Enhanced Photodegradation of Acetaminophen Using Efficient ZnO-NiO Nanofibers
Abstract
:1. Introduction
2. Results and Discussion
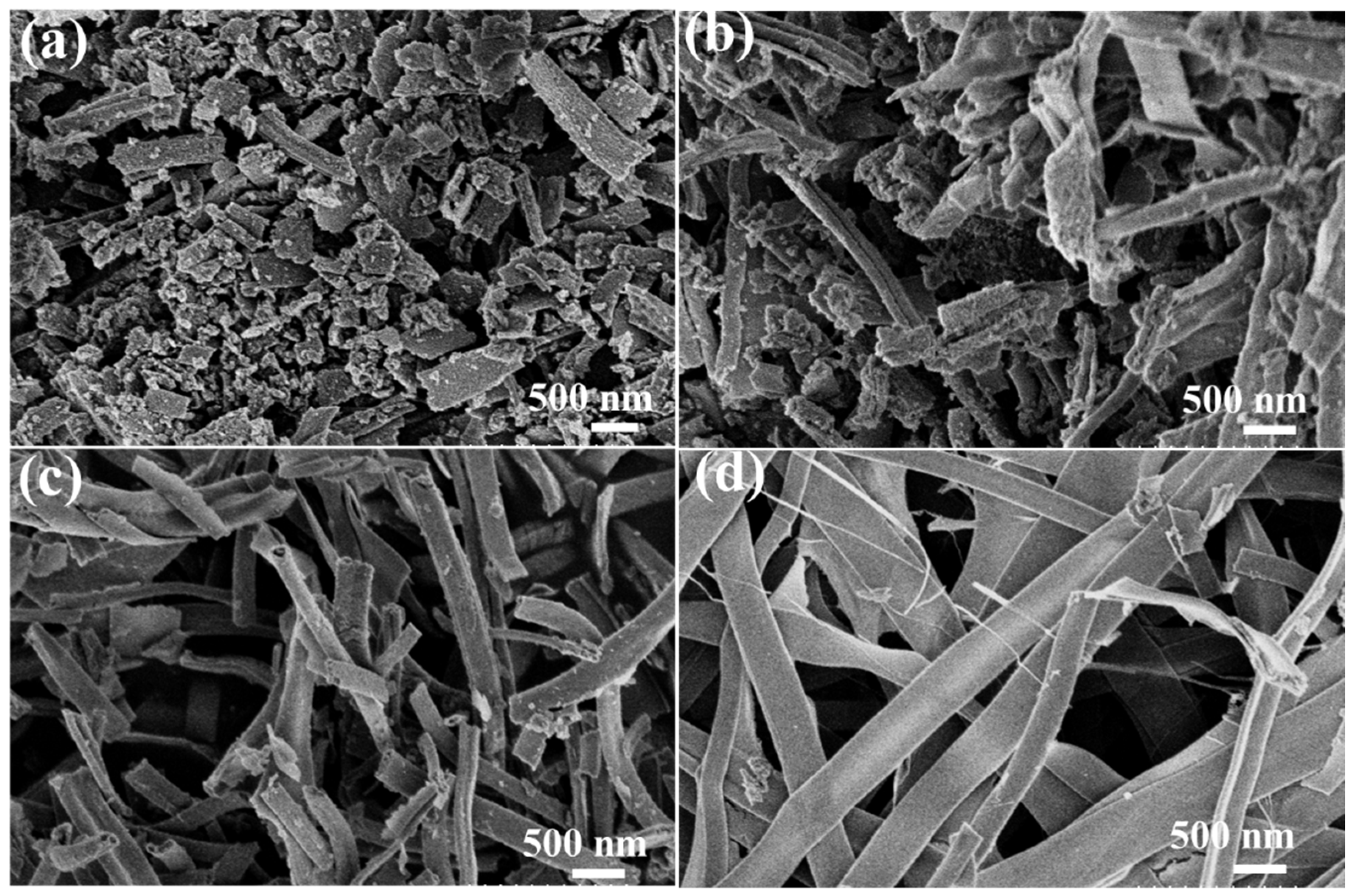
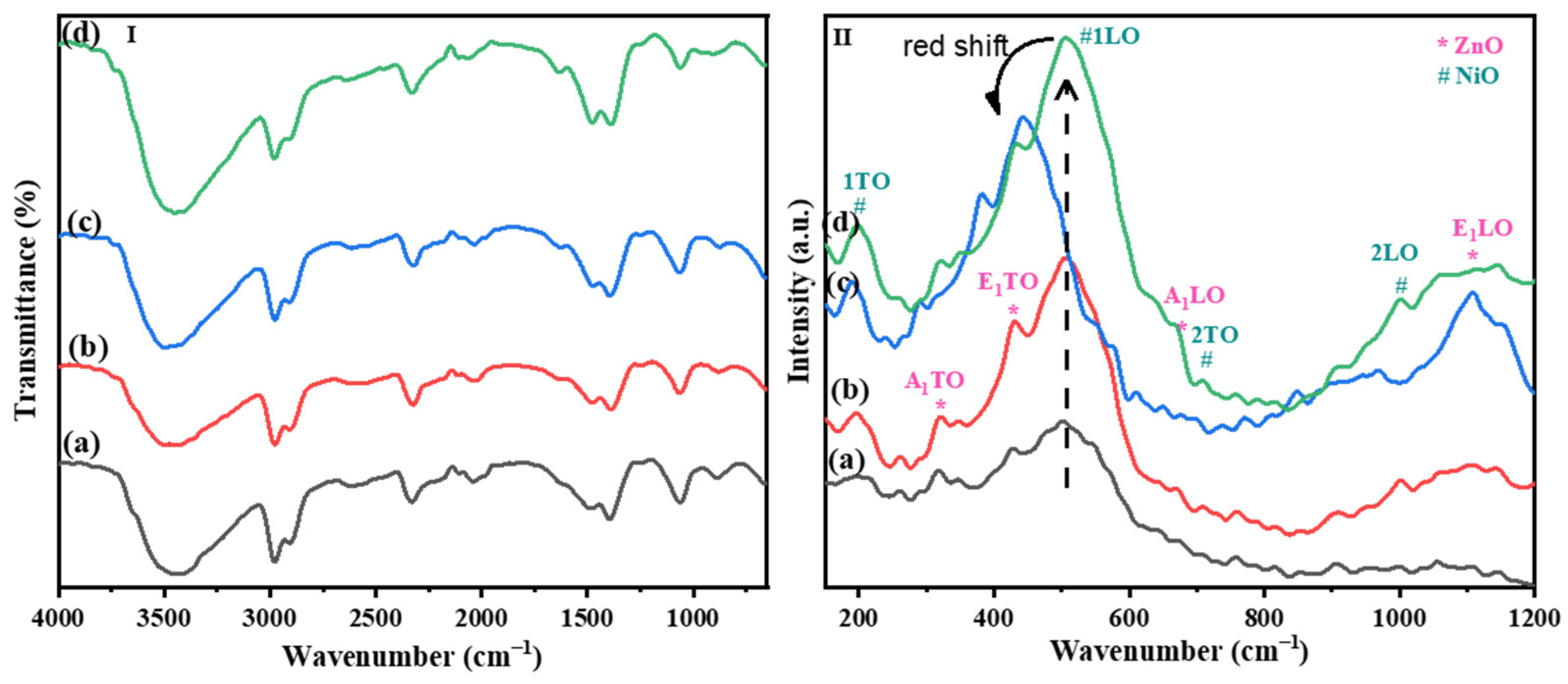
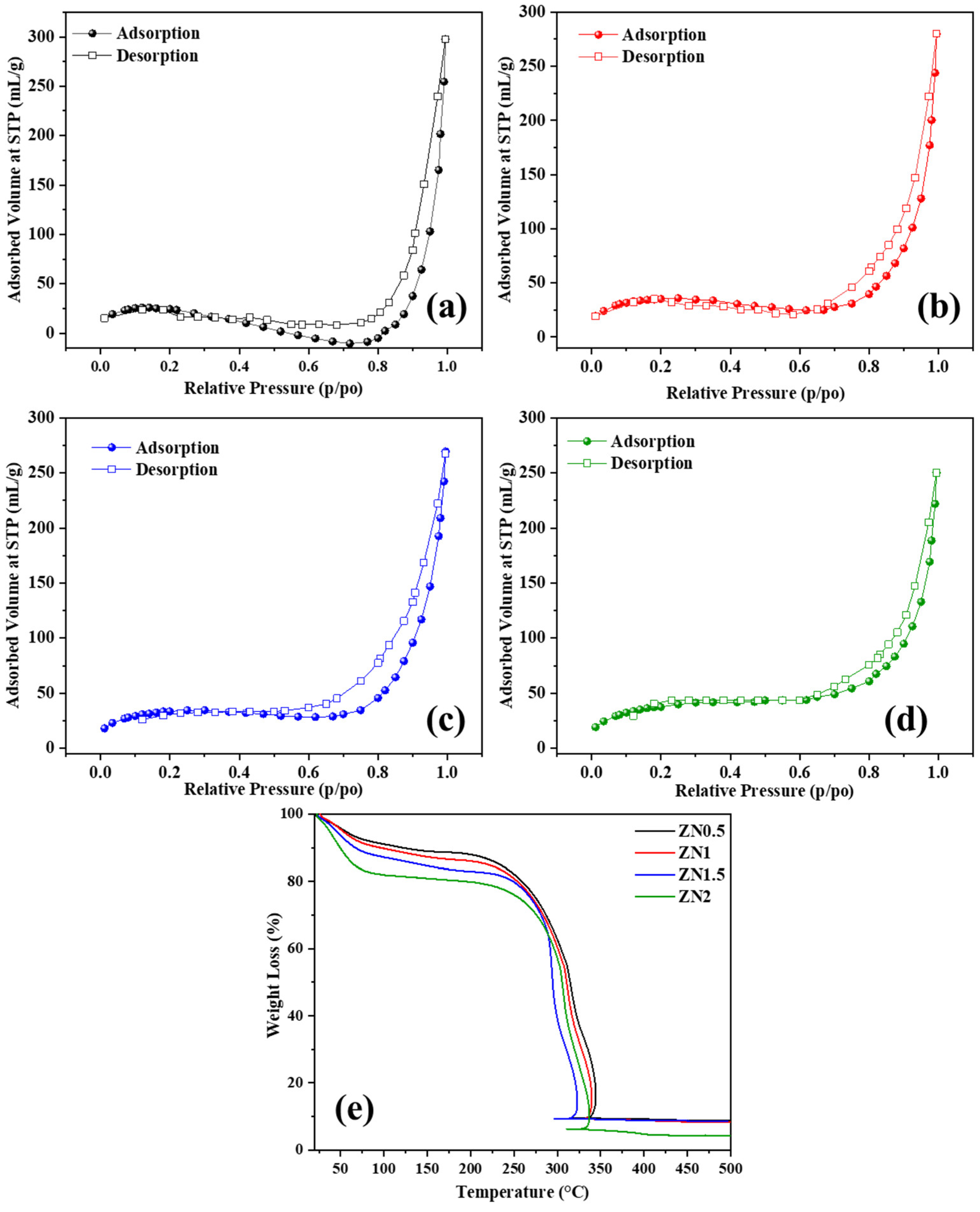
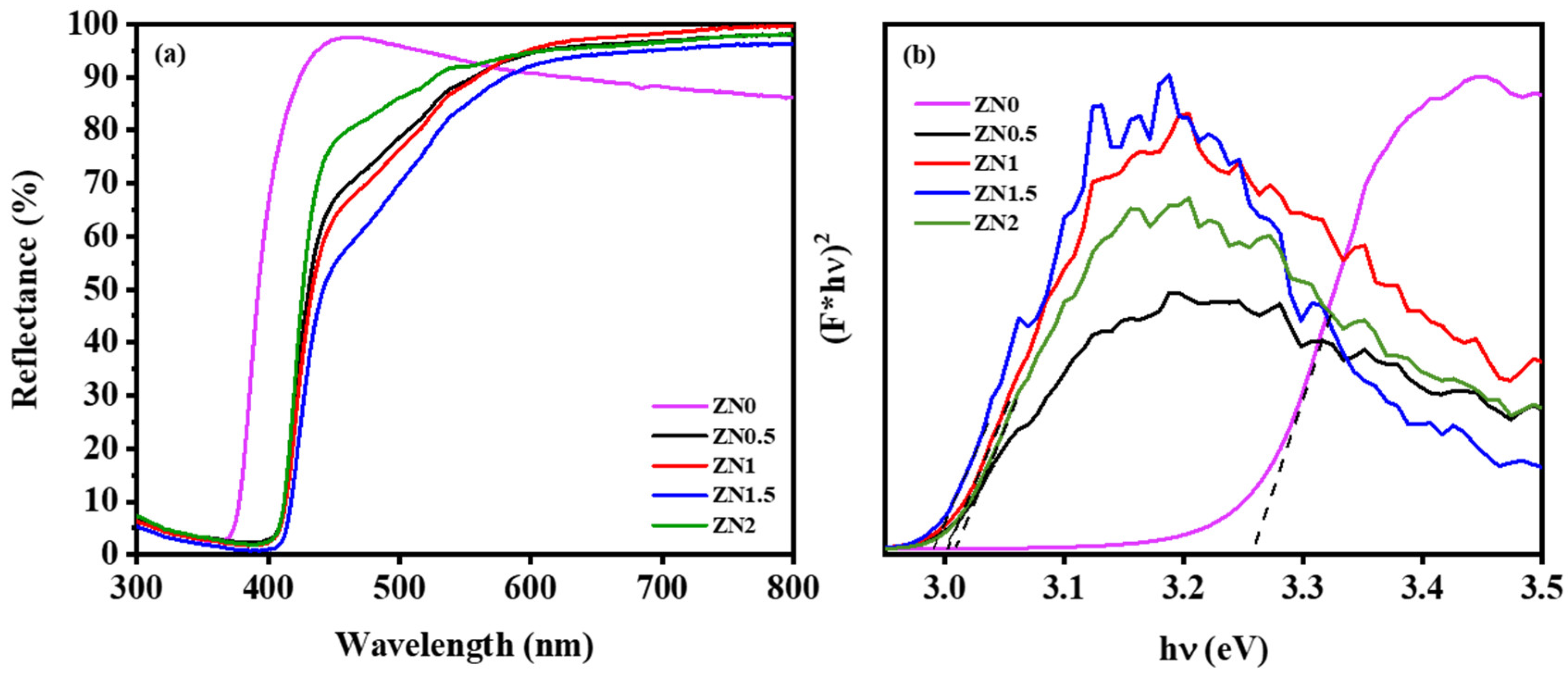
2.1. Characterization of ZnO-NiO Hybrid Nanofibers
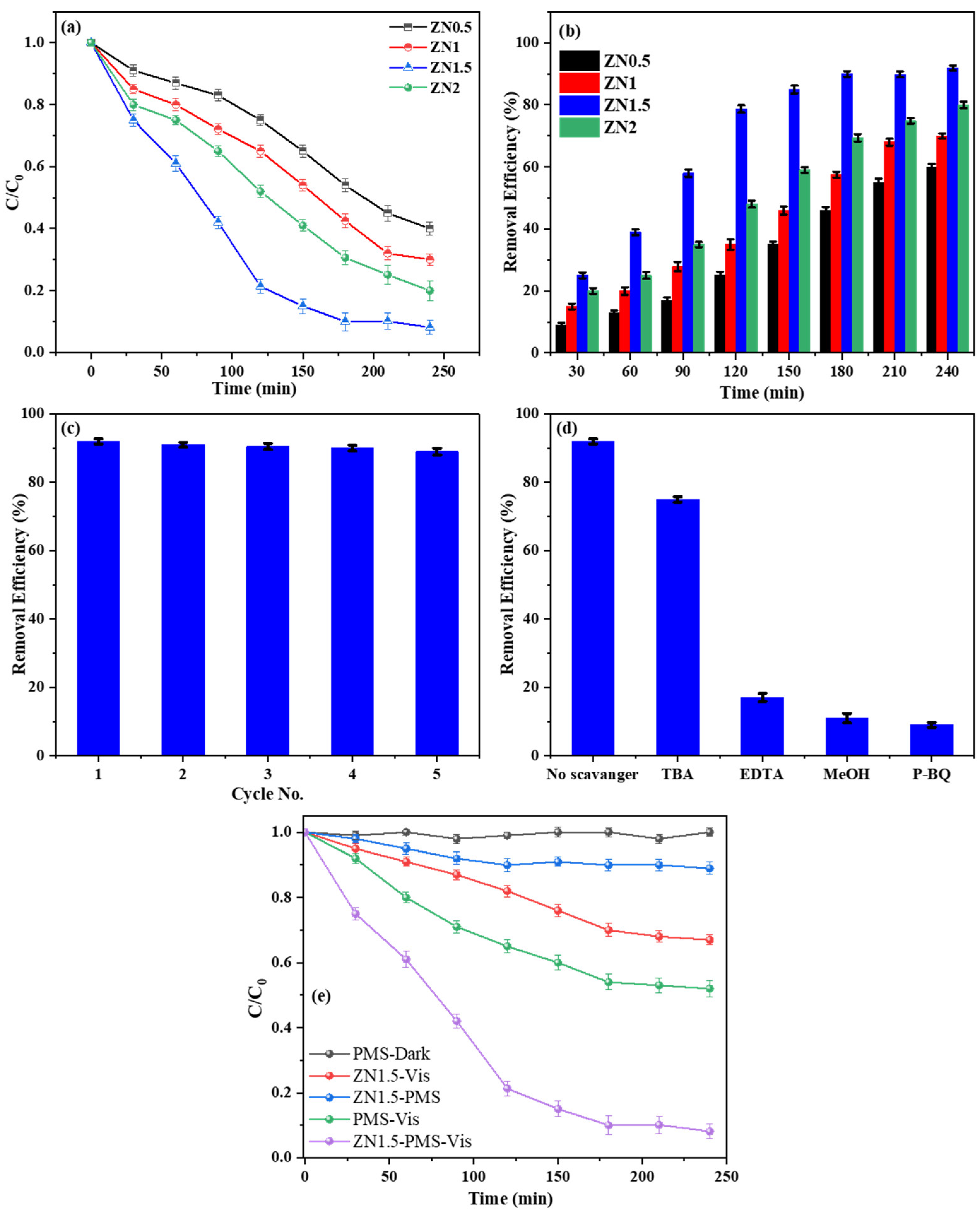
2.2. Photocatalytic Degradation of Acetaminophen Using ZnO-NiO Hybrid Nanofibers
2.3. Mechanism of ZnO/NiO Heterostructures Nanofibers
3. Materials and Experimental Methods
3.1. Chemicals and Materials
3.2. Preparation of ZnO/NiO Nanofibers
3.3. Characterizations of Materials
3.4. Photocatalytic Reaction
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ojha, S.; Tripathi, S.M.; Vishwakarma, P.K.; Mishra, S. Pharmaceuticals in the Water: Emerging Concerns and Innovative Remediation Solutions. Curr. Green Chem. 2024, 11, 50–62. [Google Scholar] [CrossRef]
- Benny, S.M.; Gupta, S.D.; Ismail, S.P.; Francis, D. Pharmaceutical Pollution of Water Bodies: Sources, Impacts, and Mitigation. In Handbook of Water Pollution; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2024; pp. 371–416. [Google Scholar]
- Vo, H.N.P.; Le, G.K.; Nguyen, T.M.H.; Bui, X.-T.; Nguyen, K.H.; Rene, E.R.; Vo, T.D.H.; Cao, N.-D.T.; Mohan, R. Acetaminophen micropollutant: Historical and current occurrences, toxicity, removal strategies and transformation pathways in different environments. Chemosphere 2019, 236, 124391. [Google Scholar]
- Negarestani, M.; Motamedi, M.; Kashtiaray, A.; Khadir, A.; Sillanpää, M. Simultaneous removal of acetaminophen and ibuprofen from underground water by an electrocoagulation unit: Operational parameters and kinetics. Groundw. Sustain. Dev. 2020, 11, 100474. [Google Scholar] [CrossRef]
