To the best of our knowledge, the reactive extrusion of PC/PMMA blends in presence of a transesterification catalyst via a continuous extrusion process up to now has not yet been reported in the scientific or patent literature. The differences of such continuous extrusion processes compared to the discontinuous experiments reported previously are lower residence times (30–90 s compared to 5–60 min) on the one hand and higher shear forces (superior mixing) during compounding on the other. In the initial continuous extrusion experiments that targeted fundamental investigation of the effects of the different catalysts on optical properties (Chapter 3.2.1), phase morphology (Chapter 3.2.2), as well as transesterification and polymer degradation (Chapter 3.3.3), the small-scale twin-screw extruder (Process 11) was used with a residence time of 90 s.
3.2.1. Influence of Catalysts on Optical Properties (Visually Phenomenological Effects)
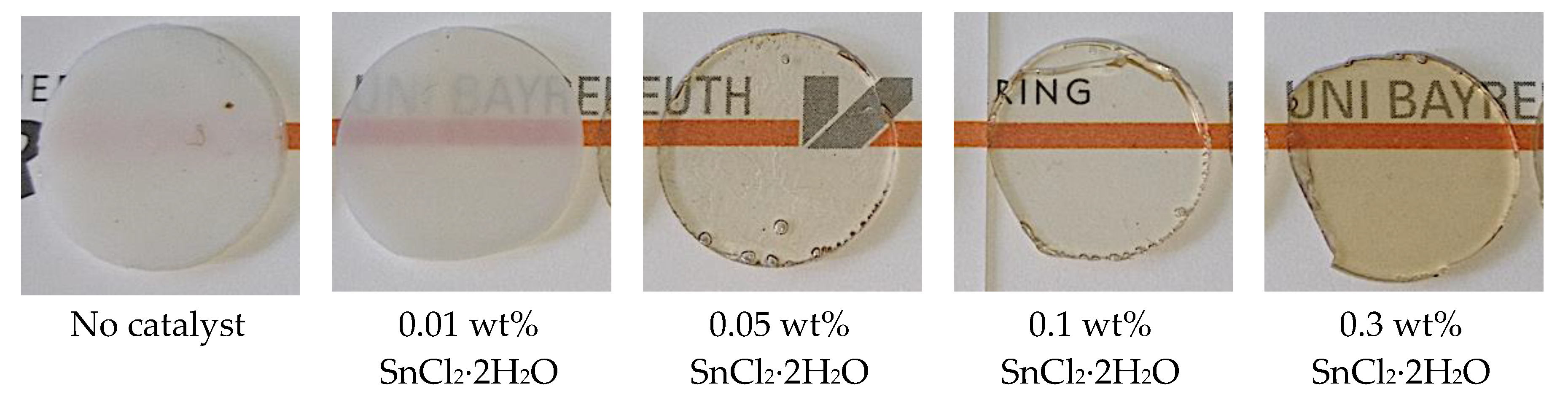
First, we investigated the effect of the catalyst content on the achieved transparency of the polymer blends, in order to define an optimum concentration of the catalyst to be used in the further catalyst screening. For this purpose, we tested the benchmark catalyst SnCl
2∙2H
2O [
6] at concentrations at 0.01, 0.05, 0.1, and 0.3 wt%.
Figure 2 shows 1 mm thick hot-melt pressed specimens of the produced PC/PMMA 50/50 blends.
The PC/PMMA 50/50 blend produced in absence of any catalyst turns out opaque. Already the addition of only 0.01 wt% SnCl2∙2H2O leads to a slightly translucent blend, which becomes transparent at an increased catalyst concentration of 0.05 wt% and above. While transparency is not further improved at catalyst contents above 0.05 wt%, the color of the blends turns increasingly brownish. The steady-state torque of the extruder in the compounding process decreases with catalyst addition. This is consistent with the results of the axial force changes previously observed in the discontinuous microcompounder experiments upon catalyst addition.
As the increase in color at higher catalyst contents is undesired for industrial applications of the blends, we decided to use a catalyst content of 0.05 wt%. At this content, the benchmark catalyst provided the optimum balance of high transparency and lowest possible color for the further catalyst screening study. In this screening study, we used SnCl
2∙2H
2O and zinc acetate as reference catalysts already reported in the scientific and patent literature, respectively, for transesterification of PC and PMMA. Starting from these two references and considering other knowledge about substances that are in principal useful transesterification catalysts for different purposes, we systematically varied cation, anion, and hydration water content in the choice of the investigated catalysts in order to allow assessment of the effects of those parameters (
Table 1). In addition, a weakly acidic phosphonium salt was added to the catalyst screening.
