancreatic Neuroendocrine Tumors (PanNETs). Clin. Cancer Res. 2019, 25, 2644–2655. [Google Scholar] [CrossRef]
- Vesely, C.; Wong, Y.N.S.; Childs, A.; Akarca, A.U.; Dhami, P.; Vaikkinen, H.; Conde, L.; Herrero, J.; Ogunbiyi, O.; Gander, A.; et al. Systematic Evaluation of the Immune Environment of Small Intestinal Neuroendocrine Tumors. Clin. Cancer Res. 2022, 28, 2657–2668. [Google Scholar] [CrossRef]
- Tsunokake, J.; Fujishima, F.; Watanabe, H.; Sato, I.; Miura, K.; Sakamoto, K.; Suzuki, H.; Sawai, T.; Itakura, Y.; Hoshi, T.; et al. Tumor Microenvironment in Mixed Neuroendocrine Non-Neuroendocrine Neoplasms: Interaction between Tumors and Immune Cells, and Potential Effects of Neuroendocrine Differentiation on the Tumor Microenvironment. Cancers 2022, 14, 2152. [Google Scholar] [CrossRef]
- Massironi, S.; Facciotti, F.; Cavalcoli, F.; Amoroso, C.; Rausa, E.; Centonze, G.; Cribiù, F.M.; Invernizzi, P.; Milione, M. Intratumor Microbiome in Neuroendocrine Neoplasms: A New Partner of Tumor Microenvironment? A Pilot Study. Cells 2022, 11, 692. [Google Scholar] [CrossRef]
- He, X.; Xu, C. Immune checkpoint signaling and cancer immunotherapy. Cell Res. 2020, 30, 660–669. [Google Scholar] [CrossRef] [PubMed]
- Chmiel, P.; Gęca, K.; Michalski, A.; Kłosińska, M.; Kaczyńska, A.; Polkowski, W.P.; Pelc, Z.; Skórzewska, M. Vista of the Future: Novel Immunotherapy Based on the Human V-Set Immunoregulatory Receptor for Digestive System Tumors. Int. J. Mol. Sci. 2023, 24, 9945. [Google Scholar] [CrossRef] [PubMed]
- Rotte, A. Combination of CTLA-4 and PD-1 blockers for treatment of cancer. J. Exp. Clin. Cancer Res. 2019, 38, 255. [Google Scholar] [CrossRef]
- Zhang, H.; Dai, Z.; Wu, W.; Wang, Z.; Zhang, N.; Zhang, L.; Zeng, W.-J.; Liu, Z.; Cheng, Q. Regulatory mechanisms of immune checkpoints PD-L1 and CTLA-4 in cancer. J. Exp. Clin. Cancer Res. 2021, 40, 184. [Google Scholar] [CrossRef] [PubMed]
- Wolchok, J.D.; Saenger, Y. The Mechanism of Anti-CTLA-4 Activity and the Negative Regulation of T-Cell Activation. Oncologist 2008, 13, 2–9. [Google Scholar] [CrossRef]
- Yokosuka, T.; Takamatsu, M.; Kobayashi-Imanishi, W.; Hashimoto-Tane, A.; Azuma, M.; Saito, T. Programmed cell death 1 forms negative costimulatory microclusters that directly inhibit T cell receptor signaling by recruiting phosphatase SHP2. J. Exp. Med. 2012, 209, 1201–1217. [Google Scholar] [CrossRef]
- Sharpe, A.H.; Pauken, K.E. The diverse functions of the PD1 inhibitory pathway. Nat. Rev. Immunol. 2018, 18, 153–167. [Google Scholar] [CrossRef]
- Fan, Y.; Ma, K.; Wang, C.; Ning, J.; Hu, Y.; Dong, D.; Dong, X.; Geng, Q.; Li, E.; Wu, Y. Prognostic value of PD-L1 and PD-1 expression in pulmonary neuroendocrine tumors. OncoTargets Ther. 2016, 9, 6075–6082. [Google Scholar] [CrossRef]
- Jenkins, R.W.; Barbie, D.A.; Flaherty, K.T. Mechanisms of resistance to immune checkpoint inhibitors. Br. J. Cancer 2018, 118, 9–16. [Google Scholar] [CrossRef]
- Patel, S.P.; Mayerson, E.; Chae, Y.K.; Strosberg, J.; Wang, J.; Konda, B.; Hayward, J.; McLeod, C.M.; Chen, H.X.; Sharon, E.; et al. A phase II basket trial of Dual Anti–CTLA–4 and Anti–PD–1 Blockade in Rare Tumors (DART) SWOG S1609: High-grade neuroendocrine neoplasm cohort. Cancer 2021, 127, 3194–3201. [Google Scholar] [CrossRef] [PubMed]
- Yi, M.; Jiao, D.; Xu, H.; Liu, Q.; Zhao, W.; Han, X.; Wu, K. Biomarkers for predicting efficacy of PD-1/PD-L1 inhibitors. Mol. Cancer 2018, 17, 129. [Google Scholar] [CrossRef] [PubMed]
