Effect of Non-Thermal Food Processing Techniques on Selected Packaging Materials
Abstract
:1. Introduction
2. Short Overview of Non-Thermal Food Processing Techniques Commonly Applied on Food Packaging Materials
3. Impact of Non-Thermal Food Processing on Selected Packaging Materials
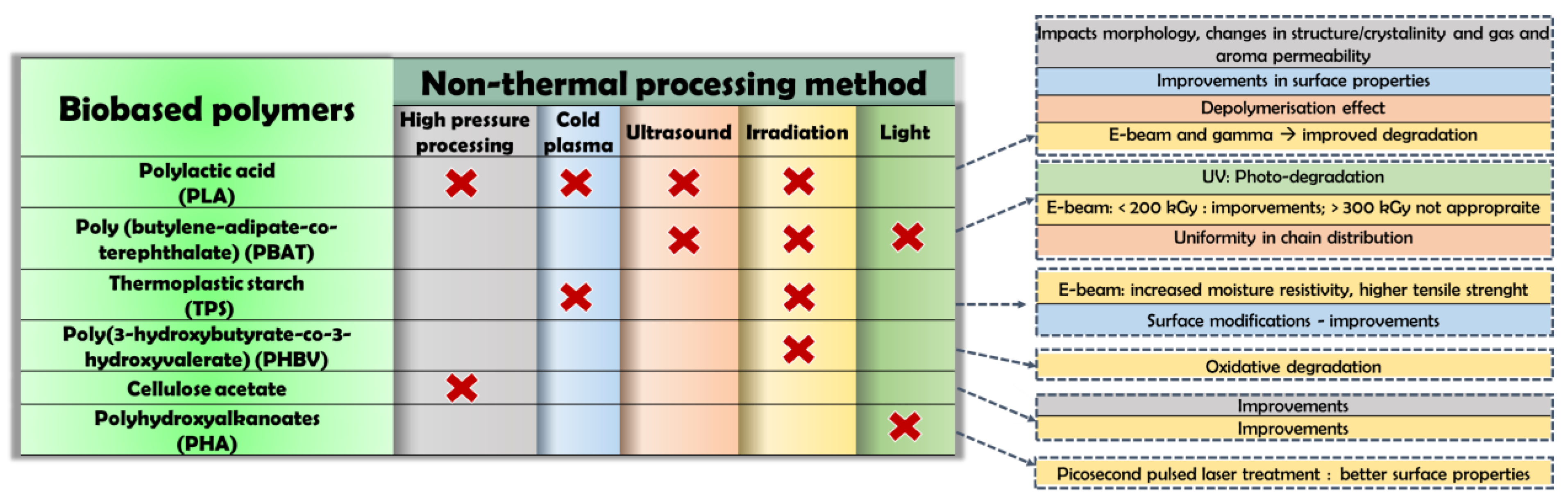
3.1. Biobased Polymers
3.1.1. Poly (Lactic Acid) (PLA)
3.1.2. Poly (Butylene-Adipate-Co-Terephthalate)
3.1.3. Thermoplastic Starch
3.1.4. Poly(3-Hydroxybutyrate-Co-3-Hydroxyvalerate)
3.1.5. Cellulose Acetate
3.1.6. Polyhydroxyalkanoates
3.1.7. Edible Coatings
3.2. Nanomaterials
4. Active Packaging
5. Safety Issues
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
List of Abbreviations
| Abbreviations | Description |
|---|---|
| AM | Antimicrobial |
| AO | Antioxidant |
| AP | Active packaging |
| CA | Cellulose acetate |
| EAB | Elongation at break |
| EC | Edible coating |
| EFSA | European Food Safety Authority |
| EOs | Essential oils |
| EVAC (EVA) | Ethylene vinyl acetate |
| EVOH (EVAL) | Ethylene vinyl alcohol |
| FCMs | Food contact materials |
| FDA | Food and Drug Administration |
| HPP | High pressure processing |
| HPPMP/HPP | Paneer prepared with HPP treated milk |
| HTMP | Heat treated milk paneer |
| HTMP/LAB | HTMP/lactic acid bacteria |
| IR | Infrared light |
| LLDPE | Linear low-density polyethylene |
| MMT | Montmorillonite |
| MW | Molecular weight |
| NPs | Nanoparticles |
| NTP | Non-thermal processing technologies |
| OMMT | Organomodified MMT |
| OTR | Oxygen transmission rate |
| P3HB | Poly-3-hydroxybutyrate |
| P3HB4HB | Poly(3-hydroxybutyrate-co-4-hydroxybutyrate) |
| P3HBHHX | P3HB-co-3-hydroxyhexanoate |
| PA | Polyamide (Nylon) |
| PBAT | Poly (butylene adipate-co-terephthalate) |
| PCL | Polycaprolactone |
| PE | Polyethylene |
| PEF | Pulsed electric field |
| PET | Poly(ethylene-terephthalate) |
| PHA | Polyhydroxyalkanoates |
| PHB | Polyhydroxybutyrate |
| PHBV | Poly(3-hydroxybutyrate-co-3-hydroxyvalerate), |
| PLA | Polylactic acid |
| PP | Polypropylene |
| PT | Phlorotannin |
| PVOH (PVAL, PVA) | Poly(vinyl alcohol) |
| TPS | Thermoplastic starch |
| TS | Tensile strength |
| UV | Ultraviolet |
| UV-C | UV light with wavelengths between 200–280 nm |
| WVP | Water vapour permeability |
| WVTR | Water vapour transmission rate |
References
- Abad, J.; Valencia-Chamorro, S.; Castro, A.; Vasco, C. Studying the effect of combining two nonconventional treatments, gamma irradiation and the application of an edible coating, on the postharvest quality of tamarillo (Solanum betaceum Cav.) fruits. Food Control 2017, 72, 319–323. [Google Scholar] [CrossRef]
- Baek, N.; Han, J.H.; Pascall, M.A. Packaging for nonthermal processing of food. In Packaging for Nonthermal Processing of Food; John Wiley & Sons: New York, NY, USA, 2018; pp. 1–13. [Google Scholar]
