Au Supported on Bovine-Bone-Derived Hydroxyapatite Catalyzes CO2 Photochemical Reduction toward Methanol
Abstract
:1. Introduction
2. Results and Discussion
2.1. Catalysts Characterization
2.1.1. Crystallographic Structure
2.1.2. Optical Characteristics
2.1.3. Electronic Transmission Microscopy (TEM)
2.1.4. N2 Physisorption
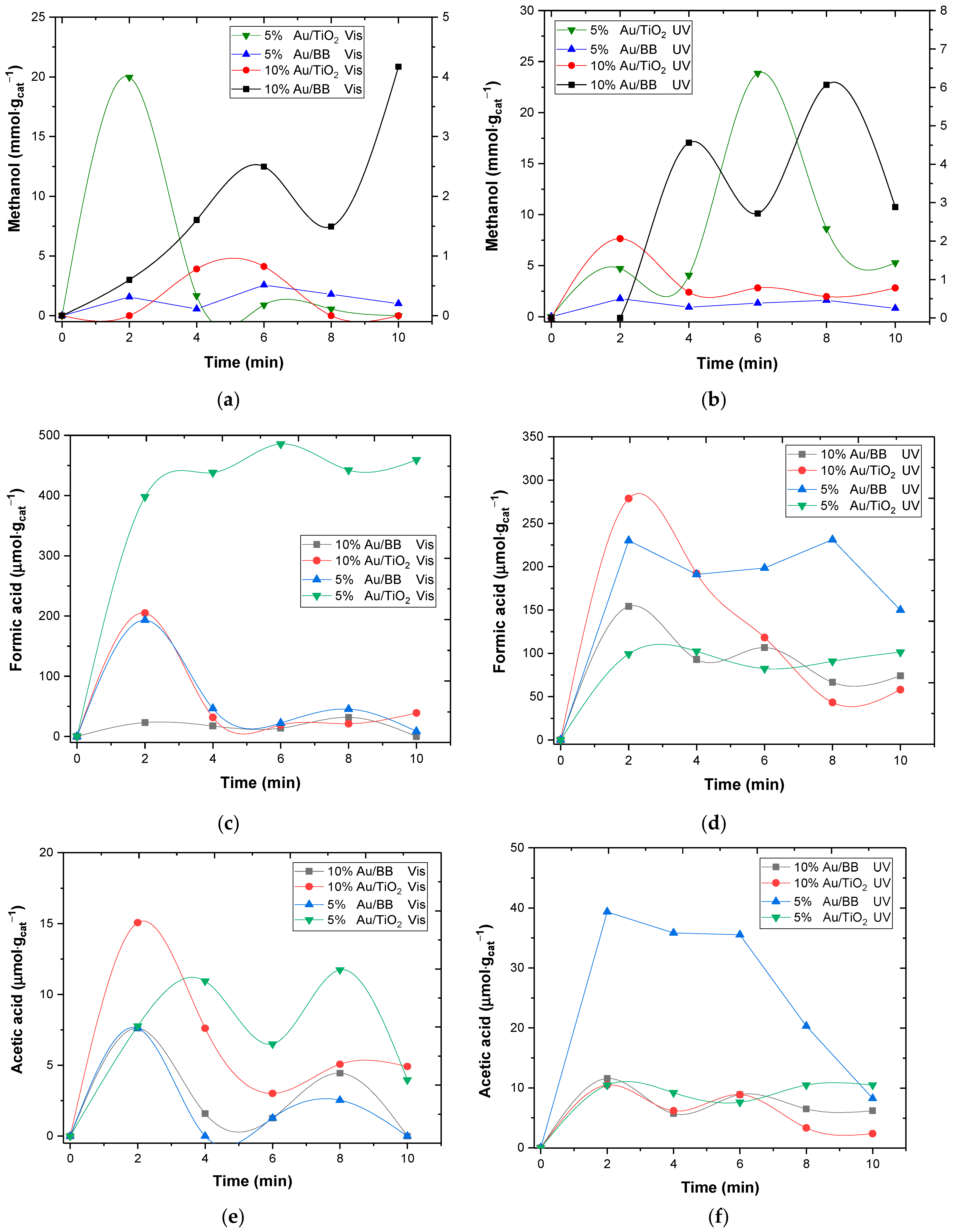
2.2. Photo-Catalyzed CO2 Chemical Reduction

3. Materials and Methods
3.1. Catalysts Synthesis
3.2. Catalyst Characterization
3.3. Photocatalyzed CO2 Chemical Reduction
3.4. Analytical Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arnau, M.; Turon, P.; Alemán, C.; Sans, J. Hydroxyapatite-based catalysts for CO2 fixation with controlled selectivity towards C2 products. Phenomenal support or active catalyst? J. Mater. Chem. A 2022, 11, 1324–1334. [Google Scholar] [CrossRef]
- Sans, J.; Arnau, M.; Turon, P.; Alemán, C. Permanently polarized hydroxyapatite, an outstanding catalytic material for carbon and nitrogen fixation. Mater. Horizons 2022, 9, 1566–1576. [Google Scholar] [CrossRef]
- Sans, J.; Revilla-López, G.; Sanz, V.; Puiggalí, J.; Turon, P.; Alemán, C. Permanently polarized hydroxyapatite for selective electrothermal catalytic conversion of carbon dioxide into ethanol. Chem. Commun. 2021, 57, 5163–5166. [Google Scholar] [CrossRef] [PubMed]
- Lv, C.; Liang, H.; Chen, H.; Wu, L. Hydroxyapatite supported Co3O4 catalyst for enhanced degradation of organic contaminants in aqueous solution: Synergistic visible-light photo-catalysis and sulfate radical oxidation process. Microchem. J. 2019, 149, 103959. [Google Scholar] [CrossRef]
- Shu, K.; Chuaicham, C.; Noguchi, Y.; Xu, L.; Sasaki, K. In-situ hydrothermal synthesis of Fe-doped hydroxyapatite photocatalyst derived from converter slag toward xanthate photodegradation and Cr(VI) reduction under visible-light irradiation. Chem. Eng. J. 2023, 459, 141474. [Google Scholar] [CrossRef]
