A Peptide Derived from Nectin-4 Increases Cisplatin Cytotoxicity in Cell Lines and Cells from Ovarian Cancer Patients’ Ascites
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Patient Sample/Ascites Processing
2.3. Peptide Synthesis
2.4. Flow Cytometry
2.5. Spheroid Formation Assay
2.6. Cell Viability
3. Results
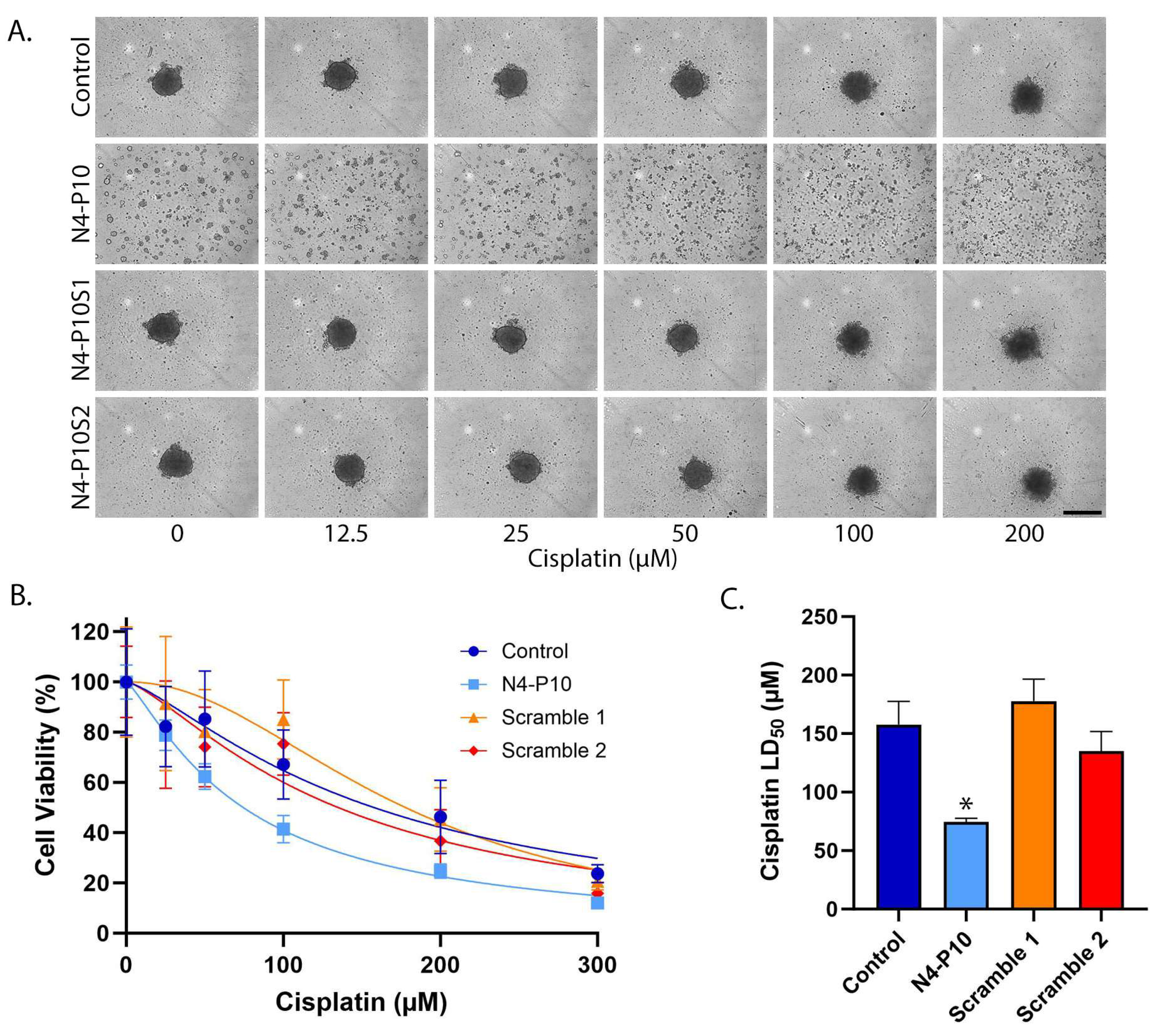
3.1. Effect of Nectin-4 Peptide on Cisplatin Cytotoxicity of NIH:OVCAR5 Cell Line
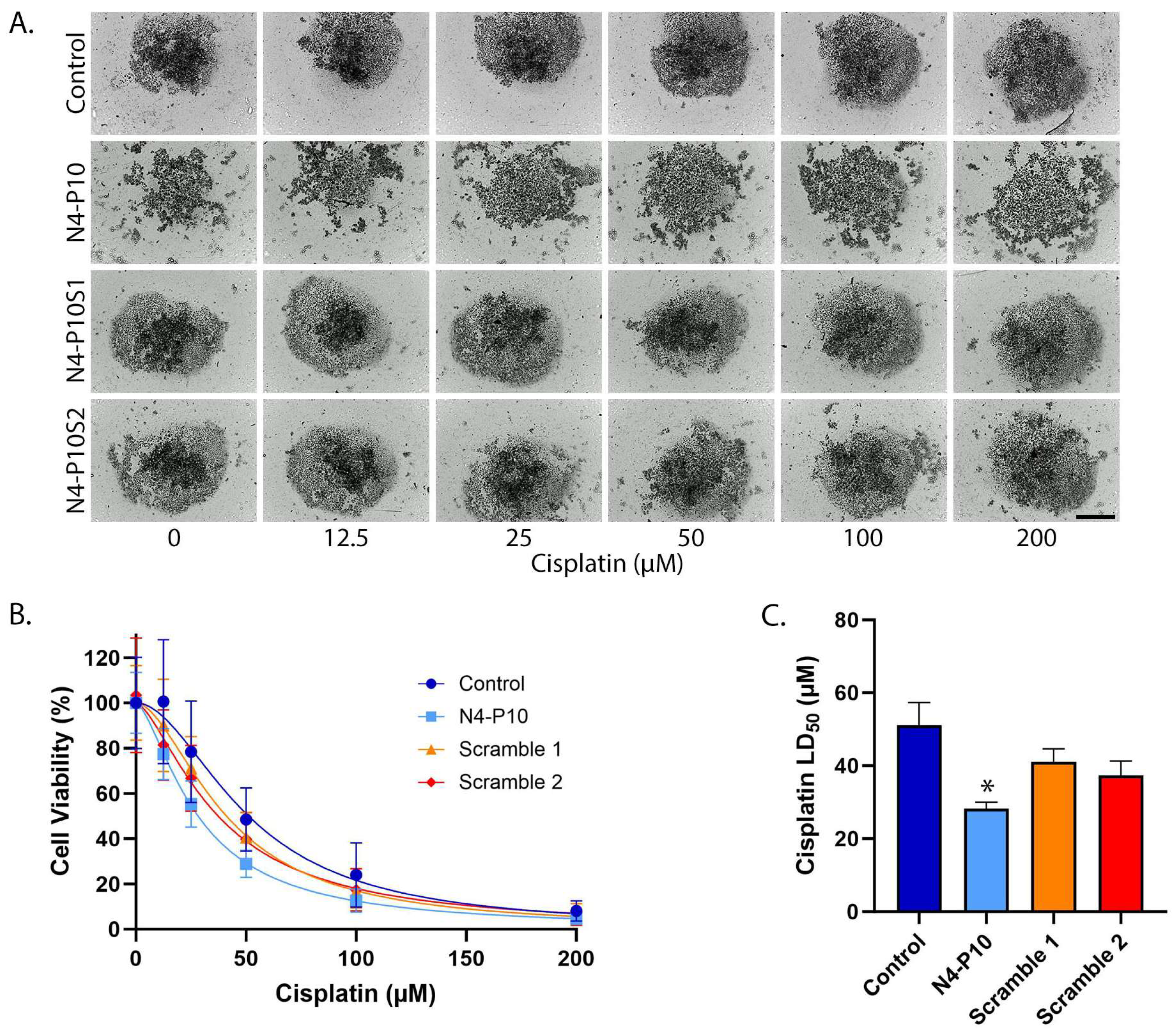
3.2. Effect of Nectin-4 Peptide on Cisplatin Cytotoxicity of CAOV3 Cell Line
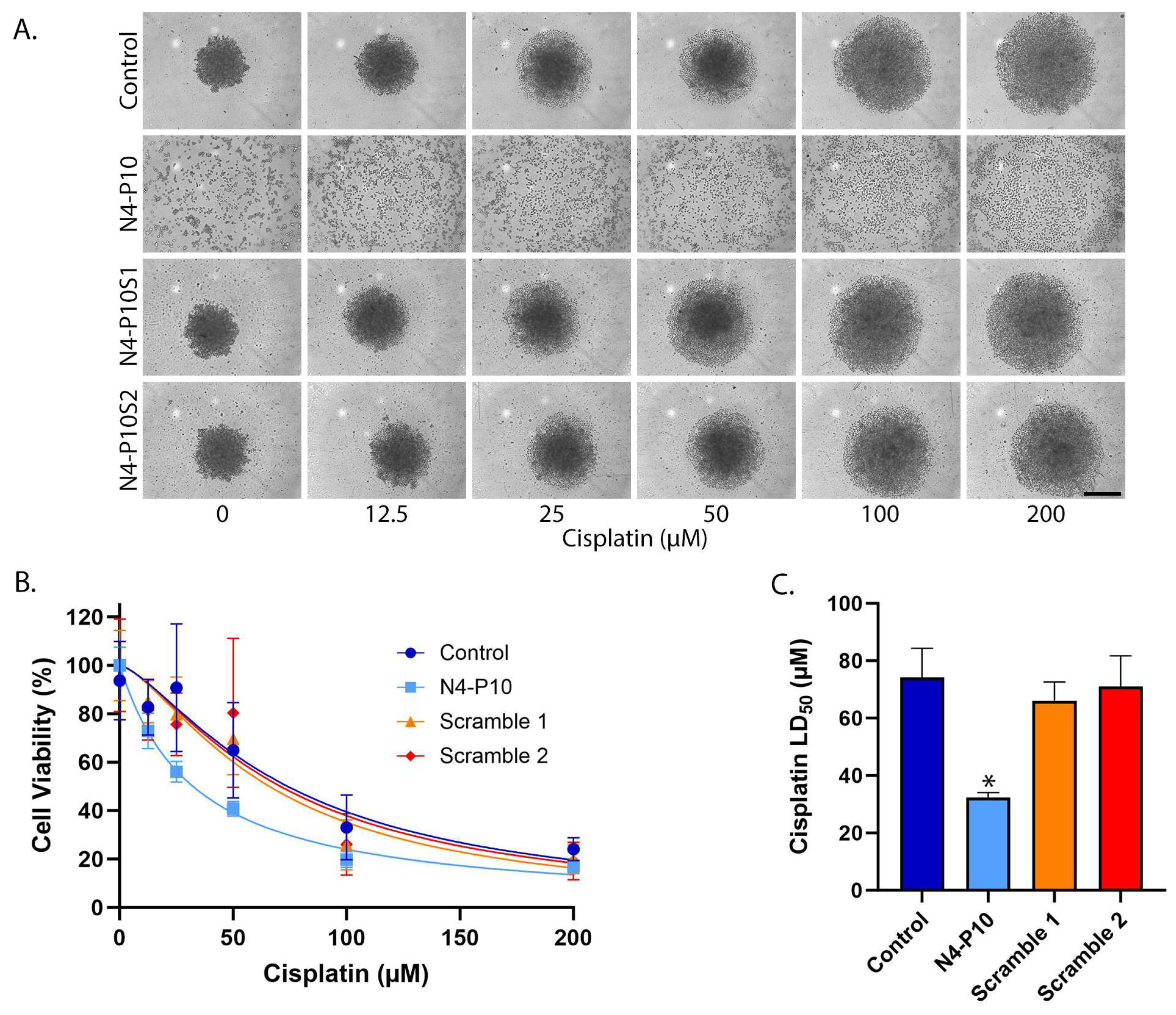
3.3. Effect of Nectin-4 Peptide on Cisplatin Cytotoxicity of ME-180 Cell Line
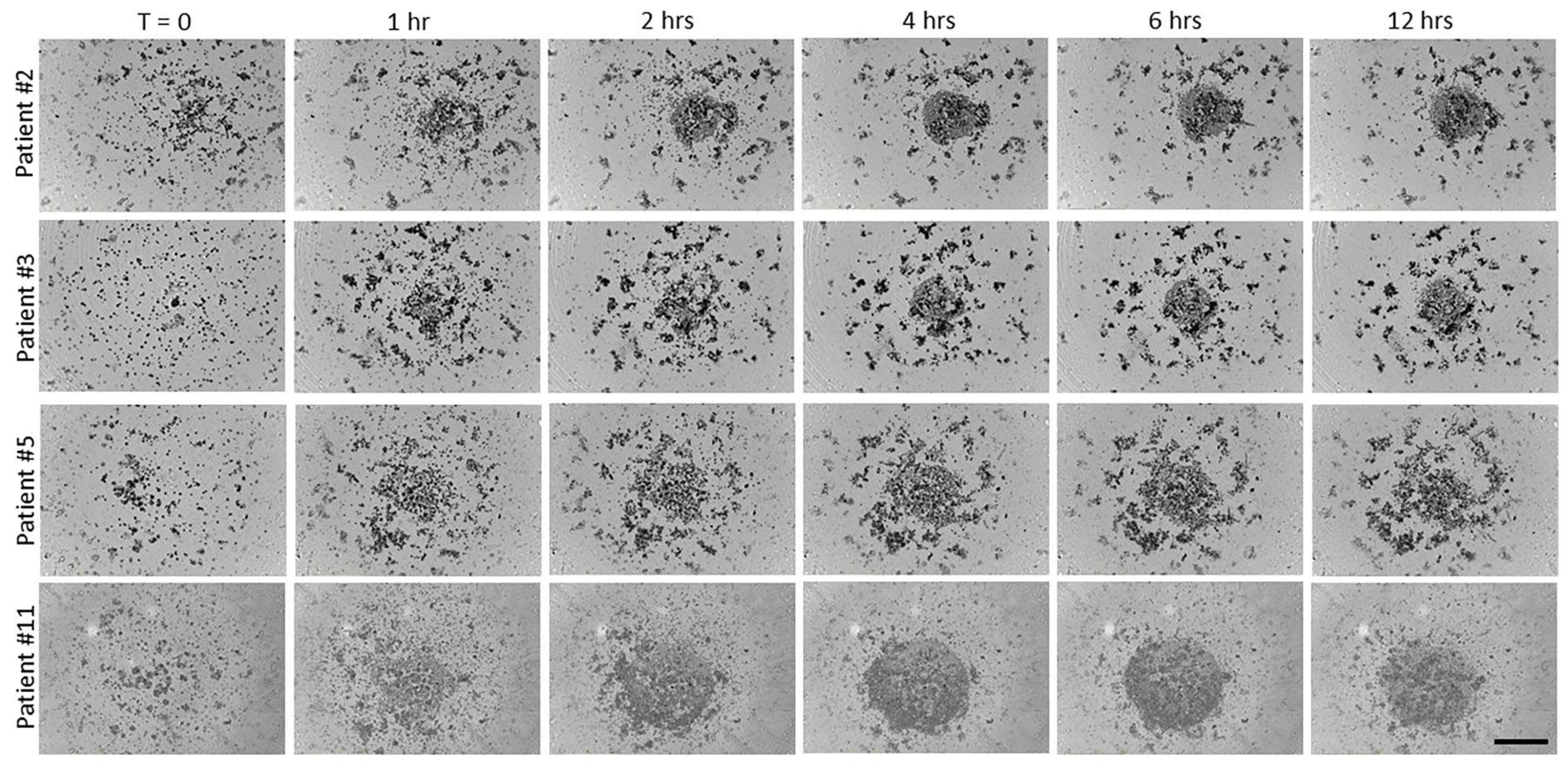
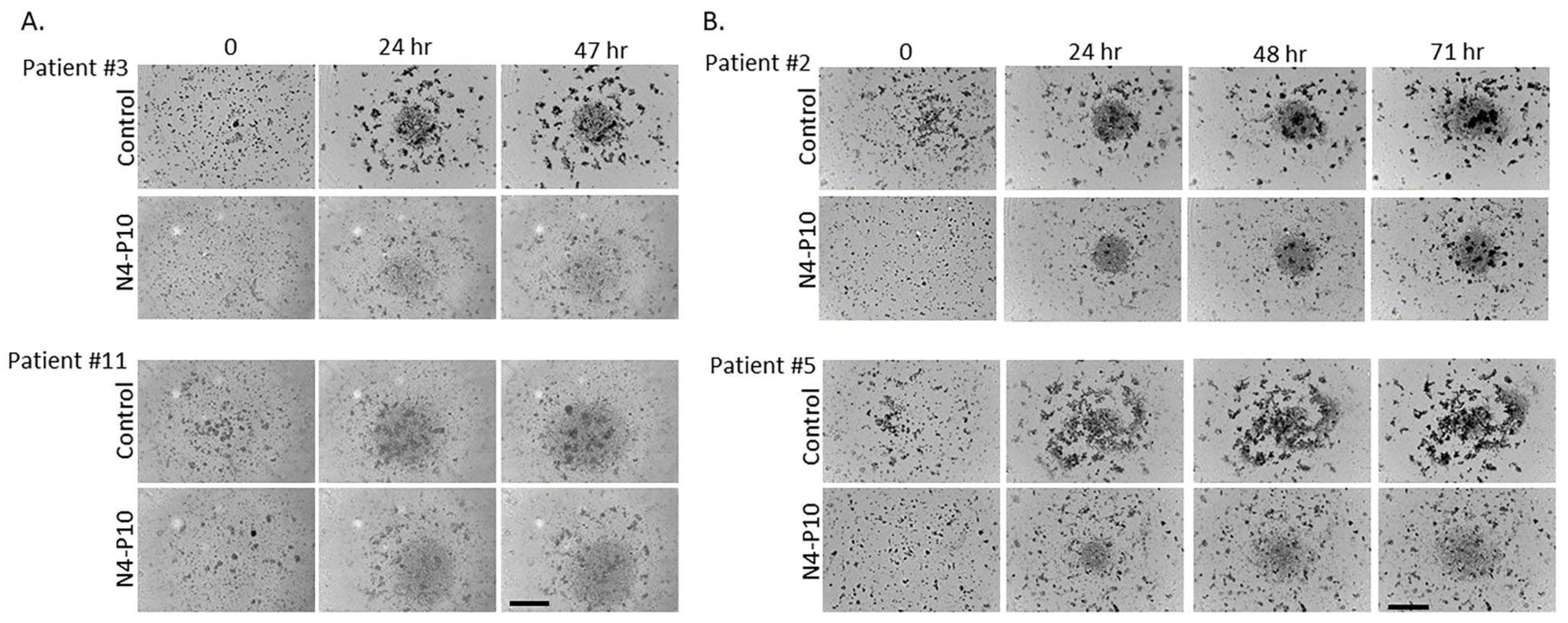
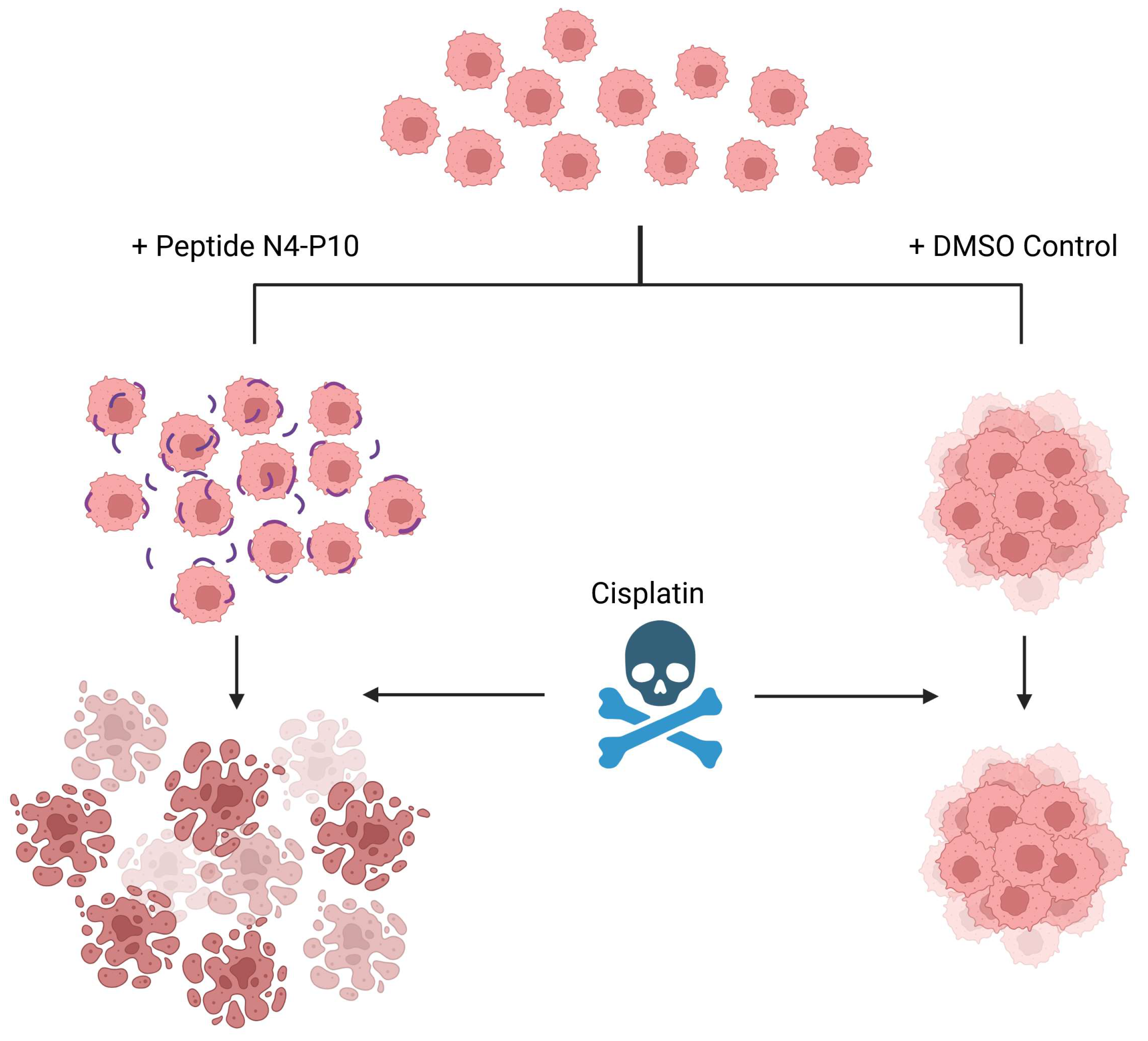
3.4. Effect of Nectin-4 Peptide on Spheroid Formation of Ovarian Cancer Patients’ Ascites Cells
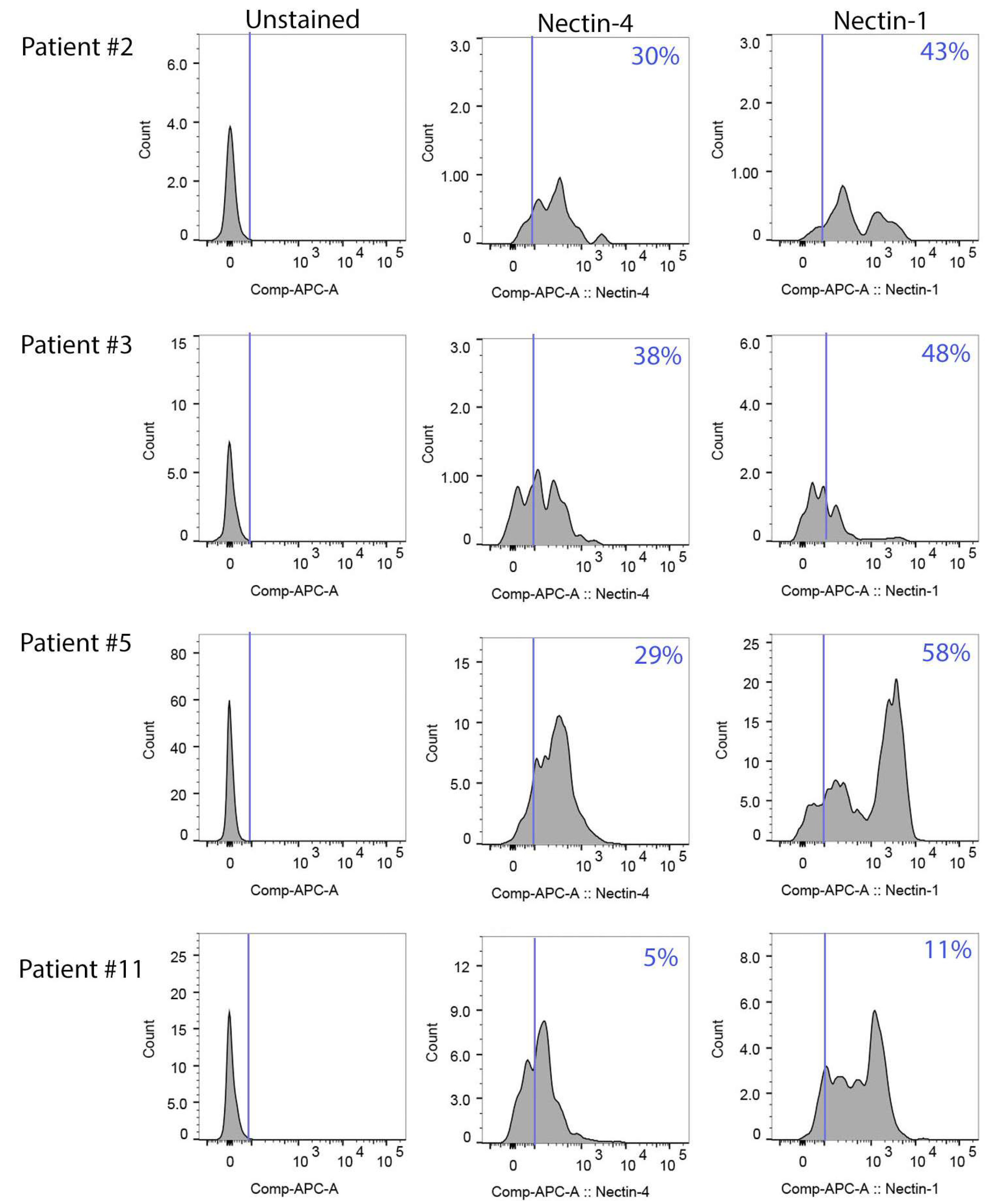
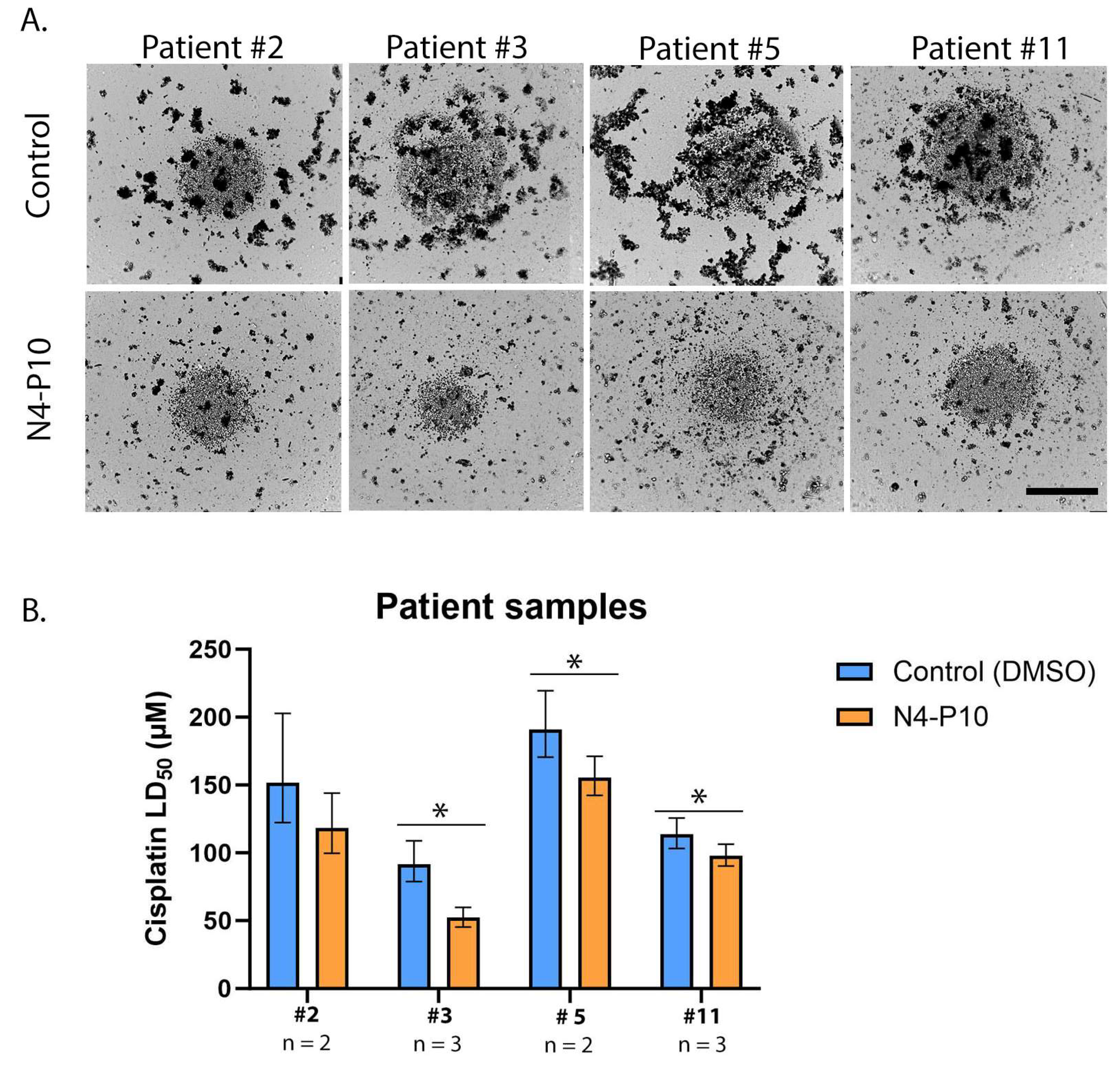
3.5. Effect of Nectin-4 Peptide on Cisplatin Cytotoxicity of Ovarian Cancer Patients’ Ascites Cells
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| N4-P10 | Nectin-4 peptide 10 |
| N4-P10S1 | Nectin-4 peptide 10 scramble 1 |
| N4-P10S2 | Nectin-4 peptide 10 scramble 2 |
| IP | Intraperitoneal |
| FBS | Fetal bovine serum |
| DMSO | Dimethyl sulfoxide |
| PBS | Phosphate-buffered saline |
| LD50 | Lethal dose 50 |
| CI | Confidence interval |
| µm | Micron |
| µM | Micromolar |
| SD | Standard deviation |
| CA125 | Cancer antigen 125 |
| HGSOC | High-grade serous ovarian cancer |
References
- Torre, L.A.; Trabert, B.; DeSantis, C.E.; Miller, K.D.; Samimi, G.; Runowicz, C.D.; Gaudet, M.M.; Jemal, A.; Siegel, R.L. Ovarian cancer statistics, 2018. CA Cancer J. Clin. 2018, 68, 284. [Google Scholar] [CrossRef] [PubMed]
- Marchetti, C.; De Felice, F.; Romito, A.; Iacobelli, V.; Sassu, C.M.; Corrado, G.; Ricci, C.; Scambia, G.; Fagotti, A. Chemotherapy resistance in epithelial ovarian cancer: Mechanisms and emerging treatments. Semin. Cancer Biol. 2021, 77, 144. [Google Scholar] [CrossRef] [PubMed]
