Management and Outcome of Recurring Low-Grade Intramedullary Astrocytomas
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
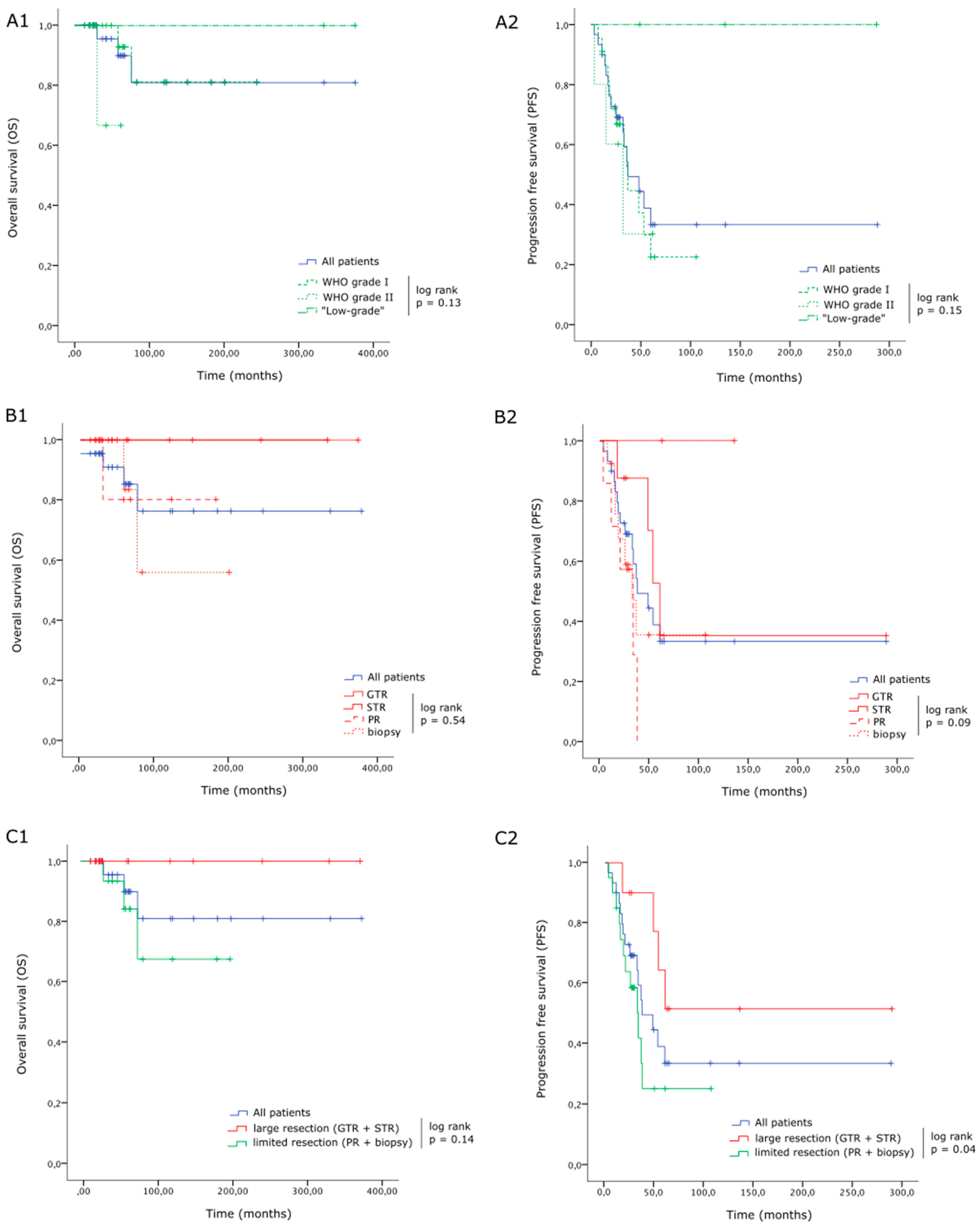
3. Results
3.1. Patients
3.1.1. Screening
3.1.2. Baseline Features
3.2. Surgery
3.2.1. Extent of Resection
3.2.2. Anatomopathological Results
3.2.3. Early Postoperative Outcome
3.3. Follow-Up
3.3.1. Adjuvant Treatment
3.3.2. Recurrences
3.3.3. Last Follow-Up
4. Discussion
4.1. Main Findings
4.2. Recurrences
4.3. Adjuvant Therapies
4.4. Molecular Profiling
4.5. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nagoshi, N.; Tsuji, O.; Suzuki, S.; Nori, S.; Yagi, M.; Okada, E.; Okita, H.; Fujita, N.; Ishii, K.; Matsumoto, M.; et al. Clinical Outcomes and a Therapeutic Indication of Intramedullary Spinal Cord Astrocytoma. Spinal Cord. 2022, 60, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Eroes, C.A.; Zausinger, S.; Kreth, F.-W.; Goldbrunner, R.; Tonn, J.-C. Intramedullary Low Grade Astrocytoma and Ependymoma. Surgical Results and Predicting Factors for Clinical Outcome. Acta Neurochir. 2010, 152, 611–618. [Google Scholar] [CrossRef] [PubMed]
- Abd-El-Barr, M.M.; Huang, K.T.; Chi, J.H. Infiltrating Spinal Cord Astrocytomas: Epidemiology, Diagnosis, Treatments and Future Directions. J. Clin. Neurosci. 2016, 29, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Ogunlade, J.; Wiginton, J.G.; Elia, C.; Odell, T.; Rao, S.C. Primary Spinal Astrocytomas: A Literature Review. Cureus 2019, 11, e5247. [Google Scholar] [CrossRef] [PubMed]
- Butenschoen, V.M.; Hubertus, V.; Janssen, I.K.; Onken, J.; Wipplinger, C.; Mende, K.C.; Eicker, S.O.; Kehl, V.; Thomé, C.; Vajkoczy, P.; et al. Surgical Treatment and Neurological Outcome of Infiltrating Intramedullary Astrocytoma WHO II-IV: A Multicenter Retrospective Case Series. J. Neurooncol. 2021, 151, 181–191. [Google Scholar] [CrossRef] [PubMed]
- Hersh, A.M.; Jallo, G.I.; Shimony, N. Surgical Approaches to Intramedullary Spinal Cord Astrocytomas in the Age of Genomics. Front. Oncol. 2022, 12, 982089. [Google Scholar] [CrossRef] [PubMed]