- Liu, X.; Ma, J.; Yang, J. Visible-light-driven amorphous Fe(III)-substituted hydroxyapatite photocatalyst: Characterization and photocatalytic activity. Mater. Lett. 2014, 137, 256–259. [Google Scholar] [CrossRef]
- Kalita, J.; Das, B.; Dhar, S.S. Synergistic effect of iron and copper in hydroxyapatite nanorods for Fenton-like oxidation of organic dye. Colloids Surfaces A Physicochem. Eng. Asp. 2022, 643, 128750. [Google Scholar] [CrossRef]
- de Medeiros, F.G.M.; Farzi, F.; Achouri, I.E.; Lotfi, S.; de Vasconcelos, B.R. Performance of Hydroxyapatite-Supported Catalysts for Methane Production Via CO2 Hydrogenation on Semi-Pilot Scale. Waste Biomass-Valorization 2023, 14, 3429–3444. [Google Scholar] [CrossRef]
- Sans, J.; Sanz, V.; Turon, P.; Alemán, C. Enhanced CO2 Conversion into Ethanol by Permanently Polarized Hydroxyapatite through C–C Coupling. ChemCatChem 2021, 13, 5025–5033. [Google Scholar] [CrossRef]
- Dos Santos Silva, D.; Villegas, A.E.C.; Bonfim, R.D.P.F.; Salim, V.M.M.; De Resende, N.S. Iron-substituted hydroxyapatite as a potential photocatalyst for selective reduction of CO2 with H2. J. CO2 Util. 2022, 63, 102102. [Google Scholar] [CrossRef]
- Tran, N.D.T.; Che, T.N.H.; Van Nguyen, T.T.; Do, B.L.; Ho, T.G.T.; Nguyen, P.A.; Pham, T.T.P.; Tri, N.; Ha, H.K.P. Fishbone derived-hydroxyapatite supported Ni-Zr nanocatalyst for CO2 methanation: Synergistic effects of support and zirconia. Arab. J. Chem. 2023, 16, 105307. [Google Scholar] [CrossRef]
- Phan, T.S.; Sane, A.R.; de Vasconcelos, B.R.; Nzihou, A.; Sharrock, P.; Grouset, D.; Minh, D.P. Hydroxyapatite supported bimetallic cobalt and nickel catalysts for syngas production from dry reforming of methane. Appl. Catal. B 2018, 224, 310–321. Available online: https://hal.science/hal-01630309 (accessed on 24 February 2024). [CrossRef]
- Rego de Vasconcelos, B.; Pham Minh, D.; Martins, E.; Germeau, A.; Sharrock, P.; Nzihou, A. Highly-efficient hydroxyapatite-supported nickel catalysts for dry reforming of methane. Int. J. Hydrog. Energy 2020, 45, 18502–18518. [Google Scholar] [CrossRef]