- Shield, K.; Ackland, M.L.; Ahmed, N.; Rice, G.E. Multicellular spheroids in ovarian cancer metastases: Biology and pathology. Gynecol. Oncol. 2009, 113, 143. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, K.; Nagao, S.; Tan, D.; Hasegawa, K. Intraperitoneal chemotherapy is now back for ovarian cancer. Int. J. Clin. Oncol. 2025, 30, 427. [Google Scholar] [CrossRef]
- Kumar, K.P.; Madhusoodanan, M.; Pangath, M.; Menon, D. Innovative landscapes in intraperitoneal therapy of ovarian cancer. Drug. Deliv. Transl. Res. 2025. [Google Scholar] [CrossRef]
- Frankel, A.; Buckman, R.; Kerbel, R.S. Abrogation of Taxol-induced G2-M arrest and apoptosis in human ovarian cancer cells grown as multicellular tumor spheroids. Cancer Res. 1997, 57, 2388. [Google Scholar]
- Bapat, S.A.; Mali, A.M.; Koppikar, C.B.; Kurrey, N.K. Stem and progenitor-like cells contribute to the aggressive behavior of human epithelial ovarian cancer. Cancer Res. 2005, 65, 3025. [Google Scholar] [CrossRef]
- Hu, L.; McArthur, C.; Jaffe, R.B. Ovarian cancer stem-like side-population cells are tumourigenic and chemoresistant. Br. J. Cancer 2010, 102, 1276. [Google Scholar] [CrossRef]
- Zhang, S.; Balch, C.; Chan, M.W.; Lai, H.C.; Matei, D.; Schilder, J.M.; Yan, P.S.; Huang, T.H.; Nephew, K.P. Identification and characterization of ovarian cancer-initiating cells from primary human tumors. Cancer Res. 2008, 68, 4311. [Google Scholar] [CrossRef]
- Irie, K.; Shimizu, K.; Sakisaka, T.; Ikeda, W.; Takai, Y. Roles and modes of action of nectins in cell-cell adhesion. Semin. Cell Dev. Biol. 2004, 15, 643. [Google Scholar] [CrossRef]
- Takai, Y.; Nakanishi, H. Nectin and afadin: Novel organizers of intercellular junctions. J. Cell Sci. 2003, 116 Pt 1, 17. [Google Scholar] [CrossRef] [PubMed]
- Takai, Y.; Irie, K.; Shimizu, K.; Sakisaka, T.; Ikeda, W. Nectins and nectin-like molecules: Roles in cell adhesion, migration, and polarization. Cancer Sci. 2003, 94, 655. [Google Scholar] [CrossRef]