- Taube, J.M.; Klein, A.; Brahmer, J.R.; Xu, H.; Pan, X.; Kim, J.H.; Chen, L.; Pardoll, D.M.; Topalian, S.L.; Anders, R.A. Association of PD-1, PD-1 Ligands, and Other Features of the Tumor Immune Microenvironment with Response to Anti–PD-1 Therapy. Clin. Cancer Res. 2014, 20, 5064–5074. [Google Scholar] [CrossRef]
- Shen, X.; Zhao, B. Efficacy of PD-1 or PD-L1 inhibitors and PD-L1 expression status in cancer: Meta-analysis. BMJ 2018, 362, k3529. [Google Scholar] [CrossRef] [PubMed]
- Balar, A.V.; Castellano, D.; O’Donnell, P.H.; Grivas, P.; Vuky, J.; Powles, T.; Plimack, E.R.; Hahn, N.M.; de Wit, R.; Pang, L.; et al. First-line pembrolizumab in cisplatin-ineligible patients with locally advanced and unresectable or metastatic urothelial cancer (KEYNOTE-052): A multicentre, single-arm, phase 2 study. Lancet Oncol. 2017, 18, 1483–1492. [Google Scholar] [CrossRef]
- de Visser, K.E.; Joyce, J.A. The evolving tumor microenvironment: From cancer initiation to metastatic outgrowth. Cancer Cell 2023, 41, 374–403. [Google Scholar] [CrossRef]
- Chen, X.; Song, E. Turning foes to friends: Targeting cancer-associated fibroblasts. Nat. Rev. Drug Discov. 2019, 18, 99–115. [Google Scholar] [CrossRef]
- Marcus, L.; Lemery, S.J.; Keegan, P.; Pazdur, R. FDA Approval Summary: Pembrolizumab for the Treatment of Microsatellite Instability-High Solid Tumors. Clin. Cancer Res. 2019, 25, 3753–3758. [Google Scholar] [CrossRef]
- Denkert, C.; von Minckwitz, G.; Darb-Esfahani, S.; Lederer, B.; Heppner, B.I.; Weber, K.E.; Budczies, J.; Huober, J.; Klauschen, F.; Furlanetto, J.; et al. Tumour-infiltrating lymphocytes and prognosis in different subtypes of breast cancer: A pooled analysis of 3771 patients treated with neoadjuvant therapy. Lancet Oncol. 2018, 19, 40–50. [Google Scholar] [CrossRef]
- Conlon, K.C.; Miljkovic, M.D.; Waldmann, T.A. Cytokines in the Treatment of Cancer. J. Interf. Cytokine Res. 2019, 39, 6–21. [Google Scholar] [CrossRef]
- Bonati, L.; Tang, L. Cytokine engineering for targeted cancer immunotherapy. Curr. Opin. Chem. Biol. 2021, 62, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Propper, D.J.; Balkwill, F.R. Harnessing cytokines and chemokines for cancer therapy. Nat. Rev. Clin. Oncol. 2022, 19, 237–253. [Google Scholar] [CrossRef] [PubMed]
- Fisher, D.T.; Appenheimer, M.M.; Evans, S.S. The two faces of IL-6 in the tumor microenvironment. Semin. Immunol. 2014, 26, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Weber, R.; Groth, C.; Lasser, S.; Arkhypov, I.; Petrova, V.; Altevogt, P.; Utikal, J.; Umansky, V. IL-6 as a major regulator of MDSC activity and possible target for cancer immunotherapy. Cell. Immunol. 2021, 359, 104254. [Google Scholar] [CrossRef]
- Karakasheva, T.A.; Lin, E.W.; Tang, Q.; Qiao, E.; Waldron, T.J.; Soni, M.; Klein-Szanto, A.J.; Sahu, V.; Basu, D.; Ohashi, S.; et al. IL-6 Mediates Cross-Talk between Tumor Cells and Activated Fibroblasts in the Tumor Microenvironment. Cancer Res. 2018, 78, 4957–4970. [Google Scholar] [CrossRef]
- Kuo, I.-Y.; Yang, Y.-E.; Yang, P.-S.; Tsai, Y.-J.; Tzeng, H.-T.; Cheng, H.-C.; Kuo, W.-T.; Su, W.-C.; Chang, C.-P.; Wang, Y.-C. Converged Rab37/IL-6 trafficking and STAT3/PD-1 transcription axes elicit an immunosuppressive lung tumor microenvironment. Theranostics 2021, 11, 7029–7044. [Google Scholar] [CrossRef]