Figure 3 shows 1 mm thick hot-melt pressed specimens of the PC/PMMA 50/50 blends produced with the different catalysts.
Of the investigated catalysts only the SnCl
2∙2H
2O reference catalyst (
Figure 3(2)), the anhydrous SnCl
2 (
Figure 3(3)), and the phosphonium salt (
Figure 3(8)) resulted in transparent blends. However, while the blends produced with both tin chloride catalysts show brownish color, only the phosphonium salt resulted in a completely transparent and, at the same time, colorless blend. Torque of the extruder was reduced for all the compositions resulting in transparent blends compared to the compositions produced with catalysts resulting in opaque blends.
The second catalyst, namely tin(II) ethyl hexanoate, reported by Singh et al. [
21] to result in transparent PC/PMMA 80/20 blends when used at a content of 0.5 wt% in the discontinuous extrusion process that he investigated, did not result in a transparent PC/PMMA 50/50 blend at the lower catalyst content of 0.05 wt% in the continuous extrusion process used here (
Figure 3(5)). Additionally, zinc acetate disclosed as catalyst for transesterification of PC and PMMA in the patent literature [
30,
31] did not result in transparent blends under our experimental conditions (
Figure 3(7)). However, this is in line with the fact that the claims of that patent did actually exclude the PC/PMMA ratio of 50/50 that is used in our current study.
3.2.2. Influence of the Catalysts on Phase Morphology and Polymer Miscibility (Mesoscopic Level Effects)
The transparency can be considered as an indicator for a fully miscible PC/PMMA blend. To proof this interpretation, TEM and DSC investigations were performed.
Figure 4 shows a comparison of the TEM images of the PC/PMMA 50/50 blends produced in absence of a catalyst (
Figure 4a, opaque) and in presence of 0.05 wt% of the phosphonium salt (
Figure 4b, transparent).
While the opaque blend produced in absence of any catalyst is showing phase separation of PC and PMMA resulting in a cocontinuous morphology that is actually expected at the 50/50 ratio of immiscible blend partners (phase domain sizes in the range of several microns), the transparent blend produced with the phosphonium salt clearly shows only a single phase. Even at increased magnification, no phase separation can be detected. We consider this finding as very strong indication that complete miscibility of the two polymers has been achieved in this blend as a consequence of use of this specific catalyst at the chosen concentration. If there was any potential remaining phase separation that cannot be detected at the maximum magnification used in our TEM investigations, the resulting phase domain sizes would have been at least dramatically decreased versus the blend produced in absence of any catalyst (i.e., down to sizes <10 nm, which is below the half of the wavelength of visible light and thus explains the observed transparency of the according blend).
The results of DSC of the transparent (
Figure 5a) and opaque blends (
Figure 5b) are in line with this interpretation of the TEM images. The DSC curves of all transparent blends show only a single
Tg as is expected in case of a completely miscible polymer blend. In contrast, the DSC curves of the opaque blends, with the exception of the blend produced with zinc acetate, show two well-separated glass transitions in line with the presence of a two-phase blend system. The values of the single
Tg’s of the transparent PC/PMMA blends are; however, shifted towards lower temperatures compared to the values expected at the 50/50 ratio of blend partners from Fox equation [
33]. This can be explained by degradation of the PC M
w (see GPC measurement below) during compounding in the presence of those catalysts resulting in transparent blends, which results in a significant reduction of the glass transition temperature of the PC component. Such PC degradation is indicated by lower axial force (in the micro compounder experiments) and lower steady-state torque (in the continuous extrusion experiments) observed during compounding of the transparent vs. the opaque PC/PMMA blends of the same 50/50 ratio.
From the opacity of the blend produced with zinc acetate, it obviously has to be concluded that this material exhibits a two-phase morphology. The single glass transition observed in the DSC in this case is thus not a consequence of complete polymer miscibility but can be considered rather to be related to a reduction of the Tg of the PC caused by PC degradation to a value that cannot anymore be separated in the DSC from the Tg of the PMMA. An alternative interpretation is that there is still a small percentage of immiscible blend partners in the material, which is not detectable by DSC (single Tg) but still is detectable by light scattering (opacity).