- Liu, Z.; Ma, Q.; Dai, L.; Dang, Z. Occurrence, removal and risk evaluation of ibuprofen and acetaminophen in municipal wastewater treatment plants: A critical review. Sci. Total Environ. 2023, 891, 164600. [Google Scholar]
- Lee, W.J.; Goh, P.S.; Lau, W.J.; Ismail, A.F. Removal of pharmaceutical contaminants from aqueous medium: A state-of-the-art review based on paracetamol. Arab. J. Sci. Eng. 2020, 45, 7109–7135. [Google Scholar] [CrossRef]
- Igwegbe, C.A.; Aniagor, C.O.; Oba, S.N.; Yap, P.-S.; Iwuchukwu, F.U.; Liu, T.; de Souza, E.C.; Ighalo, J.O. Environmental protection by the adsorptive elimination of acetaminophen from water: A comprehensive review. J. Ind. Eng. Chem. 2021, 104, 117–135. [Google Scholar] [CrossRef]
- Acero, J.L.; Benitez, F.J.; Real, F.J.; Teva, F. Coupling of adsorption, coagulation, and ultrafiltration processes for the removal of emerging contaminants in a secondary effluent. Chem. Eng. J. 2012, 210, 1–8. [Google Scholar] [CrossRef]
- Rigobello, E.S.; Dantas, A.D.B.; Di Bernardo, L.; Vieira, E.M. Removal of diclofenac by conventional drinking water treatment processes and granular activated carbon filtration. Chemosphere 2013, 92, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Skwarczynska-Wojsa, A.; Puszkarewicz, A. Removal of Acetaminophen from Aqueous Solutions in an Adsorption Process. Materials 2024, 17, 431. [Google Scholar] [CrossRef]
- Chauhan, A.; Sillu, D.; Agnihotri, S. Removal of pharmaceutical contaminants in wastewater using nanomaterials: A comprehensive review. Curr. Drug Metab. 2019, 20, 483–505. [Google Scholar] [CrossRef]
- Al-Rub, F.A.A.; Fares, M.M.; Al-Banna, L.N. Toward Cleaner Ecosystems; Elimination of Paracetamol Drug via Mesoporous Activated Carbon Date Pits. Chem. Sci. Int. J. 2024, 33, 1–21. [Google Scholar] [CrossRef]
- Peralta-Hernández, J.M.; Brillas, E. A critical review over the removal of paracetamol (acetaminophen) from synthetic waters and real wastewaters by direct, hybrid catalytic, and sequential ozonation processes. Chemosphere 2023, 313, 137411. [Google Scholar] [CrossRef] [PubMed]