- Wang, Q.-N.; Weng, X.-F.; Zhou, B.-C.; Lv, S.-P.; Miao, S.; Zhang, D.; Han, Y.; Scott, S.L.; Schüth, F.; Lu, A.-H. Direct, Selective Production of Aromatic Alcohols from Ethanol Using a Tailored Bifunctional Cobalt–Hydroxyapatite Catalyst. ACS Catal. 2019, 9, 7204–7216. [Google Scholar] [CrossRef]
- Hua, M.; Song, J.; Xie, C.; Wu, H.; Hu, Y.; Huang, X.; Han, B. Ru/hydroxyapatite as a dual-functional catalyst for efficient transfer hydrogenolytic cleavage of aromatic ether bonds without additional bases. Green Chem. 2019, 21, 5073–5079. [Google Scholar] [CrossRef]
- Li, S.; Li, Y.; Shen, W.; Bai, Y.; Kong, L. Hydroxyapatite-based catalysis in environmental decontamination. J. Clean. Prod. 2022, 380, 134961. [Google Scholar] [CrossRef]
- Wakamura, M.; Tanaka, H.; Naganuma, Y.; Yoshida, N.; Watanabe, T. Surface structure and visible light photocatalytic activity of titanium–calcium hydroxyapatite modified with Cr(III). Adv. Powder Technol. 2011, 22, 498–503. [Google Scholar] [CrossRef]
- Zou, R.; Xu, T.; Lei, X.; Wu, Q.; Xue, S. Novel and efficient red phosphorus/hollow hydroxyapatite microsphere photocatalyst for fast removal of antibiotic pollutants. J. Phys. Chem. Solids 2020, 139, 109353. [Google Scholar] [CrossRef]
- Kandori, K.; Yamaguchi, Y.; Wakamura, M. Photodecomposition of surfactants using Ti(IV)-doped calcium hydroxyapatite particles. Colloid Polym. Sci. 2017, 295, 1079–1087. [Google Scholar] [CrossRef]
- Nishikawa, H. A high active type of hydroxyapatite for photocatalytic decomposition of dimethyl sulfide under UV irradiation. J. Mol. Catal. A Chem. 2004, 207, 149–153. [Google Scholar] [CrossRef]
- Nishikawa, H.; Kato, S.; Ando, T. Rapid and complete oxidation of acetaldehyde on TiO2 photocatalytic filter supported by photo-induced activated hydroxyapatite. J. Mol. Catal. A Chem. 2005, 236, 145–148. [Google Scholar] [CrossRef]