- Boylan, K.L.M.; Buchanan, P.C.; Manion, R.D.; Shukla, D.M.; Braumberger, K.; Bruggemeyer, C.; Skubitz, A.P.N. The expression of Nectin-4 on the surface of ovarian cancer cells alters their ability to adhere, migrate, aggregate, and proliferate. Oncotarget 2017, 8, 9717. [Google Scholar] [CrossRef] [PubMed]
- Das, D.; Satapathy, S.R.; Siddharth, S.; Nayak, A.; Kundu, C.N. NECTIN-4 increased the 5-FU resistance in colon cancer cells by inducing the PI3K-AKT cascade. Cancer Chemother. Pharmacol. 2015, 76, 471. [Google Scholar] [CrossRef]
- Lattanzio, R.; Ghasemi, R.; Brancati, F.; Sorda, R.L.; Tinari, N.; Perracchio, L.; Iacobelli, S.; Mottolese, M.; Natali, P.G.; Piantelli, M. Membranous Nectin-4 expression is a risk factor for distant relapse of T1-T2, N0 luminal-A early breast cancer. Oncogenesis 2014, 3, e118. [Google Scholar] [CrossRef]
- Ma, J.; Sheng, Z.; Lv, Y.; Liu, W.; Yao, Q.; Pan, T.; Xu, Z.; Zhang, C.; Xu, G. Expression and clinical significance of Nectin-4 in hepatocellular carcinoma. OncoTargets Ther. 2016, 9, 183. [Google Scholar]
- Nishiwada, S.; Sho, M.; Yasuda, S.; Shimada, K.; Yamato, I.; Akahori, T.; Kinoshita, S.; Nagai, M.; Konishi, N.; Nakajima, Y. Nectin-4 expression contributes to tumor proliferation, angiogenesis and patient prognosis in human pancreatic cancer. J. Exp. Clin. Cancer Res. CR 2015, 34, 30. [Google Scholar] [CrossRef]
- Pavlova, N.N.; Pallasch, C.; Elia, A.E.; Braun, C.J.; Westbrook, T.F.; Hemann, M.; Elledge, S.J. A role for PVRL4-driven cell-cell interactions in tumorigenesis. eLife 2013, 2, e00358. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, P.; Yin, W.; Ji, Y.; Shen, Q.; Ni, Q. Nectin-4 promotes gastric cancer progression via the PI3K/AKT signaling pathway. Hum. Pathol. 2018, 72, 107. [Google Scholar] [CrossRef]
- Boylan, K.L.M.; Manion, R.D.; Shah, H.; Skubitz, K.M.; Skubitz, A.P.N. Inhibition of ovarian cancer cell spheroid formation by synthetic peptides derived from Nectin-4. Int. J. Mol. Sci. 2020, 21, 4637. [Google Scholar] [CrossRef]
