A Review of Recent Advances in Computer-Aided Detection Methods Using Hyperspectral Imaging Engineering to Detect Skin Cancer
Abstract
:Simple Summary
Abstract
1. Introduction
2. Related Studies
3. Methodology
3.1. Study Selection Criteria
3.2. QUADAS-2 Results
4. Results
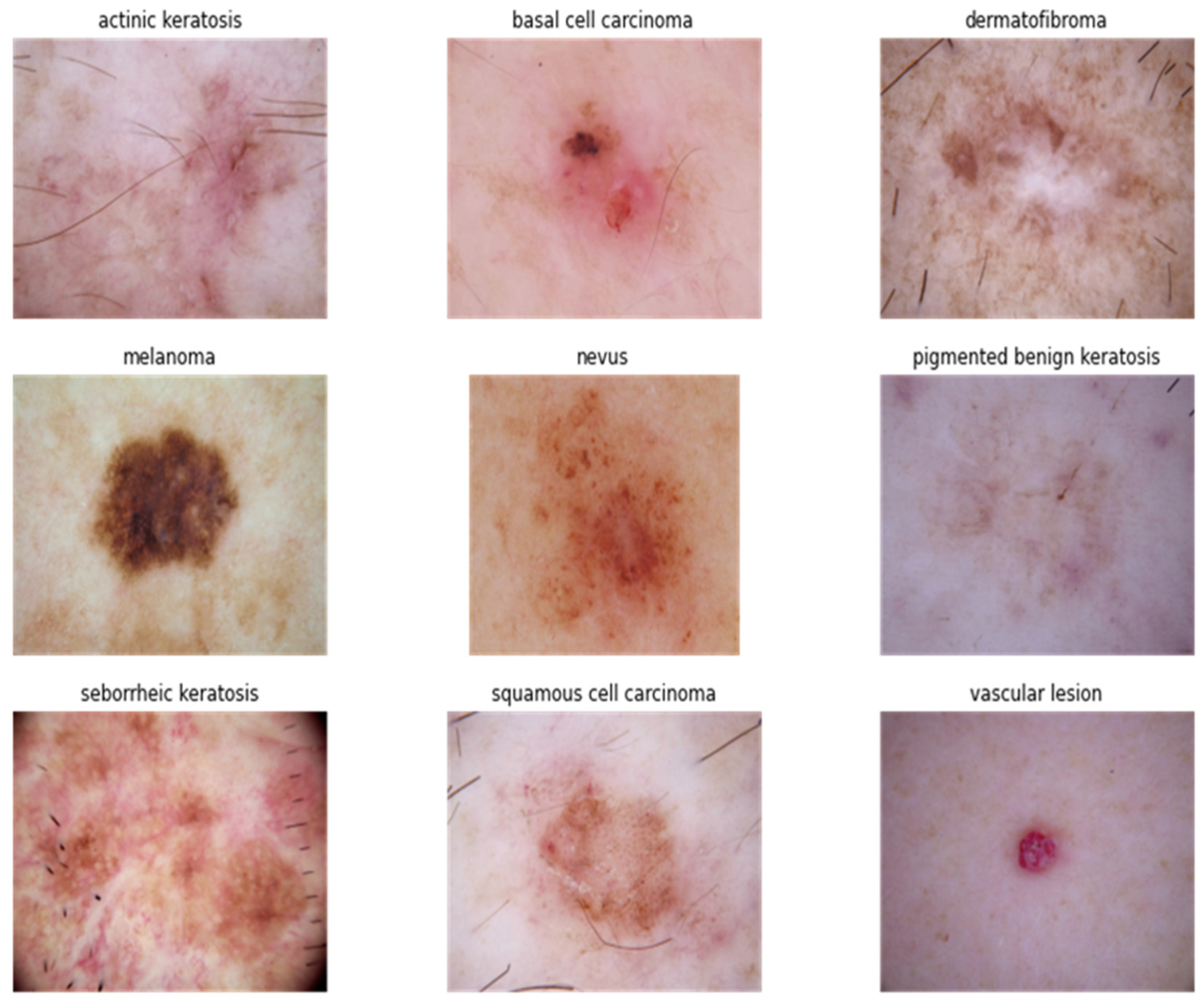
4.1. Studies under Clinical Feature Observation
4.2. Meta-Analysis of the Studies
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Gupta, A.K.; Bharadwaj, M.; Mehrotra, R. Skin cancer concerns in people of color: Risk factors and prevention. Asian Pac. J. Cancer Prev. APJCP 2016, 17, 5257. [Google Scholar]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Leiter, U.; Keim, U.; Garbe, C. Epidemiology of skin cancer: Update 2019. In Sunlight, Vitamin D and Skin Cancer; Springer: Cham, Switzerland, 2020; pp. 123–139. [Google Scholar]
- Fahradyan, A.; Howell, A.C.; Wolfswinkel, E.M.; Tsuha, M.; Sheth, P.; Wong, A.K. Updates on the management of non-melanoma skin cancer (NMSC). Healthcare 2017, 5, 82. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Mathers, C.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int. J. Cancer 2019, 144, 1941–1953. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.N.; Wang, Q.; Idrees, B.S.; Xiangli, W.; Teng, G.; Cui, X.; Zhao, Z.; Wei, K.; Abrar, M. A review on laser-induced breakdown spectroscopy in different cancers diagnosis and classification. Front. Phys. 2022, 10, 10. [Google Scholar] [CrossRef]
- Stevens, V.W.; Stenehjem, D.D.; Patterson, O.V.; Kamauu, A.W.; Yim, Y.M.; Morlock, R.J.; DuVall, S.L. Characterization and survival of patients with metastatic basal cell carcinoma in the Department of Veterans Affairs: A retrospective electronic health record review. Arch. Dermatol. Res. 2018, 310, 505–513. [Google Scholar] [CrossRef] [PubMed]
- Stiegel, E.; Lam, C.; Schowalter, M.; Somani, A.-K.; Lucas, J.; Poblete-Lopez, C. Correlation between original biopsy pathology and Mohs intraoperative pathology. Dermatol. Surg. 2018, 44, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Khazaei, Z.; Ghorat, F.; Jarrahi, A.; Adineh, H.; Sohrabivafa, M.; Goodarzi, E. Global incidence and mortality of skin cancer by histological subtype and its relationship with the human development index (HDI)—An ecology study in 2018. World Cancer Res. J. 2019, 6, e13. [Google Scholar]
- Ciążyńska, M.; Kamińska-Winciorek, G.; Lange, D.; Lewandowski, B.; Reich, A.; Sławińska, M.; Pabianek, M.; Szczepaniak, K.; Hankiewicz, A.; Ułańska, M. The incidence and clinical analysis of non-melanoma skin cancer. Sci. Rep. 2021, 11, 4337. [Google Scholar] [CrossRef]
- Popescu, D.; El-Khatib, M.; El-Khatib, H.; Ichim, L. New Trends in Melanoma Detection Using Neural Networks: A Systematic Review. Sensors 2022, 22, 496. [Google Scholar] [CrossRef]
- Ahlgrimm-Siess, V.; Laimer, M.; Arzberger, E.; Hofmann-Wellenhof, R. New diagnostics for melanoma detection: From artificial intelligence to RNA microarrays. Future Oncol. 2012, 8, 819–827. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, D.L.; Saladi, R.N.; Fox, J.L. Ultraviolet radiation and skin cancer. Int. J. Dermatol. 2010, 49, 978–986. [Google Scholar] [CrossRef] [PubMed]
- Federico, M.B. SDG 3 Good Health and Well-Being. In Actioning the Global Goals for Local Impact; Springer: Berlin/Heidelberg, Germany, 2020; pp. 39–55. [Google Scholar]
- Umar, S.A.; Tasduq, S.A. Ozone Layer Depletion and Emerging Public Health Concerns-An Update on Epidemiological Perspective of the Ambivalent Effects of Ultraviolet Radiation Exposure. Front. Oncol. 2022, 12, 866733. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.-C.; Lee, H.-C. Skin cancer dermoscopy images classification with meta data via deep learning ensemble. In Proceedings of the 2020 International Computer Symposium (ICS), Tainan, Taiwan, 17–19 December 2020; pp. 237–241. [Google Scholar]
- Kim, H.S.; Kim, H.J.; Hong, E.S.; Kim, K.B.; Lee, J.D.; Kang, T.U.; Ahn, H.S. The incidence and survival of melanoma and nonmelanoma skin cancer in patients with vitiligo: A nationwide population-based matched cohort study in Korea. Br. J. Dermatol. 2020, 182, 907–915. [Google Scholar] [CrossRef] [PubMed]
- Giaquinto, A.N.; Miller, K.D.; Tossas, K.Y.; Winn, R.A.; Jemal, A.; Siegel, R.L. Cancer statistics for African American/Black People 2022. CA A Cancer J. Clin. 2022, 72, 202–229. [Google Scholar] [CrossRef] [PubMed]
- Islami, F.; Guerra, C.E.; Minihan, A.; Yabroff, K.R.; Fedewa, S.A.; Sloan, K.; Wiedt, T.L.; Thomson, B.; Siegel, R.L.; Nargis, N. American Cancer Society’s report on the status of cancer disparities in the United States, 2021. CA A Cancer J. Clin. 2022, 72, 112–143. [Google Scholar] [CrossRef] [PubMed]
- Iwagami, M.; Caplin, B.; Smeeth, L.; Tomlinson, L.A.; Nitsch, D. Clinical Codelist—Read Codes for Hypothyroidism; Data Collection; London School of Hygiene & Tropical Medicine: London, UK, 2018. [Google Scholar] [CrossRef]