- Bharadwaz, A.; Jayasuriya, A.C. Recent trends in the application of widely used natural and synthetic polymer nanocomposites in bone tissue regeneration. Mater. Sci. Eng. C 2020, 110, 110698. [Google Scholar] [CrossRef] [PubMed]
- Camargo, P.H.C.; Satyanarayana, K.G.; Wypych, F. Nanocomposites: Synthesis, structure, properties and new application opportunities. Mater. Res. 2009, 12, 1–39. [Google Scholar] [CrossRef]
- Lateef, A.; Nazir, R. Metal Nanocomposites: Synthesis, Characterization and their Applications. Sci. Appl. Tailored Nanostructures 2017, 239–256. [Google Scholar]
- Ali, A.; Shah, T.; Ullah, R.; Zhou, P.; Guo, M.; Ovais, M.; Tan, Z.; Rui, Y. Review on Recent Progress in Magnetic Nanoparticles: Synthesis, Characterization, and Diverse Applications. Front. Chem. 2021, 9, 629054. [Google Scholar] [CrossRef] [PubMed]
- Mikhailova, E.O. Gold Nanoparticles: Biosynthesis and Potential of Biomedical Application. J. Funct. Biomater. 2021, 12, 70. [Google Scholar] [CrossRef] [PubMed]
- Chopra, H.; Bibi, S.; Singh, I.; Hasan, M.M.; Khan, M.S.; Yousafi, Q.; Baig, A.A.; Rahman, M.; Islam, F.; Bin Emran, T.; et al. Green Metallic Nanoparticles: Biosynthesis to Applications. Front. Bioeng. Biotechnol. 2022, 10, 874742. [Google Scholar] [CrossRef] [PubMed]
- Saravanan, A.; Kumar, P.S.; Karishma, S.; Vo, D.V.N.; Jeevanantham, S.; Yaashikaa, P.R.; George, C.S. A review on biosynthesis of metal nanoparticles and its environmental applications. Chemosphere 2021, 264, 128580. [Google Scholar] [CrossRef] [PubMed]
- Hing, K.A.; Best, S.M.; Bonfield, W. Characterization of porous hydroxyapatite. J. Mater. Sci. Mater. Med. 1999, 10, 135–145. [Google Scholar] [CrossRef]
- Gama-Lara, S.A.; Mendoza, M.S.P.; Vilchis-Nestor, A.R.; Natividad, R. Bionanotechnology: Silver Nanoparticles Supported on Bovine Bone Powder Used as Bactericide. Materials 2020, 13, 462. [Google Scholar] [CrossRef]