- Korch, C.; Spillman, M.A.; Jackson, T.A.; Jacobsen, B.M.; Murphy, S.K.; Lessey, B.A.; Jordan, V.C.; Bradford, A.P. DNA profiling analysis of endometrial and ovarian cell lines reveals misidentification, redundancy and contamination. Gynecol. Oncol. 2012, 127, 241. [Google Scholar] [CrossRef] [PubMed]
- Burleson, K.M.; Boente, M.P.; Pambuccian, S.E.; Skubitz, A.P.N. Disaggregation and invasion of ovarian carcinoma ascites spheroids. J. Transl. Med. 2006, 4, 6. [Google Scholar] [CrossRef] [PubMed]
- Buchanan, P.C.; Boylan, K.L.M.; Walcheck, B.; Heinze, R.; Geller, M.A.; Argenta, P.A.; Skubitz, A.P.N. Ectodomain shedding of the cell adhesion molecule Nectin-4 in ovarian cancer is mediated by ADAM10 and ADAM17. J. Biol. Chem. 2017, 292, 6339. [Google Scholar] [CrossRef] [PubMed]
- Derycke, M.S.; Pambuccian, S.E.; Gilks, C.B.; Kalloger, S.E.; Ghidouche, A.; Lopez, M.; Bliss, R.L.; Geller, M.A.; Argenta, P.A.; Harrington, K.M.; et al. Nectin 4 overexpression in ovarian cancer tissues and serum: Potential role as a serum biomarker. Am. J. Clin. Pathol. 2010, 134, 835. [Google Scholar] [CrossRef]
- Green, S.K.; Francia, G.; Isidoro, C.; Kerbel, R.S. Antiadhesive antibodies targeting E-cadherin sensitize multicellular tumor spheroids to chemotherapy in vitro. Mol. Cancer Ther. 2004, 3, 149. [Google Scholar] [CrossRef]
- Yoshida, Y.; Kurokawa, T.; Nishikawa, Y.; Orisa, M.; Kleinman, H.K.; Kotsuji, F. Laminin-1-derived scrambled peptide AG73T disaggregates laminin-1-induced ovarian cancer cell spheroids and improves the efficacy of cisplatin. Int. J. Oncol. 2008, 32, 673. [Google Scholar] [CrossRef][Green Version]
- Gunay, G.; Kirit, H.A.; Kamatar, A.; Baghdasaryan, O.; Hamsici, S.; Acar, H. The effects of size and shape of the ovarian cancer spheroids on the drug resistance and migration. Gynecol. Oncol. 2020, 159, 563. [Google Scholar] [CrossRef]