- Rodrigues, C.; Souza, V.G.L.; Coelhoso, I.; Fernando, A.L. Bio-based sensors for smart food packaging—Current applications and future trends. Sensors 2021, 21, 2148. [Google Scholar] [CrossRef] [PubMed]
- Salgado, P.R.; Di Giorgio, L.; Musso, Y.S.; Mauri, A.N. Recent developments in smart food packaging focused on biobased and biodegradable polymers. Front. Sustain. Food Syst. 2021, 5, 630393. [Google Scholar] [CrossRef]
- Carina, D.; Sharma, S.; Jaiswal, A.K.; Jaiswal, S. Seaweeds polysaccharides in active food packaging: A review of recent progress. Trends Food Sci. Technol. 2021, 110, 559–572. [Google Scholar] [CrossRef]
- Zhao, Y.-M.; de Alba, M.; Sun, D.-W.; Tiwari, B. Principles and recent applications of novel non-thermal processing technologies for the fish industry—a review. Crit. Rev. Food Sci. Nutr. 2018, 59, 728–742. [Google Scholar] [CrossRef] [PubMed]
- Abera, G.; Yildiz, F. Review on high-pressure processing of foods. Cogent Food Agric. 2019, 5, 1568725. [Google Scholar] [CrossRef]
- Chacha, J.S.; Zhang, L.; Ofoedu, C.E.; Suleiman, R.A.; Dotto, J.M.; Roobab, U.; Agunbiade, A.O.; Duguma, H.T.; Mkojera, B.T.; Hossaini, S.M.; et al. Revisiting non-thermal food processing and preservation methods—Action mechanisms, pros and cons: A technological update (2016–2021). Foods 2021, 10, 1430. [Google Scholar] [CrossRef]
- Huang, H.-W.; Wu, S.-J.; Lu, J.-K.; Shyu, Y.-T.; Wang, C.-Y. Current status and future trends of high-pressure processing in food industry. Food Control 2017, 72, 1–8. [Google Scholar] [CrossRef]
- Ahmed, J.; Varshney, S.K.; Ramaswamy, H.S. Effect of high pressure treatment on thermal and rheological properties of lentil flour slurry. LWT-Food Sci. Technol. 2009, 42, 1538–1544. [Google Scholar] [CrossRef]
- Pankaj, S.K.; Bueno-Ferrer, C.; Misra, N.N.; Milosavljević, V.; O’Donnell, C.P.; Bourke, P.; Keener, K.M.; Cullen, P.J. Applications of cold plasma technology in food packaging. Trends Food Sci. Technol. 2014, 35, 5–17. [Google Scholar] [CrossRef]
- Jordá-Vilaplana, A.; Fombuena, V.; García-García, D.; Samper, M.D.; Sánchez-Nácher, L. Surface modification of polylactic acid (PLA) by air atmospheric plasma treatment. Eur. Polym. J. 2014, 58, 23–33. [Google Scholar] [CrossRef]
- Pankaj, S.; Wan, Z.; Keener, K. Effects of cold plasma on food quality: A review. Foods 2018, 7, 4. [Google Scholar] [CrossRef] [Green Version]
- Misra, N.N.; Yepez, X.; Xu, L.; Keener, K. In-package cold plasma technologies. J. Food Eng. 2019, 244, 21–31. [Google Scholar] [CrossRef]
- Moutiq, R.; Misra, N.N.; Mendonça, A.; Keener, K. In-package decontamination of chicken breast using cold plasma technology: Microbial, quality and storage studies. Meat Sci. 2020, 159, 107942. [Google Scholar] [CrossRef]