- Roslan, N.N.; Lau, H.L.H.; Suhaimi, N.A.A.; Shahri, N.N.M.; Verinda, S.B.; Nur, M.; Lim, J.-W.; Usman, A. Recent Advances in Advanced Oxidation Processes for Degrading Pharmaceuticals in Wastewater—A Review. Catalysts 2024, 14, 189. [Google Scholar] [CrossRef]
- Lakshmi, C.N.; Singh, N. Removal of pharmaceutical compounds: Overview of treatment methods. In New Trends in Emerging Environmental Contaminants; Springer: Singapore, 2022; pp. 161–180. [Google Scholar]
- Inobeme, A.; Ajai, A.I.; Adetunji, C.O.; Munirat, M.; Tsado, M.J.; Mann, A.; Osarenre, J.E.; Inobeme, J.; Mathew, A.; Chinenye, E. Oxidation and advanced oxidation processes in pharmaceutical wastewater treatment. In Development in Wastewater Treatment Research and Processes; Elsevier: Amsterdam, The Netherlands, 2024; pp. 157–169. [Google Scholar]
- Gupta, S.; Singh, A.; Sharma, T.; Kaur, R.; Khandelwal, V.; Rawat, K.D.; Pathak, S.; Sharma, M.K.; Singh, J.; Shah, M.P.; et al. Applications of ultrafiltration, nanofiltration, and reverse osmosis in pharmaceutical wastewater treatment. In Development in Wastewater Treatment Research and Processes; Elsevier: Amsterdam, The Netherlands, 2024; pp. 33–49. [Google Scholar]
- Vara, S.; Konni, M.; Karnena, M.K. Membrane technology for treatment of pharmaceutical wastewaters: A novel approach. In Handbook of Research on Resource Management for Pollution and Waste Treatment; IGI Global: Hershey, PA, USA, 2020; pp. 502–530. [Google Scholar]
- Sanjeev, N.O.; Valsan, A.E. Photocatalytic removal of acetaminophen using green synthesized zinc oxide nanoparticle. In Proceedings of the International Conference on Emerging Trends in Engineering, Hyderabad, India, 28–30 April 2023. [Google Scholar]
