Abstract
The objective of the research was to prepare Fe-based materials for use as oxygen carriers (OCs) and investigate their reactivity in terms of their applicability to energy systems. The performance of ZrO2 supported Fe-Mn oxide oxygen carriers with hydrogen/air in an innovative combustion technology known as chemical looping combustion (CLC) was analyzed. The influence of manganese addition (15–30 wt.%) on reactivity and other physical properties of oxygen carriers was discussed. Thermogravimetric analyses (TGA) were conducted to evaluate their performance. Multi-cycle tests were conducted in TGA with oxygen carriers utilizing gaseous fuel. The effect of redox cycle number and temperature on stability and oxygen transport capacity and redox reaction rate were also evaluated. Physical-chemical analysis such as phase composition was investigated by XRD, while morphology by SEM-EDS and surface area analyses were investigated by the BET method. For screening purposes, the reduction and oxidation were carried out from 800 °C to 1000 °C. Three-cycle TGA tests at the selected temperature range indicated that all novel oxygen carriers exhibited stable chemical looping combustion performance, apart from the reference material, i.e., Fe/Zr oxide. A stable reactivity of bimetallic OCs, together with complete H2 combustion without signs of FeMn/Zr oxide agglomeration, were proved. Oxidation reaction was significantly faster than the reduction reaction for all oxygen carriers. Furthermore, the obtained data indicated that the materials have a low cost of production, with superior reactivity towards hydrogen and air, making them perfect matching carriers for industrial applications for power generation.
1. Introduction
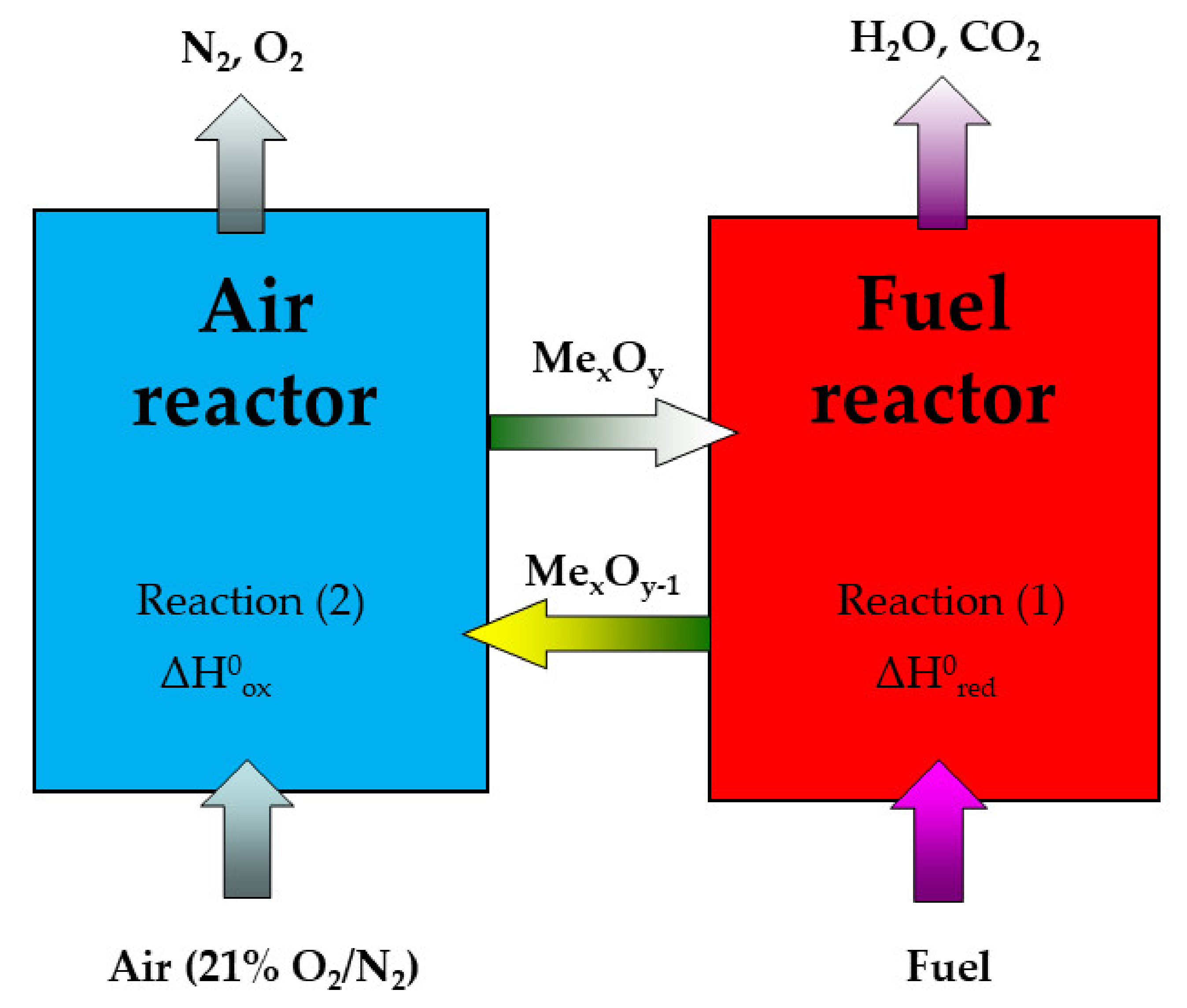
CO2, known as the primary greenhouse gas for possible global climate warming, is mainly produced from fossil fuel combustion processes. Recently, the commercial CO2 separation technologies have unfortunately displayed this withdrawal of considerable energy penalty. Nevertheless, chemical looping combustion (CLC) uses an oxygen evolved from the structure of oxygen carrier (OC), typically a metal oxide. OC transports the oxygen required for combustion from the air to the fuel in power systems, leading to the avoidance of direct contact between air and a fuel in fuel and regeneration reactors. The main advantage of a CLC power system is that a concentrated CO2 stream can be obtained from the combustion gas stream after water condensation, without requiring any energy for carbon dioxide separation or its purification [1]. For this reason, CLC is believed to be an innovative technology that may be a solution for the mentioned energy penalty problem [1,2,3]. In essence, the CLC system is usually composed of two fluidized bed reactors, as shown in Figure 1. One of them is a fuel reactor, where fuel reacts with an oxygen carrier, usually made of a metal oxide, to produce CO2 and H2O (Equation (1)). The second is an air reactor, where the reduced oxygen carrier is re-oxidized back to its original state (Equation (2)). The total amount of heat released from both fuel and air reactors is equal to the heat released from the ordinary combustion process.
CnH2m + (2n + m)MexOy ↔ nCO2 + mH2O + (2n + m)MexOy−1
2MexOy−1 + O2(g) ↔ 2MexOy
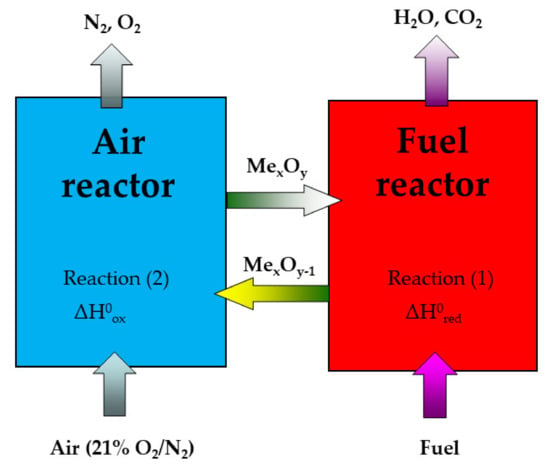
Figure 1.
Scheme for the CLC process, depicting two reactors with circulating oxygen carriers.
Furthermore, in the CLC process, NOx production is significantly reduced. Although the chemical looping with oxygen uncoupling (CLOU) technique is also a promising CO2 capture ready energy technology, it applies oxygen carriers with given thermodynamic properties enabling them spontaneously to liberate gaseous O2 in the fuel reactor, where it can react directly with solid fuels. After water condensation, carbon dioxide can be compressed and transported to an appropriate storage location. Another way is its utilization. CO2 is known to be both a thermodynamically stable compound with very low reactivity, whichever way it might be used, for example, for the synthesis of liquid fuels [4]. The flue gases from the air reactor mainly consist of nitrogen and a small amount of oxygen, which can be released into the atmosphere.
Many simple transition metal oxides such as, for example, CuO, Mn2O3, Fe2O3, etc., have been investigated as oxygen carriers in the past [5,6].
Recently, an increased interest in mixed metal oxides as OC materials has been observed. There are examples of perovskite-type compounds, for example, SrMn1−xNixO3 [7], and so-called bimetallic compounds such as supported Fe2O3-CuO and Fe-Mn oxides [8,9,10,11,12] that have been examined. The second metal oxide is typically added to improve the reactivity of Fe or the stability of Ni oxide-based oxygen carriers. The proof of this was demonstrated in a paper [13], where the bimetallic Co-Ni/Al2O3 sample displayed both high reactivity and stable behavior over multiple reductions and oxidations. Earlier investigations showed that the stabilization effect by adding CuO (20 wt.%) to Fe2O3 (60 wt.%) was observed [10] for the methane combustion process. Fe2O3–CuO oxygen carriers were supported on some inerts, such as sepiolite, bentonite, TiO2, Al2O3, and SiO2. The positive effect on the stability of Fe oxides during multiple reduction and oxidation cycles was most profound at higher temperatures (~950 °C). The observed stable and increased reactivity of Fe2O3-CuO OCs was ascribed to the formation of active phases such as spinel type CuFe2O4 and Fe2O3.
Furthermore, in several works [8,9,14] the combination of iron oxide with other d-transition metal oxides such as manganese oxide was also shown; these may give interesting thermodynamic properties, making them suitable for chemical looping. Nevertheless, it was found that the physical stability of the examined materials was low. To tackle the problem, it has been suggested that improved stability could possibly be achieved by the addition of inert material.
Oxygen carriers supply the oxygen required for fuel combustion and are composed of transition metal oxides or their mixtures. Literature shows that some potential OCs also contain inert materials based on Si, Al, Zr, Ti oxides, etc. Inerts, usually few to dozens of wt.%, are added to extend the OCs’ lifetime by reducing their attrition and improving their thermal resistivity. A suitable OC for the CLC process must show, for example, high reactivity, high selectivity for CO2 and H2O, and suitable oxygen transport capacity. Furthermore, an effective OC material must meet these expectations during multicycle redox reactions at high temperatures in a CLC power plant. Additionally, many challenges to obtaining suitable OC are associated with direct coal or biomass CLC processes, because of the possible loss of OC during interaction with the coal/biomass ash, which is of both economic and environmental concern [15,16,17,18].
For the purposes of improving the reduction performance of Fe2O3 OCs, which is known to be rather poor, substantial research has also been performed. For example, [9] showed that combined oxides of manganese and iron have interesting thermodynamic properties for CLC. However, they still found that the physical stability of the examined materials was low. The solution for this problem might be using some dopants such as support material as inert, which could enhance the redox stability of the system. Indeed, in earlier investigations [15] it was examined that stability for bimetallic Fe-Mn carriers could be successfully achieved by the addition of an inert material such as sepiolite, Al2O3, or ZrO2. The supported Fe-Mn oxides proved that, based on the comparison of Fe-Mn data with that of monometallic oxygen carriers such as Fe oxide/ZrO2 and Mn oxide/ZrO2, the addition of Mn oxide had an encouraging effect on the stability over multicycle reduction-oxidations, specifically at 900 °C. Furthermore, the presence of 4042 ppm of H2S in the synthesis gas (using simulated synthesis gas obtained from steam gasification of hard coals) did not appear to affect the stability during the five-cycle test. That is crucial since thermodynamic analyses indicated that there is an interaction between H2S and the metal oxide of an oxygen carrier. Furthermore, we have previously reported the interaction of H2S with some metal oxide oxygen carriers supported on bentonite or sepiolite. It was concluded that 60 wt.%Fe2O3−20 wt.%MnO2with 20 wt.% of inert additives were effective carriers for synthesis gas CLC both at 800 and 900 °C [15]. Subsequently, in other investigations it was proved that other iron-manganese oxygen carriers manufactured with the addition of inert Ti, for example Mn66FeTi7, may show magnetic properties [19,20]. This might be an additional benefit to the previously discussed increasing reaction rates or improving stability over redox cycling. That feature may be practically attractive because it would allow for the recovery of the oxygen carrier particles from coal or biomass ash, otherwise OCs may be lost in the ash stream when a solid fuel is burnt [21,22]. For this reason, the magnetic properties may be a key issue enabling the reduction of the amount of replaced OC particles in such a case.