- Hu, F.; Song, D.; Yan, Y.; Huang, C.; Shen, C.; Lan, J.; Chen, Y.; Liu, A.; Wu, Q.; Sun, L.; et al. IL-6 regulates autophagy and chemotherapy resistance by promoting BECN1 phosphorylation. Nat. Commun. 2021, 12, 3651. [Google Scholar] [CrossRef]
- Mace, T.A.; Shakya, R.; Pitarresi, J.R.; Swanson, B.; McQuinn, C.W.; Loftus, S.; Nordquist, E.; Cruz-Monserrate, Z.; Yu, L.; Young, G.; et al. IL-6 and PD-L1 antibody blockade combination therapy reduces tumour progression in murine models of pancreatic cancer. Gut 2018, 67, 320–332. [Google Scholar] [CrossRef]
- Kumari, N.; Dwarakanath, B.S.; Das, A.; Bhatt, A.N. Role of interleukin-6 in cancer progression and therapeutic resistance. Tumor Biol. 2016, 37, 11553–11572. [Google Scholar] [CrossRef]
- Balkwill, F. TNF-α in promotion and progression of cancer. Cancer Metastasis Rev. 2006, 25, 409–416. [Google Scholar] [CrossRef]
- Zidi, I.; Mestiri, S.; Bartegi, A.; Amor, N. Ben TNF-α and its inhibitors in cancer. Med. Oncol. 2010, 27, 185–198. [Google Scholar] [CrossRef]
- Qu, Y.; Wang, X.; Bai, S.; Niu, L.; Zhao, G.; Yao, Y.; Li, B.; Li, H. The effects of TNF-α/TNFR2 in regulatory T cells on the microenvironment and progression of gastric cancer. Int. J. Cancer 2022, 150, 1373–1391. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Ao, X.; Shen, Z.; Ao, L.; Wu, X.; Pu, C.; Guo, W.; Xing, W.; He, M.; Yuan, H.; et al. TNF-α augments CXCL10/CXCR3 axis activity to induce Epithelial-Mesenchymal Transition in colon cancer cell. Int. J. Biol. Sci. 2021, 17, 2683–2702. [Google Scholar] [CrossRef] [PubMed]
- Dixit, A.; Sarver, A.; Zettervall, J.; Huang, H.; Zheng, K.; Brekken, R.A.; Provenzano, P.P. Targeting TNF-α–producing macrophages activates antitumor immunity in pancreatic cancer via IL-33 signaling. JCI Insight 2022, 7, 153242. [Google Scholar] [CrossRef]
- Chen, Y.; McAndrews, K.M.; Kalluri, R. Clinical and therapeutic relevance of cancer-associated fibroblasts. Nat. Rev. Clin. Oncol. 2021, 18, 792–804. [Google Scholar] [CrossRef]
- Mao, X.; Xu, J.; Wang, W.; Liang, C.; Hua, J.; Liu, J.; Zhang, B.; Meng, Q.; Yu, X.; Shi, S. Crosstalk between cancer-associated fibroblasts and immune cells in the tumor microenvironment: New findings and future perspectives. Mol. Cancer 2021, 20, 131. [Google Scholar] [CrossRef]
- Pei, L.; Liu, Y.; Liu, L.; Gao, S.; Gao, X.; Feng, Y.; Sun, Z.; Zhang, Y.; Wang, C. Roles of cancer-associated fibroblasts (CAFs) in anti- PD-1/PD-L1 immunotherapy for solid cancers. Mol. Cancer 2023, 22, 29. [Google Scholar] [CrossRef] [PubMed]
- Elebiyo, T.C.; Rotimi, D.; Evbuomwan, I.O.; Maimako, R.F.; Iyobhebhe, M.; Ojo, O.A.; Oluba, O.M.; Adeyemi, O.S. Reassessing vascular endothelial growth factor (VEGF) in anti-angiogenic cancer therapy. Cancer Treat. Res. Commun. 2022, 32, 100620. [Google Scholar] [CrossRef]
- Saif, M.W. Anti-angiogenesis therapy in pancreatic carcinoma. JOP 2006, 7, 163–173. [Google Scholar]
- Ferrara, N.; Hillan, K.J.; Novotny, W. Bevacizumab (Avastin), a humanized anti-VEGF monoclonal antibody for cancer therapy. Biochem. Biophys. Res. Commun. 2005, 333, 328–335. [Google Scholar] [CrossRef]
- Bruix, J.; Qin, S.; Merle, P.; Granito, A.; Huang, Y.-H.; Bodoky, G.; Pracht, M.; Yokosuka, O.; Rosmorduc, O.; Breder, V.; et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017, 389, 56–66. [Google Scholar] [CrossRef]
- Llovet, J.M.; Ricci, S.; Mazzaferro, V.; Hilgard, P.; Gane, E.; Blanc, J.-F.; de Oliveira, A.C.; Santoro, A.; Raoul, J.-L.; Forner, A.; et al. Sorafenib in Advanced Hepatocellular Carcinoma. N. Engl. J. Med. 2008, 359, 378–390. [Google Scholar] [CrossRef]