- Arnold, M.; Singh, D.; Laversanne, M.; Vignat, J.; Vaccarella, S.; Meheus, F.; Cust, A.E.; de Vries, E.; Whiteman, D.C.; Bray, F. Global burden of cutaneous melanoma in 2020 and projections to 2040. JAMA Dermatol. 2022, 158, 495–503. [Google Scholar] [CrossRef] [PubMed]
- Di Carlo, V.; Stiller, C.A.; Eisemann, N.; Bordoni, A.; Matz, M.; Curado, M.P.; Daubisse-Marliac, L.; Valkov, M.; Bulliard, J.L.; Morrison, D. Does the morphology of cutaneous melanoma help explain the international differences in survival? Results from 1,578,482 adults diagnosed during 2000–2014 in 59 countries (CONCORD-3). Br. J. Dermatol. 2022, 187, 364–380. [Google Scholar] [CrossRef]
- Perez, E.; Ventura, S. Multi-view Deep Neural Networks for multiclass skin lesion diagnosis. In Proceedings of the 2022 IEEE International Conference on Omni-layer Intelligent Systems (COINS), Barcelona, Spain, 1–3 August 2022; pp. 1–6. [Google Scholar]
- Siegel, R.; Ward, E.; Brawley, O.; Jemal, A. Cancer statistics, 2011: The impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA A Cancer J. Clin. 2011, 61, 212–236. [Google Scholar] [CrossRef]
- Gochi, A.; Orita, K.; Fuchimoto, S.; Tanaka, N.; Ogawa, N. The prognostic advantage of preoperative intratumoral injection of OK-432 for gastric cancer patients. Br. J. Cancer 2001, 84, 443–451. [Google Scholar] [CrossRef]
- Ishihara, K.; Saida, T.; Otsuka, F.; Yamazaki, N. Statistical profiles of malignant melanoma and other skin cancers in Japan: 2007 update. Int. J. Clin. Oncol. 2008, 13, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Gloster, H.M., Jr.; Brodland, D.G. The epidemiology of skin cancer. Dermatol. Surg. 1996, 22, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Reilly, P.; Cosh, J.; Maddison, P.; Rasker, J.; Silman, A. Mortality and survival in rheumatoid arthritis: A 25 year prospective study of 100 patients. Ann. Rheum. Dis. 1990, 49, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Tseng, W.-P. Effects and dose-response relationships of skin cancer and blackfoot disease with arsenic. Environ. Health Perspect. 1977, 19, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Phadke, J.G. Survival pattern and cause of death in patients with multiple sclerosis: Results from an epidemiological survey in north east Scotland. J. Neurol. Neurosurg. Psychiatry 1987, 50, 523–531. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiya, K.; Aoyama, T.; Ju, M.; Atsumi, Y.; Kazama, K.; Numata, M.; Tamagawa, H.; Yukawa, N.; Rino, Y. A Case of Rectal Cancer with Brain and Skin Metastasis with Long-Term Survival Managed by Multidisciplinary Therapy. Gan Kagaku Ryoho 2022, 49, 1148–1150. [Google Scholar]
- Celebi, M.E.; Iyatomi, H.; Schaefer, G.; Stoecker, W.V. Lesion border detection in dermoscopy images. Comput. Med. Imaging Graph. 2009, 33, 148–153. [Google Scholar] [CrossRef]
- Fernandes, S.L.; Chakraborty, B.; Gurupur, V.P.; Prabhu, G.A. Early skin cancer detection using computer aided diagnosis techniques. J. Integr. Des. Process. Sci. 2016, 20, 33–43. [Google Scholar] [CrossRef]
- Adla, D.; Reddy, G.; Nayak, P.; Karuna, G. Deep learning-based computer aided diagnosis model for skin cancer detection and classification. Distrib. Parallel Databases 2022, 40, 717–736. [Google Scholar] [CrossRef]
- Tsai, C.L.; Mukundan, A.; Chung, C.S.; Chen, Y.H.; Wang, Y.K.; Chen, T.H.; Tseng, Y.S.; Huang, C.W.; Wu, I.C.; Wang, H.C. Hyperspectral Imaging Combined with Artificial Intelligence in the Early Detection of Esophageal Cancer. Cancers 2021, 13, 4593. [Google Scholar] [CrossRef]
- Xu, Z.; Sheykhahmad, F.R.; Ghadimi, N.; Razmjooy, N. Computer-aided diagnosis of skin cancer based on soft computing techniques. Open Med. 2020, 15, 860–871. [Google Scholar] [CrossRef] [PubMed]
- Jaleel, J.A.; Salim, S.; Aswin, R. Computer aided detection of skin cancer. In Proceedings of the 2013 International Conference on Circuits, Power and Computing Technologies (ICCPCT), Nagercoil, India, 20–21 March 2013; pp. 1137–1142. [Google Scholar]
- Kumar, K.A.; Vanmathi, C. Optimization driven model and segmentation network for skin cancer detection. Comput. Electr. Eng. 2022, 103, 108359. [Google Scholar] [CrossRef]
- Filali, Y.; EL Khoukhi, H.; Sabri, M.A.; Aarab, A. Efficient fusion of handcrafted and pre-trained CNNs features to classify melanoma skin cancer. Multimed. Tools Appl. 2020, 79, 31219–31238. [Google Scholar] [CrossRef]
- Mohanty, S.P.; Kougianos, E. Biosensors: A tutorial review. Ieee Potentials 2006, 25, 35–40. [Google Scholar] [CrossRef]
- Malibari, A.A.; Alzahrani, J.S.; Eltahir, M.M.; Malik, V.; Obayya, M.; Al Duhayyim, M.; Neto, A.V.L.; de Albuquerque, V.H.C. Optimal deep neural network-driven computer aided diagnosis model for skin cancer. Comput. Electr. Eng. 2022, 103, 108318. [Google Scholar] [CrossRef]
- Bratchenko, I.A.; Bratchenko, L.A.; Moryatov, A.A.; Khristoforova, Y.A.; Artemyev, D.N.; Myakinin, O.O.; Orlov, A.E.; Kozlov, S.V.; Zakharov, V.P. In vivo diagnosis of skin cancer with a portable Raman spectroscopic device. Exp. Dermatol. 2021, 30, 652–663. [Google Scholar] [CrossRef] [PubMed]