- Gama-Lara, S.A.; Natividad, R.; Vilchis-Nestor, A.R.; López-Castañares, R.; García-Orozco, I.; Gonzalez-Pedroza, M.G.; Morales-Luckie, R.A. Ultra-Small Platinum Nanoparticles with High Catalytic Selectivity Synthesized by an Eco-friendly Method Supported on Natural Hydroxyapatite. Catal. Lett. 2019, 149, 3447–3453. [Google Scholar] [CrossRef]
- Natividad, R. CO2 photoconversion catalyzed by nanoparticles supported on TiO2. In Nanoparticles in Green Organic Synthesis: Strategy towards Sustainability; Elsevier: Amsterdam, The Netherlands, 2023; pp. 421–452. [Google Scholar]
- Dey, A.; Silveira, V.R.; Vadell, R.B.; Lindblad, A.; Lindblad, R.; Shtender, V.; Görlin, M.; Sá, J. Exploiting hot electrons from a plasmon nanohybrid system for the photoelectroreduction of CO2. Commun. Chem. 2024, 7, 59. [Google Scholar] [CrossRef]
- Rossi, T.P.; Erhart, P.; Kuisma, M. Hot-carrier generation in plasmonic nanoparticles: The importance of atomic structure. ACS Nano 2020, 14, 9963–9971. [Google Scholar] [CrossRef] [PubMed]
- Mariappan, A.; Pandi, P.; Rani, K.B.; Rajeswarapalanichamy; Neyvasagam, K. Study of the photocatalytic and antibacterial effect of Zn and Cu doped hydroxyapatite. Inorg. Chem. Commun. 2021, 136, 109128. [Google Scholar] [CrossRef]
- Sarina, S.; Waclawik, E.R.; Zhu, H. Photocatalysis on supported gold and silver nanoparticles under ultraviolet and visible light irradiation. Green Chem. 2013, 15, 1814–1833. [Google Scholar] [CrossRef]
- Tsvetkov, M.; Zaharieva, J.; Milanova, M. Ferrites, modified with silver nanoparticles, for photocatalytic degradation of malachite green in aqueous solutions. Catal. Today 2019, 357, 453–459. [Google Scholar] [CrossRef]
- Patel, S.; Han, J.; Qiu, W.; Gao, W. Synthesis and characterisation of mesoporous bone char obtained by pyrolysis of animal bones, for environmental application. J. Environ. Chem. Eng. 2015, 3, 2368–2377. [Google Scholar] [CrossRef]
- De Witte, K.; Ribbens, S.; Meynen, V.; De Witte, I.; Ruys, L.; Cool, P.; Vansant, E.F. Photocatalytic study of P25 and mesoporous titania in aqueous and gaseous environment. Catal. Commun. 2008, 9, 1787–1792. [Google Scholar] [CrossRef]