- Roodenburg, B. Pulsed Electric Field treatment of packaged food. Ph.D. Thesis, Technische Universiteit Delft, Delft, The Netherlads, 7 December 2011. [Google Scholar]
- Gabrić, D.; Barba, F.; Roohinejad, S.; Gharibzahedi, S.M.T.; Radojčin, M.; Putnik, P.; Bursać Kovačević, D. Pulsed electric fields as an alternative to thermal processing for preservation of nutritive and physicochemical properties of beverages: A review. J. Food Process Eng. 2018, 41, 12638. [Google Scholar] [CrossRef]
- Arshad, R.N.; Abdul-Malek, Z.; Jusoh, Y.M.M.; Radicetti, E.; Tedeschi, P.; Mancinelli, R.; Lorenzo, J.M.; Aadil, R.M. Sustainable electroporator for continuous pasteurisation: Design and performance evaluation with orange juice. Sustainability 2022, 14, 1896. [Google Scholar] [CrossRef]
- Galić, K.; Ščetar, M.; Kurek, M. The benefits of processing and packaging. Trends Food Sci. Technol. 2011, 22, 127–137. [Google Scholar] [CrossRef]
- Abida, J.; Rayees, B.; Masoodi, F.A. Pulsed light technology: A novel method for food preservation. Int. Food Res. J. 2014, 21, 839–848. [Google Scholar]
- Gómez-López, V.M.; Ragaert, P.; Debevere, J.; Devlieghere, F. Pulsed light for food decontamination: A review. Trends Food Sci. Technol. 2007, 18, 464–473. [Google Scholar] [CrossRef]
- John, D.; Ramaswamy, H.S. Pulsed light technology to enhance food safety and quality: A mini-review. Curr. Opin. Food Sci. 2018, 23, 70–79. [Google Scholar] [CrossRef]
- Alarcon-Rojo, A.D.; Peña-González, E.; García- Galicia, I.; Carrillo-López, L.; Huerta-Jiménez, M.; Reyes- Villagrana, R.; Janacua-Vidales, H. Ultrasound application to improve meat quality. In Descriptive Food Science; Intech Open: London, UK, 2018; pp. 153–172. [Google Scholar]
- Beikzadeh, S.; Ghorbani, M.; Shahbazi, N.; Izadi, F.; Pilevar, Z.; Mortazavian, A.M. The effects of novel thermal and nonthermal technologies on the properties of edible food packaging. Food Eng. Rev. 2020, 12, 333–345. [Google Scholar] [CrossRef]
- Ščetar, M.; Daniloski, D.; Tinjić, M.; Kurek, M.; Galić, K. Effect of ultrasound treatment on barrier changes of polymers before and after exposure to food simulants. Polymers 2022, 14, 990. [Google Scholar] [CrossRef] [PubMed]
- Fabbri, P.; Viaggi, D.; Cavani, F.; Bertin, L.; Michetti, M.; Carnevale, E.; Ochoa, J.V.; Martinez, G.A.; Degli Esposti, M.; Fischer, P.K.; et al. Top Emerging Bio-Based Products, Their Properties and Industrial Applications. Available online: https://www.ecologic.eu/sites/default/files/publication/2018/3513-top-emerging-bio-based-products.pdf (accessed on 18 July 2022).
- Conn, R.E.; Kolstad, J.J.; Borzelleca, J.F.; Dixler, D.S.; Filer, L.J.; Ladu, B.N.; Pariza, M.W. Safety assessment of polylactide (PLA) for use as a food-contact polymer. Food Chem. Toxicol. 1995, 33, 273–283. [Google Scholar] [CrossRef] [PubMed]