- Zhou, Y.; Ren, X.; Hou, Z.; Wang, N.; Jiang, Y.; Luan, Y. Engineering a photosensitizer nanoplatform for amplified photodynamic immunotherapy via tumor microenvironment modulation. Nanoscale Horizons 2021, 6, 120–131. [Google Scholar] [CrossRef] [PubMed]
- Cigrovski Berković, M.; Čačev, T.; Catela Ivković, T.; Marout, J.; Ulamec, M.; Zjačić-Rotkvić, V.; Kapitanović, S. High VEGF serum values are associated with locoregional spread of gastroenteropancreatic neuroendocrine tumors (GEP-NETs). Mol. Cell. Endocrinol. 2016, 425, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Iida, N.; Dzutsev, A.; Stewart, C.A.; Smith, L.; Bouladoux, N.; Weingarten, R.A.; Molina, D.A.; Salcedo, R.; Back, T.; Cramer, S.; et al. Commensal Bacteria Control Cancer Response to Therapy by Modulating the Tumor Microenvironment. Science 2013, 342, 967–970. [Google Scholar] [CrossRef] [PubMed]
- Karin, M.; Jobin, C.; Balkwill, F. Chemotherapy, immunity and microbiota—A new triumvirate? Nat. Med. 2014, 20, 126–127. [Google Scholar] [CrossRef]
- Hodi, F.S.; O’Day, S.J.; McDermott, D.F.; Weber, R.W.; Sosman, J.A.; Haanen, J.B.; Gonzalez, R.; Robert, C.; Schadendorf, D.; Hassel, J.C.; et al. Improved Survival with Ipilimumab in Patients with Metastatic Melanoma. N. Engl. J. Med. 2010, 363, 711–723. [Google Scholar] [CrossRef]
- Motzer, R.J.; Escudier, B.; McDermott, D.F.; George, S.; Hammers, H.J.; Srinivas, S.; Tykodi, S.S.; Sosman, J.A.; Procopio, G.; Plimack, E.R.; et al. Nivolumab versus Everolimus in Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2015, 373, 1803–1813. [Google Scholar] [CrossRef]
- Magalhães, D.S.T.; Magalhães, H.M.; Mesquita, A.S.A. Long lasting complete response with immunotherapy in a metastatic bladder carcinoma: A case report. Porto Biomed. J. 2021, 6, e127. [Google Scholar] [CrossRef]
- Powles, T.; Albiges, L.; Bex, A.; Grünwald, V.; Porta, C.; Procopio, G.; Schmidinger, M.; Suárez, C.; de Velasco, G. ESMO Clinical Practice Guideline update on the use of immunotherapy in early stage and advanced renal cell carcinoma. Ann. Oncol. 2021, 32, 1511–1519. [Google Scholar] [CrossRef]
- Koessler, T.; Alsina, M.; Arnold, D.; Ben-Aharon, I.; Collienne, M.; Lutz, M.P.; Neuzillet, C.; Obermannova, R.; Peeters, M.; Sclafani, F.; et al. ESMO Congress 2021: Highlights from the EORTC gastrointestinal tract cancer group’s perspective. ESMO Open 2022, 7, 100392. [Google Scholar] [CrossRef]
- Marabelle, A.; Le, D.T.; Ascierto, P.A.; Di Giacomo, A.M.; De Jesus-Acosta, A.; Delord, J.-P.; Geva, R.; Gottfried, M.; Penel, N.; Hansen, A.R.; et al. Efficacy of Pembrolizumab in Patients With Noncolorectal High Microsatellite Instability/Mismatch Repair–Deficient Cancer: Results From the Phase II KEYNOTE-158 Study. J. Clin. Oncol. 2020, 38, 1–10. [Google Scholar] [CrossRef]
- Shitara, K.; Özgüroğlu, M.; Bang, Y.-J.; Di Bartolomeo, M.; Mandalà, M.; Ryu, M.-H.; Fornaro, L.; Olesiński, T.; Caglevic, C.; Chung, H.C.; et al. Pembrolizumab versus paclitaxel for previously treated, advanced gastric or gastro-oesophageal junction cancer (KEYNOTE-061): A randomised, open-label, controlled, phase 3 trial. Lancet 2018, 392, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Mehnert, J.M.; Bergsland, E.; O’Neil, B.H.; Santoro, A.; Schellens, J.H.M.; Cohen, R.B.; Doi, T.; Ott, P.A.; Pishvaian, M.J.; Puzanov, I.; et al. Pembrolizumab for the treatment of programmed death–ligand 1–positive advanced carcinoid or pancreatic neuroendocrine tumors: Results from the KEYNOTE-028 study. Cancer 2020, 126, 3021–3030. [Google Scholar] [CrossRef]