- Khalid, S.; Kelly, R.; Carlton, A.; Wu, R.; Peta, A.; Melville, P.; Maasarani, S.; Meyer, H.; Adogwa, O. Adult Intradural Intramedullary Astrocytomas: A Multicenter Analysis. J. Spine Surg. 2019, 5, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Nagashima, Y.; Nishimura, Y.; Eguchi, K.; Yamaguchi, J.; Haimoto, S.; Ohka, F.; Takayasu, M.; Saito, R. Recent Molecular and Genetic Findings in Intramedullary Spinal Cord Tumors. Neurospine 2022, 19, 262–271. [Google Scholar] [CrossRef]
- Benes, V.; Barsa, P.; Benes, V.; Suchomel, P. Prognostic Factors in Intramedullary Astrocytomas: A Literature Review. Eur. Spine J. 2009, 18, 1397–1422. [Google Scholar] [CrossRef]
- Golpayegani, M.; Edalatfar, M.; Ahmadi, A.; Sadeghi-Naini, M.; Salari, F.; Hanaei, S.; Shokraneh, F.; Ghodsi, Z.; Vaccaro, A.R.; Rahimi-Movaghar, V. Complete Versus Incomplete Surgical Resection in Intramedullary Astrocytoma: Systematic Review with Individual Patient Data Meta-Analysis. Glob. Spine J. 2023, 13, 227–241. [Google Scholar] [CrossRef]
- Parker, F.; Campello, C.; Lejeune, J.-P.; David, P.; Herbrecht, A.; Aghakhani, N.; Messerer, M. Intramedullary astrocytomas: A French retrospective multicenter study. Neurochirurgie 2017, 63, 402–409. [Google Scholar] [CrossRef] [PubMed]
- Fakhreddine, M.H.; Mahajan, A.; Penas-Prado, M.; Weinberg, J.; McCutcheon, I.E.; Puduvalli, V.; Brown, P.D. Treatment, Prognostic Factors, and Outcomes in Spinal Cord Astrocytomas. Neuro Oncol. 2013, 15, 406–412. [Google Scholar] [CrossRef] [PubMed]
- Seki, T.; Hida, K.; Yano, S.; Aoyama, T.; Koyanagi, I.; Sasamori, T.; Hamauch, S.; Houkin, K. Clinical Factors for Prognosis and Treatment Guidance of Spinal Cord Astrocytoma. Asian Spine J. 2016, 10, 748–754. [Google Scholar] [CrossRef] [PubMed]
- Lebrun, L.; Meléndez, B.; Blanchard, O.; De Nève, N.; Van Campenhout, C.; Lelotte, J.; Balériaux, D.; Riva, M.; Brotchi, J.; Bruneau, M.; et al. Clinical, Radiological and Molecular Characterization of Intramedullary Astrocytomas. Acta Neuropathol. Commun. 2020, 8, 128. [Google Scholar] [CrossRef] [PubMed]