- Szafranska, Natural Controlled-Releasing Antifungal Film ANTIPACKTM AF. Available online: https://www.handary.com/product-show-Antipack-AF-Bio-degradable-active-antifungal-film (accessed on 29 July 2022).
- Jem, K.J.; Tan, B. The development and challenges of poly (lactic acid) and poly (glycolic acid). Adv. Ind. Eng. Polym. Res. 2020, 3, 60–70. [Google Scholar] [CrossRef]
- Fleckenstein, B.S.; Sterr, J.; Langowski, H.-C. The effect of high pressure processing on the integrity of polymeric packaging - analysis and categorization of occurring defects. Packag. Technol. Sci. 2014, 27, 83–103. [Google Scholar] [CrossRef]
- Ahmed, J.; Mulla, M.; Arfat, Y.A. Application of high-pressure processing and polylactide/cinnamon oil packaging on chicken sample for inactivation and inhibition of Listeria monocytogenes and Salmonella Typhimurium, and post-processing film properties. Food Control 2017, 78, 160–168. [Google Scholar] [CrossRef]
- Ahmed, J.; Mulla, M.Z.; Vahora, A.; Bher, A.; Auras, R. Morphological, barrier and thermo-mechanical properties of high-pressure treated polylactide graphene oxide reinforced composite films. Food Packag. Shelf Life 2021, 29, 100702. [Google Scholar] [CrossRef]
- Mauricio-Iglesias, M.; Peyron, S.; Chalier, P.; Gontard, N. Scalping of four aroma compounds by one common (LDPE) and one biosourced (PLA) packaging materials during high pressure treatments. J. Food Eng. 2011, 102, 9–15. [Google Scholar] [CrossRef]
- Galotto, M.J.; Ulloa, P.A.; Hernández, D.; Fernández-Martín, F.; Gavara, R.; Guarda, A. Mechanical and thermal behaviour of flexible food packaging polymeric films materials under high pressure/temperature treatments. Packag. Technol. Sci. 2008, 21, 297–308. [Google Scholar] [CrossRef]
- Cubeddu, A.; Fava, P.; Pulvirenti, A.; Haghighi, H.; Licciardello, F. Suitability assessment of PLA bottles for high-pressure processing of apple juice. Foods 2021, 10, 295. [Google Scholar] [CrossRef] [PubMed]
- Benetto, E.; Jury, C.; Igos, E.; Carton, J.; Hild, P.; Vergne, C.; Di Martino, J. Using atmospheric plasma to design multilayer film from polylactic acid and thermoplastic starch: A screening Life Cycle Assessment. J. Clean. Prod. 2015, 87, 953–960. [Google Scholar] [CrossRef]
- Heidemann, H.M.; Dotto, M.E.R.; Laurindo, J.B.; Carciofi, B.A.M.; Costa, C. Cold plasma treatment to improve the adhesion of cassava starch films onto PCL and PLA surface. Colloids Surf. Physicochem. Eng. Aspects 2019, 580, 123739. [Google Scholar] [CrossRef]
- Chen, G.; Ali, F.; Dong, S.; Yin, Z.; Li, S.; Chen, Y. Preparation, characterization and functional evaluation of chitosan-based films with zein coatings produced by cold plasma. Carbohydr. Polym. 2018, 202, 39–46. [Google Scholar] [CrossRef]