- Strosberg, J.; Mizuno, N.; Doi, T.; Grande, E.; Delord, J.-P.; Shapira-Frommer, R.; Bergsland, E.; Shah, M.; Fakih, M.; Takahashi, S.; et al. Efficacy and Safety of Pembrolizumab in Previously Treated Advanced Neuroendocrine Tumors: Results From the Phase II KEYNOTE-158 Study. Clin. Cancer Res. 2020, 26, 2124–2130. [Google Scholar] [CrossRef]
- Lu, M.; Zhang, P.; Zhang, Y.; Li, Z.; Gong, J.; Li, J.; Li, J.; Li, Y.; Zhang, X.; Lu, Z.; et al. Efficacy, Safety, and Biomarkers of Toripalimab in Patients with Recurrent or Metastatic Neuroendocrine Neoplasms: A Multiple-Center Phase Ib Trial. Clin. Cancer Res. 2020, 26, 2337–2345. [Google Scholar] [CrossRef]
- Patel, S.P.; Othus, M.; Chae, Y.K.; Giles, F.J.; Hansel, D.E.; Singh, P.P.; Fontaine, A.; Shah, M.H.; Kasi, A.; Baghdadi, T.A.; et al. A Phase II Basket Trial of Dual Anti–CTLA-4 and Anti–PD-1 Blockade in Rare Tumors (DART SWOG 1609) in Patients with Nonpancreatic Neuroendocrine Tumors. Clin. Cancer Res. 2020, 26, 2290–2296. [Google Scholar] [CrossRef]
- Haanen, J.; Obeid, M.; Spain, L.; Carbonnel, F.; Wang, Y.; Robert, C.; Lyon, A.R.; Wick, W.; Kostine, M.; Peters, S.; et al. Management of toxicities from immunotherapy: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2022, 33, 1217–1238. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.A. New NCCN Guidelines: Recognition and Management of Immunotherapy-Related Toxicity. J. Natl. Compr. Cancer Netw. 2018, 16, 594–596. [Google Scholar] [CrossRef] [PubMed]
- Bertrand, A.; Kostine, M.; Barnetche, T.; Truchetet, M.-E.; Schaeverbeke, T. Immune related adverse events associated with anti-CTLA-4 antibodies: Systematic review and meta-analysis. BMC Med. 2015, 13, 211. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhou, S.; Yang, F.; Qi, X.; Wang, X.; Guan, X.; Shen, C.; Duma, N.; Vera Aguilera, J.; Chintakuntlawar, A.; et al. Treatment-Related Adverse Events of PD-1 and PD-L1 Inhibitors in Clinical Trials. JAMA Oncol. 2019, 5, 1008. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.Y.; Salem, J.-E.; Cohen, J.V.; Chandra, S.; Menzer, C.; Ye, F.; Zhao, S.; Das, S.; Beckermann, K.E.; Ha, L.; et al. Fatal Toxic Effects Associated With Immune Checkpoint Inhibitors. JAMA Oncol. 2018, 4, 1721. [Google Scholar] [CrossRef] [PubMed]
- Chiang, C.; Chan, S.; Lee, S.; Wong, I.O.; Choi, H.C. Cost-effectiveness of Pembrolizumab as a Second-Line Therapy for Hepatocellular Carcinoma. JAMA Netw. Open 2021, 4, e2033761. [Google Scholar] [CrossRef]
- Zeng, D.; Li, M.; Zhou, R.; Zhang, J.; Sun, H.; Shi, M.; Bin, J.; Liao, Y.; Rao, J.; Liao, W. Tumor Microenvironment Characterization in Gastric Cancer Identifies Prognostic and Immunotherapeutically Relevant Gene Signatures. Cancer Immunol. Res. 2019, 7, 737–750. [Google Scholar] [CrossRef]
- Jiang, Y.; Xie, J.; Huang, W.; Chen, H.; Xi, S.; Han, Z.; Huang, L.; Lin, T.; Zhao, L.-Y.; Hu, Y.-F.; et al. Tumor Immune Microenvironment and Chemosensitivity Signature for Predicting Response to Chemotherapy in Gastric Cancer. Cancer Immunol. Res. 2019, 7, 2065–2073. [Google Scholar] [CrossRef]
- Mino-Kenudson, M.; Schalper, K.; Cooper, W.; Dacic, S.; Hirsch, F.R.; Jain, D.; Lopez-Rios, F.; Tsao, M.S.; Yatabe, Y.; Beasley, M.B.; et al. Predictive Biomarkers for Immunotherapy in Lung Cancer: Perspective From the International Association for the Study of Lung Cancer Pathology Committee. J. Thorac. Oncol. 2022, 17, 1335–1354. [Google Scholar] [CrossRef]