The Aim
Consequently, the purpose of this work is to investigate the reduction-oxidation performance of Fe2O3-MnO2, supported on ZrO2 by thermogravimetric analyses (TGA) during cycling tests. This is because the global importance of CO2, being a part of greenhouse gas emissions from fossil fuel power stations, drives the need for developing new materials that can be used for CO2 adsorption conversion or its storage. There are many advantages of using Fe and Mn oxides as oxygen carriers. The reason for this is because both oxides are widely available, they have an attractive low cost, and are characterized by only minimal health and environmental concerns. They also contribute to high oxygen transport capacity and lend significant physical strength to the prepared carrier. Mixing a monometallic iron oxygen carrier with another metal oxide can improve physical stability over multiple reduction-oxidation cycles. Moreover, the modification of Fe-based oxygen carriers by introducing foreign dopants may be an important strategy to improve the reduction-oxidation performance in chemical looping combustion processes. Manganese oxides have displayed better reduction-oxidation kinetics, so it is believed that they might improve Fe2O3 kinetics, which are usually slow [15,16,23]. Additionally, thermodynamic calculations show that the formation of sulfides and sulfates with Fe2O3 is minimal within the temperature range of the CLC process; thus, Fe2O3 is likely to be more resistant to sulfur poisoning than oxygen carriers, such as copper oxide [24].
Zirconia support has been chosen based on our previous research results that have shown that the support type had a significant effect on both reduction and oxidation reaction rate. Furthermore, the use of zirconium oxide in the synthesis of oxygen carriers has not been thoroughly investigated, which creates many possibilities for research on the composition and method of synthesis of carriers, characterized by good stability and oxygen transfer capacity. The advantages of using supported mixed metal oxides on their performance will be discussed in detail. We have decided to add some inert materials to the mixture of active metal oxides to extend the OCs’ lifetime by reducing their attrition and possibly by improving their thermal resistivity. The phase compositions of unreacted and reacted samples were analyzed by X-ray diffraction (XRD).
2. Results
2.1. Phase Composition, BET Surface Area, Pore Analysis for Oxygen Carriers
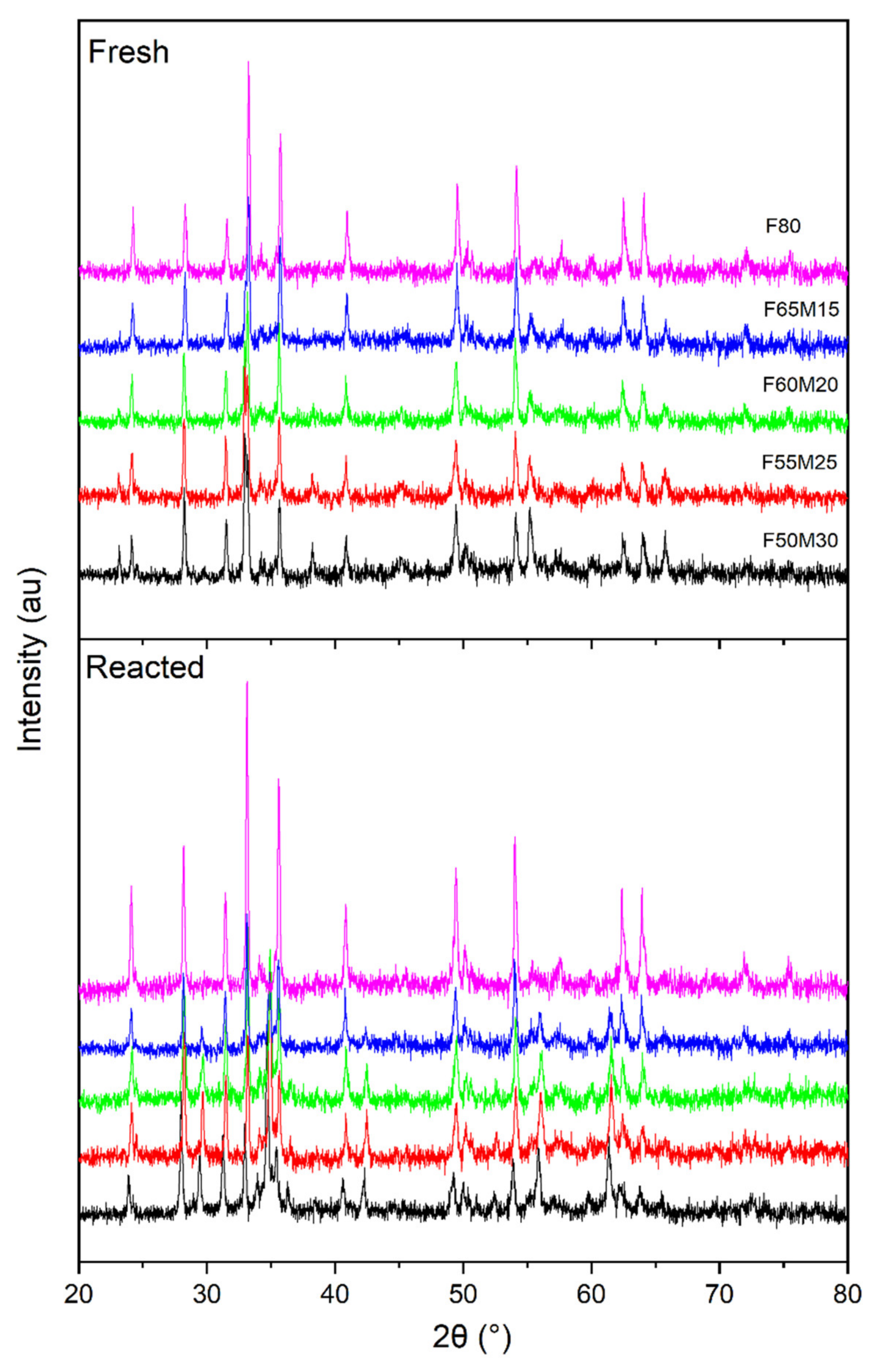
Phase composition investigations indicated that the samples were well crystalized ceramics (Figure 2). The phases detected were mostly Fe2O3 (37%–55%) and ZrO2 (about 20%), with varying amounts of bixbyite oxides (24.6%–43.2%) with a general formula of (Mn,Fe)2O3 (Table 1). For the reference material, i.e., F80 sample, Fe2O3 and ZrO2 were detected. The quantitative analysis was performed by using of The Reference Intensity Ratio (RIR)method.
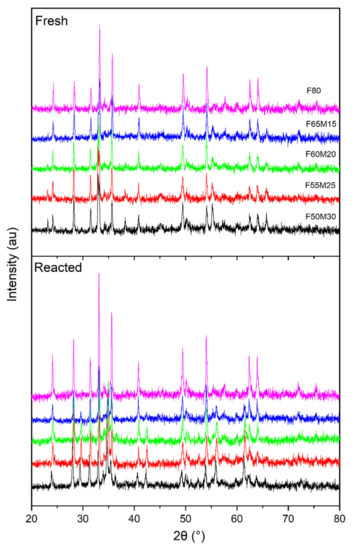
Figure 2.
Comparison of X-ray diffraction pattern between fresh and reacted samples.

Table 1.
Phase composition data for fresh and reacted OCs.
Figure 2 shows the XRD patterns obtained for the five oxygen carriers of the Fe-Mn-Zr-O system, which were collected at room temperature. Additionally, phase identification was also carried out for the reactions with gaseous fuel samples (reacted over 15 cycles), which were further treated with air to be regenerated.
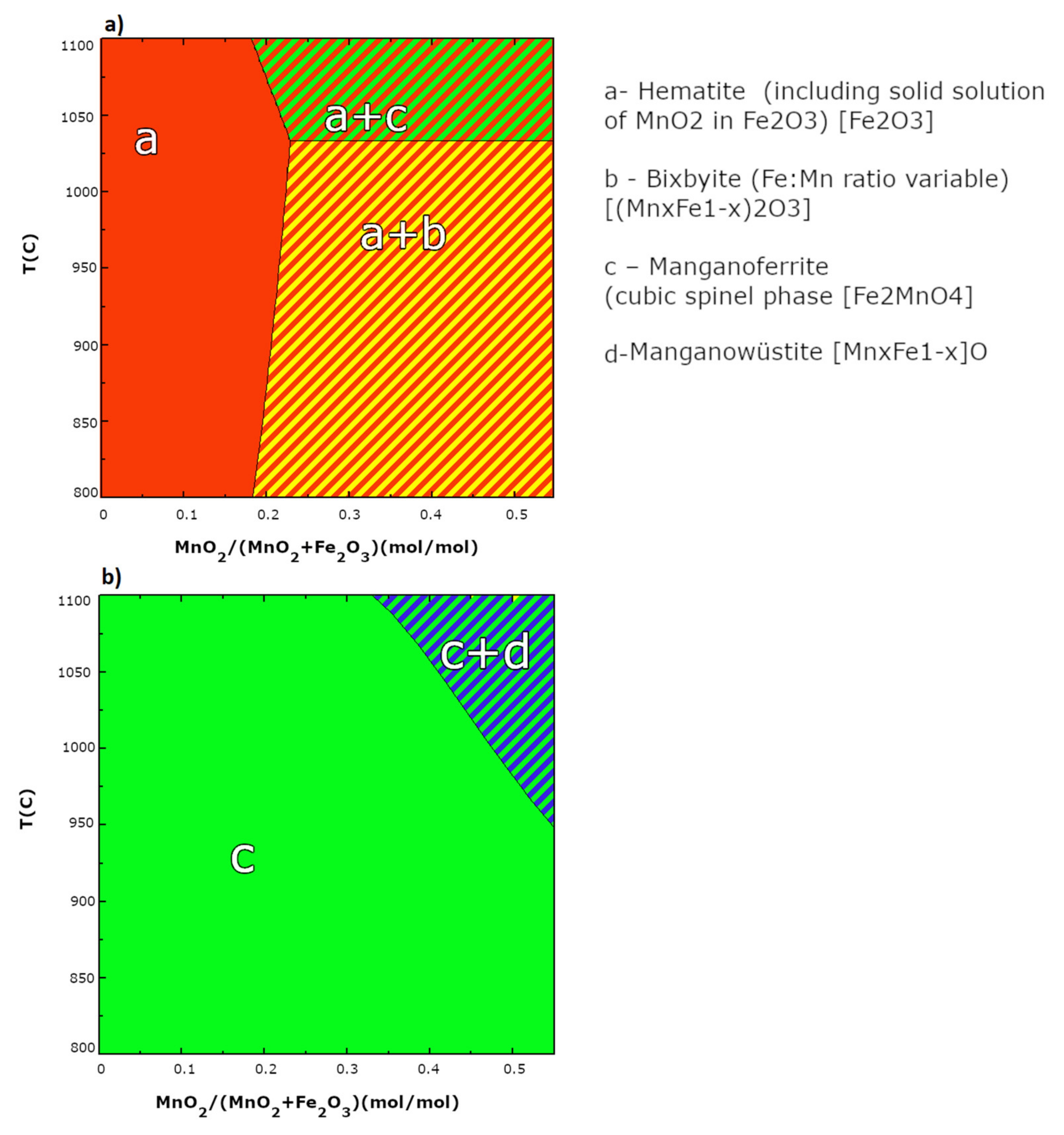
Different forms of bixbyite of general (Mn,Fe)2O3 composition were formed during the synthesis of oxygen carriers, for example, (Fe0.42Mn0.58)2O3 was specifically detected. Formation of bixbyite phase is a consequence of the interaction between active Mn and Fe oxides. The occurrence of formation of that phase was also predicted by simulation by FactSage software (Figure 3), and additionally confirmed by XRD tests. Certainly, the calculated phase diagram of the Fe2O3-MnO2 system under partial oxygen pressure of 0.20 atm (Figure 3a) shows that at synthesis temperature, i.e., 950 °C, hematite and bixbyite with variable ratio of Fe:Mn are predictable. While in the atmosphere poor in oxygen content (Figure 3b), manganoferrite with the chemical formula of Fe2MnO4 should be observed. As a result, the obtained XRD experimental data corelate well with the theoretical data.
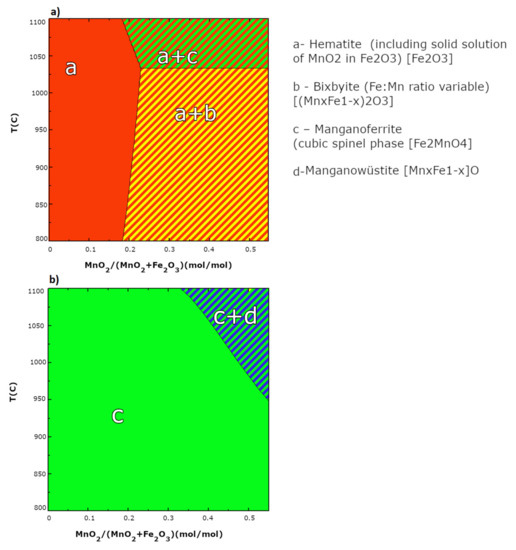
Figure 3.
Calculated phase diagrams of Fe2O3-MnO2 system: under oxygen partial pressure of 0.20 atm aerobic condition (a) and under anaerobic conditions (b).
Based on this, it is expected that different forms of oxides obtained within the synthesis stage (Mn,Fe)2O3 and hematite (Fe2O3) will be actively participating in CLC reactions, while ZrO2 will be responsible for the fine distribution of active oxides, and the improvement of redox stable performance over multiply reduction-oxidation cycles. Furthermore, the formation of new phases such as bixbyite type might be hypothetically additionally beneficial.
Table 2 presents a summary of the obtained results for the analyzed oxygen carriers. The surface area increases from 5.29 to 7.52 m2 × 10−1/g with the increasing of Fe2O3 content in the sample. The total pore volume remains similar for all samples, which is approximately 1.96–2.5 cm3 × 10−3/g. Initially, with a decrease in MnO2 content, the total pore volume in the material increases (except for F55M25 sample), while for the compared sample (F80) it reaches 2.350 cm3 × 10−3/g. The average pore diameter remains at a similar level for all doped manganese oxide samples (ca 15 nm), except for F80 material, which is the lowest of 12.51 nm. It is clear that even a small addition of MnO2 has a positive effect on the pore diameter of OC, and it was improved by 18 to 27%. Nitrogen adsorption and desorption isotherms (not shown) for oxygen carriers indicate that the observed loops of hysteresis are characteristic of the H1 type, which are cylindrical pores with a constant cross-section. This type of pore may be helpful for evolving the oxygen from oxide samples to the fuel. The specific surface area was found to be of less importance in the context of O2 adsorption/desorption than the contribution of manganese ion concentration in the OC particles.

Table 2.
Phase composition and surface parameters.
2.2. Oxygen Transport Capacity
One of the most important factors for OC selection for practical industrial application is their capacity to transport oxygen to the fuel. Therefore, the oxygen transport capacity was evaluated in this work based on TGA data. Additionally, theoretical oxygen transport capacity of the synthesized OCs was calculated for different reduction states and is shown in Table 3. These were calculated as the amount of oxygen that can be provided by the solid based on the amount of active metal oxides such as Fe2O3 and MnO2 during the reaction with a fuel. Accordingly, different final reductions of the oxygen carrier were considered. The presence of Fe2O3 in the ternary mixed compound contributes to CLC capacity, while the presence of MnO2 may contribute to both CLC and CLOU capacity. It is clear that iron oxide is the main source of oxygen in the prepared samples and depending on its content (50–65 wt.%), the analyzed oxygen carriers can transfer from 2.5 up to 19.5 wt.%, depending on the reactions involved in the oxygen transfer, as shown in Table 3, The addition of manganese oxide may further affect the amount of oxygen released, from 1.4 wt.% for the F65M15 sample to 11.0 wt.% for F50M30. Altogether the F50M30-F65M15 series of materials can transfer at least 4.65 to 26.1 wt.% maximum in total. Due to slight changes in the concentration of manganese (IV) oxide added to iron (III) oxide (15–30 wt.%) for a given reaction path, a slight increase in oxygen capacity within the series can be achieved. For example, F65M15 can release from 4.6 to 25.1 wt.%, while F50M30 is able to deliver from 5.3 to 26.1 wt.% of oxygen. The comparative sample that contains one active metal oxide, iron oxide, can release from 4.0 to 24 wt.%. These data will be used and compared further with experimentally observed capacities.

Table 3.
Predicted oxygen transfer capacity of the oxygen carriers under different reduction states.
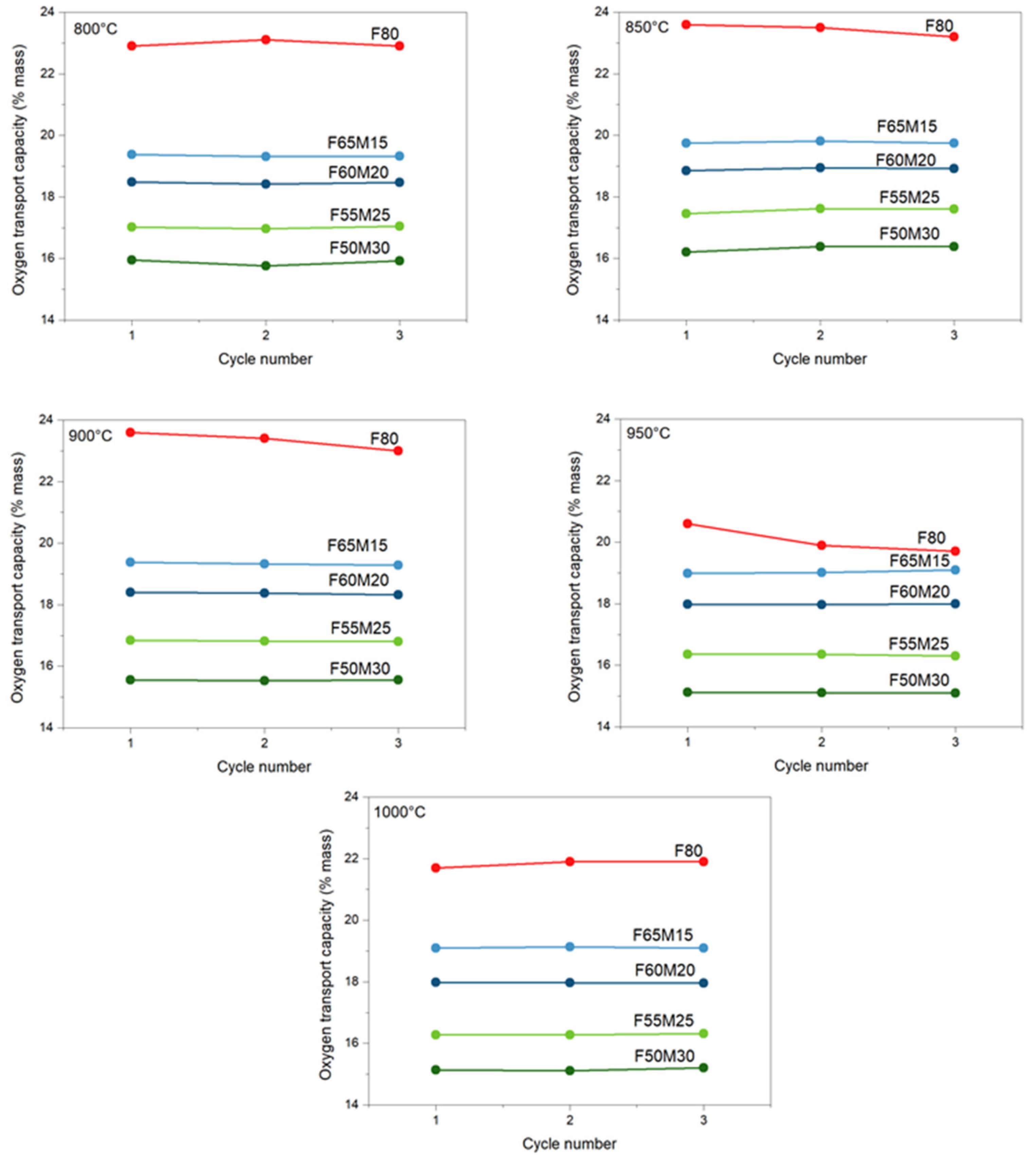
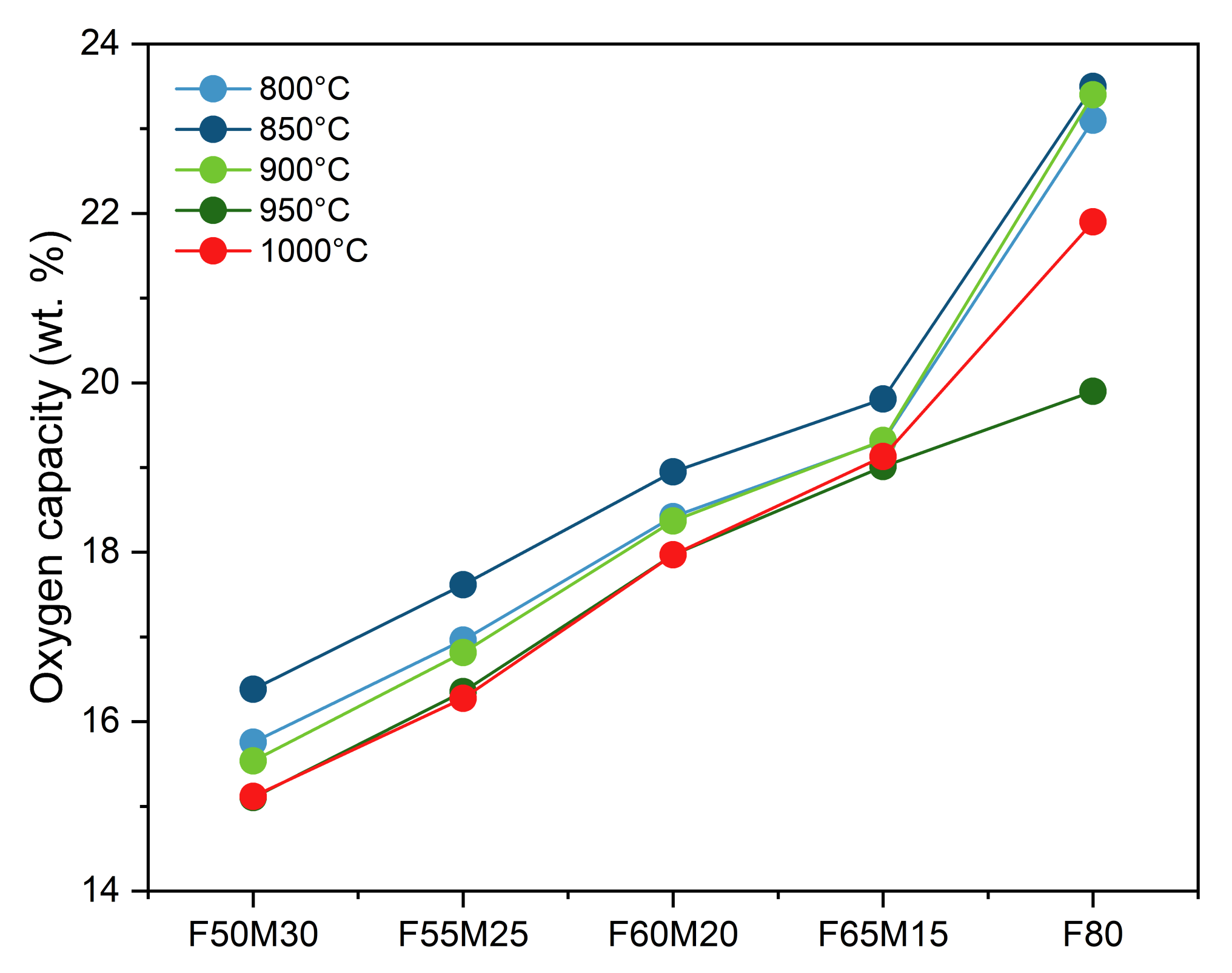
As shown in Figure 4, for iron-manganese materials containing 15, 20, 25, and 30 wt.% of manganese (IV) oxide, the experimental oxygen transport capacities determined via TGA were all close to the predicted values (Table 3). Interestingly, for all iron oxides doped with manganese, stable performance is observed with an increasing cycling number (1–3 cycles). At 800 °C, the lowest capacities were observed for the F65M15 sample: 15.95 wt.% was observed for the first cycle, then this capacity was maintained through cycling. Similar behavior is observed also for F60M20, F55M25, and F50M30 materials. The highest capacity was observed for the F80 sample, and at 800 °C for the first cycle it was 22.9 wt.%.
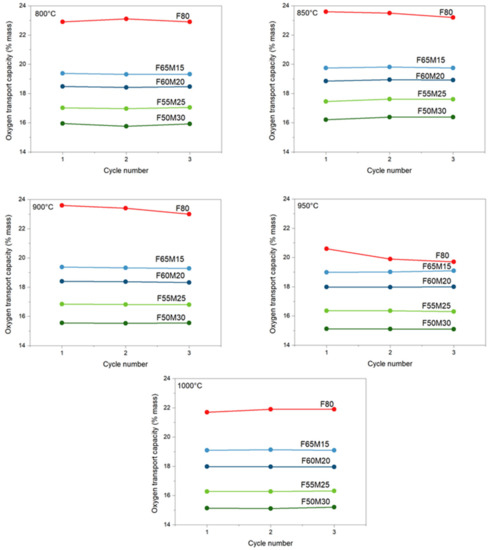
Figure 4.
Stability of measured oxygen capacity for OCs at different temperatures.
When increasing temperature, the stable oxygen evolving from the oxide structure to the fuel was observed and kept stable with the cycling number. This behavior supports the benefits of manganese addition to iron oxide. In contrast, for pure Fe2O3/ZrO2 OC a profound decrease with cycling number is observed, especially with the increase in temperature, i.e., 850 °C, 900 °C and 950 °C. For Fe2O3/ZrO2 at 800 °C, the stage with the greatest reduction potential according to the observed and compared to the predicted capacities is the reduction of Fe2O3 to Fe (mostly) with some traces of FeO, since the experimental capacity was 22.9 wt.% where the theoretical maximum extent of mass reduction calculated was 24 wt.%. For mixed metal OCs, observed capacities are well suited for Fe2O3+MnO2/Fe+Mn2O3 reduction for F50M30 and F55M25 OCs, while for F60M20 and F65M15 it is reduction to Fe+Mn3O4. Because of the fact that no pure manganese oxides, for example, neither Mn2O3 nor MnO2, were detected in XRD pattern (Figure 2), an additional contribution in chemical looping oxygen capacity may be attributed to bixbyite mixed manganese-iron oxides. Certainly, FactSage calculation proved that in CLC conditions (Figure 3b) those mixed oxides should follow the transformation of bixbyite to manganoferrite with the evolving of some oxide to the fuel.
Another point is that the addition of Mn is further beneficial since an improved stable CLC performance is clearly observed when comparing that behavior with the behavior of the undoped sample. For the F80 sample, a decrease in capacity is observed with an increase in redox cycle number for wide process temperature ranges.
The relatively high oxygen capacities in this work, obtained and proved via TGA, enable a lower required bed inventory in realistic CLC applications, which is an advantage.
Figure 5 shows a summary of the capacity observation for the selected 2-nd redox cycle for the given chemical compositions. For 800 °C, an increase in capacity is observed when increasing iron oxide content, reaching the maximum value for the pure F80 sample (light blue line). Increasing the temperature to 850 °C shows a simultaneous capacity increase of all samples of about 0.5 wt.%. This means the temperature here has a positive effect on capacity. However, further heating to 900 °C (light green line) leads to a decrease in capacities, which are similar in value to those observed experimentally for 800 °C. For 950 °C and 1000 °C (dark green and red lines), capacities are the same. Based on that behavior in terms of the best oxygen carrier capacities, which were achieved at 850 °C, this is the best CLC temperature enabled using almost all available capacities. This observation may be due to the difficulties in reoxidation that the manganese-based oxides face at higher temperatures. This was observed previously elsewhere [8,20].
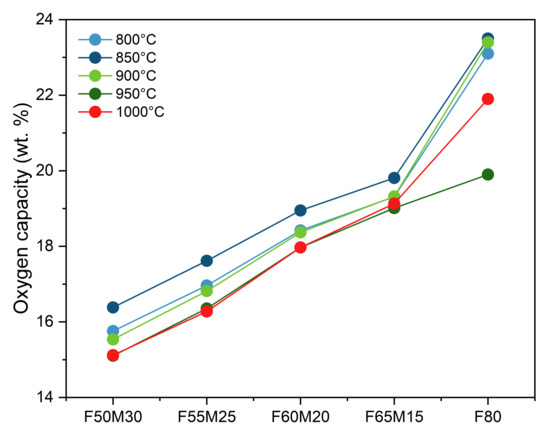
Figure 5.
Oxygen transport capacity as a function of temperature and chemical composition for Fe- Mn/Zr oxides OCs.
For the pure F80 sample, this behavior may be related to the agglomeration tendency of iron particles, which is known from the literature [15,16].
2.3. Reactivity with Fuel and Air and Thermal Stability
The characterization of the oxygen carriers was based on the evaluation of their reduction and oxidation rates through TGA experiments at temperatures suitable for the CLC processes (i.e., 800–1000 °C). TGA testing was also performed to learn about: (a) the temperature that is needed to efficiently combust the fuel with the application of Fe-Mn-Zr oxides, (b) thermal stability of each sample, (c) the effect of the chemical composition of OC on its reactivity.
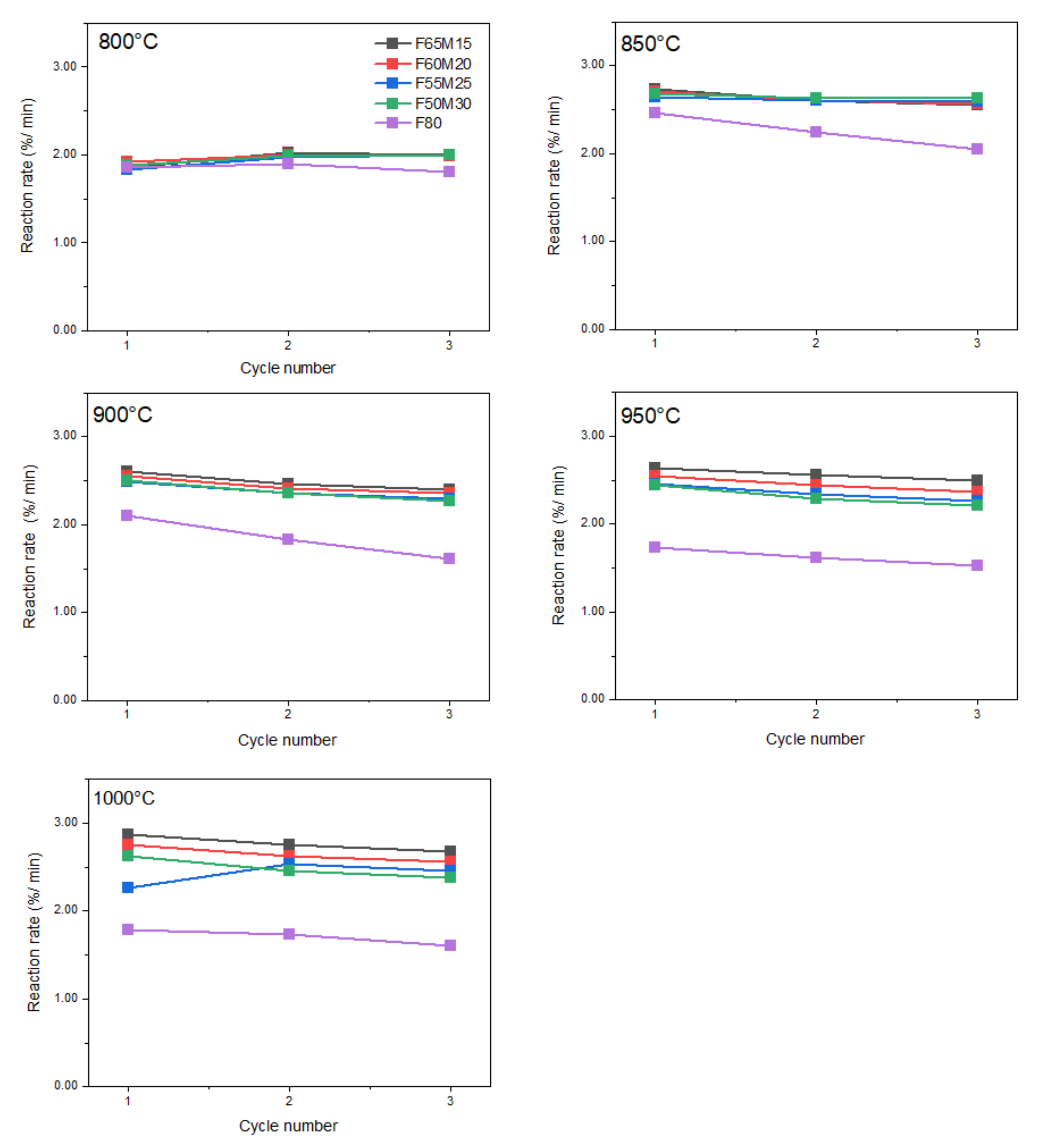
Reduction reaction rate values in function of cycling number and chemical composition are shown in Figure 6, where the reduction rates are of a similar order for all samples. At the lowest experimental temperature, i.e., 800 °C, the lowest reduction reaction rate was observed for F80 at 1.81%/min, while the highest of 2.0%/min was recorded for doped samples. The rate was maintained with the cycling numbers 1 to 3. When the process temperature was systematically increased, a continuous increase in reduction rates was observed for all doped samples, except for the undoped F80 material. For Fe-Mn samples, the rate clearly increased to approximately 2.87%/min at 1000 °C. However, for the undoped sample, different behavior was observed. At the very beginning, an increase in rate of 2.46%/min was observed at 850 °C, and then the rate systematically decreased with each 50 °C step, and was finally 1.61%/min at 1000 °C.
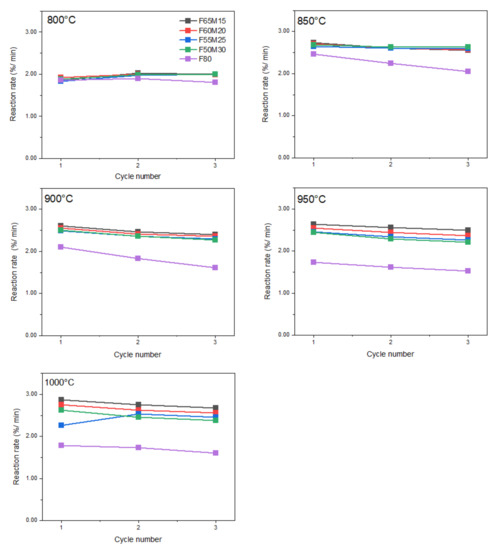
Figure 6.
Reduction reaction rates for OCs versus cycle number at given temperatures 800–1000 °C.
Subsequently, we evaluated the oxygen carrier reactivity towards H2 and its potential for oxygen release through isothermal redox cycles in TGA. The reduction reaction rates are concluded to be associated with chemical and phase composition. Considering the influence of the Mn oxide addition, the highest rate values were found for Mn-rich materials. In other words, the faster hydrogen combustion reaction was observed for the sample doped with manganese oxide compared to the un-doped sample. This is because for those samples besides Fe2O3, (Mn,Fe)2O3 such as (Fe0.42Mn0.58)2O3 are also present. For these reasons, a measurable increase in fuel combustion is detected. In [15], the strong positive temperature effect on the reduction rate was found in a similar sample of 60 wt.%Fe−20 wt.% Mn oxides/20 wt.% ZrO2 composition when the synthesis gas was combusted. However, by using other supports, sepiolite or Al2O3 and the same molar ratios, a small decrease in the reduction reaction rate was observed with an increasing temperature. Our data confirm that the inert material selection was right. For this reason, in this work a temperature effect was evaluated. Obtained data show that the fuel combustion rates increased, this being especially clear within 800–850 °C. However, the rates for doped samples negligibly changed within an increase in Mn dopant in the material in this temperature range. Furthermore, between 900 and 1000 °C the positive effect of temperature was more profound. However, surprisingly, that expected rate indeed increased for doped samples in general. Nevertheless, the phase transition between bixbyite and some spinel phases that is supposed to occur under anaerobic conditions could be an explanation for the lower than expected reaction rate in certain temperature ranges. On the other hand, the initial increase in F80 OC could be attributed to the pore reorganization (for example, an increase in pore size), and the final decrease may be due to the melting surface, which will be discussed through further analyzing SEM microphotographs. The surface areas and pore volumes for all oxygen carriers are shown in Table 1. F80 has the highest surface area of 7.52 m2 × 10−1/g, and FM samples have a slightly lower surface area of 5.29–6.28 m2 × 10−1/g. This correlates well with the oxidation performance at the very beginning, i.e., at 800 °C, but not with the reduction performance.
We anticipated that Mn oxide introduction to the Fe-Zr oxides would improve OC material performance. Our reactivity experiments proved this, because the stabilization effect was observed.
Considering the reoxidation in the air reactor, previously reacted with fuel, oxygen carriers were subjected to oxidation reactions in TGA. These was processed to examine the regeneration potential of OCs at conditions similar to those of the air reactor conditions. To evaluate the oxygen transport capability of the prepared materials, in this work, TGA experiments were carried out by oxidizing the samples at 800 °C, 850 °C, 900 °C, 950 °C and finally at 1000 °C in air.
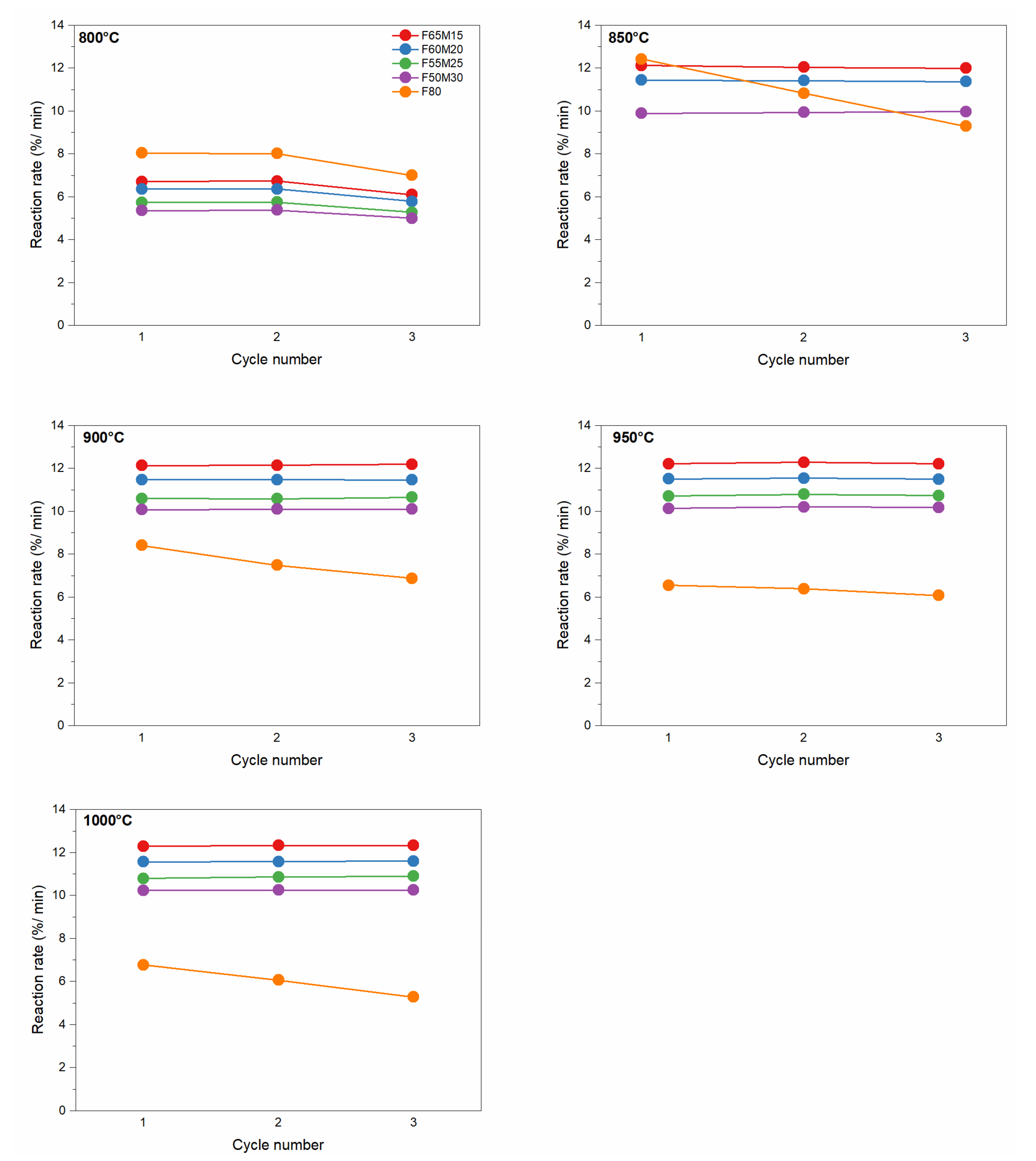
Oxidation reaction rates in function of cycling number and chemical composition are also shown in Figure 7. The stability of regeneration reaction rates for OCs is shown for the given temperatures. The pronounced stability of regeneration cycle numbers for all studied temperatures can be concluded based on the observation. Compared with the reduction reaction, the regeneration rates are significantly higher. At 800 °C the rate is F80 > F65M15 > F60M20 > F55M25 > F50M30. When the oxidation temperature was increased to 850 °C the significant ease of oxidation of the synthesized OC materials is observed—it was almost doubled. For example, for F50M30, the maximum regeneration reaction rate was 5.38, 9.93, 10.09, 10.20, and 10.25%/min for 800, 850, 900, 950 and 1000 °C, respectively. For F65M15, the maximum regeneration reaction rate was 6.73, 12.03, 12.14, 12.28, and 12.32%/min. In addition, the oxidation rates are considerably high in the analyzed temperature range compared to the reduction reaction. Further heating from 850 °C to 900 °C further shows the stable oxidation for all doped Fe-Mn oxides within the cycling number and increase in Mn content. It is also clear that temperature did not significantly affect the oxidation reaction rates above 850 °C.
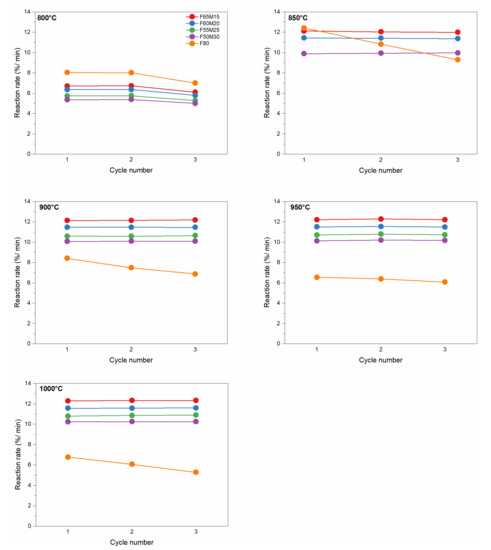
Figure 7.
Oxidation reaction rate for OCs versus cycle number at given temperatures.
Nevertheless, a similar behavior in terms of reactivity with fuel and air for pure F80 OC was observed. Firstly, this is because the lowest reduction with hydrogen was detected. Secondly, gradually decreased regeneration rates with air were observed for the reference OC F80 material. For example, at 800 °C a rate of 8.01%/min was observed, which gradually decreased with an increase in regeneration temperature, down to 6.06%/min at 1000 °C. For the monometallic material, regeneration processed slowly both with temperature and with each cycle handled. For this reason, the conclusion that a small Mn addition to 50–60 wt.% Fe2O3/20 wt.% ZrO2 significantly improved the oxidation reaction rates, because oxidation rate increased by a factor of 2, can be withdrawn.
Using the proper inert material as an addition to active metal oxide is known to influence the OCs’ stability. Additionally, it may also influence the reactivity, enabling, for example, its improvement. This can be supported by data shown elsewhere [15], where the strong positive temperature effect on regeneration ability was found in a 60 wt.% Fe2O3−20 wt.% MnO2/20 wt.% ZrO2 sample. However, for the same cases for both 60 wt.% Fe2O3−20 wt.% MnO2/20 wt.% sepiolite and 60 wt.% Fe2O3−20 wt.% MnO2/20 wt.% Al2O3, the oxidation rate may remain the same with an increase in the temperature. The obtained results of reactivity in the present paper further support the claim that Zr support was a good choice for mixed metal Fe-Mn oxides
2.4. Characterization after Redox Tests
Determination of the morphology of the obtained materials and distribution of elements was performed with a scanning electron microscope (SEM) and energy dispersion X-ray spectrometer (EDS) for both as-synthesized and spent samples. It is important to notice that SEM images supported the size of the particles (ca 125–180 µm) and their oval form (Figure 8), as required.

Figure 8.
Scanning electron microscope photomicrographs of as-synthesized FeMn OC samples (F50M30, F55M25, F60M20 and F65M15).