- Hu, S.; Li, P.; Wei, Z.; Wang, J.; Wang, H.; Wang, Z. Antimicrobial activity of nisin-coated polylactic acid film facilitated by cold plasma treatment. J. Appl. Polym. Sci. 2018, 135, 46844. [Google Scholar] [CrossRef]
- Grewell, D.; Srinivasan, G.; Cochran, E. Depolymerization of post-consumer polylactic acid products. J. Renew. Mater. 2014, 2, 157–165. [Google Scholar] [CrossRef] [Green Version]
- Zhang, R.; Lan, W.; Ji, T.; Sameen, D.E.; Ahmed, S.; Qin, W.; Liu, Y. Development of polylactic acid/ZnO composite membranes prepared by ultrasonication and electrospinning for food packaging. LWT 2021, 135, 110072. [Google Scholar] [CrossRef]
- Jin, T.Z.; Guo, M.; Yang, R. Combination of pulsed electric field processing and antimicrobial bottle for extending microbiological shelf-life of pomegranate juice. Innov. Food Sci. Emerg. Technol. 2014, 26, 153–158. [Google Scholar] [CrossRef]
- Madera-Santana, T.J.; Meléndrez, R.; González-García, G.; Quintana-Owen, P.; Pillai, S.D. Effect of gamma irradiation on physicochemical properties of commercial poly(lactic acid) clamshell for food packaging. Radiat. Phys. Chem. 2016, 123, 6–13. [Google Scholar] [CrossRef]
- Salvatore, M.; Marra, A.; Duraccio, D.; Shayanfar, S.; Pillai, S.D.; Cimmino, S.; Silvestre, C. Effect of electron beam irradiation on the properties of polylactic acid/montmorillonite nanocomposites for food packaging applications. J. Appl. Polym. Sci. 2016, 133, 42219. [Google Scholar] [CrossRef]
- Zivanovic, S. Electron beam processing to improve the functionality of biodegradable food packaging. In Electron Beam Pasteurization and Complementary Food Processing Technologies; Elsevier: Amsterdam, The Netherlands, 2015; pp. 279–294. [Google Scholar]
- Cairns, M.-L.; Sykes, A.; Dickson, G.R.; Orr, J.F.; Farrar, D.; Dumba, A.; Buchanan, F.J. Through-thickness control of polymer bioresorption via electron beam irradiation. Acta Biomater. 2011, 7, 548–557. [Google Scholar] [CrossRef]
- Huang, Y.; Gohs, U.; Müller, M.T.; Zschech, C.; Wiessner, S. Electron beam treatment of polylactide at elevated temperature in nitrogen atmosphere. Radiat. Phys. Chem. 2019, 159, 166–173. [Google Scholar] [CrossRef]
- Krul’, L.P.; Butovskaya, G.V.; Fedorenko, A.A.; Roginets, L.P.; Sal’nikova, I.A. Gamma- and electron beam radiation-induced degradation of poly-L-lactide. High Energ. Chem. 2020, 54, 136–141. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, Q.; Wang, B.; Wang, Y.; Liu, C.; Shen, C. Effect of electron beam irradiation dose on the properties of commercial biodegradable poly(lactic acid), poly(butylenes adipate-co-terephthalate) and their blends. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2020, 478, 131–136. [Google Scholar] [CrossRef]