| References | Authors | Samples | Technique | Neoplasm Grading | Study Group | Additional Information |
|---|---|---|---|---|---|---|
| [45] | Sampedro-Núñez et al. | formalin-fixed paraffin-embedded (FFPE) blocks | IHC (immunohistochemistry); IF (immunofluorescence) | G1; G2; G3 | 164 | Analysis of PD-1 and PD-L1 expression in the TME, in addition to the characterization of the tumor immune cell, infiltrates. |
| [46] | Cives et al. | FFPE | IHC | G1; G2 | 102 | Analysis of PD-1 and PD-L1 expression in the TME, in addition to the characterization of the tumor immune cell, infiltrates. |
| [47] | Rosery et al. | FFPE | IHC; mIF | G3; GEP-NECs | 37 | Analysis of PD-1 and PD-L1 expression in the TME. Focus on cytotoxic T-cells and TAMs. |
| [48] | Cho et al. | FFPE | Artificial intelligence (AI)-powered hematoxylin and eosin (H & E) analyzer | All | 218 | Analysis of TILs density and PD-L1 expression. Correlation between mentioned above factors. |
| [49] | Yuan et al. | Postoperative samples stored at −80 °C; samples from modified mice cohort | Multiple techniques for evaluating different markers: IHC; IF; RT-PCR; Western blot; among others | undefined | 37 and additional mice cohort | Evaluation of expression of B7 immune-checkpoints: HERV-H LTR-Associating Protein 2 (HHLA2) and B7 Family Member X (B7x), hypoxia-inducible factor 1 alpha (HIF-1α). |
| [50] | Hiltunen et al. | FFPE | IHC | G1; G2 | 132 | Analysis of the density of CD3+/CD8+/CD4+ and FOXP3+ T-cells. |
| [51] | Imam et al. | FFPE | IHC | G1; G2; G3 | 47 | Analysis of the role of CD47 expression and CD163+ TAMs in panNETs |
| [52] | Da Silva et al. | FFPE | IHC; RNA sequencing | G1; G2; G3 | 95 | Expression of PD-1; PD-L1 and PD-L2, profiling T-cell subsets in the TME. Additional RNA sequencing for further characterization of PD-L1 and PD-L2 expression. |
| [53] | Rösner et al. | FFPE | IHC | All | 457 | Evaluation of PD-1 and PD-L1 expression. |
| [54] | Roberts et al. | FFPE | IHC | GEP-NECs | 37 | PD-1 and PD-L1 expression in poorly differentiated NECs. |
| [55] | Wei et al. | FFPE; Fresh frozen NETs samples | IHC; RT-PCR; IF | G1; G2; G3 | 158 | Analysis of TME with the determination of 14 immune signatures affecting patients’ prognoses. |
| [56] | Mo et al. | FFPE | IHC | All | 187 | Expression of CD117+ mast cells and CD68+ macrophages; CD15+ neutrophils; and CD3+, CD4+, and CD8+ T cells in panNENs. |
| [57] | Pereira et al. | FFPE | IHC | G1; G2 | 39 | Expression of IL-6, Ki-67, FOXM1, and IGF1R in GEP-NETs. |
| [58] | Bösch et al. | FFPE | IHC | G1; G2; G3 | 244 | Expression of PD-1/PD-L1 and characterization of TILs in GEP-NENs |
| [59] | Young et al. | Fresh frozen panNETs samples | RNA sequencing | G1; G2; G3 | 207 | Analysis of expression of immune-related genes in panNETs |
| [60] | Busse et al. | FFPE | IHC; mRNA immunoprofiling | All | 78 | Expression of immune-related factors in the TME. |
| [61] | Sato et al. | FFPE | IHC | G1; G2; G3 | 16 | Analysis of TILs and human leukocyte antigen (HLA) class I and other factors. |
| [62] | Herman Mahečić et al. | FFPE | IHC | G1; G2; G3 | 43 | Analysis of the role of tumor necrosis factor alpha (TNF-α), interleukin 1 beta (IL-1β), IL-2, and IL-6 in GEP-NENs |
| [63] | Milione et al. | FFPE | IHC | All | 315 | Analysis of immune-, inflammatory-, angiogenesis-, proliferation-, and fibroblast-related biomarkers |