- Bohunicky, B.; Mousa, S. Biosensors: The new wave in cancer diagnosis. Nanotechnol. Sci. Appl. 2010, 4, 1–10. [Google Scholar]
- Keshavarz, A.; Vafapour, Z. Water-based terahertz metamaterial for skin cancer detection application. IEEE Sens. J. 2018, 19, 1519–1524. [Google Scholar] [CrossRef]
- Lalitha, K.; Lakshmi, K. An overview on biosensors. Int. J. Pharm. Chem. Biol. Sci. 2017, 7, 293–302. [Google Scholar]
- Ashraf, R.; Afzal, S.; Rehman, A.U.; Gul, S.; Baber, J.; Bakhtyar, M.; Mehmood, I.; Song, O.-Y.; Maqsood, M. Region-of-interest based transfer learning assisted framework for skin cancer detection. IEEE Access 2020, 8, 147858–147871. [Google Scholar] [CrossRef]
- Alheejawi, S.; Xu, H.; Berendt, R.; Jha, N.; Mandal, M. Novel lymph node segmentation and proliferation index measurement for skin melanoma biopsy images. Comput. Med. Imaging Graph. 2019, 73, 19–29. [Google Scholar] [CrossRef]
- Vocaturo, E.; Perna, D.; Zumpano, E. Machine learning techniques for automated melanoma detection. In Proceedings of the 2019 IEEE International Conference on Bioinformatics and Biomedicine (BIBM), San Diego, CA, USA, 18–21 November 2019; pp. 2310–2317. [Google Scholar]
- Rey-Barroso, L.; Peña-Gutiérrez, S.; Yáñez, C.; Burgos-Fernández, F.J.; Vilaseca, M.; Royo, S. Optical technologies for the improvement of skin cancer diagnosis: A review. Sensors 2021, 21, 252. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Li, H.; Jin, Z. A visually interpretable deep learning framework for histopathological image-based skin cancer diagnosis. IEEE J. Biomed. Health Inform. 2021, 25, 1483–1494. [Google Scholar] [CrossRef] [PubMed]
- Saba, T. Computer vision for microscopic skin cancer diagnosis using handcrafted and non-handcrafted features. Microsc. Res. Tech. 2021, 84, 1272–1283. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Alshehri, M.; AlGhamdi, R.; Sharma, P.; Deep, V. A de-ann inspired skin cancer detection approach using fuzzy c-means clustering. Mob. Netw. Appl. 2020, 25, 1319–1329. [Google Scholar] [CrossRef]
- Premaladha, J.; Ravichandran, K. Novel approaches for diagnosing melanoma skin lesions through supervised and deep learning algorithms. J. Med. Syst. 2016, 40, 96. [Google Scholar] [CrossRef] [PubMed]
- Afifi, S.; GholamHosseini, H.; Sinha, R. SVM classifier on chip for melanoma detection. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2017, 2017, 270–274. [Google Scholar] [CrossRef] [PubMed]
- Thiem, D.G.; Römer, P.; Blatt, S.; Al-Nawas, B.; Kämmerer, P.W. New Approach to the Old Challenge of Free Flap Monitoring—Hyperspectral Imaging Outperforms Clinical Assessment by Earlier Detection of Perfusion Failure. J. Pers. Med. 2021, 11, 1101. [Google Scholar] [CrossRef]
- Ghamisi, P.; Rasti, B.; Yokoya, N.; Wang, Q.; Hofle, B.; Bruzzone, L.; Bovolo, F.; Chi, M.; Anders, K.; Gloaguen, R. Multisource and multitemporal data fusion in remote sensing: A comprehensive review of the state of the art. IEEE Geosci. Remote Sens. Mag. 2019, 7, 6–39. [Google Scholar] [CrossRef]
- Li, Q.; He, X.; Wang, Y.; Liu, H.; Xu, D.; Guo, F. Review of spectral imaging technology in biomedical engineering: Achievements and challenges. J. Biomed. Opt. 2013, 18, 100901. [Google Scholar] [CrossRef]
- Lu, G.; Fei, B. Medical hyperspectral imaging: A review. J. Biomed. Opt. 2014, 19, 010901. [Google Scholar] [CrossRef]
- Zakian, C.M.; Pretty, I.A.; Ellwood, R. Near-infared hyperspectral imaging of teeth for dental caries detection. J. Biomed. Opt. 2009, 14, 064047. [Google Scholar] [CrossRef] [PubMed]
- Tromberg, B.J.; Shah, N.; Lanning, R.; Cerussi, A.; Espinoza, J.; Pham, T.; Svaasand, L.; Butler, J. Non-invasive in vivo characterization of breast tumors using photon migration spectroscopy. Neoplasia 2000, 2, 26–40. [Google Scholar] [CrossRef] [PubMed]
- Cerussi, A.E.; Berger, A.J.; Bevilacqua, F.; Shah, N.; Jakubowski, D.; Butler, J.; Holcombe, R.F.; Tromberg, B.J. Sources of absorption and scattering contrast for near-infrared optical mammography. Acad. Radiol. 2001, 8, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Bi, D.; Zhu, D.; Sheykhahmad, F.R.; Qiao, M. Computer-aided skin cancer diagnosis based on a New meta-heuristic algorithm combined with support vector method. Biomed. Signal Process. Control 2021, 68, 102631. [Google Scholar] [CrossRef]
- Barducci, A.; Guzzi, D.; Marcoionni, P.; Pippi, I. Aerospace wetland monitoring by hyperspectral imaging sensors: A case study in the coastal zone of San Rossore Natural Park. J. Environ. Manag. 2009, 90, 2278–2286. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.-W. Hyperspectral Imaging for Food Quality Analysis and Control; Elsevier: Amsterdam, The Netherlands, 2010. [Google Scholar]
- Lu, B.; Dao, P.D.; Liu, J.; He, Y.; Shang, J. Recent advances of hyperspectral imaging technology and applications in agriculture. Remote Sens. 2020, 12, 2659. [Google Scholar] [CrossRef]