- Al-Khadhuri, A.; Al-Sabahi, J.; Kyaw, H.H.; Myint, M.T.Z.; Al-Farsi, B.; Al-Abri, M. Photocatalytic degradation toward pharmaceutical pollutants using supported zinc oxide nanorods catalyzed visible light system. Int. J. Environ. Sci. Technol. 2023, 20, 10021–10030. [Google Scholar] [CrossRef]
- Zyoud, A.H.; Zubi, A.; Hejjawi, S.; Helal, M.H.; Zyoud, S.H.; Qamhieh, N.; Hajamohideen, A.; Hilal, H.S. Removal of acetaminophen from water by simulated solar light photodegradation with ZnO and TiO2 nanoparticles: Catalytic efficiency assessment for future prospects. J. Environ. Chem. Eng. 2020, 8, 104038. [Google Scholar] [CrossRef]
- Ahmad, W.; Kaur, N.; Joshi, H.C. Photocatalytic behavior of NiO nanoparticles towards photocatalytic degradation of paracetamol. Mater. Today Proc. 2023, 73, 36–40. [Google Scholar] [CrossRef]
- Ali, S.M.; Hussein, E.H.; Dakhil, O.A.A. Photocatalytic activity of ZnO/NiO nano-heterojunction synthesized by modified-chemical bath deposition. Nano Futures 2021, 5, 35001. [Google Scholar] [CrossRef]
- Yousaf, S.; Zulfiqar, S.; Din, M.I.; Agboola, P.O.; Aboud, M.F.A.; Warsi, M.F.; Shakir, I. Solar light irradiated photocatalytic activity of ZnO–NiO/rGO nanocatalyst. J. Mater. Res. Technol. 2021, 12, 999–1009. [Google Scholar] [CrossRef]
- Dorneanu, P.P.; Airinei, A.; Olaru, N.; Homocianu, M.; Nica, V.; Doroftei, F. Preparation and characterization of NiO, ZnO and NiO–ZnO composite nanofibers by electrospinning method. Mater. Chem. Phys. 2014, 148, 1029–1035. [Google Scholar] [CrossRef]
- Liu, Y.; Jia, J.; Li, Y.V.; Hao, J.; Pan, K. Novel ZnO/NiO Janus-like nanofibers for effective photocatalytic degradation. Nanotechnology 2018, 29, 435704. [Google Scholar] [CrossRef]
- Li, J.; Zhao, F.; Zhang, L.; Zhang, M.; Jiang, H.; Li, S.; Li, J. Electrospun hollow ZnO/NiO heterostructures with enhanced photocatalytic activity. RSC Adv. 2015, 5, 67610–67616. [Google Scholar] [CrossRef]