| [64] | Centozone et al. | FFPE | IHC | G3; NECs | 45 | Analysis of myeloid markers—CD33, CD163, and Arginase in High-Grade GEP-NETs. |
| [65] | De Hosson et al. | FFPE | IHC | G1; G2 | 41 | Analysis of PD-L1, T-cells, indoleamine 2,3-dioxygenase (IDO) and tryptophan 2,3-dioxygenase (TDO), mismatch repair proteins (MMRp), and activated fibroblasts. |
| [66] | Ali et al. | FFPE | IHC | G3 | 136 | Analysis of expression of PD-L1 in G3 GEP-NENs. |
| [67] | Takahashi et al. | FFPE | IHC with multispectral imaging | All | 52 | Analysis of TILs, macrophages, and PD-1/PD-L1 expression. |
| [68] | Baretii et al. | FFPE | IHC | undefined | 36 | Analysis of CD3, CD8, PD-1, PD-L1, IDO expression |
| [69] | Cai et al. | FFPE | IHC | G1; G2 | 104 | Analysis of TAMs and HLA-I/II, PD-L1, B7-H3 expression |
| [70] | Vesely et al. | FFPE; fresh frozen NETs samples | IHC; FCM | G1; G2; G3 | 40 | Analysis of T-cell subsets in the TME, characterization of expression of immune checkpoint molecules. |
| [71] | Tsunokake et al. | FFPE | IHC | NECs | 33 | Analysis of immune microenvironment in addition to comparing TILs, TAMs, and other relevant factors in the components of the same tumor. |
| [72] | Massironi et al. | FFPE | fluorescent in situ hybridization (FISH) by confocal microscopy | G1; G2; G3 | 40 | Analysis of GEP-NENs microbiome and its correlation with the immune microenvironment. |
| References | Key Findings |
|---|---|
| [45] | PD-1/PD-L1 were expressed in 1 to 8% of GEP-NEs and can be correlated with disease progression. |
| [46] | Expression of PD-L1 was higher in duodenal NETs than in ileal/jejunal. One-third of tumors were immunologically ignorant and unsuitable for immune checkpoint blockade. |
| [47] | Intense PD-1+ CD8+ immune cell infiltration showed the most favorable median overall survival (OS). |
| [48] | TIL density and PD-L1 expression were both significantly higher in high-grade NENs. |
| [49] | Higher expression of B7x and HHLA2 correlated with higher grade and higher incidence of nodal and distal spread. Furthermore, expression of the above factors was correlated with hypoxia and HIF-1α. |
| [50] | There was no correlation between CD3+, CD4+, CD8+, and FOXP3+ T-cells density in TME and patients’ prognosis. |
| [51] | CD47 was overexpressed in panNETs; moreover, CD163+ TAMs were correlated with higher grade and distal spread. |
| [52] | No significant difference in the PD-1, PD-L1, and T-cell infiltrate levels was spotted between G1, G2, and G3 tumors. Expression of immune checkpoints was rare in GEP-NETs. |
| [53] | PD-L1 expression was common in GEP-NENs and increased with grading. |
| [54] | PD-1 and PD-L1 expression was a common event in poorly differentiated NECs. |
| [55] | T-cells and macrophages were dominant infiltrates in panNETs, CCL19, IL-16, CD163, IRF4, and CD8 and can be possible predictors of immune responses. |
| [56] | CD117+ mast cells showed a protective role in panNENs. High mast cell infiltration was correlated with elevated CD4+ T-cells. |
| [57] | IL-6 expression in GEP-NETs can be correlated with disease progression. Furthermore, patients with low HDL cholesterol expression had higher IL-6 peritumoral expression. |
| [58] | High TILs and PD-1 expression were significantly associated with shorter patient survival and higher grading in GEP-NENs. PD-L1 expression showed a trend of shorter patient survival. |