- Al Habyan, S.; Kalos, C.; Szymborski, J.; McCaffrey, L. Multicellular detachment generates metastatic spheroids during intra-abdominal dissemination in epithelial ovarian cancer. Oncogene 2018, 37, 5127. [Google Scholar] [CrossRef]
- Micek, H.M.; Rosenstock, L.; Ma, Y.; Hielsberg, C.; Montemorano, L.; Gari, M.K.; Ponik, S.M.; Kreeger, P.K. Model of collective detachment in high-grade serous ovarian cancer demonstrates that tumor spheroids produce ECM to support metastatic processes. APL Bioeng. 2023, 7, 016111. [Google Scholar] [CrossRef]
- Auer, K.; Bachmayr-Heyda, A.; Aust, S.; Grunt, T.W.; Pils, D. Comparative transcriptome analysis links distinct peritoneal tumor spread types, miliary and non-miliary, with putative origin, tubes and ovaries, in high grade serous ovarian cancer. Cancer Lett. 2017, 388, 158. [Google Scholar] [CrossRef]
- Izar, B.; Tirosh, I.; Stover, E.H.; Wakiro, I.; Cuoco, M.S.; Alter, I.; Rodman, C.; Leeson, R.; Su, M.J.; Shah, P.; et al. A single-cell landscape of high-grade serous ovarian cancer. Nat. Med. 2020, 26, 1271–1279. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Gotimer, K.; De Souza, C.; Tepper, C.G.; Karnezis, A.N.; Leiserowitz, G.S.; Chien, J.; Smith, L.H. Short-term organoid culture for drug sensitivity testing of high-grade serous carcinoma. Gynecol. Oncol. 2020, 157, 783. [Google Scholar] [CrossRef] [PubMed]
- Velletri, T.; Villa, C.E.; Cilli, D.; Barzaghi, B.; Riso, P.L.; Lupia, M.; Luongo, R.; Lopez-Tobon, A.; De Simone, M.; Bonnal, R.J.P.; et al. Single cell-derived spheroids capture the self-renewing subpopulations of metastatic ovarian cancer. Cell Death Differ. 2022, 29, 614. [Google Scholar] [CrossRef]
- Honda, T.; Shimizu, K.; Kawakatsu, T.; Yasumi, M.; Shingai, T.; Fukuhara, A.; Ozaki-Kuroda, K.; Irie, K.; Nakanishi, H.; Takai, Y. Antagonistic and agonistic effects of an extracellular fragment of nectin on formation of E-cadherin-based cell-cell adhesion. Genes Cells 2003, 8, 51. [Google Scholar] [CrossRef]