- Anghileri, E.; Broggi, M.; Mazzapicchi, E.; Farinotti, M.; Botturi, A.; Tramacere, I.; Marchetti, M. Therapeutic Approaches in Adult Primary Spinal Cord Astrocytoma: A Systematic Review. Cancers 2022, 14, 1292. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Aguilar, D.; ReFaey, K.; Clifton, W.; Durcanova, B.; Chen, S.G.; Deen, H.G.; Bydon, M.; Trifiletti, D.M.; Pichelmann, M.A.; Quiñones-Hinojosa, A. Prognostic Factors and Survival in Low Grade Gliomas of the Spinal Cord: A Population-Based Analysis from 2006 to 2012. J. Clin. Neurosci. 2019, 61, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Juthani, R.G.; Bilsky, M.H.; Vogelbaum, M.A. Current Management and Treatment Modalities for Intramedullary Spinal Cord Tumors. Curr. Treat. Options Oncol. 2015, 16, 39. [Google Scholar] [CrossRef]
- Karikari, I.O.; Nimjee, S.M.; Hodges, T.R.; Cutrell, E.; Hughes, B.D.; Powers, C.J.; Mehta, A.I.; Hardin, C.; Bagley, C.A.; Isaacs, R.E.; et al. Impact of Tumor Histology on Resectability and Neurological Outcome in Primary Intramedullary Spinal Cord Tumors: A Single-Center Experience with 102 Patients. Neurosurgery 2011, 68, 188–197; discussion 197. [Google Scholar] [CrossRef]
- Santino, S.F.; Salles, D.; Stávale, J.N.; Malinverni, A.C.M. Pathophysiological Evaluation of Pilocytic Astrocytoma in Adults: Histopathological and Immunohistochemical Analysis. Pathol. Res. Pract. 2023, 248, 154593. [Google Scholar] [CrossRef]
- She, D.-J.; Lu, Y.-P.; Xiong, J.; Geng, D.-Y.; Yin, B. MR Imaging Features of Spinal Pilocytic Astrocytoma. BMC Med. Imaging 2019, 19, 5. [Google Scholar] [CrossRef]
- Alturkustani, M. Infiltration in Pilocytic Astrocytoma: A Diagnostic Pitfall. Cureus 2022, 14, e27940. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Wahab, M.; Etuk, B.; Palermo, J.; Shirato, H.; Kresl, J.; Yapicier, O.; Walker, G.; Scheithauer, B.W.; Shaw, E.; Lee, C.; et al. Spinal Cord Gliomas: A Multi-Institutional Retrospective Analysis. Int. J. Radiat. Oncol. Biol. Phys. 2006, 64, 1060–1071. [Google Scholar] [CrossRef]
- Isaacson, S.R. Radiation Therapy and the Management of Intramedullary Spinal Cord Tumors. J. Neurooncol. 2000, 47, 231–238. [Google Scholar] [CrossRef]
- Assessment of Outcome in Patients Undergoing Surgery for Intradural Spinal Tumor Using the Multidimensional Patient-Rated Core Outcome Measures Index and the Modified McCormick Scale—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/26235018/ (accessed on 14 August 2023).