3.2.3. Influence of the Catalysts on Transesterification and Polymer Degradation (Molecular Level Effects)
Complete polymer miscibility that results in transparent PC/PMMA blends when selected catalysts are used in the compounding process can be the consequence of PC-g-PMMA copolymer formation by transesterification. Such copolymer acting as compatibilizer was proposed by Singh et al. [
6]. Alternatively, however, polymer miscibility can also be the consequence of degradation of one or both polymer blend partners during compounding using such selected catalysts or a consequence of a combination of both polymer degradation and PC-g-PMMA copolymer formation. That is because blends of semimiscible polymers A and B are known to transit from two-phase to one-phase morphology if 1/N
A + 1/N
B exceeds a threshold value [
34]. This threshold is a constant for a given polymer pair and temperature. N
A and N
B are the weight average numbers of monomer units in polymers A and B, respectively. In other words, two semimiscible polymers A and B become fully miscible if the molecular weights of polymers A and/or B are small enough. The disadvantage of miscible blends resulting from reduced molecular weight of either blend partner is that the low M
w of the polymer(s) will typically result in negative impacts on the mechanical material performance of the blend. As the next step, we thus investigated both PC-g-PMMA copolymer formation and polymer degradation occurring during continuous compounding of PC/PMMA 50/50 blends in the presence of the various catalysts. The target was to identify the actual root cause of the polymer miscibility in the transparent blends obtained with selected catalysts.
To prove formation of and quantify any PC-g-PMMA copolymer resulting from transesterification reaction during PC/PMMA compounding, the acetone insoluble portions of the produced blends were investigated by FTIR and
1H-NMR measurements. The collected FTIR spectra are shown in
Figure 6.
In the FTIR spectra of the acetone insoluble portions of all transparent blends, two clearly distinct carbonyl stretching vibration bends at 1720 cm
−1 (assigned to PMMA) and at 1770 cm
−1 (assigned to PC) are observed. A blend fraction that is not soluble in acetone, but nevertheless contains a significant amount of PMMA is strong indication for formation of a PC-g-PMMA copolymer by transesterification in these materials. PMMA that is not chemically bonded to PC had been proven to be completely soluble in acetone under the applied conditions (see experimental part above). On the other hand, the FTIR spectra of the acetone insoluble fractions of the opaque blends (
Figure 6b) show essentially only the bend at 1770 cm
−1, i.e., this fraction of the opaque blends contains essentially only polycarbonate. Our conclusion is that the opaque PC/PMMA blends actually do not contain any significant amounts of PC-g-PMMA copolymer, i.e., practically no transesterification occurred during their compounding. As a proof of this interpretation, FTIR investigations have also been performed on the acetone soluble fractions of the opaque PC/PMMA blends. All spectra exhibited only a single carbonyl stretching vibration bend at 1720 cm
−1 related to PMMA, so did not provide any indication for presence of PC-g-PMMA copolymer with potentially lower block length of the PC that could be imagined to be soluble in acetone. The opaque material produced with zinc acetylacetonate as catalyst (
Figure 6b(9)) likely contains a minor fraction of PC-g-PMMA copolymer as indicated by the small shoulder at 1720 cm
−1 in the respective FTIR spectrum of its acetone insoluble fraction. Obviously, the amount of the PC-g-PMMA copolymer generated via transesterification in the presence of zinc acetylacetonate is; however, insufficient to result in a transparent blend—at least at the molecular weights of the PC and PMMA resulting in this particular blend upon compounding.
FTIR is easy and comfortable to provide semiquantitative proof of PC-g-PMMA copolymer formation because it can be applied on a solid, i.e., solving of the sample prior to investigation is not required. However, FTIR is not the best method for quantification of the extent of the PC-g-PMMA copolymer formation since it requires a calibration and is not very precise due to overlapping IR bends related to PC and PMMA (see
Figure 6). We thus decided to do, in addition, quantitative
1H-NMR spectroscopy on the acetone insoluble part of the PC/PMMA 50/50 blend produced in the presence of 0.05 wt% of the phosphonium salt catalyst. This sample was chosen as an example because it had displayed the best performance regarding transparency and color (
Figure 3).