- Sodek, K.L.; Ringuette, M.J.; Brown, T.J. Compact spheroid formation by ovarian cancer cells is associated with contractile behavior and an invasive phenotype. Int. J. Cancer 2009, 124, 2060. [Google Scholar] [CrossRef] [PubMed]
- Iwanicki, M.P.; Davidowitz, R.A.; Ng, M.R.; Besser, A.; Muranen, T.; Merritt, M.; Danuser, G.; Ince, T.A.; Brugge, J.S. Ovarian cancer spheroids use myosin-generated force to clear the mesothelium. Cancer Discov. 2011, 1, 144. [Google Scholar] [CrossRef]
- Liu, Y.; Han, X.; Li, L.; Zhang, Y.; Huang, X.; Li, G.; Xu, C.; Yin, M.; Zhou, P.; Shi, F.; et al. Role of Nectin-4 protein in cancer (Review). Int. J. Oncol. 2021, 59, 93. [Google Scholar] [CrossRef]
- Baraban, E.G.; Vlachou, E.; Patel, S.; Kates, M.; Johnson, B.; Smith, A.; Shenderov, E.; Sharma, S.; Denmeade, S.R.; Brame, A.; et al. Nectin-4 expression in prostatic adenocarcinoma: An immunohistochemical study. Prostate 2025, 85, 443–447. [Google Scholar] [CrossRef]
- Fabre-Lafay, S.; Monville, F.; Garrido-Urbani, S.; Berruyer-Pouyet, C.; Ginestier, C.; Reymond, N.; Finetti, P.; Sauvan, R.; Adelaide, J.; Geneix, J.; et al. Nectin-4 is a new histological and serological tumor associated marker for breast cancer. BMC Cancer 2007, 7, 73. [Google Scholar] [CrossRef]
- Takano, A.; Ishikawa, N.; Nishino, R.; Masuda, K.; Yasui, W.; Inai, K.; Nishimura, H.; Ito, H.; Nakayama, H.; Miyagi, Y.; et al. Identification of nectin-4 oncoprotein as a diagnostic and therapeutic target for lung cancer. Cancer Res. 2009, 69, 6694. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, J.; Shen, Q.; Yin, W.; Huang, H.; Liu, Y.; Ni, Q. High expression of Nectin-4 is associated with unfavorable prognosis in gastric cancer. Oncol. Lett. 2018, 15, 8789. [Google Scholar] [CrossRef]
- Lin, X.; Hu, H.; Pan, Y.; Gao, S. The prognostic role of expression of Nectin-4 in esophageal cancer. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2019, 25, 10089. [Google Scholar] [CrossRef] [PubMed]