- Hashim, M.; Usman, M.; Ahmad, S.; Shah, R.; Ali, A.; Rahman, N.U. ZnO/NiO Nanocomposite with Enhanced Photocatalytic H2 Production. Int. J. Photoenergy 2024, 2024, 2676368. [Google Scholar] [CrossRef]
- Goel, R.; Jha, R.; Ravikant, C. Investigating the structural, electrochemical, and optical properties of p-type spherical nickel oxide (NiO) nanoparticles. J. Phys. Chem. Solids 2020, 144, 109488. [Google Scholar] [CrossRef]
- Kavosh, M.; Moallemian, H.; Molaei, H.; Mehranniya, H.; Salami, M.; Dehdashti, M.E. Study of optical and structural properties of zinc oxide nanofibers by using Zn (ac) 2/PVA precursors. Synth. React. Inorg. Met. Nano-Metal Chem. 2016, 46, 225–229. [Google Scholar] [CrossRef]
- Singh, V.; Sapehia, R.; Dhiman, V. Removal of methylene blue dye by green synthesized NiO/ZnO nanocomposites. Inorg. Chem. Commun. 2024, 162, 112267. [Google Scholar] [CrossRef]
- Moon, J.; Park, J.-A.; Lee, S.-J.; Lim, S.C.; Zyung, T. Structure and electrical properties of electrospun ZnO–NiO mixed oxide nanofibers. Curr. Appl. Phys. 2009, 9, S213–S216. [Google Scholar] [CrossRef]
- Qiao, L.; Wang, X.; Sun, X.; Li, X.; Zheng, Y.; He, D. Single electrospun porous NiO–ZnO hybrid nanofibers as anode materials for advanced lithium-ion batteries. Nanoscale 2013, 5, 3037–3042. [Google Scholar] [CrossRef]
- Truong, T.K.; Nguyen, T.Q.; La, H.P.P.; Le, H.V.; Van Man, T.; Cao, T.M.; Van Pham, V. Insight into the degradation of p-nitrophenol by visible-light-induced activation of peroxymonosulfate over Ag/ZnO heterojunction. Chemosphere 2021, 268, 129291. [Google Scholar] [CrossRef]
- Bhatia, P.; Nath, M. Green synthesis of p-NiO/n-ZnO nanocomposites: Excellent adsorbent for removal of congo red and efficient catalyst for reduction of 4-nitrophenol present in wastewater. J. Water Process Eng. 2020, 33, 101017. [Google Scholar] [CrossRef]