Figure 7 shows the recorded NMR spectrum of this sample as compared to the NMR reference spectra of the neat PC and PMMA feedstock polymers. For the quantitative analysis, the NMR signals at 7.1–7.3 ppm attributed to the eight aromatic protons of the bisphenol-A units in the PC and at 3.6 ppm attributed to the three methyl ester protons of the PMMA were integrated. A content of 10 mol% of PMMA repetition units, corresponding to 4 wt% of PMMA as part of a PC-g-PMMA copolymer was calculated to be present in the acetone insoluble part of the investigated PC/PMMA 50/50 blend. No significant bend at 1770 cm
−1 attributed to PC had been observed in the acetone soluble part of this blend, so we conclude that no PC-g-PMMA copolymer was extracted by the acetone. We thus estimate that the total content of PC-g-PMMA copolymer that has formed upon reactive compounding in the original PC/PMMA 50/50 blend via transesterification with the phosphonium salt catalyst is in the order of about 2 wt%.
Although the results of the FTIR and
1H-NMR investigations confirm presence of PC-g-PMMA copolymer in the transparent PC/PMMA blends, this does not necessarily mean that this copolymer is (exclusively) responsible for the observed transparency. The mechanism of transesterification as proposed by Singh et al. [
6] results in scissoring of one PC chain per formed PC-g-PMMA copolymer molecule. Although Singh did not explicitly report according experimental results, such transesterification, hence, must result in a significant reduction of PC M
w as a side effect, which actually might be the real root cause, or at least a secondary prerequisite beyond the PC-g-PMMA copolymer formation, for obtaining transparent PC/PMMA blends.
In order to allow judgement about the real root cause of the transparency, we thus performed GPC measurements. Molecular weight distributions determined on the acetone insoluble parts of the transparent PC/PMMA blends are shown in
Figure 8. Furthermore, the molecular weights at the peak (maximum) position of the molecular weight distribution, M
peak determined for the acetone insoluble parts of both the transparent and opaque PC/PMMA 50/50 compounds are summarized in
Table 2. Molecular weight distributions determined on the acetone soluble parts of the transparent blends were essentially identical with the molecular weight distribution of the pure PMMA raw material. This acetone soluble part of a PC/PMMA blend, based on our previous investigation on a physical PC/PMMA mixture compounded in the absence of any catalyst (see experimental part), can be assigned to the unreacted PMMA portions within the reactively compounded products. It thus can be concluded that no degradation of the PMMA polymer has occurred during the compounding process.
In contrast to what is observed for the vast majority of the opaque PC/PMMA blends, in case of the transparent blends the molecular weight distributions of the acetone insoluble parts, i.e., of the fractions consisting of PC and any potentially formed PC-g-PMMA copolymer, in general show strong shifts towards lower molecular weights compared to the pure PC raw material used in the preparation of the blends (
Figure 8 and
Table 2). This PC molecular weight decrease is particularly severe with SnCl
2∙2H
2O. It can be explained by chain scissoring during transesterification according to the mechanism of Singh et al. [
6], who proposed a cross-transesterification of a carbonate group in the PC polymer chain with the methyl ester groups in the PMMA side groups. As a consequence of such reaction, the PC molecules involved will be divided into two parts of lower molecular weights that add up to the molecular weight of the initial PC molecule. Only one part will be chemically bonded to a PMMA molecule during the transesterification, while the other will remain as a part of the free PC phase and thus reduce its average molecular weight. However, molecular weight decrease could also be, at least partially, due to hydrolytic degradation of the PC via reaction with residual moisture that was not completely removed by the predrying of the polymer raw materials and/or is introduced by the catalyst (SnCl
2∙2H
2O). Our observation that PC molecular weight degradation measured by GPC is also observed upon compounding of the pure PC with the respective catalysts even in the absence of any PMMA, but not to the same extent as observed in the PC/PMMA 50/50 blends (also see
Figure 1b), proves that actually both types of reactions likely contribute to the observed total molecular weight decreases. This can also explain why the PC molecular weight decrease is less severe when anhydrous SnCl
2 is used as catalyst instead of SnCl
2∙2H
2O. The latter introduces some additional water into the blend mixture and thus can increase the contribution of hydrolysis reaction to the total decrease of PC molecular weight. The shoulders/second peak at high molecular weight that are observed in the GPC of the acetone insoluble fraction of the transparent PC/PMMA blends are most likely related to the PC-g-PMMA copolymer formed during reactive extrusion (containing both contributions of the PMMA with M
w=130.000 g/mol and of the PC with M
w = 46.000 g/mol). While these GPC contributions of the PC-g-PMMA copolymer in samples (3) and (8) are visible as shoulders, in sample (2) it appears as a well resolved second peak because of the more severe degradation of the PC in this case.