- Fei, B. Hyperspectral imaging in medical applications. In Data Handling in Science and Technology; Elsevier: Amsterdam, The Netherlands, 2020; Volume 32, pp. 523–565. [Google Scholar]
- Lee, C.-H.; Mukundan, A.; Chang, S.-C.; Wang, Y.-L.; Lu, S.-H.; Huang, Y.-C.; Wang, H.-C. Comparative Analysis of Stress and Deformation between One-Fenced and Three-Fenced Dental Implants Using Finite Element Analysis. J. Clin. Med. 2021, 10, 3986. [Google Scholar] [CrossRef]
- Hege, E.K.; O’Connell, D.; Johnson, W.; Basty, S.; Dereniak, E.L. Hyperspectral imaging for astronomy and space surveillance. Imaging Spectrom. IX 2004, 5159, 380–391. [Google Scholar]
- Courtenay, L.A.; González-Aguilera, D.; Lagüela, S.; Del Pozo, S.; Ruiz-Mendez, C.; Barbero-García, I.; Román-Curto, C.; Cañueto, J.; Santos-Durán, C.; Cardeñoso-Álvarez, M.E. Hyperspectral imaging and robust statistics in non-melanoma skin cancer analysis. Biomed. Opt. Express 2021, 12, 5107–5127. [Google Scholar] [CrossRef]
- Tsai, T.-J.; Mukundan, A.; Chi, Y.-S.; Tsao, Y.-M.; Wang, Y.-K.; Chen, T.-H.; Wu, I.-C.; Huang, C.-W.; Wang, H.-C. Intelligent Identification of Early Esophageal Cancer by Band-Selective Hyperspectral Imaging. Cancers 2022, 14, 4292. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.-J.; Mukundan, A.; Tsao, Y.-M.; Huang, C.-W.; Wang, H.-C. Identification of Early Esophageal Cancer by Semantic Segmentation. J. Pers. Med. 2022, 12, 1204. [Google Scholar] [CrossRef] [PubMed]
- Aboughaleb, I.H.; Aref, M.H.; El-Sharkawy, Y.H. Hyperspectral imaging for diagnosis and detection of ex-vivo breast cancer. Photodiagnosis Photodyn. Ther. 2020, 31, 101922. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Yu, T.; Hu, B.; Hou, X.; Zhang, Z.; Liu, X.; Liu, J.; Wang, X.; Zhong, J.; Tan, Z. Uav-borne hyperspectral imaging remote sensing system based on acousto-optic tunable filter for water quality monitoring. Remote Sens. 2021, 13, 4069. [Google Scholar] [CrossRef]
- Liu, H.; Bruning, B.; Garnett, T.; Berger, B. Hyperspectral imaging and 3D technologies for plant phenotyping: From satellite to close-range sensing. Comput. Electron. Agric. 2020, 175, 105621. [Google Scholar] [CrossRef]
- Shrestha, S.; Knapič, M.; Žibrat, U.; Deleuran, L.C.; Gislum, R. Single seed near-infrared hyperspectral imaging in determining tomato (Solanum lycopersicum L.) seed quality in association with multivariate data analysis. Sens. Actuators B Chem. 2016, 237, 1027–1034. [Google Scholar] [CrossRef]
- Wu, N.; Liu, F.; Meng, F.; Li, M.; Zhang, C.; He, Y. Rapid and accurate varieties classification of different crop seeds under sample-limited condition based on hyperspectral imaging and deep transfer learning. Front. Bioeng. Biotechnol. 2021, 9, 696292. [Google Scholar] [CrossRef]
- Hsiao, Y.-P.; Mukundan, A.; Chen, W.-C.; Wu, M.-T.; Hsieh, S.-C.; Wang, H.-C. Design of a Lab-On-Chip for Cancer Cell Detection through Impedance and Photoelectrochemical Response Analysis. Biosensors 2022, 12, 405. [Google Scholar] [CrossRef]
- Stuart, M.B.; McGonigle, A.J.; Willmott, J.R. Hyperspectral imaging in environmental monitoring: A review of recent developments and technological advances in compact field deployable systems. Sensors 2019, 19, 3071. [Google Scholar] [CrossRef]
- Chen, C.-W.; Tseng, Y.-S.; Mukundan, A.; Wang, H.-C. Air Pollution: Sensitive Detection of PM2.5 and PM10 Concentration Using Hyperspectral Imaging. Appl. Sci. 2021, 11, 4543. [Google Scholar] [CrossRef]
- Huang, S.-Y.; Mukundan, A.; Tsao, Y.-M.; Kim, Y.; Lin, F.-C.; Wang, H.-C. Recent Advances in Counterfeit Art, Document, Photo, Hologram, and Currency Detection Using Hyperspectral Imaging. Sensors 2022, 22, 7308. [Google Scholar] [CrossRef] [PubMed]
- Mukundan, A.; Tsao, Y.-M.; Lin, F.-C.; Wang, H.-C. Portable and low-cost hologram verification module using a snapshot-based hyperspectral imaging algorithm. Sci. Rep. 2022, 12, 18475. [Google Scholar] [CrossRef] [PubMed]
- Mukundan, A.; Wang, H.-C.; Tsao, Y.-M. A Novel Multipurpose Snapshot Hyperspectral Imager used to Verify Security Hologram. In Proceedings of the 2022 International Conference on Engineering and Emerging Technologies (ICEET), Kuala Lumpur, Malaysia, 27–28 October 2022; pp. 1–3. [Google Scholar]
- Mukundan, A.; Tsao, Y.-M.; Cheng, W.-M.; Lin, F.-C.; Wang, H.-C. Automatic Counterfeit Currency Detection Using a Novel Snapshot Hyperspectral Imaging Algorithm. Sensors 2023, 23, 2026. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, S.J.; Lodder, R.A. Hyperspectral imaging technology for pharmaceutical analysis. Biomed. Nanotechnol. Archit. Appl. 2002, 4626, 136–147. [Google Scholar]
- Chang, L.; Zhang, M.; Li, W. A coarse-to-fine approach for medical hyperspectral image classification with sparse representation. AOPC 2017 Opt. Spectrosc. Imaging 2017, 10461, 136–144. [Google Scholar]