- Aghakhani, N.; Messerer, M.; David, P.; Herbrecht, A.; Parker, F. Intramedullary ependymomas: A French retrospective multicenter study of 221 cases. Neurochirurgie 2017, 63, 391–397. [Google Scholar] [CrossRef]
- Zhang, D.; Fan, T.; Fan, W.; Zhao, X.; Liang, C.; Wang, Y.; Wu, K. Clinical Characteristics and Treatment Outcomes of Long-Level Intramedullary Spinal Cord Tumors: A Consecutive Series of 43 Cases. Neurospine 2023, 20, 231–239. [Google Scholar] [CrossRef]
- Hongo, H.; Takai, K.; Komori, T.; Taniguchi, M. Intramedullary Spinal Cord Ependymoma and Astrocytoma: Intraoperative Frozen-Section Diagnosis, Extent of Resection, and Outcomes. J. Neurosurg. Spine 2018, 30, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Chaskis, E.; Minichini, V.; Luce, S.; Devriendt, D.; Goldman, S.; De Witte, O.; Sadeghi, N.; Lefranc, F. Contribution of temozolomide chemotherapy for intramedullary grade II spinal cord astrocytomas in adults: Our experience. Neurochirurgie 2017, 63, 297–301. [Google Scholar] [CrossRef]
- Zhang, M.; Iyer, R.R.; Azad, T.D.; Wang, Q.; Garzon-Muvdi, T.; Wang, J.; Liu, A.; Burger, P.; Eberhart, C.; Rodriguez, F.J.; et al. Genomic Landscape of Intramedullary Spinal Cord Gliomas. Sci. Rep. 2019, 9, 18722. [Google Scholar] [CrossRef] [PubMed]
- Chai, R.C.; Yan, H.; An, S.Y.; Pang, B.; Chen, H.Y.; Mu, Q.H.; Zhang, K.N.; Zhang, Y.W.; Liu, Y.Q.; Liu, X.; et al. Genomic profiling and prognostic factors of H3 K27M-mutant spinal cord diffuse glioma. Brain Pathol. 2023, 33, e13153. [Google Scholar] [CrossRef]
- Chai, R.C.; Zhang, Y.W.; Liu, Y.Q.; Chang, Y.Z.; Pang, B.; Jiang, T.; Jia, W.Q.; Wang, Y.Z. The molecular characteristics of spinal cord gliomas with or without H3 K27M mutation. Acta Neuropathol. Commun. 2020, 8, 40. [Google Scholar] [CrossRef]
- Biczok, A.; Strübing, F.L.; Eder, J.M.; Egensperger, R.; Schnell, O.; Zausinger, S.; Neumann, J.E.; Herms, J.; Tonn, J.-C.; Dorostkar, M.M. Molecular Diagnostics Helps to Identify Distinct Subgroups of Spinal Astrocytomas. Acta Neuropathol. Commun. 2021, 9, 119. [Google Scholar] [CrossRef] [PubMed]

| Clinical Features | Number (Total n = 30) | |
|---|---|---|
| Mean age (years, (min–max)) | 28.5 (16–66) | |
| Sex ratio (F/M) | 18/12 | |
| Mean delay between symptoms onset and surgery (months, (min–max)) | 18 (1–120) | |
| Preoperative McCormick scale | I | 5 (16.7%) |
| II | 19 (63.3%) | |
| III | 5 (16.7%) | |
| IV | 1 (3.3%) | |
| V | 0 | |
| Preoperative symptoms | Pain | 25 (83.3%) |
| Motor | 23 (76.7%) | |
| Sensory | 27 (90%) | |
| Sphincter | 13 (43.3%) | |
| Radiological features | ||
| Vertebral levels involved | Mean (min–max) | 3 (1–9) |
| Cervical | 8 (26.7%) | |
| Cervicodorsal | 2 (6.7%) | |
| Dorsal | 18 (60%) | |
| Dorsolumbar | 2 (6.7%) | |
| Cone | 0 | |
| Panmedullary | 0 | |
| T1-weighted sequence signal (%) | Hypo | 11/23(47.8%) |
| Iso | 10/23 (43.5%) | |
| Hyper | 2/23 (8.7%) | |
| T2-weighted sequence signal (%) | Hypo | 2/24 (8.3%) |
| Iso | 0 | |
| Hyper | 22/24 (91.7%) | |
| Gadolinium enhancement | 26 (86.7%) | |
| Peritumoral cyst | 18/29 (62%) | |