The M
peak values of the acetone insoluble fractions of the opaque PC/PMMA blends (
Table 2) show no or significantly smaller shifts towards lower levels compared to the transparent blends. The only exception in this context is the blend produced with zinc acetate, which results in a similar molecular weight reduction as the phosphonium salt with which a transparent blend was achieved. The exceptionally severe PC degradation observed with zinc acetate can explain the also exceptional observation of only a single
Tg in the DSC of this blend (see above). Obviously, the zinc acetate does catalyze the hydrolytic cleavage of the PC by residual water, but not the transesterification of the PC with PMMA. Thus, the blend produced with zinc acetate is opaque due to absence of PC-g-PMMA copolymer, but the
Tg of the PC is reduced to a value similar to that of the PMMA, so that both glass transitions cannot anymore be resolved in the DSC experiment.
3.2.4. Mechanical Performance of the Reactively Compatibilized PC/PMMA Blends
Because effective reactive compatibilization of PC/PMMA blends resulted not only in the targeted formation of PC-g-PMMA copolymer, but inherently also in PC M
w degradation as an undesired side-effect, we assessed the mechanical properties of the produced blends to investigate the impact of the combination of both effects on the overall technical performance of the materials. While the formation of PC-g-PMMA copolymer is hoped to result in improved mechanical properties due to phase morphology stabilization, the PC degradation is expected to drive performance in the opposite direction. Previous investigations so far had not reported any mechanical properties of the produced transparent PC/PMMA blends, because the discontinuous lab-scale compounding did not provide sufficient material to do so. In order to allow testing of material properties in accordance with industrially relevant DIN EN ISO standards, we had to scale up the reactive extrusion process to a technical scale to allow production of material quantities sufficient for injection molding of standardized test specimens. For this purpose, a 26 mm twin-screw extruder (ZSK26 MC-18) with a throughput of 20 kg/h and a residence time of 30 s was used to produce PC/PMMA blends in absence of a catalyst as well as in presence of 0.05 wt% SnCl
2∙2H
2O, zinc acetate (previously reported benchmarks) and the phosphonium salt. All property data determined on the constituent PC and PMMA raw materials as well as the three produced PC/PMMA 50/50 blends are summarized in
Table 3. The different visual phenomenological behaviors of the three blends (level of transparency and color) previously observed on these blends when produced on a laboratory-scale extruder were 1:1 reproduced in this scale-up.
The mechanical, thermal, and rheological properties of the PC/PMMA 50/50 blend produced in absence of any catalyst as expected are all in between the according properties of the constituent polymers. Most of these properties, however, are more or less shifted from the numeric average of both polymer blend constituents to the level observed for PMMA, i.e., the blend behaves more similar to PMMA than might be expected at the 50/50 polymer ratio. The only exception is the tensile strain at yield, which, within accuracy of the measurement, for the PC/PMMA blend is at the same level as for the PC raw material. The blend produced with zinc acetate as catalyst, not only with respect to the visual (optical) properties, but also with regards to the other technical properties, behaves quite similar to the blend produced in absence of any catalyst. The minor differences in terms of melt viscosity and Vicat B/120 are related to the PC Mw degradation observed with use of this catalyst (see above). The high haze values of the blends produced in absence of a catalyst (99.4%) and in presence of zinc acetate (98.7%) confirm the visual impression of complete opacity of these materials. The haze determined for the two blends produced with transesterification-effective catalysts also confirm the visual impression of transparency. But moreover, it shows that the phosphonium catalyst results in a more optically clear material compared to the reference catalyst SnCl2∙2H2O, which, based on this optical measurement, rather has to be considered a translucent than a transparent material. The optical measurements (yellowness index) also confirm the superior color of the transparent blend produced with the phosphonium salt.