- Yang, K.-Y.; Fang, Y.-J.; Karmakar, R.; Mukundan, A.; Tsao, Y.-M.; Huang, C.-W.; Wang, H.-C. Assessment of Narrow Band Imaging Algorithm for Video Capsule Endoscopy Based on Decorrelated Color Space for Esophageal Cancer. Cancers 2023, 15, 4715. [Google Scholar] [CrossRef] [PubMed]
- de la Ossa, M.Á.F.; Amigo, J.M.; García-Ruiz, C. Detection of residues from explosive manipulation by near infrared hyperspectral imaging: A promising forensic tool. Forensic Sci. Int. 2014, 242, 228–235. [Google Scholar] [CrossRef] [PubMed]
- Favreau, P.F.; Hernandez, C.; Lindsey, A.S.; Alvarez, D.F.; Rich, T.C.; Prabhat, P.; Leavesley, S.J. Thin-film tunable filters for hyperspectral fluorescence microscopy. J. Biomed. Opt. 2013, 19, 011017. [Google Scholar] [CrossRef]
- Xu, D.; Ni, G.; Jiang, T.; Jiang, L.; Chi, M. Integration of field work and hyperspectral data for oil and gas exploration. In Proceedings of the 2007 IEEE International Geoscience and Remote Sensing Symposium, Barcelona, Spain, 23–28 July 2007; pp. 3194–3197. [Google Scholar]
- Gowen, A.A.; Feng, Y.; Gaston, E.; Valdramidis, V. Recent applications of hyperspectral imaging in microbiology. Talanta 2015, 137, 43–54. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, X.; Hou, Z.; Ning, J.; Zhang, Z. Discrimination of nitrogen fertilizer levels of tea plant (Camellia sinensis) based on hyperspectral imaging. J. Sci. Food Agric. 2018, 98, 4659–4664. [Google Scholar] [CrossRef]
- Huang, S.; Wang, L.; Chen, W.; Lin, D.; Huang, L.; Wu, S.; Feng, S.; Chen, R. Non-invasive optical detection of esophagus cancer based on urine surface-enhanced Raman spectroscopy. In Proceedings of the Twelfth International Conference on Photonics and Imaging in Biology and Medicine (PIBM 2014), Wuhan, China, 17 September 2014; pp. 537–542. [Google Scholar]
- Zabalza, J.; Ren, J.; Wang, Z.; Marshall, S.; Wang, J. Singular spectrum analysis for effective feature extraction in hyperspectral imaging. IEEE Geosci. Remote Sens. Lett. 2014, 11, 1886–1890. [Google Scholar] [CrossRef]
- Fabelo, H.; Ortega, S.; Lazcano, R.; Madroñal, D.; Callicó, G.M.; Juárez, E.; Salvador, R.; Bulters, D.; Bulstrode, H.; Szolna, A. An intraoperative visualization system using hyperspectral imaging to aid in brain tumor delineation. Sensors 2018, 18, 430. [Google Scholar] [CrossRef] [PubMed]
- More, S.S.; Beach, J.M.; McClelland, C.; Mokhtarzadeh, A.; Vince, R. In vivo assessment of retinal biomarkers by hyperspectral imaging: Early detection of Alzheimer’s disease. ACS Chem. Neurosci. 2019, 10, 4492–4501. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-I.; Wu, C.-C.; Liu, K.-H.; Chen, H.-M.; Chen, C.C.-C.; Wen, C.-H. Progressive band processing of linear spectral unmixing for hyperspectral imagery. IEEE J. Sel. Top. Appl. Earth Obs. Remote Sens. 2014, 8, 2583–2597. [Google Scholar] [CrossRef]
- Akbari, H.; Halig, L.V.; Zhang, H.; Wang, D.; Chen, Z.G.; Fei, B. Detection of cancer metastasis using a novel macroscopic hyperspectral method. In Proceedings of the Medical Imaging 2012: Biomedical Applications in Molecular, Structural, and Functional Imaging, San Diego, CA, USA, 14 April 2012; pp. 299–305. [Google Scholar]
- Senan, E.M.; Jadhav, M.E. Classification of dermoscopy images for early detection of skin cancer—A review. Int. J. Comput. Appl. 2019, 975, 8887. [Google Scholar]
- Esteva, A.; Kuprel, B.; Novoa, R.A.; Ko, J.; Swetter, S.M.; Blau, H.M.; Thrun, S. Dermatologist-level classification of skin cancer with deep neural networks. Nature 2017, 542, 115–118. [Google Scholar] [CrossRef] [PubMed]
- Ortega, S.; Fabelo, H.; Iakovidis, D.K.; Koulaouzidis, A.; Callico, G.M. Use of hyperspectral/multispectral imaging in gastroenterology. Shedding some–different–light into the dark. J. Clin. Med. 2019, 8, 36. [Google Scholar] [CrossRef]
- Nachbar, F.; Stolz, W.; Merkle, T.; Cognetta, A.B.; Vogt, T.; Landthaler, M.; Bilek, P.; Braun-Falco, O.; Plewig, G. The ABCD rule of dermatoscopy: High prospective value in the diagnosis of doubtful melanocytic skin lesions. J. Am. Acad. Dermatol. 1994, 30, 551–559. [Google Scholar] [CrossRef]
- Tsao, H.; Olazagasti, J.M.; Cordoro, K.M.; Brewer, J.D.; Taylor, S.C.; Bordeaux, J.S.; Chren, M.-M.; Sober, A.J.; Tegeler, C.; Bhushan, R. Early detection of melanoma: Reviewing the ABCDEs. J. Am. Acad. Dermatol. 2015, 72, 717–723. [Google Scholar] [CrossRef]
- Peters, M.S.; Winkelmann, R.K. The biopsy. Dermatol. Clin. 1984, 2, 209–217. [Google Scholar] [CrossRef]
- Wollina, U.; Burroni, M.; Torricelli, R.; Gilardi, S.; Dell’Eva, G.; Helm, C.; Bardey, W. Digital dermoscopy in clinical practise: A three-centre analysis. Ski. Res. Technol. 2007, 13, 133–142. [Google Scholar] [CrossRef]
- Tudor, A.; Feldman, J.; Diamandis, C. Why the ABCDE Rule Is Not Helpful but Dangerous in Skin Cancer Prevention. Zenodo, 27 November 2021. Available online: https://www.scanoma.com/blog/why-the-abcde-rule-is-not-helpful-but-dangerous-in-skin-cancer-prevention (accessed on 26 November 2023).