| Peritumoral hemorrhage | 9/28 (32.1%) | |
| Surgery | Number (Total n = 30) | |
|---|---|---|
| Cleavage plan described | 4 (13.3%) | |
| Residue according to surgeon | 28 (93.3%) | |
| Residue visible on first post-op MRI | 27 (90%) | |
| Extent of resection according to surgeon | Gross total resection | 2 (6.7%) |
| Subtotal resection | 8 (26.7%) | |
| Partial resection | 7 (23.3%) | |
| Biopsy | 13 (43.3%) | |
| Pathology | ||
| Ki67 levels | <5% | 20/22 (90.9%) |
| ≥5% | 2/22 (9.1%) | |
| Clinical | ||
| Early postoperative McCormick scale | I | 5 (16.7%) |
| II | 13 (43.3%) | |
| III | 7 (23.3%) | |
| IV | 4 (13.3%) | |
| V | 1 (3.3%) | |
| Complications | Epidural hematoma | 1 (3.3%) |
| Sex | Age | Spinal Segment | PreopMCS | Extent of Resection | WHO Grade | Ki-67 (%) | Postop MCS | Early Adjuvant Treatment | Delay for Progression (Months) | Management of Progression |
|---|---|---|---|---|---|---|---|---|---|---|
| M | 37 | Dorsal | III | Biopsy | I | 5 | IV | No | 7 | Surgery |
| F | 33 | Cervical | I | Biopsy | I | 10 | I | No | 36 | Surgery + RT + CT |
| F | 22 | Cervical | II | PR | I | 3 | III | No | 53 | RT |
| F | 44 | Cervical | II | PR | I | 1 | I | No | 33 | CT |
| F | 47 | Cervico-dorsal | II | PR | I | 1 | III | No | 11 | RT + CT |
| F | 24 | Dorsal | II | STR | I | 1 | II | No | 48 | Surgery |
| M | 23 | Dorsal | II | PR | I | 4 | II | No | 37 | No treatment |
| F | 49 | Dorso-lumbar | II | Biopsy | I | 2 | III | No | 25 | RT + CT |
| M | 30 | Dorsal | II | Biopsy | I | - | IV | CT | 18 | RT + CT |
| F | 44 | Cervical | II | STR | I | 3 | III | No | 60 | No treatment |
| F | 16 | Dorsal | II | Biopsy | I | 5 | II | CT | 14 | Surgery + CT |
| F | 37 | Dorsal | II | STR | I | - | II | No | 17 | No treatment |
| F | 40 | Dorsal | II | PR | I | 2 | II | No | 20 | Surgery |
| M | 27 | Cervical | III | STR | II | 10 | III | No | 3 | RT |
| M | 58 | Dorsal | II | Biopsy | II | 1 | II | RT | 15 | No treatment |
| F | 16 | Dorsal | IV | Biopsy | II | 6 | IV | RT | 32 | CT |
| Number (Total n = 30) | ||
|---|---|---|
| Follow-up | Mean (in months) | 90.3 |
| Median (min–max) | 59 (13–376) | |
| McCormick scale at last follow-up | I | 3 (10%) |
| II | 12 (40%) | |
| III | 8 (26.7%) | |
| IV | 4 (13.3%) | |
| V | 3 (10%) | |
| Tumor progression | PFS (in months) | 28.5 (3–288) |
| PFS (in years) | 2 (0–24) | |
| Radiological | 16 (53.3%) | |
| Symptomatic | 12 (40%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chaskis, E.; Silvestri, M.; Aghakhani, N.; Parker, F.; Knafo, S. Management and Outcome of Recurring Low-Grade Intramedullary Astrocytomas. Cancers 2024, 16, 2417. https://doi.org/10.3390/cancers16132417
Chaskis E, Silvestri M, Aghakhani N, Parker F, Knafo S. Management and Outcome of Recurring Low-Grade Intramedullary Astrocytomas. Cancers. 2024; 16(13):2417. https://doi.org/10.3390/cancers16132417
Chicago/Turabian StyleChaskis, Elly, Martina Silvestri, Nozar Aghakhani, Fabrice Parker, and Steven Knafo. 2024. "Management and Outcome of Recurring Low-Grade Intramedullary Astrocytomas" Cancers 16, no. 13: 2417. https://doi.org/10.3390/cancers16132417