- Mansouri, M.; Berrayah, A.; Beyens, C.; Rosenauer, C.; Jama, C.; Maschke, U. Effects of electron beam irradiation on thermal and mechanical properties of poly(lactic acid) films. Polym. Degrad. Stab. 2016, 133, 293–302. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, Y.; Chen, Y. Modified Brittle poly(lactic acid) by biodegradable hyperbranched poly(ester amide). Iran. Polym. J. 2008, 17, 891–898. [Google Scholar]
- Tábi, T. The application of the synergistic effect between the crystal structure of poly(lactic acid) (PLA) and the presence of ethylene vinyl acetate copolymer (EVA) to produce highly ductile PLA/EVA blends. J. Therm. Anal. Calorim. 2019, 138, 1287–1297. [Google Scholar] [CrossRef] [Green Version]
- Luchian-Lupu, A.-M.; Zaharescu, T.; Lungulescu, E.-M.; Râpă, M.; Iovu, H. Availability of PLA/SIS blends for packaging and medical applications. Radiat. Phys. Chem. 2020, 172, 108696. [Google Scholar] [CrossRef]
- Jian, J.; Xiangbin, Z.; Xianbo, H. An overview on synthesis, properties and applications of poly(butylene-adipate-co-terephthalate)–PBAT. Adv. Ind. Eng. Polym. Res. 2020, 3, 19–26. [Google Scholar] [CrossRef]
- Hwang, I.-T.; Jung, C.-H.; Kuk, I.-S.; Choi, J.-H.; Nho, Y.-C. Electron beam-induced crosslinking of poly(butylene adipate-co-terephthalate). Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2010, 268, 3386–3389. [Google Scholar] [CrossRef]
- Iuliano, A.; Nowacka, M.; Rybak, K.; Rzepna, M. The effects of electron beam radiation on material properties and degradation of commercial PBAT/PLA blend. J. Appl. Polym. Sci. 2019, 137, 48462. [Google Scholar] [CrossRef]
- Samantaray, P.K.; Ellingford, C.; Farris, S.; O’Sullivan, D.; Tan, B.; Sun, Z.; McNally, T.; Wan, C. Electron beam-mediated cross-linking of blown film-extruded biodegradable PGA/PBAT blends toward high toughness and low oxygen permeation. ACS Sustain. Chem. Eng. 2022, 10, 1267–1276. [Google Scholar] [CrossRef]
- Falcão, G.A.M.; Almeida, T.G.; Bardi, M.A.G.; Carvalho, L.H.; Canedo, E.L. PBAT/organoclay composite films—part 2: Effect of UV aging on permeability, mechanical properties and biodegradation. Polym. Bull. 2018, 76, 291–301. [Google Scholar] [CrossRef]
- Xie, J.; Yan, Y.; Fan, S.; Min, X.; Wang, L.; You, X.; Jia, X.; Waterhouse, G.I.N.; Wang, J.; Xu, J. Prediction model of photodegradation for PBAT/PLA mulch films: Strategy to fast evaluate service life. Environ. Sci. Technol. 2022, 56, 9041–9051. [Google Scholar] [CrossRef]
- Kim, H.-J.; Lee, C.-L.; Yoon, K.-S.; Rhim, J.-W. Synergistic effect of UV-C LED irradiation and PLA/PBAT-based antimicrobial packaging film on fresh-cut vegetables. Food Control 2022, 138, 109027. [Google Scholar] [CrossRef]
- Kirsh, I.; Frolova, Y.; Beznaeva, O.; Bannikova, O.; Gubanova, M.; Tveritnikova, I.; Romanova, V.; Filinskaya, