Band Gap Engineering in Quadruple-Layered Sillén–Aurivillius Perovskite Oxychlorides Bi7Fe2Ti2O17X (X = Cl, Br, I) for Enhanced Photocatalytic Performance
Abstract
1. Introduction
2. Results and Discussion
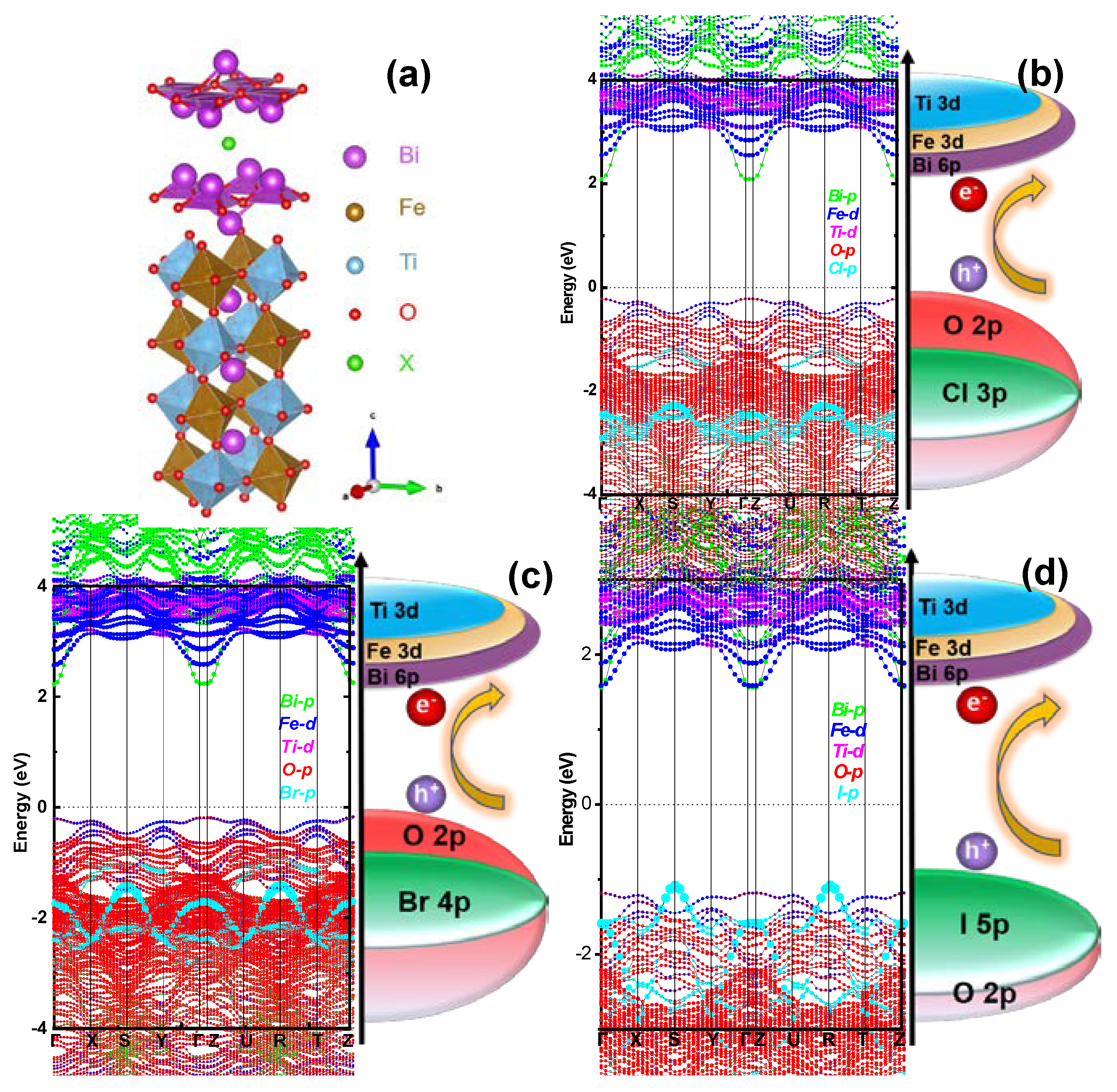
2.1. DFT Calculations
2.2. Material Properties of BFTOX (X = Cl, Br, and I)
2.3. Photocatalytic CO2 Reduction Performance for BFTOX Materials
2.4. Photocatalytic Activity Enhancement Mechanism for the Photodegradation of Organic Pollutants
3. Materials and Methods
3.1. Materials
3.2. Preparation of Photocatalysts
3.2.1. Preparation of BiOX (X = Cl, Br, and I)
3.2.2. Preparation of Bi7Fe2Ti2O17X (X = Cl, Br, and I)
3.3. Characterization
3.4. Photocatalytic Experiments
3.5. Electrochemical Measurement
3.6. Theoretical Calculations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yan, J.; Wang, C.; Ma, H.; Li, Y.; Liu, Y.; Suzuki, N.; Terashima, C.; Fujishima, A.; Zhang, X. Photothermal synergic enhancement of direct Z-scheme behavior of Bi4TaO8Cl/W18O49 heterostructure for CO2 reduction. Appl. Catal. B Environ. 2020, 268, 118401. [Google Scholar] [CrossRef]
- Zhang, Y.; Lin, S.; Cheng, S.; He, Z.; Hu, Z.; Zhou, Z.; Liu, W.; Sun, Y. Boosting Cu(In,Ga)Se2 Thin Film Growth in Low-Temperature Rapid-Deposition Processes: An Improved Design for the Single-Heating Knudsen Effusion Cell. Engineering 2021, 7, 534–541. [Google Scholar] [CrossRef]
- Katsumata, H.; Islam Molla, M.A.; Islam, J.B.; Tateishi, I.; Furukawa, M.; Kaneco, S. Dual Z-scheme heterojunction g-C3N4/Ag3PO4/AgBr photocatalyst with enhanced visible-light photocatalytic activity. Ceram. Int. 2022, 48, 21898–21905. [Google Scholar] [CrossRef]
- Lu, X.; Quan, L.; Hou, H.; Qian, J.; Liu, Z.; Zhang, Q. Fabrication of 1D/2D Y-doped CeO2/ZnIn2S4 S-scheme photocatalyst for enhanced photocatalytic H2 evolution. J. Alloy. Compd. 2022, 925, 166552. [Google Scholar] [CrossRef]
- Melchionna, M.; Fornasiero, P. Updates on the Roadmap for Photocatalysis. ACS Catal. 2020, 10, 5493–5501. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, A.; Krishnan, V. Perovskite Oxide Based Materials for Energy and Environment-Oriented Photocatalysis. ACS Catal. 2020, 10, 10253–10315. [Google Scholar] [CrossRef]
- Kumar, A.; Pathania, D.; Gupta, N.; Raj, P.; Sharma, A. Photo-degradation of noxious pollutants from water system using Cornulaca monacantha stem supported ZnFe2O4 magnetic bio-nanocomposite. Sustain. Chem. Pharm. 2020, 18, 100290. [Google Scholar] [CrossRef]
- Arush, S.; Deepak, P.; Ajay, K. Bio-Polymer Based Tragacanth Gum (TG) Loaded Fe3O4 Nanocomposite for the Sequestration of Tenacious Congo Red Dye from Waste Water. J. Mater. Sci. Technol. Res. 2020, 7, 92–100. [Google Scholar] [CrossRef]
- Sun, C.; Yang, J.; Xu, M.; Cui, Y.; Ren, W.; Zhang, J.; Zhao, H.; Liang, B. Recent intensification strategies of SnO2-based photocatalysts: A review. Chem. Eng. J. 2022, 427, 131564. [Google Scholar] [CrossRef]
- Xu, K.; Wang, L.; Xu, X.; Dou, S.X.; Hao, W.; Du, Y. Two dimensional bismuth-based layered materials for energy-related applications. Energy Storage Mater. 2019, 19, 446–463. [Google Scholar] [CrossRef]
- Lu, X.; Liu, Z.; Zhao, X.; Xu, W.; Hou, H.; Qian, J. CdS Nanoparticles Decorated 1D CeO2 Nanorods for Enhanced Photocatalytic Desulfurization Performance. Catalysts 2022, 12, 1478. [Google Scholar] [CrossRef]
- Chandel, M.; Thakur, M.; Sharma, A.; Pathania, D.; Kumar, A.; Singh, L. Chlorophyll sensitized (BiO)2CO3/ CdWO4/rGO nano-hybrid assembly for solar assisted photo-degradation of chlorzoxazone. Chemosphere 2022, 305, 135472. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Gao, M.; Zhang, Y.; Zhang, Z.; Cao, X.; Wang, B.; Wang, X. Photocatalytic reduction of CO2 on BiOX: Effect of halogen element type and surface oxygen vacancy mediated mechanism. Appl. Catal. B: Environ. 2020, 274, 119063. [Google Scholar] [CrossRef]
- Zhang, L.; Li, Y.; Li, Q.; Fan, J.; Carabineiro, S.A.C.; Lv, K. Recent advances on Bismuth-based Photocatalysts: Strategies and mechanisms. Chem. Eng. J. 2021, 419, 129484. [Google Scholar] [CrossRef]
- He, R.; Cao, S.; Zhou, P.; Yu, J. Recent advances in visible light Bi-based photocatalysts. Chin. J. Catal. 2014, 35, 989–1007. [Google Scholar] [CrossRef]
- Miao, Z.; Wang, Q.; Zhang, Y.; Meng, L.; Wang, X. In situ construction of S-scheme AgBr/BiOBr heterojunction with surface oxygen vacancy for boosting photocatalytic CO2 reduction with H2O. Appl. Catal. B Environ. 2022, 301, 120802. [Google Scholar] [CrossRef]
- Meng, X.; Zhang, Z. Bismuth-based photocatalytic semiconductors: Introduction, challenges and possible approaches. J. Mol. Catal. A Chem. 2016, 423, 533–549. [Google Scholar] [CrossRef]
- Divya, J.; Shivaramu, N.J.; Roos, W.D.; Purcell, W.; Swart, H.C. Synthesis, surface and photoluminescence properties of Sm3+ doped α-Bi2O3. J. Alloy. Compd. 2021, 854, 157221. [Google Scholar] [CrossRef]
- Zhao, Q.; Liu, X.; Xing, Y.; Liu, Z.; Du, C. Synthesizing Bi2O3/BiOCl heterojunctions by partial conversion of BiOCl. J. Mater. Sci. 2016, 52, 2117–2130. [Google Scholar] [CrossRef]
- Shang, J.; Chen, T.; Wang, X.; Sun, L.; Su, Q. Facile fabrication and enhanced photocatalytic performance: From BiOCl to element-doped BiOCl. Chem. Phys. Lett. 2018, 706, 483–487. [Google Scholar] [CrossRef]
- Han, N.; Zhang, Q. Stoichiometry-dependent photocatalytic performance of bismuth-based oxychlorides BiOyCl. Appl. Surf. Sci. 2021, 562, 150215. [Google Scholar] [CrossRef]
- He, H.; Yin, J.; Li, Y.; Zhang, Y.; Qiu, H.; Xu, J.; Xu, T.; Wang, C. Size controllable synthesis of single-crystal ferroelectric Bi4Ti3O12 nanosheet dominated with {0 0 1} facets toward enhanced visible-light-driven photocatalytic activities. Appl. Catal. B Environ. 2014, 156–157, 35–43. [Google Scholar] [CrossRef]
- Xie, Z.; Tang, X.; Shi, J.; Wang, Y.; Yuan, G.; Liu, J.-M. Excellent piezo-photocatalytic performance of Bi4Ti3O12 nanoplates synthesized by molten-salt method. Nano Energy 2022, 98, 107247. [Google Scholar] [CrossRef]
- Bhat, S.S.M.; Sundaram, N.G. Photocatalysis of Bi4NbO8Cl hierarchical nanostructure for degradation of dye under solar/UV irradiation. New J. Chem. 2015, 39, 3956–3963. [Google Scholar] [CrossRef]
- Fujito, H.; Kunioku, H.; Kato, D.; Suzuki, H.; Higashi, M.; Kageyama, H.; Abe, R. Layered Perovskite Oxychloride Bi4NbO8Cl: A Stable Visible Light Responsive Photocatalyst for Water Splitting. J. Am. Chem. Soc. 2016, 138, 2082–2085. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wang, C.; Li, D.; Xing, Y.; Zhang, X.; Liu, Y. Bi4TaO8Cl/Bi heterojunction enables high-selectivity photothermal catalytic conversion of CO2-H2O flow to liquid alcohol. Chem. Eng. J. 2022, 435, 135133. [Google Scholar] [CrossRef]
- Sun, J.; Han, N.; Gu, Y.; Lu, X.; Si, L.; Zhang, Q. Hole Doping to Enhance the Photocatalytic Activity of Bi4NbO8Cl. Catalysts 2020, 10, 1425. [Google Scholar] [CrossRef]
- Zhong, C.; Kato, D.; Takeiri, F.; Fujii, K.; Yashima, M.; Nishiwaki, E.; Fujii, Y.; Koreeda, A.; Tassel, C.; Abe, R.; et al. Single Crystal Growth of Sillén–Aurivillius Perovskite Oxyhalides Bi4NbO8X (X = Cl, Br). Inorganics 2018, 6, 41. [Google Scholar] [CrossRef]
- Kunioku, H.; Higashi, M.; Tomita, O.; Yabuuchi, M.; Kato, D.; Fujito, H.; Kageyama, H.; Abe, R. Strong hybridization between Bi-6s and O-2p orbitals in Sillén–Aurivillius perovskite Bi4MO8X (M = Nb, Ta; X = Cl, Br), visible light photocatalysts enabling stable water oxidation. J. Mater. Chem. A 2018, 6, 3100–3107. [Google Scholar] [CrossRef]
- Sharma, A.; Kanth, S.K.; Xu, S.; Han, N.; Zhu, L.; Fan, L.; Liu, C.; Zhang, Q. Visible light driven g-C3N4/Bi4NbO8X (X Cl, Br) heterojunction photocatalyst for the degradation of organic pollutants. J. Alloy. Compd. 2022, 895, 162576. [Google Scholar] [CrossRef]
- Kato, D.; Hongo, K.; Maezono, R.; Higashi, M.; Kunioku, H.; Yabuuchi, M.; Suzuki, H.; Okajima, H.; Zhong, C.; Nakano, K.; et al. Valence Band Engineering of Layered Bismuth Oxyhalides toward Stable Visible-Light Water Splitting: Madelung Site Potential Analysis. J. Am. Chem. Soc. 2017, 139, 18725–18731. [Google Scholar] [CrossRef]
- Tao, X.; Zhao, Y.; Mu, L.; Wang, S.; Li, R.; Li, C. Bismuth Tantalum Oxyhalogen: A Promising Candidate Photocatalyst for Solar Water Splitting. Adv. Energy Mater. 2018, 8, 1701392. [Google Scholar] [CrossRef]
- Majumdar, A.; Ghosh, U.; Pal, A. Novel 2D/2D g-C(3)N(4)/Bi(4)NbO(8)Cl nano-composite for enhanced photocatalytic degradation of oxytetracycline under visible LED light irradiation. J. Colloid. Interface Sci. 2021, 584, 320–331. [Google Scholar] [CrossRef] [PubMed]
- Qu, X.; Liu, M.; Zhang, W.; Sun, Z.; Meng, W.; Shi, L.; Du, F. A facile route to construct NiTiO3/Bi4NbO8Cl heterostructures for enhanced photocatalytic water purification. J. Mater. Sci. 2020, 55, 9330–9342. [Google Scholar] [CrossRef]
- Shi, L.; Wu, X.; Xu, C.; Bai, Q.; Nie, Z.; Ji, C.; Zhang, Y.; Yin, Z.; Zhang, S.; Qu, X.; et al. Defect-engineering of Pt/Bi4NbO8Br heterostructures for synergetic promotional photocatalytic removal of versatile organic contaminants. J. Mater. Chem. C 2021, 9, 2784–2792. [Google Scholar] [CrossRef]
- Feng, Z.; Li, G.; Yuan, X.; Zhang, Z.; Tang, M.; Zhang, R. Improved photocatalytic activity of Bi4TaO8Cl by Gd3+ doping. J. Am. Ceram. Soc. 2018, 102, 2390–2397. [Google Scholar] [CrossRef]
- Gu, Y.; Yu, F.; Chen, J.; Zhang, Q. Facile Synthesis of Sillén-Aurivillius Layered Oxide Bi7Fe2Ti2O17Cl with Efficient Photocatalytic Performance for Degradation of Tetracycline. Catalysts 2022, 12, 221. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, X.; Xian, T.; Liu, G.; Yang, H. Photocatalytic purification of simulated dye wastewater in different pH environments by using BaTiO3/Bi2WO6 heterojunction photocatalysts. Opt. Mater. 2021, 113, 110853. [Google Scholar] [CrossRef]
- Shao, L.; Xia, X.; Wei, G.; Qin, J.; Liu, Y. A dramatic enhancement of antibiotic photodegradation catalyzed by red mud-derived Bi5FeTi3O15. Sep. Purif. Technol. 2021, 275, 119244. [Google Scholar] [CrossRef]
- Yin, X.; Li, X.; Liu, H.; Gu, W.; Zou, W.; Zhu, L.; Fu, Z.; Lu, Y. Realizing selective water splitting hydrogen/oxygen evolution on ferroelectric Bi3TiNbO9 nanosheets. Nano Energy 2018, 49, 489–497. [Google Scholar] [CrossRef]
- Wang, Z.; Li, C.; Domen, K. Recent developments in heterogeneous photocatalysts for solar-driven overall water splitting. Chem. Soc. Rev. 2019, 48, 2109–2125. [Google Scholar] [CrossRef] [PubMed]
- Li, K.-L.; Lee, W.W.; Lu, C.-S.; Dai, Y.-M.; Chou, S.-Y.; Chen, H.-L.; Lin, H.-P.; Chen, C.-C. Synthesis of BiOBr, Bi3O4Br, and Bi12O17Br2 by controlled hydrothermal method and their photocatalytic properties. J. Taiwan Inst. Chem. Eng. 2014, 45, 2688–2697. [Google Scholar] [CrossRef]
- Lin, H.-P.; Lee, W.W.; Huang, S.-T.; Chen, L.-W.; Yeh, T.-W.; Fu, J.-Y.; Chen, C.-C. Controlled hydrothermal synthesis of PbBiO2Br/BiOBr heterojunction with enhanced visible-driven-light photocatalytic activities. J. Mol. Catal. A Chem. 2016, 417, 168–183. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, Z.; Lin, S.; Cheng, S.; He, Z.; Wang, C.; Zhou, Z.; Sun, Y.; Liu, W. Facile Silver-Incorporated Method of Tuning the Back Gradient of Cu(In,Ga)Se2 Films. ACS Appl. Energy Mater. 2020, 3, 9963–9971. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, Z.; Lin, S.; Wang, C.; Cheng, S.; He, Z.; Zhou, Z.; Sun, Y.; Liu, W. Silver Surface Treatment of Cu(In,Ga)Se2 (CIGS) Thin Film: A New Passivation Process for the CdS/CIGS Heterojunction Interface. Sol. RRL 2020, 4, 2000290. [Google Scholar] [CrossRef]
- Zhang, Y.; Gao, Q.; Ao, J.; Zhang, Y.; Bi, J.; Guo, J.; Han, Y.; Sun, G.; Zhang, Y.; Liu, W.; et al. Enhancing Surface Properties for Electrodeposited Cu(In,Ga)Se2 Films by (NH4)2S Solution at Room Temperature. ACS Appl. Energy Mater. 2021, 4, 3822–3831. [Google Scholar] [CrossRef]
- Qing, G.; Ao, J.; Zhang, Y.; Zhang, Y.; Guo, J.; Sun, G.; Liu, W.; Liu, F.; Zhang, Y. Pulse Selenization in Cu(In,Ga)Se2 Solar Cells: A Promising Approach to Achieve High Efficiency by Electrodeposition. ACS Appl. Energy Mater. 2021, 4, 8322–8329. [Google Scholar] [CrossRef]
- Makula, P.; Pacia, M.; Macyk, W. How To Correctly Determine the Band Gap Energy of Modified Semiconductor Photocatalysts Based on UV-Vis Spectra. J. Phys. Chem. Lett. 2018, 9, 6814–6817. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, S.; Mu, Z.; Liu, K.; Chen, J.; Zhou, C.; Yao, Y.; Chen, X.; Shi, L.; Wang, Z.; et al. Simulation analysis of Cd-free Cu(In,Ga)Se2 solar cells with novel BiOX (X=Cl, Br) buffer layers. Vacuum 2022, 206, 111569. [Google Scholar] [CrossRef]
- Xu, S.; Zhang, Y.; Wang, Y.; Chen, J.; Zhou, C.; Mu, Z.; Zhang, Z.; Zhang, J.; Wang, J.; Zhang, Q. Coupled Ferroelectric Polarization in Novelty Sillén–Aurivillius BaBi4TiNbO11Cl Material for Photocatalysis. J. Alloy. Compd. 2023, 954, 169932. [Google Scholar] [CrossRef]
- Han, N.; Xu, S.; Zhang, Q. Flux synthesis of Bi2MO4Cl (M = Gd and Nd) nanosheets for high-efficiency photocatalytic oxygen evolution under visible light. J. Mater. Sci. 2022, 57, 2870–2882. [Google Scholar] [CrossRef]
- Wang, S.; Teng, F.; Zhao, Y. Effect of the molecular structure and surface charge of a bismuth catalyst on the adsorption and photocatalytic degradation of dye mixtures. RSC Adv. 2015, 5, 76588–76598. [Google Scholar] [CrossRef]
- Kresse, G.; Hafner, J. Ab initio molecular dynamics for liquid metals. Phys. Rev. B Condens. Matter. 1993, 47, 558–561. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865. [Google Scholar] [CrossRef] [PubMed]
- Heyd, J.; Scuseria, G.E.; Ernzerhof, M.J. Hybrid functionals based on a screened Coulomb potential. J. Chem. Phys. 2003, 118, 8207–8215. [Google Scholar] [CrossRef]








| BFTOC | BFTOB | BFTOI | ||||
|---|---|---|---|---|---|---|
| Effective Mass | Electron | Hole | Electron | Hole | Electron | Hole |
| mxx (Γ−X) | 0.023 | 0.181 | 0.013 | 0.131 | 0.053 | |
| myy (Γ−Y) | 0.035 | 0.099 | 0.018 | 0.098 | 0.054 | |
| mzz (Γ−Z) | 9.879 | 7.904 | 19.004 | 6.228 | 5.137 | |
| mxx (S−Y) | 0.035 | |||||
| myy (S−X) | 0.031 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.; Gu, Y.; Xu, S.; Zhang, Y.; Zhang, Z.; Shi, L.; Mu, Z.; Zhou, C.; Zhang, J.; Zhang, Q. Band Gap Engineering in Quadruple-Layered Sillén–Aurivillius Perovskite Oxychlorides Bi7Fe2Ti2O17X (X = Cl, Br, I) for Enhanced Photocatalytic Performance. Catalysts 2023, 13, 751. https://doi.org/10.3390/catal13040751
Chen J, Gu Y, Xu S, Zhang Y, Zhang Z, Shi L, Mu Z, Zhou C, Zhang J, Zhang Q. Band Gap Engineering in Quadruple-Layered Sillén–Aurivillius Perovskite Oxychlorides Bi7Fe2Ti2O17X (X = Cl, Br, I) for Enhanced Photocatalytic Performance. Catalysts. 2023; 13(4):751. https://doi.org/10.3390/catal13040751
Chicago/Turabian StyleChen, Jikun, Yan Gu, Shishi Xu, Yunxiang Zhang, Zhe Zhang, Lin Shi, Zhichao Mu, Chenliang Zhou, Jiali Zhang, and Qinfang Zhang. 2023. "Band Gap Engineering in Quadruple-Layered Sillén–Aurivillius Perovskite Oxychlorides Bi7Fe2Ti2O17X (X = Cl, Br, I) for Enhanced Photocatalytic Performance" Catalysts 13, no. 4: 751. https://doi.org/10.3390/catal13040751
APA StyleChen, J., Gu, Y., Xu, S., Zhang, Y., Zhang, Z., Shi, L., Mu, Z., Zhou, C., Zhang, J., & Zhang, Q. (2023). Band Gap Engineering in Quadruple-Layered Sillén–Aurivillius Perovskite Oxychlorides Bi7Fe2Ti2O17X (X = Cl, Br, I) for Enhanced Photocatalytic Performance. Catalysts, 13(4), 751. https://doi.org/10.3390/catal13040751