- Paul, D.; Maiti, S.; Sethi, D.P.; Neogi, S. Bi-functional NiO-ZnO nanocomposite: Synthesis, characterization, antibacterial and photo assisted degradation study. Adv. Powder Technol. 2021, 32, 131–143. [Google Scholar] [CrossRef]
- Hallaji, H.; Keshtkar, A.R.; Moosavian, M.A. A novel electrospun PVA/ZnO nanofiber adsorbent for U (VI), Cu (II) and Ni (II) removal from aqueous solution. J. Taiwan Inst. Chem. Eng. 2015, 46, 109–118. [Google Scholar] [CrossRef]
- Pantò, F.; Dahrouch, Z.; Saha, A.; Patanè, S.; Santangelo, S.; Triolo, C. Photocatalytic degradation of methylene blue dye by porous zinc oxide nanofibers prepared via electrospinning: When defects become merits. Appl. Surf. Sci. 2021, 557, 149830. [Google Scholar] [CrossRef]
- Xu, Y.; Yan, H.; Chen, T. Application of ZnO/WO3 Composite Nanofiber Photocatalysts in Textile Wastewater Treatment. Separations 2023, 10, 339. [Google Scholar] [CrossRef]
- You, J.; Sun, W.; Su, S.; Ao, Z.; Liu, C.; Yao, G.; Lai, B. Degradation of bisphenol A by peroxymonosulfate activated with oxygen vacancy modified nano-NiO-ZnO composite oxides: A typical surface-bound radical system. Chem. Eng. J. 2020, 400, 125915. [Google Scholar] [CrossRef]
- Zhang, J.; Li, J. The oxygen vacancy defect of ZnO/NiO nanomaterials improves photocatalytic performance and ammonia sensing performance. Nanomaterials 2022, 12, 433. [Google Scholar] [CrossRef] [PubMed]
- Puttaraju, T.D.; Soundarya, T.L.; Nagaraju, G.; Lingaraju, K.; Manjula, M.V.; Devaraja, S.; Manjunatha, M. Biogenic approach for synthesis of ZnO/NiO nanocomposites as a highly efficient photocatalyst and evaluation of their biological properties. Braz. J. Chem. Eng. 2023, 1–14. [Google Scholar] [CrossRef]
- Thampy, U.S.U.; Mahesh, A.; Sibi, K.S.; Jawahar, I.N.; Biju, V. Enhanced photocatalytic activity of ZnO–NiO nanocomposites synthesized through a facile sonochemical route. SN Appl. Sci. 2019, 1, 1478. [Google Scholar] [CrossRef]