- Challita-Eid, P.M.; Satpayev, D.; Yang, P.; An, Z.; Morrison, K.; Shostak, Y.; Raitano, A.; Nadell, R.; Liu, W.; Lortie, D.R.; et al. Enfortumab vedotin antibody-drug conjugate targeting Nectin-4 is a highly potent therapeutic agent in multiple preclinical cancer models. Cancer Res. 2016, 76, 3003. [Google Scholar] [CrossRef] [PubMed]
- Chang, E.; Weinstock, C.; Zang, L.; Charlab, R.; Dorff, S.E.; Gong, Y.; Hsu, V.; Li, F.; Ricks, T.K.; Song, P.; et al. FDA Approval Summary: Enfortumab vedotin for locally advanced or metastatic urothelial carcinoma. Clin. Cancer Res. 2021, 27, 922. [Google Scholar] [CrossRef] [PubMed]
- Cho, W.C.; Saade, R.; Nagarajan, P.; Aung, P.P.; Milton, D.R.; Marques-Piubelli, M.L.; Hudgens, C.; Ledesma, D.; Nelson, K.; Ivan, D.; et al. Nectin-4 expression in a subset of cutaneous adnexal carcinomas: A potential target for therapy with enfortumab vedotin. J. Cutan. Pathol. 2024, 51, 360. [Google Scholar] [CrossRef]
- Hanna, K.S. Advancements in therapy for bladder cancer: Enfortumab vedotin. J. Adv. Pract. Oncol. 2020, 11, 412. [Google Scholar]
- Hoffman-Censits, J.; Maldonado, L. Targeted treatment of locally advanced and metastatic urothelial cancer: Enfortumab vedotin in context. OncoTargets Ther. 2022, 15, 1519. [Google Scholar] [CrossRef]
- Maas, M.; Stuhler, V.; Walz, S.; Stenzl, A.; Bedke, J. Enfortumab vedotin—Next game-changer in urothelial cancer. Expert Opin. Biol. Ther. 2021, 21, 801. [Google Scholar] [CrossRef]
- M-Rabet, M.; Cabaud, O.; Josselin, E.; Finetti, P.; Castellano, R.; Farina, A.; Agavnian-Couquiaud, E.; Saviane, G.; Collette, Y.; Viens, P.; et al. Nectin-4: A new prognostic biomarker for efficient therapeutic targeting of primary and metastatic triple-negative breast cancer. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. ESMO 2017, 28, 769. [Google Scholar] [CrossRef]
- Zhou, W.; Fang, P.; Yu, D.; Ren, H.; You, M.; Yin, L.; Mei, F.; Zhu, H.; Wang, Z.; Xu, H.; et al. Preclinical evaluation of 9MW2821, a site-specific monomethyl auristatin E-based antibody-drug conjugate for treatment of Nectin-4-expressing cancers. Mol. Cancer Ther. 2023, 22, 913. [Google Scholar] [CrossRef]