- Neatu, S.; Maciá-Agulló, J.A.; Concepción, P.; Garcia, H. Gold-copper nanoalloys supported on TiO2 as photocatalysts for CO2 reduction by water. J. Am. Chem. Soc. 2014, 136, 15969–15976. [Google Scholar] [CrossRef]
- Yin, S.; Zhou, Y.; Liu, Z.; Wang, H.; Zhao, X.; Zhu, Z.; Yan, Y.; Huo, P. Elucidating protonation pathways in CO2 photoreduction using the kinetic isotope effect. Nat. Commun. 2024, 15, 437. [Google Scholar] [CrossRef]
- Morales, D.O.; Regalado-Méndez, A.; Pérez-Alonso, C.; Natividad, R. Physical and reactive absorption of CO2 in capillaries: Mass transfer, modelling and produced chemical species. Chem. Eng. Res. Des. 2023, 198, 247–258. [Google Scholar] [CrossRef]
- Buxton, G.V.; Elliott, A.J. Rate Constant for Reaction of Hydroxyl Radicals with Bicarbonate Ions? Int. J. Radiat. Appl. Instrum. Part C. Radiat. Phys. Chem. 1986, 27, 241–243. [Google Scholar] [CrossRef]
- Karamian, E.; Sharifnia, S. On the general mechanism of photocatalytic reduction of CO2. J. CO2 Util. 2016, 16, 194–203. [Google Scholar] [CrossRef]
- Bittencourt, A.F.B.; Mendes, P.C.D.; Valença, G.P.; Da Silva, J.L.F. Acid-base properties of hydroxyapatite(0001) by the adsorption of probe molecules: An ab initio investigation. Phys. Rev. Mater. 2021, 5, 075003. [Google Scholar] [CrossRef]
- Nishikawa, H. Photo-Induced Catalytic Activity of Hydroxyapatite Based on Photo-Excitation. Phosphorus Res. Bull. 2007, 21, 97–102. [Google Scholar] [CrossRef]
- Bystrov, V.; Piccirillo, C.; Tobaldi, D.; Castro, P.; Coutinho, J.; Kopyl, S.; Pullar, R. Oxygen vacancies, the optical band gap (Eg) and photocatalysis of hydroxyapatite: Comparing modelling with measured data. Appl. Catal. B Environ. 2016, 196, 100–107. [Google Scholar] [CrossRef]
- Diallo-Garcia, S.; Osman, M.B.; Krafft, J.-M.; Casale, S.; Thomas, C.; Kubo, J.; Costentin, G. Identification of Surface Basic Sites and Acid–Base Pairs of Hydroxyapatite. J. Phys. Chem. C 2014, 118, 12744–12757. [Google Scholar] [CrossRef]