- Sabzehmeidani, M.M.; Karimi, H.; Ghaedi, M. Electrospinning preparation of NiO/ZnO composite nanofibers for photodegradation of binary mixture of rhodamine B and methylene blue in aqueous solution: Central composite optimization. Appl. Organomet. Chem. 2018, 32, e4335. [Google Scholar] [CrossRef]
- Kohantorabi, M.; Moussavi, G.; Mohammadi, S.; Oulego, P.; Giannakis, S. Photocatalytic activation of peroxymonosulfate (PMS) by novel mesoporous Ag/ZnO@NiFe2O4 nanorods, inducing radical-mediated acetaminophen degradation under UVA irradiation. Chemosphere 2021, 277, 130271. [Google Scholar] [CrossRef]
- Tanos, F.; Makhoul, E.; Nada, A.A.; Bekheet, M.F.; Riedel, W.; Kawrani, S.; Belaid, H.; Petit, E.; Viter, R.; Fedorenko, V.; et al. Graphene oxide-induced CuO reduction in TiO2/CaTiO3/Cu2O/Cu composites for photocatalytic degradation of drugs via peroxymonosulfate activation. Appl. Surf. Sci. 2024, 656, 159698. [Google Scholar] [CrossRef]
- Chen, K.; Wang, X.; Xia, P.; Xie, J.; Wang, J.; Li, X.; Tang, Y.; Li, L. Efficient removal of 2,2′,4,4′-tetrabromodiphenyl ether with a Z-scheme Cu2O-(rGO-TiO2) photocatalyst under sunlight irradiation. Chemosphere 2020, 254, 126806. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Hu, R.; Liu, C.; Xing, C.; Qian, C.; Zuo, X.; Nan, J.; Wang, L. Facile large-scale synthesis of β-Bi2O3 nanospheres as a highly efficient photocatalyst for the degradation of acetaminophen under visible light irradiation. Appl. Catal. B Environ. 2013, 140, 433–443. [Google Scholar] [CrossRef]
- Chau, J.H.F.; Lai, C.W.; Leo, B.F.; Juan, J.C.; Johan, M.R. Advanced photocatalytic degradation of acetaminophen using Cu2O/WO3/TiO2 ternary composite under solar irradiation. Catal. Commun. 2022, 163, 106396. [Google Scholar] [CrossRef]