- Rigby, M.; Bennett, G.; Chen, L.; Mudd, G.E.; Harrison, H.; Beswick, P.J.; Van Rietschoten, K.; Watcham, S.M.; Scott, H.S.; Brown, A.N.; et al. BT8009; A Nectin-4 targeting bicycle toxin conjugate for treatment of solid tumors. Mol. Cancer Ther. 2022, 21, 1747. [Google Scholar] [CrossRef] [PubMed]
- Bekos, C.; Muqaku, B.; Dekan, S.; Horvat, R.; Polterauer, S.; Gerner, C.; Aust, S.; Pils, D. NECTIN4 (PVRL4) as putative therapeutic target for a specific subtype of high grade serous ovarian cancer-An integrative multi-omics approach. Cancers 2019, 11, 698. [Google Scholar] [CrossRef] [PubMed]
- Chalif, J.; Wegner, L.; Backes, F.; Chambers, L.M. Hyperthermic intraperitoneal chemotherapy in the management of ovarian, fallopian tube and peritoneal carcinomas. Surg. Oncol. Clin. 2025, 34, 265. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boylan, K.L.M.; Walz, C.; Schefter, A.M.; Skubitz, A.P.N. A Peptide Derived from Nectin-4 Increases Cisplatin Cytotoxicity in Cell Lines and Cells from Ovarian Cancer Patients’ Ascites. Cancers 2025, 17, 901. https://doi.org/10.3390/cancers17050901
Boylan KLM, Walz C, Schefter AM, Skubitz APN. A Peptide Derived from Nectin-4 Increases Cisplatin Cytotoxicity in Cell Lines and Cells from Ovarian Cancer Patients’ Ascites. Cancers. 2025; 17(5):901. https://doi.org/10.3390/cancers17050901
Chicago/Turabian StyleBoylan, Kristin L. M., Caitlin Walz, Alexandra M. Schefter, and Amy P. N. Skubitz. 2025. "A Peptide Derived from Nectin-4 Increases Cisplatin Cytotoxicity in Cell Lines and Cells from Ovarian Cancer Patients’ Ascites" Cancers 17, no. 5: 901. https://doi.org/10.3390/cancers17050901
APA StyleBoylan, K. L. M., Walz, C., Schefter, A. M., & Skubitz, A. P. N. (2025). A Peptide Derived from Nectin-4 Increases Cisplatin Cytotoxicity in Cell Lines and Cells from Ovarian Cancer Patients’ Ascites. Cancers, 17(5), 901. https://doi.org/10.3390/cancers17050901