- Wang, L.; Li, B.; Dionysiou, D.D.; Chen, B.; Yang, J.; Li, J. Overlooked Formation of H2O2 during the Hydroxyl Radical-Scavenging Process When Using Alcohols as Scavengers. Environ. Sci. Technol. 2022, 56, 3386–3396. [Google Scholar] [CrossRef]
- Ding, L.; Li, M.; Zhao, Y.; Zhang, H.; Shang, J.; Zhong, J.; Sheng, H.; Chen, C.; Zhao, J. The vital role of surface Brönsted acid/base sites for the photocatalytic formation of free ·OH radicals. Appl. Catal. B Environ. 2020, 266, 118634. [Google Scholar] [CrossRef]
- Mondal, S.; Reyes, M.E.; Pal, U. Plasmon induced enhanced photocatalytic activity of gold loaded hydroxyapatite nanoparticles for methylene blue degradation under visible light. RSC Adv. 2017, 7, 8633–8645. [Google Scholar] [CrossRef]
- Qian, K.; Sweeny, B.C.; Johnston-Peck, A.C.; Niu, W.; Graham, J.O.; DuChene, J.S.; Qiu, J.; Wang, Y.-C.; Engelhard, M.H.; Su, D.; et al. Surface Plasmon-Driven Water Reduction: Gold Nanoparticle Size Matters. J. Am. Chem. Soc. 2014, 136, 9842–9845. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Wang, W.; Sun, S.; Zhang, L.; Zheng, Y. Equilibrating the Plasmonic and Catalytic Roles of Metallic Nanostructures in Photocatalytic Oxidation over Au-Modified CeO2. ACS Catal. 2014, 5, 613–621. [Google Scholar] [CrossRef]
- Makuła, P.; Pacia, M.; Macyk, W. How To Correctly Determine the Band Gap Energy of Modified Semiconductor Photocatalysts Based on UV–Vis Spectra. J. Phys. Chem. Lett. 2018, 9, 6814–6817. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Ali, S.A.; Mahur, A.; Virk, H.; Singh, F.; Khan, S.; Avasthi, D.; Prasad, R. Study of optical band gap and carbonaceous clusters in swift heavy ion irradiated polymers with UV–Vis spectroscopy. Nucl. Instrum. Methods Phys. Res. Sect. B 2008, 266, 1788–1792. [Google Scholar] [CrossRef]
- Escobedo-Morales, A.; Ruiz-López, I.I.; Ruiz-Peralta, M.D.; Tepech-Carrillo, L.; Sánchez-Cantú, M.; Moreno-Orea, J.E. Automated method for the determination of the band gap energy of pure and mixed powder samples using diffuse reflectance spectroscopy. Heliyon 2019, 5, e01505. [Google Scholar] [CrossRef]
- Morales, A.E.; Mora, E.S.; Pal, U. Use of diffuse reflectance spectroscopy for optical characterization of un-supported nanostructures. Rev. Mex. Fis. 2006, 53, 18–22. [Google Scholar]




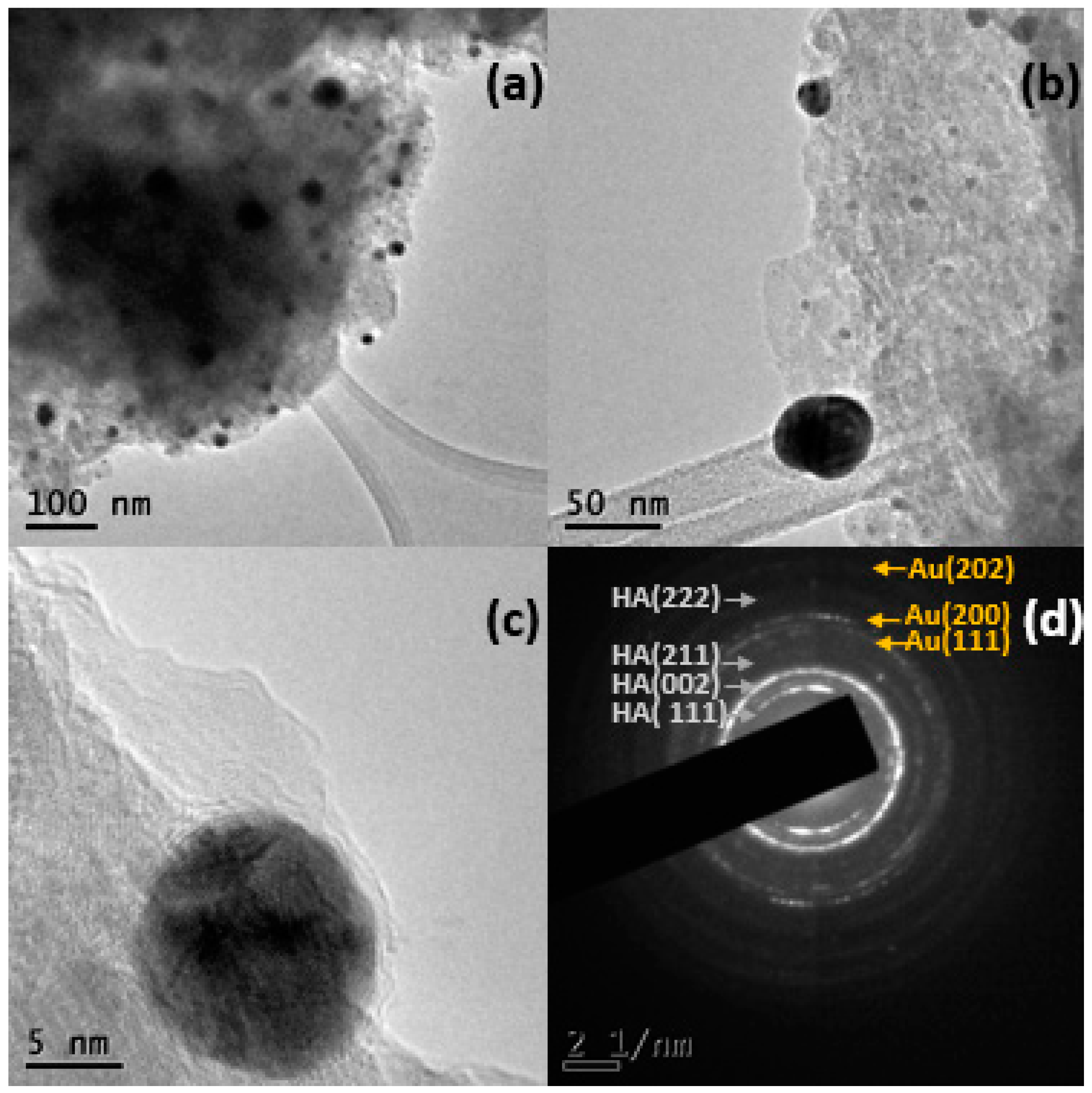

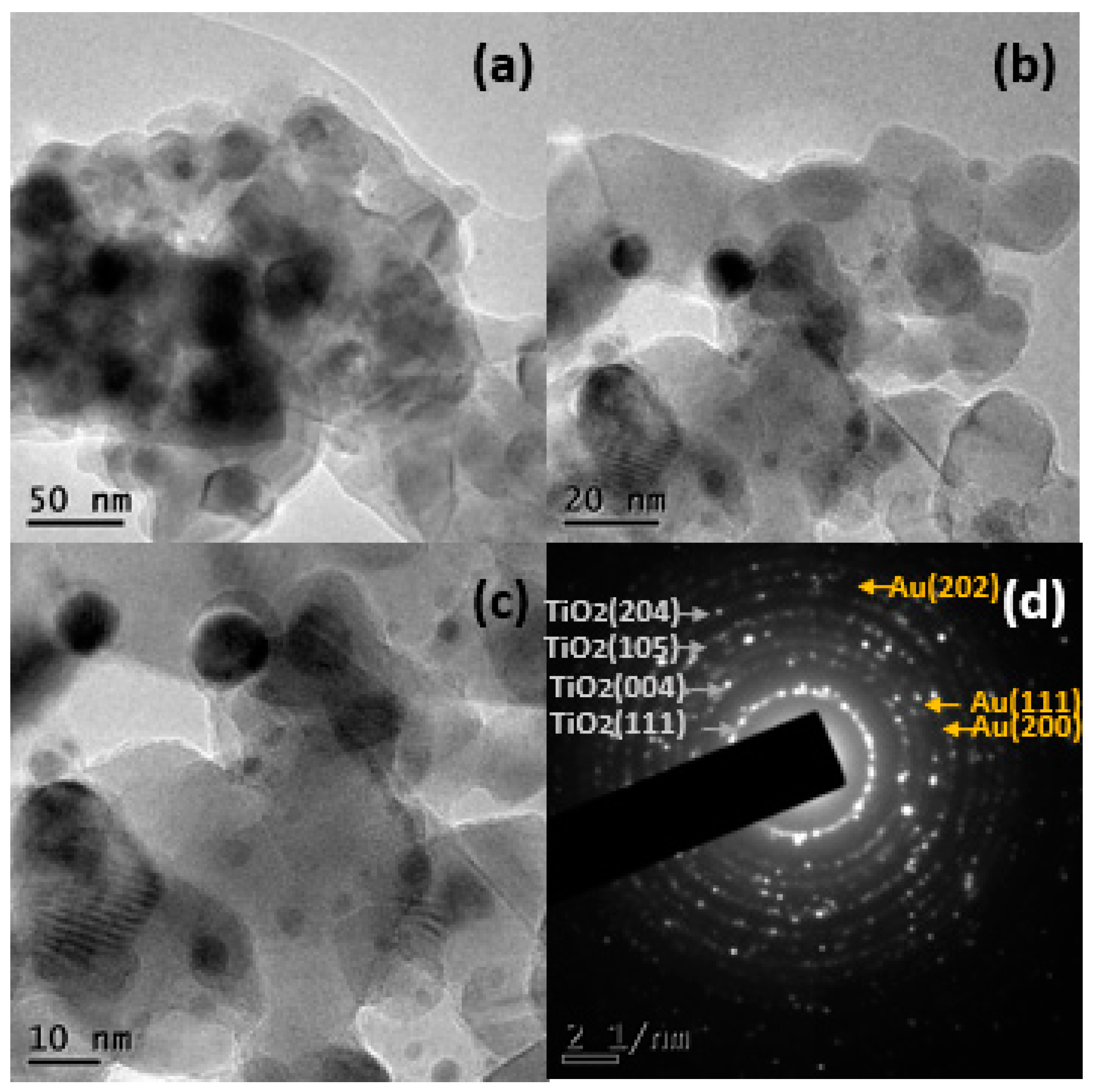
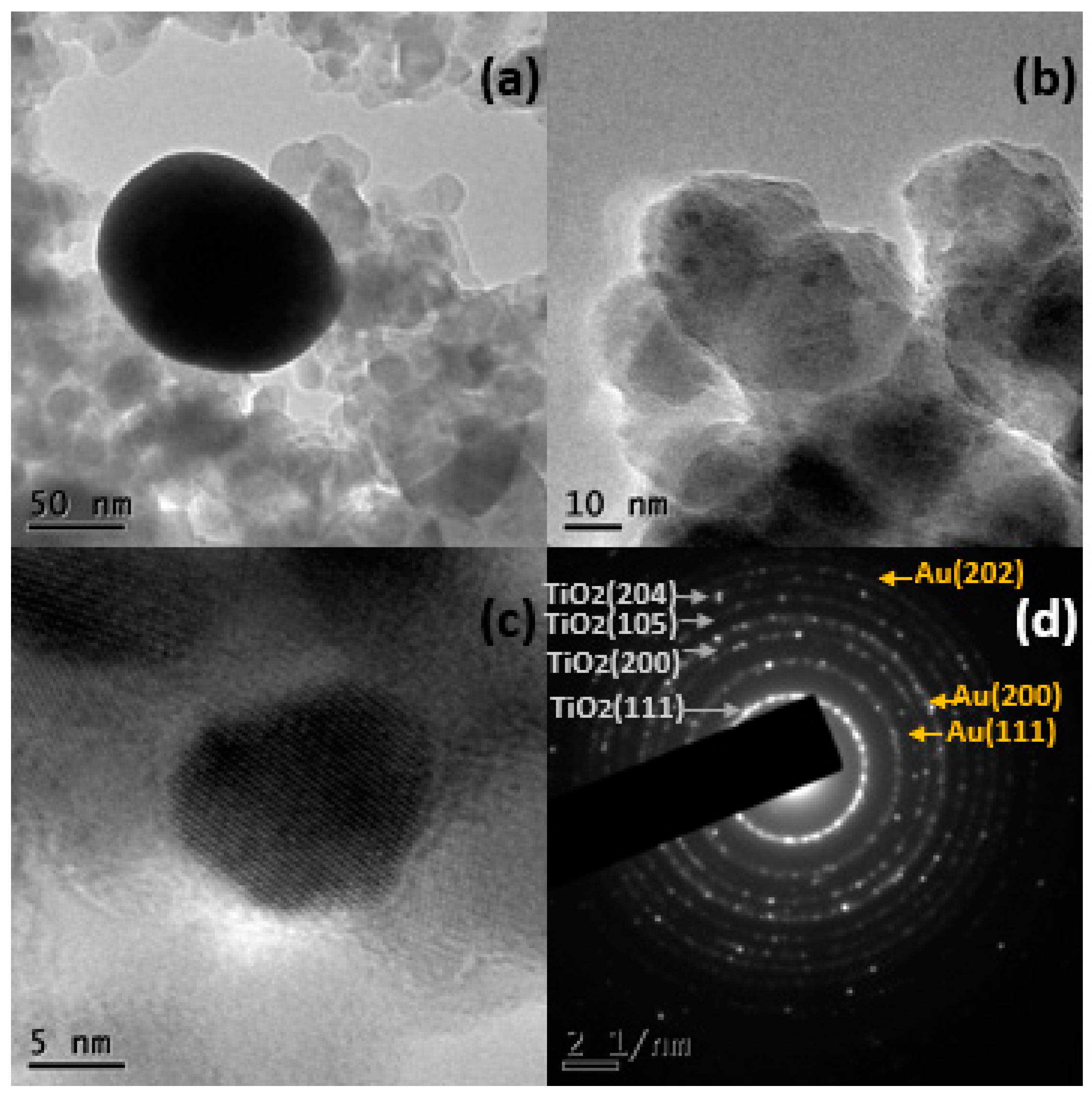
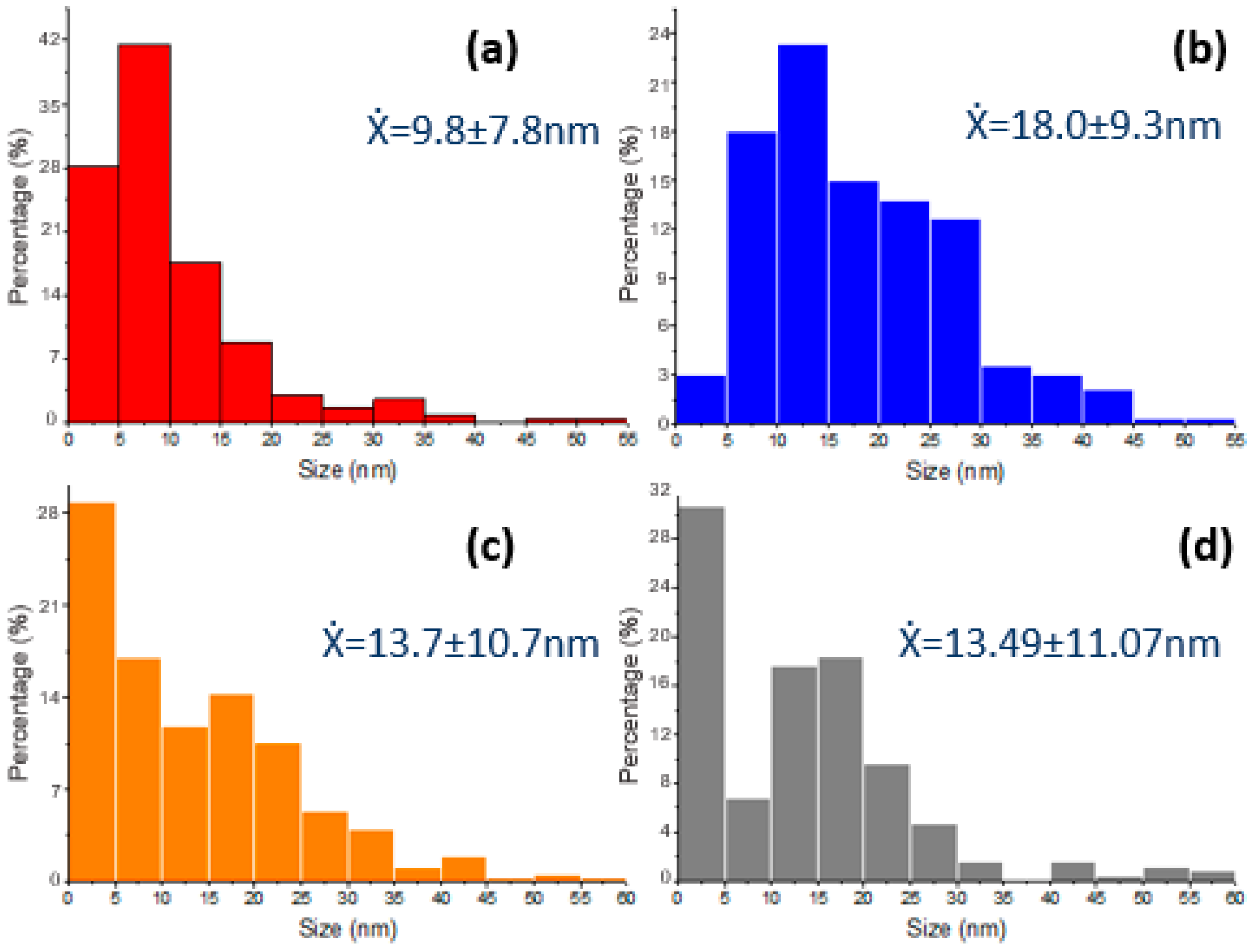


| Name | % Au | Support | Immersion Time |
|---|---|---|---|
| BB | 0 | Bovine bone powder | 30 s |
| 5% Au/BB | 5 | Bovine bone powder | |
| 10% Au/BB | 10 | Bovine bone powder | |
| 5% Au/TiO2 | 5 | Titanium dioxide | |
| 10% Au/TiO2 | 10 | Titanium dioxide |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gama-Lara, S.A.; Vilchis-Néstor, A.R.; Amado-Piña, D.; Natividad, R. Au Supported on Bovine-Bone-Derived Hydroxyapatite Catalyzes CO2 Photochemical Reduction toward Methanol. Catalysts 2024, 14, 417. https://doi.org/10.3390/catal14070417
Gama-Lara SA, Vilchis-Néstor AR, Amado-Piña D, Natividad R. Au Supported on Bovine-Bone-Derived Hydroxyapatite Catalyzes CO2 Photochemical Reduction toward Methanol. Catalysts. 2024; 14(7):417. https://doi.org/10.3390/catal14070417
Chicago/Turabian StyleGama-Lara, Sergio Arturo, Alfredo Rafael Vilchis-Néstor, Deysi Amado-Piña, and Reyna Natividad. 2024. "Au Supported on Bovine-Bone-Derived Hydroxyapatite Catalyzes CO2 Photochemical Reduction toward Methanol" Catalysts 14, no. 7: 417. https://doi.org/10.3390/catal14070417
APA StyleGama-Lara, S. A., Vilchis-Néstor, A. R., Amado-Piña, D., & Natividad, R. (2024). Au Supported on Bovine-Bone-Derived Hydroxyapatite Catalyzes CO2 Photochemical Reduction toward Methanol. Catalysts, 14(7), 417. https://doi.org/10.3390/catal14070417