| [59] | Detailed information about molecular subtypes: metastasis-like primary MLP-1 and MLP-2, insulinoma-like and intermediate. MLP-1 subtype correlated with higher immune-related genes expression and immune responses in TME. |
| [60] | G1/G2 NENs differ from poorly differentiated NENs. Both NET G1/G2 and NET G3/NEC showed low expression of IFNγ-associated genes and low intratumoral T-cell infiltration. |
| [61] | CD4+, CD8+, and CD45RO+ (memory) T-cells were present in TME; simultaneously, there was no correlation between TILs and patients’ prognosis. |
| [62] | High expression of TNF-α was correlated with higher tumor grades. GEP-NENs had higher expression of IL-6 than IL-1β or IL-2. |
| [63] | G1/G2 versus G3 GEP-NENs showed divergence with immune-inflammatory markers. G1/G2 to G3 transition was associated with immune-inflammatory profile changes. |
| [64] | High-grade NENs could be divided into prognostic sub-groups based on myeloid and T-cell markers. Tumors with a high density of the abovementioned markers show a better prognosis. |
| [65] | Expression of factors correlated with immune checkpoint treatment responses were present to a limited extent or even absent. TDO and IDO were expressed in more than 50% of NETs. |
| [66] | PD-L1 expression was present in only a small subset of G3 tumors. This factor shows no correlation with clinical parameters and prognosis. |
| [67] | While NECs can be characterized by hot immune microenvironments with abundant TILs, NETs had a cold immune microenvironment with few TILs. Several intraepithelial PD-1+ T-cells and PD-L1+ macrophages were elevated according to the grade. |
| [68] | Higher intratumoral CD3+ T-cell infiltrate was associated with a better prognosis. Expression for CD3/8, IDO, and PD-1 differed among the interface and the tumor. |
| [69] | The high amount of CD8+ T-cell infiltration with low TAMs can be correlated with a positive prognosis. |
| [70] | TILs were present in less than 10% of tumors, however intratumoral TILs had higher expression of PD-1. Moreover, CD8+ TILs had higher expression of PD-1 and CTLA-4. |
| [71] | Comparing neuroendocrine and non-neuroendocrine areas, there was more angiogenic activity and a more suppressive microenvironment in neuroendocrine areas. |
| [72] | Ninety percent of NETs showed microorganisms’ infiltration, with a homogeneous microbial distribution. Bacterial localization in panNEN was observed in the proximity of blood vessels. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chmiel, P.; Rychcik-Pazyrska, P.; Stec, R. Defining Tumor Microenvironment as a Possible Target for Effective GEP-NENs Immunotherapy—A Systematic Review. Cancers 2023, 15, 5232. https://doi.org/10.3390/cancers15215232
Chmiel P, Rychcik-Pazyrska P, Stec R. Defining Tumor Microenvironment as a Possible Target for Effective GEP-NENs Immunotherapy—A Systematic Review. Cancers. 2023; 15(21):5232. https://doi.org/10.3390/cancers15215232
Chicago/Turabian StyleChmiel, Paulina, Paulina Rychcik-Pazyrska, and Rafał Stec. 2023. "Defining Tumor Microenvironment as a Possible Target for Effective GEP-NENs Immunotherapy—A Systematic Review" Cancers 15, no. 21: 5232. https://doi.org/10.3390/cancers15215232
APA StyleChmiel, P., Rychcik-Pazyrska, P., & Stec, R. (2023). Defining Tumor Microenvironment as a Possible Target for Effective GEP-NENs Immunotherapy—A Systematic Review. Cancers, 15(21), 5232. https://doi.org/10.3390/cancers15215232