- Li, K.; Chen, J.; Ao, Y.; Wang, P. Preparation of a ternary g-C3N4-CdS/Bi4O5I2 composite photocatalysts with two charge transfer pathways for efficient degradation of acetaminophen under visible light irradiation. Sep. Purif. Technol. 2021, 259, 118177. [Google Scholar] [CrossRef]
- Yan, T.; Wu, T.; Zhang, Y.; Sun, M.; Wang, X.; Wei, Q.; Du, B. Fabrication of In2S3/Zn2GeO4 composite photocatalyst for degradation of acetaminophen under visible light. J. Colloid Interface Sci. 2017, 506, 197–206. [Google Scholar] [CrossRef]


| Pollutant | C (mg/L) | Catalyst | Irradiation Source | Time of Degradation (min) | Removal Efficiency (%) | Ref. |
|---|---|---|---|---|---|---|
| Tetrabromobisphenol A | 10 | BiVO4/rGO-Cu2O | 300 W Xenon lamp | 180 | 91 | [47] |
| Acetaminophen | 10 | Beta-Bi2O3 | 1000 W Xenon lamp | 180 | 94 | [48] |
| Acetaminophen | 1 | Cu2O/WO3/TiO2 | 150 W Xenon lamp | 60 | 92 | [49] |
| Acetaminophen | 3 | g-C3N4-CdS-Bi4O5I2-3 | 300 W Xenon lamp | 25 | 80 | [50] |
| Acetaminophen | 10 | CTO-Cu-GO | 150 W linear halogen lamp | 180 | 96 | [46] |
| Acetaminophen | 5 | In2S3/Zn2GeO4 | Xenon lamp | 360 | 95 | [51] |
| Acetaminophen | 10 | ZnO-NiO | 100 W Xenon lamp | 240 | 92 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gomaa, H.E.; El-Maghrabi, H.H.; Gomaa, F.A.; Raynaud, P.; Nada, A.A. Enhanced Photodegradation of Acetaminophen Using Efficient ZnO-NiO Nanofibers. Catalysts 2024, 14, 403. https://doi.org/10.3390/catal14070403
Gomaa HE, El-Maghrabi HH, Gomaa FA, Raynaud P, Nada AA. Enhanced Photodegradation of Acetaminophen Using Efficient ZnO-NiO Nanofibers. Catalysts. 2024; 14(7):403. https://doi.org/10.3390/catal14070403
Chicago/Turabian StyleGomaa, Hassan E., Heba H. El-Maghrabi, Fatma A. Gomaa, Patrice Raynaud, and Amr A. Nada. 2024. "Enhanced Photodegradation of Acetaminophen Using Efficient ZnO-NiO Nanofibers" Catalysts 14, no. 7: 403. https://doi.org/10.3390/catal14070403