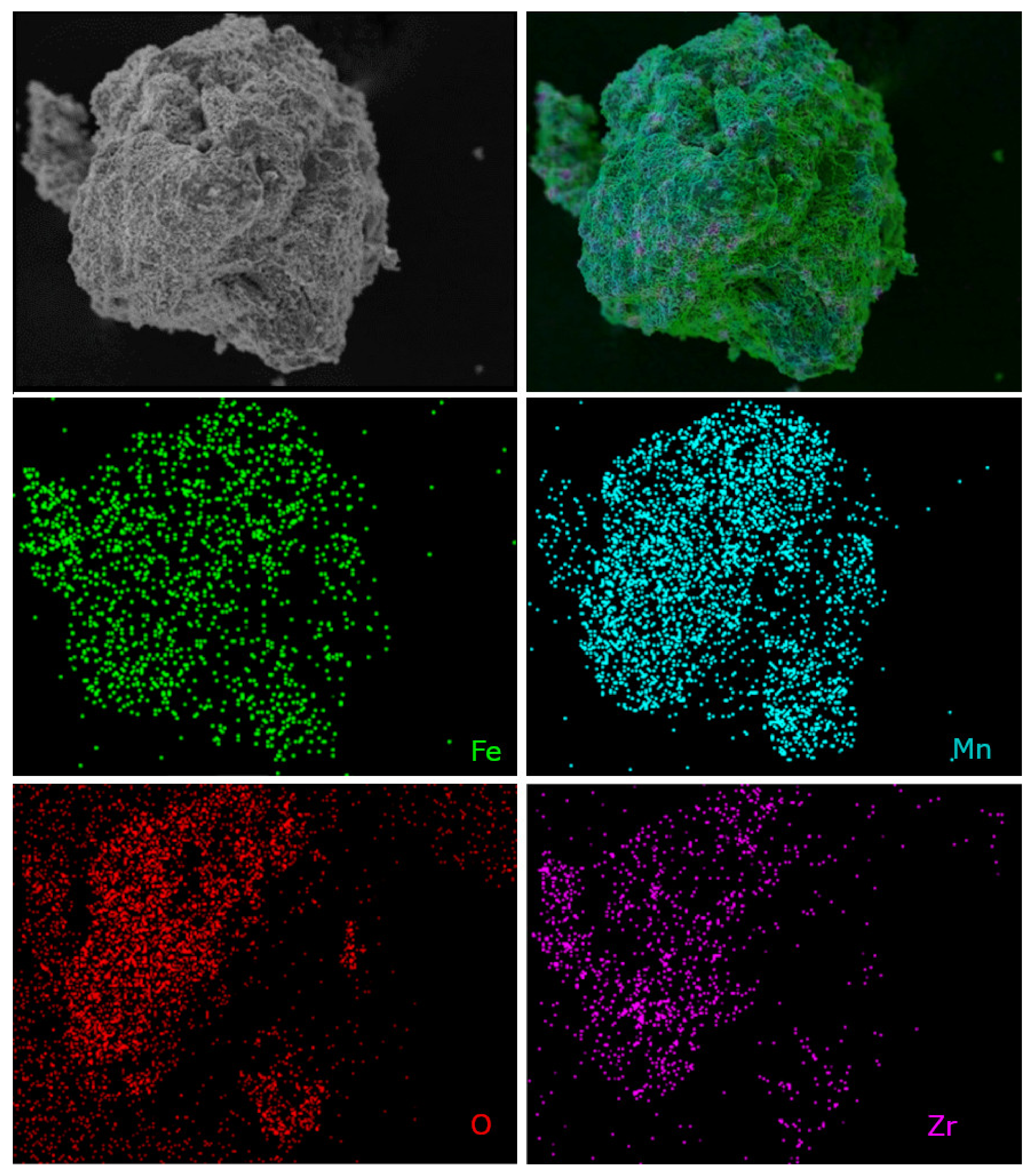
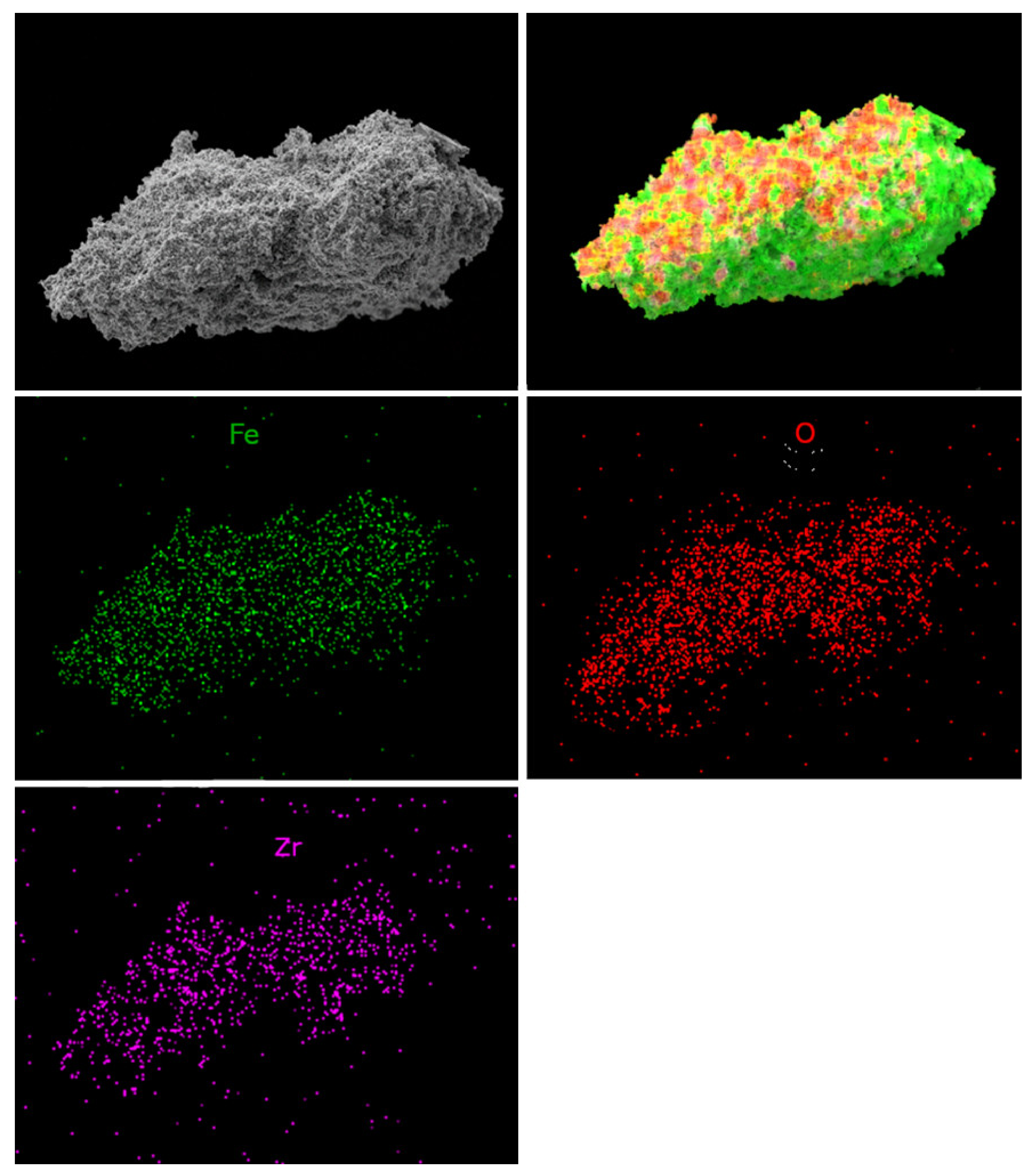
Moreover, the chemical microanalysis data show that Fe-Mn-Zr oxides obtained in this work display homogenous dispersion of the elements, as assumed. This proves the good quality of the OCs, since the dispersion of the Fe, Mn, Zr, and O of the scanned areas in samples was very good, which was shown in Figure 9 and Figure 10. The element mapping for the fresh F50Mn30Zr20 (Figure 9) sample supports the presence of porous manganese ferrite and the spots of ZrO2, as previously observed in diffractograms. Furthermore, the morphology and texture of doped and undoped samples differs, for example, Figure 10 shows that F80 sample particles are more porous and irregular compared to doped materials.
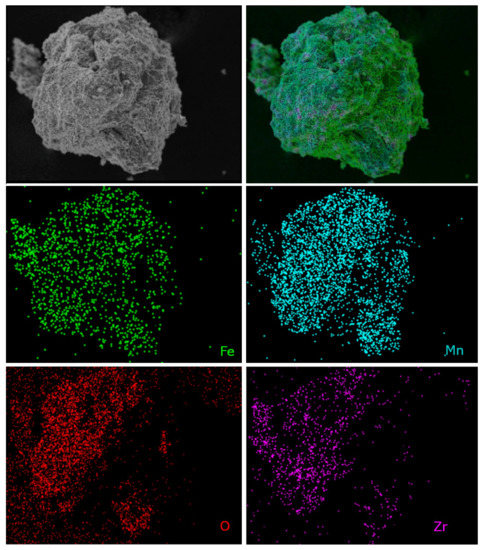
Figure 9.
Scanning electron microscope photomicrographs with element mapping for fresh F50Mn30Zr20 sample.
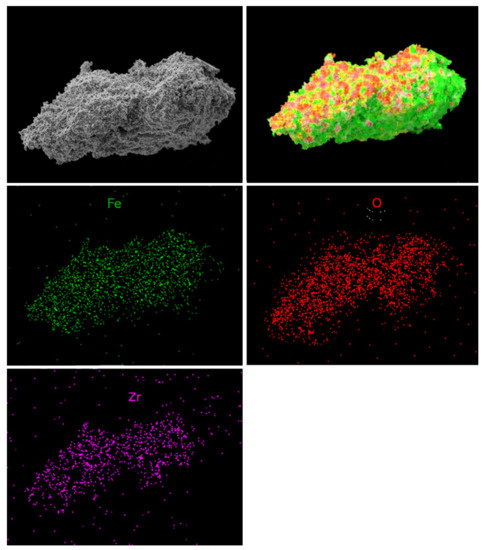
Figure 10.
Scanning electron microscope photomicrographs with element mapping for fresh F80Zr20 sample.
The XRD analyses of spent oxygen carriers (Figure 2) showed that their crystal structures have not changed significantly after cycling reactions carried out under high temperature (800–1000 °C) for about 25 h of experiments. However, profound phase analysis showed some minor crystal phase changes in the diffractograms during cyclic fuel combustion carried out at high temperatures.
In the as-synthesized samples, the peaks of Fe2O3, ZrO2 and so-called bixbyite (Fe,Mn)2O3 phases were observed. The bixbyite type reflexes such as 23.13; 32.95; 38.23; 55.18; 65.78° were specifically attributed to the presence of (Fe0.42Mn0.58)2O3 oxide, which corresponded well with the crystal data 1,543,471 from The Inorganic Crystal Structure Database (ICSD).
Furthermore, the degree of agreement between the experimentally determined crystal phase and that predicted is further seen by analyzing Figure 2 and Figure 3.
Bixbyite is a rarely occurring natural iron-manganese oxide mineral typically showing an isometric hexahedral habit and a hardness of ca. 6. It is also known that bixbyites are minerals containing from 45 to 60 mol% of Fe2O3, forming usually at high temperatures from 800 to 1000 °C. Some interesting forms of bixbyites in their natural form may be found, for example, in the seams and cavities of the rhyolitic host rock in the Thomas Mountain Range, Utah, USA. Nowadays, a world-wide phenomenon of increased interest in the mixed iron-manganese oxides can be observed. First of all, it is attributed mainly to their interesting magnetic and electrical properties. Some attracted attention due to several potential applications, such as catalysis or sensors or super capacitors.
For the spent materials among Fe2O3 and ZrO2, some (Fe,Mn)O4 type oxides were detected instead of the (Mn,Fe)2O3. The observed diffraction peaks at 2theta, such as 29.59; 34.82; 34.88; 36.47; 42.3; 42.39; 55.99; 56.03; 61.44; 61.51°, were attributed to the presence of manganoferrite(Fe1.3 Mn1.7)2O4, which is spinel-type oxide, here called iwakiite, and corresponded suitably with the crystal phase data of the ICSD number 76612.
In this work, the presence of iron-manganese oxides in the form of manganoferrites, both in fresh and spent OCs material, may have practical implications for factors such as the ease of the separation of spent oxygen carriers from the fuel due to magnetic properties, which is superior. For example, in two members of the bixbyite family, FeMnO3 and Fe0.5Mn1.5O3 oxides, an evident ferrimagnetic behavior was observed, while the origin of ferrimagnetism was ascribed to the antiparallel alignment of the unequal spins on 8a and 24d crystallographic sites. It was also observed that with increasing Fe content in Mn2O3, there was a clear change in the magnetic behavior while exploring the high-field magnetization behavior. Some crystal changes were also observed from cubic to orthorhombic structure. Since Mn is known to exist in various valence states (for example, +2; +3, etc.), for this reason the magnetic properties are enriched depending upon the composition and this phenomenon may be specifically created and used as desired.
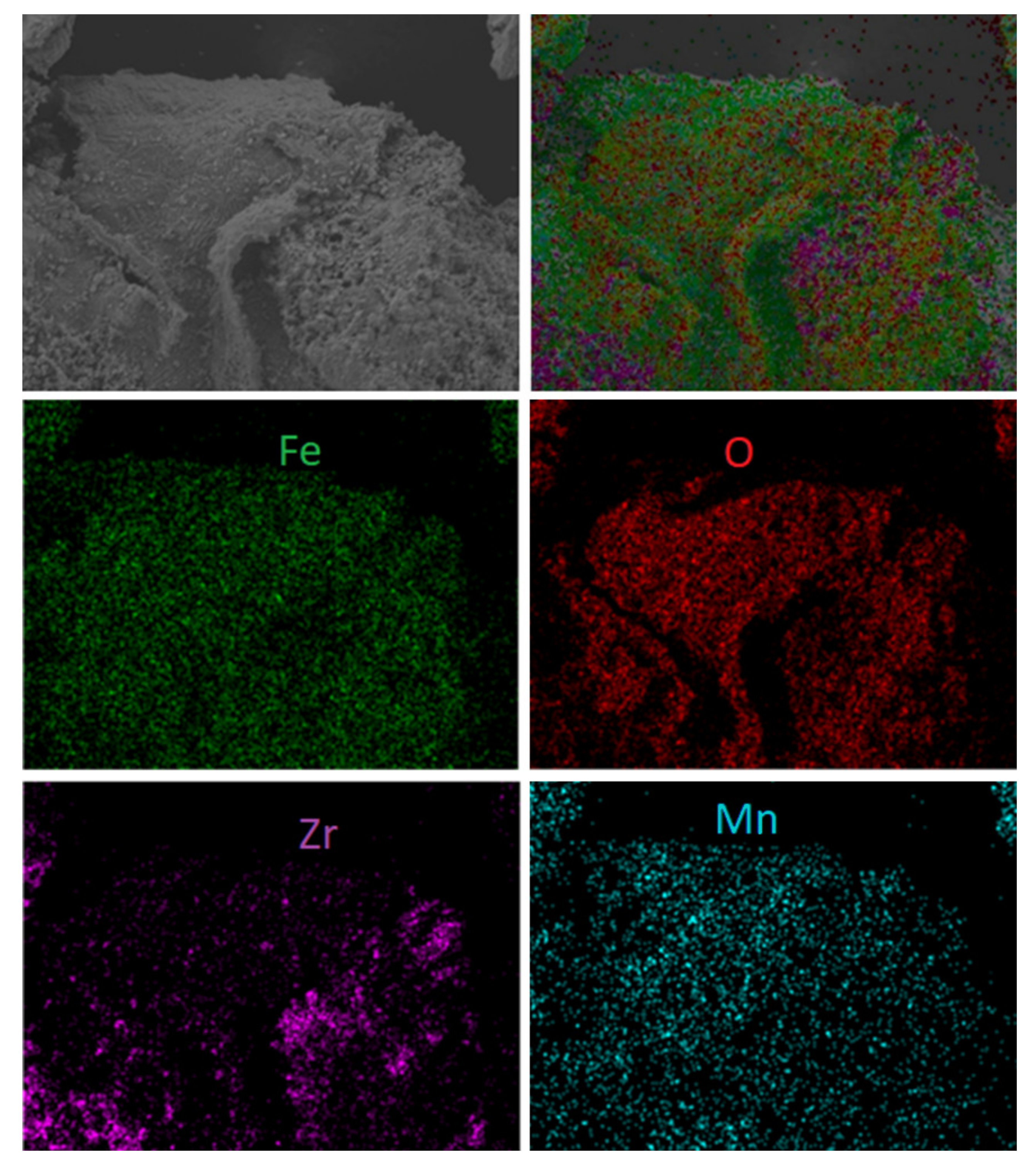
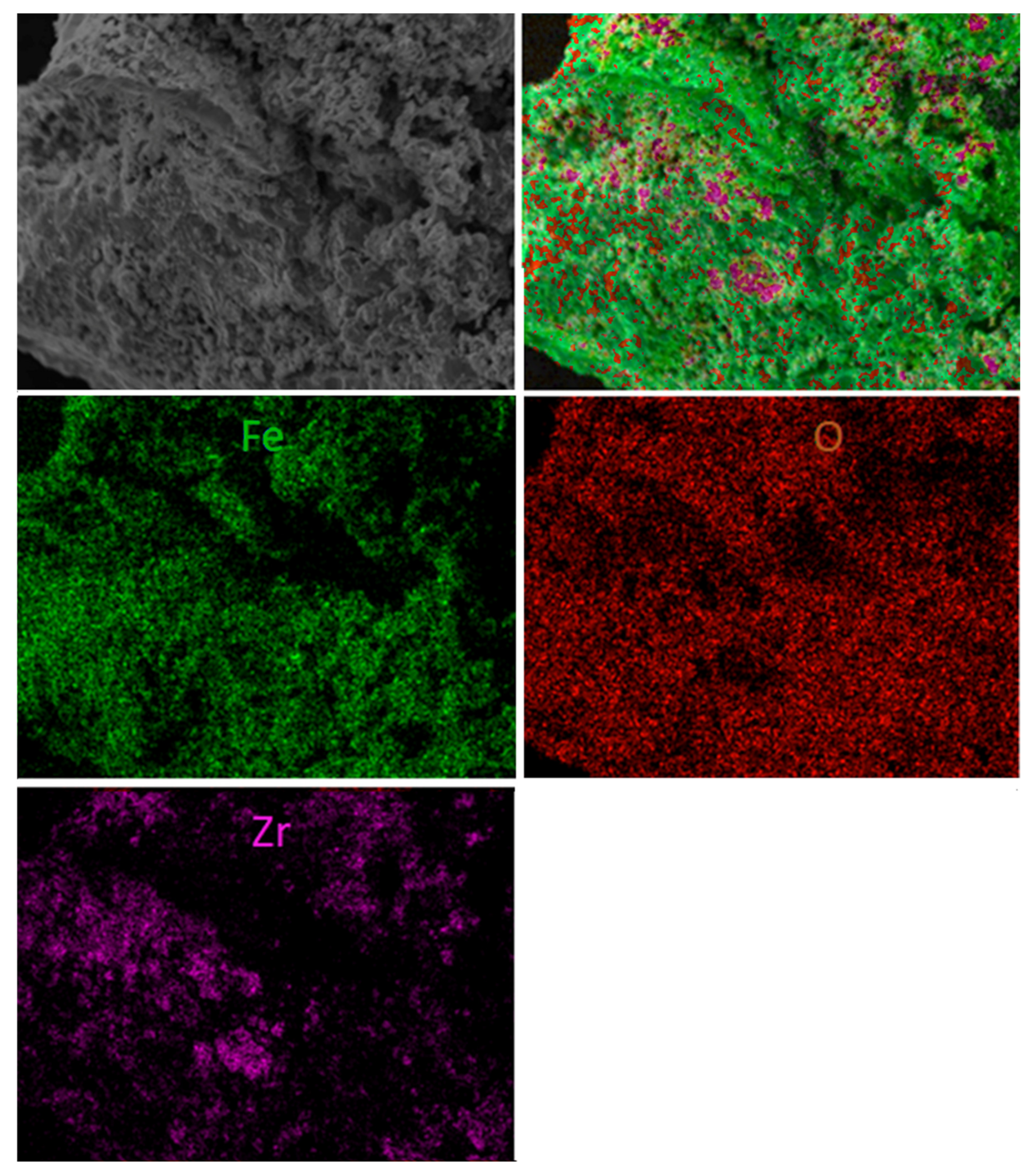
Additionally, some morphology changes were detected during the examination of spent materials. Figure 11 shows both scanning electron microscope photomicrographs with element mapping for the sample first reacted with fuel and then with regenerated F65Mn15, while Figure 12 displays reference F80 OC. The SEM analysis proved that in the spent materials a type of shell was formed on the external surface of the OC granule. The high-quality picture shows the dense and striped surface of the oval granule, with thickness of the layer estimated to be 5–10 µm and a highly porous inner core. The shell is stripped, while the stripes are arranged from top to bottom and from left to right with a medium diameter of 20 µm. Based on element mapping and powder diffraction pattern analysis, the first compact and hard layer can be attributed to the presence of manganese-iron spinel, precisely (Fe1.3 Mn1.7)2O4, and the subsequent use of hematite (Fe2O3). Opposite to manganese, the doped samples were used as the reference material. Figure 12 shows scanning electron microscope photomicrographs with element mapping for F80 sample reacted and subsequently regenerated with air. It is clear that both oxygen and iron are uniformly broadcasted on the internal and external surface of the granule, which is attributed to Fe2O3, while zirconia is rather outside, and seems to be separate small grains. The SEM image shows that the OC particle is strongly porous with some melted areas (left). This is the proof of the decreased reactivity with an increase in CLC process temperature observed during the TGA experiments.
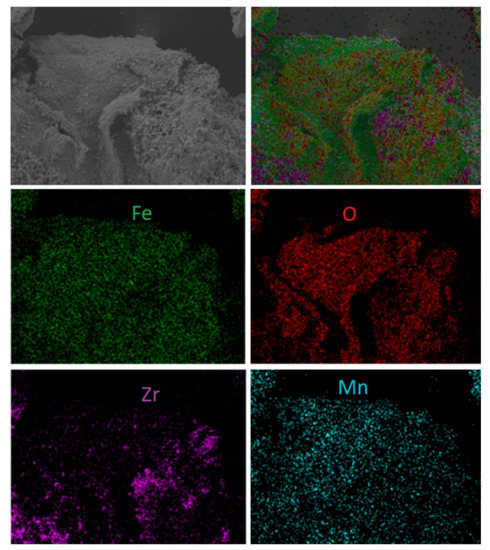
Figure 11.
Scanning electron microscope photomicrographs with element mapping for reacted and regenerated F65Mn15 sample.
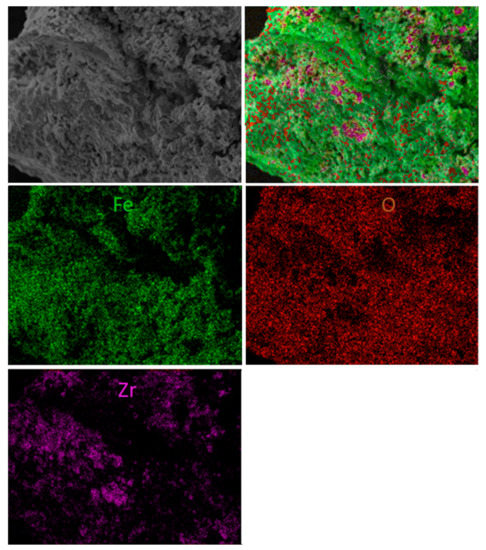
Figure 12.
Scanning electron microscope photomicrographs with element mapping for reacted and regenerated F80 sample.
From a practical point of view, high attrition resistance and low agglomeration tendency of OC particles are expected and might be advantageous [8,21]. For this reason, SEM pictures were taken for the reacted samples. No sign of agglomeration of spent doped Fe-Mn materials in tested reaction conditions was observed.
3. Conclusions
The present work was a screening of the performance of Mn-Fe-Zr based oxygen carriers, prepared with different Mn:Fe mass ratios and a fixed ZrO2 concentration. Four oxygen carriers of the Fe-Mn-Zr-O system were prepared, and for comparison purposes “pure” Fe2O3/ZrO2 was also obtained. The XRD data confirmed the presence of Fe2O3 and (Mn,Fe)2O3 bixbyite type (mostly), and ZrO2.
A comprehensive evaluation of the physical and chemical properties of the materials prepared with different Mn:Fe ratios was carried out regarding their capability to be used in iG-CLC processes. The main goal of the work was the synthesis of selected mixed compounds containing Fe2O3, MnO2, ZrO2 oxides for low-emission energy generation process applications. An approach to exchange Fe cations with Mn in Fe-Zr framework was made. The impact of manganese’s presence in the mixed oxide system on the model fuel combustion was determined. The partial goals of this work included: evaluation of the tendency to agglomeration of carrier granules; surface area analysis and its expected participation in redox processes; critical morphology analysis of synthesized mixed compounds using scanning electron microscopy (SEM); assessment of the reactivity of the selected fuel; evaluation of oxygen transfer capacity on the basis of the assumed stoichiometric compositions of the samples and TGA testing; and selecting the most promising materials for further research which are F65M15 and F60M20 materials.
XRD analyses showed that the oxygen carrier particles might be reduced-oxidized during the multicycle CLC. Successful regeneration was achieved after cyclic reaction with hydrogen; however, new manganoferrite phases were formed, specifically iwakiite (Fe1.3 Mn1.7)2O4.
4. Materials and Methods
4.1. Synthesis and Materials Quality Examination
Fe2O3-MnO2 supported on 20 wt.% ZrO2 samples with different chemical compositions and oxygen carriers were prepared by using the mechanical mixing method (Table 4). High quality powders of Fe2O3 and MnO2 (<99%, Sigma Aldrich, St. Louis, MO, USA) were mixed roughly with inert ZrO2, and then deionized water was added to obtain a paste. The paste was dried, crushed, and calcined at 950 °C in air for 8 h. A proportion of 10 wt.% of graphite was added to the mixture to improve the porosity/surface area of the samples. The graphite is oxidized to CO2 during calcination at 950 °C in air and is well known to be a porosity increasing agent. After cooling, the procedure was repeated. In other words, the sample was crushed and thoroughly mixed with water and graphite addition again. It was then calcined at 950 °C in air for 8 h. Calcined samples were subjected to sieving to isolate the desired fraction of 125–180 µm. As a result, five different oxygen carriers were obtained (Table 4). The symbol F50M30 refers to 50 wt.% Fe2O3–30 wt.% MnO2 and 20 wt.% ZrO2 sample, etc. To examine the effect of manganese addition, different concentrations of Mn oxides were prepared, and finally, F80 sample with no manganese was obtained. The procedure and temperature selected was based on our previous experience (1050 °C 20 h) [15] and a paper published elsewhere, where the best results of the carrier strength were observed at low calcination temperatures (850 °C for 4 h) [25].

Table 4.
The obtained OC samples.
Furthermore, due to the phase transition of zirconium oxide at a temperature of about 1170 °C, the calcination temperature was selected as 950 °C.
4.2. X-ray Powder Diffraction
XRD patterns of both “fresh” Fe2O3-MnO2 oxygen carriers and “reacted spent” after reaction with hydrogen/air were collected at room temperature with the application of the X-ray powder diffractometer MiniFlex600 by RIGAKU. The experiments were carried out at 40kV, 15mA, with a filtered CuKα radiation of λ = 1.54056Å. In particular, the diffraction patterns were obtained with a step of 0.02°, over the 2θ range of 20 to 80°. The crystalline phase identification and quantification were performed with the application of JCPDS data. For quantitative phase composition, RIR method was used.
4.3. Microstructure
The microstructure of the materials’ surface was studied using scanning electron microscopy, by means of the JEOL JSM-6610 LV instrument with an energy dispersion X-ray spectrometer for chemical microanalysis purposes. The surface morphology was studied by gluing carbon tape onto the samples; carbon tape was not used in the chemical analysis of the samples. The study was conducted using a low vacuum detector at an accelerating voltage of 15 kV and different magnification of image 50–1000×. The EDS Oxford Aztec Energy, with an Si(Li) X-ray detector was used for investigation of the homogeneity of the samples. The composition analysis by EDS was performed. During this study, an analysis of the composition of the entire grain surface was carried out (due to the presence of the elements: Fe, Mn, Zr, O), and a linear analysis of selected grain fragments to examine the composition and possible presence of the expected structures.
4.4. Surface Area Characterization and Porosity Analysis
Autosorb 1C (Quantachrome, Boynton Beach, FL, USA) instrument was used to determine the pore volume and surface area via N2 adsorption isotherms at 77 K. Prior to the measurements, the samples were degassed under vacuum at 350 °C for 4 h. The pore size and volume were calculated using the BET (Brunauer–Emmett–Teller) and BJH (Barrett–Joyner–Halenda) methods, respectively.
4.5. Thermogravimetric Analysis (TG)
Thermogravimetric experiments were conducted using a thermal analyzer (STA 409 PG Luxx by Netzsch, Selb, Germany) which was coupled to a QMS 403C by Aëolos. The mass spectrometer used for the evolved gas analysis could detect mass numbers of 1–300 amu in the MID mode. In the experiments, the mass changes of the metal oxide OCs were measured isothermally as a function of time. The sample was heated in an inert Ar atmosphere with a heating rate of 20 K/min. When a temperature of 800 °C was reached, the sample was held for about 10 min and flushed with synthetic air, and then the experiment was started with consecutive introduction of reducing and oxidizing gases.
After stabilization, the oxygen carrier particles were exposed to three successive reduction-oxidation cycles, conducted at atmospheric pressure to determine the reactivity.
In the CLC measurements, for each test, a sample weighing approximately 50 mg was placed on an Al2O3 plate. A mixture of 4% H2/Ar (used as a fuel agent) was used for the reduction reaction, and 20% O2/N2 was used for the oxidation reaction. The reaction gas flow rates were set at 100 mL min-1. The reduction time was set at 45 min, and the oxidation time was set at 20 min. To avoid the reduction gases mixing with air, the TG system was flushed with Ar for 10 min before and after each reduction/oxidation reaction. Furthermore, both the oxygen carrier mass and gas flow rates used in the TGA experiments were chosen to avoid limitations in the external film mass transfer and interparticle diffusion. Reduction-oxidation cycles were conducted at atmospheric pressure to determine the stability of the carriers. To understand the effect of temperature, TGA experiments of CLC were carried out at 800–1000 °C temperature range.
Hydrogen is an environmentally friendly energy carrier derived from, for example, biomass or coal. Hydrogen is also a part of the synthesis gas, which can be used as a gaseous fuel for CLC for power generation. For this reason, it was applied in this work to examine the reactivity of synthesized Fe-Mn-based OCs. Furthermore, H2 was used to explore the oxygen transfer capacity of the materials under very severe reducing conditions.
The fractional conversions (X), such as the fractional reduction and the fractional oxidation, were calculated from the thermogravimetric data. The fractional conversions are defined within the fractional reduction equation (Equation (3)) and fractional oxidation equation (Equation (4)):
Fractional reduction (X) = (Moxd ‒ M)/(Moxd ‒ Mred)
Fractional oxidation (X) = (M ‒ Mred)/(Moxd ‒ Mred)
For the hydrogen cyclic CLC testing, M denotes the instantaneous mass, while Moxd denotes the mass of a completely oxidized sample in the TG experiment (in other words, the maximum mass after oxidation) and Mred denotes the mass of a completely reduced sample in the TG experiment (the minimum mass after reduction reaction). The reaction rates were calculated by differentiating the mass data with respect to time.
Author Contributions
Conceptualization, methodology, supervision, E.K.; sample synthesis and SEM images acquiring, R.L. and E.K.; writing—original draft preparationwriting—review and editing, E.K.; visualization, R.L. Both authors have read and agreed to the published version of the manuscript.
Funding
This research was funded in part by the NATIONAL SCIENCE CENTRE, POLAND, grant number 2020/37/B/ST5/01259.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ishida, M.; Jin, H. A new advanced power generation system using chemical-looping combustion. Energy 1994, 19, 415–422. [Google Scholar] [CrossRef]
- Ryu, H.-J.; Lee, D.; Nam, H.; Hwang, B.W.; Kim, H.; Won, Y.; Ra, H.W.; Yoon, S.M.; Baek, J.-I. Combustion Characteristics of Natural Gas and Syngas Using Mass Produced Oxygen Carrier Particle in a 0.5 MWth Chemical Looping Combustion System. Trans. Korean Hydrog. New Energy Soc. 2021, 32, 134–142. [Google Scholar] [CrossRef]
- Babinski, P.; Sciazko, M.; Ksepko, E. Limitation of thermogravimetry for oxy-combustion analysis of coal chars. J. Therm. Anal. Calorim. 2017, 133, 713–725. [Google Scholar] [CrossRef] [Green Version]
- Stawowy, M.; Ciesielski, R.; Maniecki, T.; Matus, K.; Łużny, R.; Trawczynski, J.; Silvestre-Albero, J.; Łamacz, A. CO2 Hydrogenation to Methanol over Ce and Zr Containing UiO-66 and Cu/UiO-66. Catalysts 2019, 10, 39. [Google Scholar] [CrossRef] [Green Version]
- García-Labiano, F.; De Diego, L.F.; Adánez, J.; Abad, A.; Gayán, P. Temperature variations in the oxygen carrier particles during their reduction and oxidation in a chemical-looping combustion system. Chem. Eng. Sci. 2005, 60, 851–862. [Google Scholar] [CrossRef] [Green Version]
- Cho, P.; Mattisson, T.; Lyngfelt, A. Comparison of iron-, nickel-, copper- and manganese-based oxygen carriers for chemical-looping combustion. Fuel 2004, 83, 1215–1225. [Google Scholar] [CrossRef]
- Ksepko, E. Perovskite-type Sr(Mn1−xNix)O3 materials and their chemical-looping oxygen transfer properties. Int. J. Hydrogen Energy 2014, 39, 8126–8137. [Google Scholar] [CrossRef]
- Larring, Y.; Braley, C.; Pishahang, M.; Andreassen, K.A.; Bredesen, R. Evaluation of a mixed Fe-Mn oxide system for chemical looping combustion. Energy Fuels 2015, 29, 3438–3445. [Google Scholar] [CrossRef]
- Azimi, G.; Leion, H.; Mattisson, T.; Lyngfelt, A. Chemical-looping with oxygen uncoupling using combined Mn-Fe oxides, testing in batch fluidized bed. Energy Procedia 2011, 4, 370–377. [Google Scholar] [CrossRef] [Green Version]
- Siriwardane, R.V.; Ksepko, E.; Tian, H.; Poston, J.; Simonyi, T.; Sciazko, M. Interaction of iron–copper mixed metal oxide oxygen carriers with simulated synthesis gas derived from steam gasification of coal. Appl. Energy 2013, 107, 111–123. [Google Scholar] [CrossRef]
- Utsis, N.; Landau, M.V.; Erenburg, A.; Herskowitz, M. Reverse Water Gas Shift by Chemical Looping with Iron-Substituted Hexaaluminate Catalysts. Catalysts 2020, 10, 1082. [Google Scholar] [CrossRef]
- Cimino, S.; Mancino, G.; Lisi, L. Performance and Stability of Metal (Co, Mn, Cu)-Promoted La2O2SO4 Oxygen Carrier for Chemical Looping Combustion of Methane. Catalysts 2019, 9, 147. [Google Scholar] [CrossRef] [Green Version]
- Jin, H.; Ishida, M. Reactivity study on natural-gas-fueled chemical-looping combustion by a fixed-bed reactor. Ind. Eng. Chem. Res. 2002, 41, 4004–4007. [Google Scholar] [CrossRef]
- Rydén, M.; Lyngfelt, A.; Mattisson, T. Combined manganese/iron oxides as oxygen carrier for chemical looping combustion with oxygen uncoupling (CLOU) in a circulating fluidized bed reactor system. Energy Procedia 2011, 4, 341–348. [Google Scholar] [CrossRef] [Green Version]
- Ksepko, E.; Siriwardane, R.V.; Tian, H.; Simonyi, T.; Sciazko, M. Effect of H2S on Chemical Looping Combustion of Coal-Derived Synthesis Gas over Fe–Mn Oxides Supported on Sepiolite, ZrO2, and Al2O3. Energy Fuels 2012, 26, 2461–2472. [Google Scholar] [CrossRef]
- Larring, Y.; Pishahang, M.; Sunding, M.F.; Tsakalakis, K. Fe–Mn based minerals with remarkable redox characteristics for chemical looping combustion. Fuel 2015, 159, 169–178. [Google Scholar] [CrossRef]
- Moldenhauer, P.; Serrano, A.; García-Labiano, F.; de Diego, L.F.; Biermann, M.; Mattisson, T.; Lyngfelt, A. Chemical-Looping Combustion of Kerosene and Gaseous Fuels with a Natural and a Manufactured Mn–Fe-Based Oxygen Carrier. Energy Fuels 2018, 32, 8803–8816. [Google Scholar] [CrossRef]
- Dai, J.; Whitty, K. Effects of Coal Ash on CuO as an Oxygen Carrier for Chemical Looping with Oxygen Uncoupling. Energy Fuels 2018, 32, 11656–11665. [Google Scholar] [CrossRef]
- Ryden, M.; Kallen, M.; Jing, D.; Hedayati, A.; Mattisson, T.; Lyngfelt, A. (Fe1−xMnx)TiyO3 based oxygen carriers for chemical-looping combustion and chemical-looping with oxygen uncoupling. Energy Procedia 2014, 51, 85–98. [Google Scholar] [CrossRef] [Green Version]
- Abián, M.; Abad, A.; Izquierdo, M.T.; Gayán, P.; de Diego, L.F.; García-Labiano, F.; Adánez, J. Titanium substituted manganese-ferrite as an oxygen carrier with permanent magnetic properties for chemical looping combustion of solid fuels. Fuel 2017, 195, 38–48. [Google Scholar] [CrossRef] [Green Version]
- Dai, J.; Whitty, K.J. Predicting and alleviating coal ash-induced deactivation of CuO as an oxygen carrier for chemical looping with oxygen uncoupling. Fuel 2019, 241, 1214–1222. [Google Scholar] [CrossRef]
- Dai, J.; Hughey, L.; Whitty, K.J. Influence of fuel ash on the recoverability of copper from the spent material of chemical looping combustion. Fuel Process. Technol. 2020, 201, 106358. [Google Scholar] [CrossRef]
- Poelman, H.; Galvita, V.V. Intensification of Chemical Looping Processes by Catalyst Assistance and Combination. Catalysts 2021, 11, 266. [Google Scholar] [CrossRef]
- Tian, H.; Simonyi, T.; Poston, J.; Siriwardane, R. Effect of Hydrogen Sulfide on Chemical Looping Combustion of Coal-Derived Synthesis Gas over Bentonite-Supported Metal−Oxide Oxygen Carriers. Ind. Eng. Chem. Res. 2009, 48, 8418–8430. [Google Scholar] [CrossRef]
- Azimi, G.; Leion, H.; Mattisson, T.; Rydén, M.; Snijkers, F.; Lyngfelt, A. Mn–Fe Oxides with Support of MgAl2O4, CeO2, ZrO2 and Y2O3–ZrO2 for Chemical-Looping Combustion and Chemical-Looping with Oxygen Uncoupling. Ind. Eng. Chem. Res. 2014, 53, 10358–10365. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).