Both transparent blends unfortunately show a dramatic deterioration of the mechanical performance in terms of toughness related properties (impact strength, tensile strain at break, tensile strain at yield, and tensile strength) versus the opaque blend of same polymer composition produced in absence of any catalyst. Impact strengths of these two blends fall down to a level that is similar to the pure PMMA as the more brittle blend partner. Tensile strengths, strains at break, and strains at yield fall even below the values of both constituent blend partners. The same is true for the melt viscosity. The heat resistance (Vicat B/120) is negatively affected by the addition of the two catalysts as well. The effects on melt viscosity, heat resistance, and mechanical performance can all be regarded as a direct consequence of the PC Mw degradation. Obviously, the detrimental effect of PC Mw degradation overcompensates any potentially positive effect of the PC-g-PMMA copolymer formation, which is thus masked. PC/PMMA blends of that low ductility, as observed for the transparent materials obtained via reactive compatibilization, for sure have to be regarded as technically unsuitable for any industrial applications. Thus, different manufacturing process strategies that do not inherently result in PC degradation as a side effect of PC-g-PMMA copolymer formation have to be developed to potentially achieve transparent PC/PMMA blends with a useful balance of properties.
3.2.5. Structure–Properties Relationships—The Root Cause of Transparency of PC/PMMA Blends
Figure 9 recaps in form of a graphical illustration the correlation between transparency (haze) and the number averaged PC molecular weight (M
n) of the acetone insoluble portions of selected PC/PMMA 50/50 blends produced in absence of any catalyst (opaque), and in presence of 0.05 wt% of catalysts zinc acetate (opaque), phosphonium salt (transparent), and SnCl
2⋅2H
2O (transparent).
The PC molecular weights of all transparent PC/PMMA 50/50 blends obviously fall below a certain threshold limit of around 15,000 g/mol and, at the same time, contain PC-g-PMMA copolymer that could act as a phase compatibilizer. The opaque blend produced with zinc acetate as catalyst exhibits about the same PC molecular weight as the blend produced with phosphonium salt, but due to the ineffectiveness of the zinc acetate for transesterification does not contain any PC-g-PMMA copolymer. This finding shows that the PC molecular weight reduction alone is not sufficient to result in transparent PC/PMMA blends and thus supports the interpretation that the low PC molecular weight resulting from transesterification is not the root cause (at least not the sole root cause) of the transparency. In order to confirm this conclusion, we designed an additional experiment targeting production of a PC/PMMA blend with a very low molecular weight PC that definitely does not contain any PC-g-PMMA copolymer. For this experiment, a tailor-made polycarbonate feedstock was synthesized that exhibited a molecular weight (M
w = 16,700 g/mol, M
n = 7000 g/mol) comparable to the lowest level achieved in the reactively compatibilized PC/PMMA compounds (i.e., in presence of SnCl
2⋅2H
2O as catalyst) and, like the standard Makrolon
® 2408 polycarbonate feedstock used in the other experiments, exhibited a negligible phenolic hydroxyl (pOH) group content, as determined by
1H-NMR spectroscopy, of <100 ppm. This tailor-made low molecular weight PC feedstock was compounded with the standard PMMA grade (Plexiglas
® 8H)—both were thoroughly predried in vacuum at 60 °C—in the absence of any catalyst to produce a purely physical PC/PMMA 50/50 blend (gray column in
Figure 9). The blend turned out opaque, proving our previous conclusion that transparency cannot be exclusively the consequence of the PC M
w degradation, i.e., presence of the PC-g-PMMA copolymer which, in our case is the result of a transesterification, is at least a necessary requirement to achieve transparent blends. The question if transparency of PC/PMMA blends can be also achieved in presence of higher molecular weight PC in the resulting blend, or rather transparency requires a combination of both presence of PC-g-PMMA copolymer and a low M
w PC cannot be clearly answered based on the results of our current study. This is because with the currently investigated catalytic reactive compatibilization approach, we have not yet succeeded to produce any PC/PMMA blend in which PC-g-PMMA copolymer had been formed to any significant extent and in which the PC molecular weight was significantly higher than the M
n = 15,000 g/mol threshold. Starting from Makrolon
® 3108 PC feedstock with higher molecular weight than Makrolon
® 2408 failed to achieve this target. However, a reliable answer on the above question is crucial to allow a conclusion if transparent PC/PMMA blends with useful mechanical performance are at all technically feasible. Different manufacturing process strategies that do not inherently result in PC degradation as a side effect of PC-g-PMMA-copolymer formation therefore have to be developed and assessed to eventually conclude on this topic.