- Fabelo, H.; Melián, V.; Martínez, B.; Beltrán, P.; Ortega, S.; Marrero, M.; Callicó, G.M.; Sarmiento, R.; Castaño, I.; Carretero, G. Dermatologic hyperspectral imaging system for skin cancer diagnosis assistance. In Proceedings of the 2019 XXXIV Conference on Design of Circuits and Integrated Systems (DCIS), Bilbao, Spain, 20–22 November 2019; pp. 1–6. [Google Scholar]
- Jain, S.; Pise, N. Computer aided melanoma skin cancer detection using image processing. Procedia Comput. Sci. 2015, 48, 735–740. [Google Scholar] [CrossRef]
- Serao, N.; Delfino, K.; Southey, B.; Zas, S.R. Development of a transcriptomic-based index to prognosticate cancer. ISBRA 2010 2010, 2010, 42. [Google Scholar]
- Carli, P.; Quercioli, E.; Sestini, S.; Stante, M.; Ricci, L.; Brunasso, G.; De Giorgi, V. Pattern analysis, not simplified algorithms, is the most reliable method for teaching dermoscopy for melanoma diagnosis to residents in dermatology. Br. J. Dermatol. 2003, 148, 981–984. [Google Scholar] [CrossRef]
- Kasmi, R.; Mokrani, K. Classification of malignant melanoma and benign skin lesions: Implementation of automatic ABCD rule. IET Image Process. 2016, 10, 448–455. [Google Scholar] [CrossRef]
- Ali, A.-R.H.; Li, J.; Yang, G. Automating the ABCD rule for melanoma detection: A survey. IEEE Access 2020, 8, 83333–83346. [Google Scholar] [CrossRef]
- Ahnlide, I.; Bjellerup, M.; Nilsson, F.; Nielsen, K. Validity of ABCD rule of dermoscopy in clinical practice. Acta Derm. Venereol. 2016, 96, 367–372. [Google Scholar] [CrossRef] [PubMed]
- Binder, M.; Kittler, H.; Steiner, A.; Dawid, M.; Pehamberger, H.; Wolff, K. Reevaluation of the ABCD rule for epiluminescence microscopy. J. Am. Acad. Dermatol. 1999, 40, 171–176. [Google Scholar] [CrossRef]
- Milton, M.A.A. Automated skin lesion classification using ensemble of deep neural networks in ISIC 2018: Skin lesion analysis towards melanoma detection challenge. arXiv 2019, arXiv:1901.10802. [Google Scholar]
- Basov, S.; Dankner, Y.; Weinstein, M.; Katzir, A.; Platkov, M. Noninvasive mid-IR fiber-optic evanescent wave spectroscopy (FEWS) for early detection of skin cancers. Med. Phys. 2020, 47, 5523–5530. [Google Scholar] [CrossRef]
- Saba, T. Recent advancement in cancer detection using machine learning: Systematic survey of decades, comparisons and challenges. J. Infect. Public Health 2020, 13, 1274–1289. [Google Scholar] [CrossRef]
- Jerant, A.F.; Johnson, J.T.; Sheridan, C.D.; Caffrey, T.J. Early detection and treatment of skin cancer. Am. Fam. Physician 2000, 62, 357–368. [Google Scholar]
- Ragab, M.; Choudhry, H.; Al-Rabia, M.W.; Binyamin, S.S.; Aldarmahi, A.A.; Mansour, R.F. Early and accurate detection of melanoma skin cancer using hybrid level set approach. Front. Physiol. 2022, 13, 965630. [Google Scholar] [CrossRef] [PubMed]
- Masood, A.; Ali Al-Jumaily, A. Computer aided diagnostic support system for skin cancer: A review of techniques and algorithms. Int. J. Biomed. Imaging 2013, 2013, 323268. [Google Scholar] [CrossRef]
- Johr, R.H. Dermoscopy: Alternative melanocytic algorithms—The ABCD rule of dermatoscopy, menzies scoring method, and 7-point checklist. Clin. Dermatol. 2002, 20, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Kumar, Y.; Gupta, S.; Singla, R.; Hu, Y.-C. A systematic review of artificial intelligence techniques in cancer prediction and diagnosis. Arch. Comput. Methods Eng. 2021, 29, 2043–2070. [Google Scholar] [CrossRef]
- Abbasi, N.R.; Shaw, H.M.; Rigel, D.S.; Friedman, R.J.; McCarthy, W.H.; Osman, I.; Kopf, A.W.; Polsky, D. Early diagnosis of cutaneous melanoma: Revisiting the ABCD criteria. JAMA 2004, 292, 2771–2776. [Google Scholar] [CrossRef]
- Burlina, P.; Billings, S.; Joshi, N.; Albayda, J. Automated diagnosis of myositis from muscle ultrasound: Exploring the use of machine learning and deep learning methods. PLoS ONE 2017, 12, e0184059. [Google Scholar] [CrossRef] [PubMed]
- Chilamkurthy, S.; Ghosh, R.; Tanamala, S.; Biviji, M.; Campeau, N.G.; Venugopal, V.K.; Mahajan, V.; Rao, P.; Warier, P. Deep learning algorithms for detection of critical findings in head CT scans: A retrospective study. Lancet 2018, 392, 2388–2396. [Google Scholar] [CrossRef]
- Bianconi, F.; Fravolini, M.L.; Pizzoli, S.; Palumbo, I.; Minestrini, M.; Rondini, M.; Nuvoli, S.; Spanu, A.; Palumbo, B. Comparative evaluation of conventional and deep learning methods for semi-automated segmentation of pulmonary nodules on CT. Quant. Imaging Med. Surg. 2021, 11, 3286. [Google Scholar] [CrossRef]
- Javed, R.; Rahim, M.S.M.; Saba, T.; Rehman, A. A comparative study of features selection for skin lesion detection from dermoscopic images. Netw. Model. Anal. Health Inform. Bioinform. 2020, 9, 4. [Google Scholar] [CrossRef]
- Hagerty, J.R.; Stanley, R.J.; Almubarak, H.A.; Lama, N.; Kasmi, R.; Guo, P.; Drugge, R.J.; Rabinovitz, H.S.; Oliviero, M.; Stoecker, W.V. Deep learning and handcrafted method fusion: Higher diagnostic accuracy for melanoma dermoscopy images. IEEE J. Biomed. Health Inform. 2019, 23, 1385–1391. [Google Scholar] [CrossRef]
- Whiting, P.F.; Rutjes, A.W.; Westwood, M.E.; Mallett, S.; Deeks, J.J.; Reitsma, J.B.; Leeflang, M.M.; Sterne, J.A.; Bossuyt, P.M.; QUADAS-2 Group. QUADAS-2: A revised tool for the quality assessment of diagnostic accuracy studies. Ann. Intern. Med. 2011, 155, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Cook, C.; Cleland, J.; Huijbregts, P. Creation and critique of studies of diagnostic accuracy: Use of the STARD and QUADAS methodological quality assessment tools. J. Man. Manip. Ther. 2007, 15, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Mallett, S.; Takwoingi, Y.; Davenport, C.F.; Hyde, C.J.; Whiting, P.F.; Deeks, J.J.; Leeflang, M.M.; QUADAS-C Group; Bossuyt, P.M.M.; et al. QUADAS-C: A tool for assessing risk of bias in comparative diagnostic accuracy studies. Ann. Intern. Med. 2021, 174, 1592–1599. [Google Scholar] [CrossRef] [PubMed]
- Sounderajah, V.; Ashrafian, H.; Rose, S.; Shah, N.H.; Ghassemi, M.; Golub, R.; Kahn, C.E.; Esteva, A.; Karthikesalingam, A.; Mateen, B. A quality assessment tool for artificial intelligence-centered diagnostic test accuracy studies: QUADAS-AI. Nat. Med. 2021, 27, 1663–1665. [Google Scholar] [CrossRef] [PubMed]
- Schueler, S.; Schuetz, G.M.; Dewey, M. The revised QUADAS-2 tool. Ann. Intern. Med. 2012, 156, 323. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Mulder, F.; Leeflang, M.; Wolff, R.; Whiting, P.; Bossuyt, P.M. QUAPAS: An Adaptation of the QUADAS-2 Tool to Assess Prognostic Accuracy Studies. Ann. Intern. Med. 2022, 175, 1010–1018. [Google Scholar] [CrossRef]
- Mann, R.; Hewitt, C.E.; Gilbody, S.M. Assessing the quality of diagnostic studies using psychometric instruments: Applying QUADAS. Soc. Psychiatry Psychiatr. Epidemiol. 2009, 44, 300–307. [Google Scholar] [CrossRef]
- Lumbreras, B.; Porta, M.; Márquez, S.; Pollán, M.; Parker, L.A.; Hernández-Aguado, I. QUADOMICS: An adaptation of the Quality Assessment of Diagnostic Accuracy Assessment (QUADAS) for the evaluation of the methodological quality of studies on the diagnostic accuracy of ‘-omics’-based technologies. Clin. Biochem. 2008, 41, 1316–1325. [Google Scholar] [CrossRef]
- Leon, R.; Martinez-Vega, B.; Fabelo, H.; Ortega, S.; Melian, V.; Castaño, I.; Carretero, G.; Almeida, P.; Garcia, A.; Quevedo, E. Non-invasive skin cancer diagnosis using hyperspectral imaging for in-situ clinical support. J. Clin. Med. 2020, 9, 1662. [Google Scholar] [CrossRef]
- Lindholm, V.; Raita-Hakola, A.-M.; Annala, L.; Salmivuori, M.; Jeskanen, L.; Saari, H.; Koskenmies, S.; Pitkänen, S.; Pölönen, I.; Isoherranen, K. Differentiating Malignant from Benign Pigmented or Non-Pigmented Skin Tumours—A Pilot Study on 3D Hyperspectral Imaging of Complex Skin Surfaces and Convolutional Neural Networks. J. Clin. Med. 2022, 11, 1914. [Google Scholar] [CrossRef]
- Christensen, G.B.; Nagaoka, T.; Kiyohara, Y.; Johansson, I.; Ingvar, C.; Nakamura, A.; Sota, T.; Nielsen, K. Clinical performance of a novel hyperspectral imaging device for cutaneous melanoma and pigmented skin lesions in Caucasian skin. Ski. Res. Technol. 2021, 27, 803–809. [Google Scholar] [CrossRef] [PubMed]
- Hosking, A.M.; Coakley, B.J.; Chang, D.; Talebi-Liasi, F.; Lish, S.; Lee, S.W.; Zong, A.M.; Moore, I.; Browning, J.; Jacques, S.L. Hyperspectral imaging in automated digital dermoscopy screening for melanoma. Lasers Surg. Med. 2019, 51, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Pozhar, V.E.; Machikhin, A.S.; Myakinin, O.O.; Bratchenko, I.A. Application of Acousto-Optical Hyperspectral Imaging for Skin Cancer Diagnostics. In Multimodal Optical Diagnostics of Cancer; Springer: Berlin/Heidelberg, Germany, 2020; pp. 505–536. [Google Scholar]
- Pardo, A.; Gutiérrez-Gutiérrez, J.A.; Lihacova, I.; López-Higuera, J.M.; Conde, O.M. On the spectral signature of melanoma: A non-parametric classification framework for cancer detection in hyperspectral imaging of melanocytic lesions. Biomed. Opt. Express 2018, 9, 6283–6301. [Google Scholar] [CrossRef] [PubMed]
- Vinokurov, V.; Khristoforova, Y.; Myakinin, O.; Bratchenko, I.; Moryatov, A.; Machikhin, A.; Zakharov, V. Neural network classifier for hyperspectral images of skin pathologies. J. Phys. Conf. Ser. 2021, 2127, 012026. [Google Scholar] [CrossRef]
- Räsänen, J.; Salmivuori, M.; Pölönen, I.; Grönroos, M.; Neittaanmäki, N. Hyperspectral Imaging Reveals Spectral Differences and Can Distinguish Malignant Melanoma from Pigmented Basal Cell Carcinomas: A Pilot Study. Acta Derm. Venereol. 2021, 101, adv00405. [Google Scholar] [CrossRef] [PubMed]
- Nagaoka, T.; Nakamura, A.; Kiyohara, Y.; Sota, T. Melanoma screening system using hyperspectral imager attached to imaging fiberscope. In Proceedings of the 2012 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, San Diego, CA, USA, 28 August 2012–1 September 2012; pp. 3728–3731. [Google Scholar]
- Zherdeva, L.A.; Bratchenko, I.A.; Myakinin, O.O.; Moryatov, A.A.; Kozlov, S.V.; Zakharov, V.P. In vivo hyperspectral imaging and differentiation of skin cancer. In Proceedings of the Optics in Health Care and Biomedical Optics VII, Beijing, China, 31 October 2016; pp. 658–665. [Google Scholar]
- Huang, H.-Y.; Hsiao, Y.-P.; Mukundan, A.; Tsao, Y.-M.; Chang, W.-Y.; Wang, H.-C. Classification of Skin Cancer Using Novel Hyperspectral Imaging Engineering via YOLOv5. J. Clin. Med. 2023, 12, 1134. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Zeng, N.; Wang, N. Sensitivity, specificity, accuracy, associated confidence interval and ROC analysis with practical SAS implementations. NESUG Proc. Health Care Life Sci. Baltim. Md. 2010, 19, 67. [Google Scholar]
- Matinfar, M.; Shahidi, S.; Feizi, A. Incidence of nonmelanoma skin cancer in renal transplant recipients: A systematic review and meta-analysis. J. Res. Med. Sci. Off. J. Isfahan Univ. Med. Sci. 2018, 23, 14. [Google Scholar]
- Gandini, S.; Palli, D.; Spadola, G.; Bendinelli, B.; Cocorocchio, E.; Stanganelli, I.; Miligi, L.; Masala, G.; Caini, S. Anti-hypertensive drugs and skin cancer risk: A review of the literature and meta-analysis. Crit. Rev. Oncol. Hematol. 2018, 122, 1–9. [Google Scholar] [CrossRef]
- Sharon, E.; Snast, I.; Lapidoth, M.; Kaftory, R.; Mimouni, D.; Hodak, E.; Levi, A. Laser treatment for non-melanoma skin cancer: A systematic review and meta-analysis. Am. J. Clin. Dermatol. 2021, 22, 25–38. [Google Scholar] [CrossRef] [PubMed]
- Arafa, A.; Mostafa, A.; Navarini, A.A.; Dong, J.-Y. The association between smoking and risk of skin cancer: A meta-analysis of cohort studies. Cancer Causes Control 2020, 31, 787–794. [Google Scholar] [CrossRef] [PubMed]
- Jiyad, Z.; Olsen, C.; Burke, M.; Isbel, N.; Green, A.C. Azathioprine and risk of skin cancer in organ transplant recipients: Systematic review and meta-analysis. Am. J. Transplant. 2016, 16, 3490–3503. [Google Scholar] [CrossRef] [PubMed]
- Glas, A.S.; Lijmer, J.G.; Prins, M.H.; Bonsel, G.J.; Bossuyt, P.M. The diagnostic odds ratio: A single indicator of test performance. J. Clin. Epidemiol. 2003, 56, 1129–1135. [Google Scholar] [CrossRef] [PubMed]
- Duke, A.A.; Bègue, L.; Bell, R.; Eisenlohr-Moul, T. Revisiting the serotonin–aggression relation in humans: A meta-analysis. Psychol. Bull. 2013, 139, 1148. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Wan, S.; Piao, S.; Knapp, A.K.; Classen, A.T.; Vicca, S.; Ciais, P.; Hovenden, M.J.; Leuzinger, S.; Beier, C. A meta-analysis of 1119 manipulative experiments on terrestrial carbon-cycling responses to global change. Nat. Ecol. Evol. 2019, 3, 1309–1320. [Google Scholar] [CrossRef] [PubMed]
- Cartiff, B.M.; Duke, R.F.; Greene, J.A. The effect of epistemic cognition interventions on academic achievement: A meta-analysis. J. Educ. Psychol. 2021, 113, 477. [Google Scholar] [CrossRef]
- Greene, J.A.; Cartiff, B.M.; Duke, R.F. A meta-analytic review of the relationship between epistemic cognition and academic achievement. J. Educ. Psychol. 2018, 110, 1084. [Google Scholar] [CrossRef]


| Risk of Bias | Applicability Concerns | ||||||
|---|---|---|---|---|---|---|---|
| Study | Patient Selection | Index Test | Reference Standard | Flow and Timing | Patient Selection | Index Test | Reference Standard |
| Leon et al. [136] |  |  |  |  |  |  |  |
| Lindholm et al. [137] |  |  |  |  |  |  |  |
| Christensen et al. [138] |  |  |  |  |  |  |  |
| Hosking et al. [139] |  |  |  |  |  |  |  |
| Pozhar et al. [140] |  |  |  |  |  |  |  |
| Pardo et al. [141] |  |  |  |  |  |  |  |
| Vinokurov et al. [142] |  |  |  |  |  |  |  |
| Rasanen et al. [143] |  |  |  |  |  |  |  |
| T. Nagaoka et al. [144] |  |  |  |  |  |  |  |
| Zherdeva et al. [145] |  |  |  |  |  |  |  |
 Low Risk;
Low Risk;  High Risk;
High Risk;  Unclear Risk.
Unclear Risk.| Author/ Year | No. of Patients | No. of Images | Wavelength (nm) | Sensitivity (%) | Specificity (%) | Nationality of Data | Type of AI | Year of Publication | Area under Curve (AUC) |
|---|---|---|---|---|---|---|---|---|---|
| Leon et al./2020 [136] | 61 | 76 | 450–950 | 87.5 | 100 | Western | SVM | 2020 | 0.89 |
| Lindholm et al./2022 [137] | 33 | 42 | 477–891 | 87 | 93 | Western | CNN | 2022 | NA |
| Christens et al./2021 [138] | 186 | 202 | 400–800 | 96.7 | 42.1 | Asian | DI | 2021 | 0.800 |
| Hosking et al./2019 [139] | 91 | 100 | 350–950 | 100 | 36 | Western | SVM | 2019 | 1 |
| Pozhar et al./2020 [140] | 91 | 91 | 450–750 | 84 | 87 | Western | SKL | 2020 | NA |
| Pardo et al./2018 [141] | 116 | 124 | 600–900 | 96.8 | 95.7 | Asian | CNN | 2018 | NA |
| Vinokuro et al./2021 [142] | NA | 648 | 450–950 | 79 | 95 | Asian | k-NN | 2021 | NA |
| Rasanen et al./2021 [143] | 24 | 26 | 500–850 | 99.6 | 98.6 | Western | CNN | 2021 | 0.95 |
| Zherdeva et al./2012 [145] | NA | 45 | 450–750 | 84 | 87 | Asian | DI | 2012 | NA |
| T. Nagaoka et al./2012 [144] | 27 | 27 | 450–1000 | 100 | 94.4 | Asian | NA | 2012 | NA |
| Subgroup | Number of Studies | Number of Patients | Number of Images | Sensitivity (%) | Specificity (%) |
|---|---|---|---|---|---|
| Average meta-analysis of all studies | 10 | 86 | ~150 | 91.89 | 84.28 |
| Nationality of Data | |||||
| Asian | 5 | 151 | 255 | 92.12 | 85.42 |
| Western | 5 | 50 | 67 | 76.35 | 82.92 |
| Type of AI | |||||
| CNN | 3 | 74.5 | 83 | 91.9 | 94.35 |
| SVM | 2 | 76 | 88 | 94.56 | 78.1 |
| SKL | 1 | 91 | 91 | 84 | 87 |
| DI | 2 | 186 | 123 | 90.35 | 64.55 |
| Year of Publication | |||||
| 2021–2022 | 4 | 81 | ~229 | 90.57 | 82.17 |
| 2019–2020 | 3 | 81 | 89 | 90.5 | 74.33 |
| Before 2018 | 3 | 116 | ~84 | 94.25 | 93.85 |
| Type of Cancer Classification | |||||
| BCC | 3 | 81 | 90 | 94.43 | 77.9 |
| SCC | 2 | NA | 45 | 90.1 | 92.65 |
| AK | 1 | 91 | 91 | 84 | 87 |
| Melanoma | 4 | 104 | 113 | 98.62 | 73.36 |
| Band Region | |||||
| Visible (380–780 nm) | 10 | 86.5 | 175 | 80.4 | 92.66 |
| Visible near IR | 8 | 92.75 | 105 | 94.567 | 77.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, H.-Y.; Hsiao, Y.-P.; Karmakar, R.; Mukundan, A.; Chaudhary, P.; Hsieh, S.-C.; Wang, H.-C. A Review of Recent Advances in Computer-Aided Detection Methods Using Hyperspectral Imaging Engineering to Detect Skin Cancer. Cancers 2023, 15, 5634. https://doi.org/10.3390/cancers15235634
Huang H-Y, Hsiao Y-P, Karmakar R, Mukundan A, Chaudhary P, Hsieh S-C, Wang H-C. A Review of Recent Advances in Computer-Aided Detection Methods Using Hyperspectral Imaging Engineering to Detect Skin Cancer. Cancers. 2023; 15(23):5634. https://doi.org/10.3390/cancers15235634
Chicago/Turabian StyleHuang, Hung-Yi, Yu-Ping Hsiao, Riya Karmakar, Arvind Mukundan, Pramod Chaudhary, Shang-Chin Hsieh, and Hsiang-Chen Wang. 2023. "A Review of Recent Advances in Computer-Aided Detection Methods Using Hyperspectral Imaging Engineering to Detect Skin Cancer" Cancers 15, no. 23: 5634. https://doi.org/10.3390/cancers15235634
APA StyleHuang, H.-Y., Hsiao, Y.-P., Karmakar, R., Mukundan, A., Chaudhary, P., Hsieh, S.-C., & Wang, H.-C. (2023). A Review of Recent Advances in Computer-Aided Detection Methods Using Hyperspectral Imaging Engineering to Detect Skin Cancer. Cancers, 15(23), 5634. https://doi.org/10.3390/cancers15235634









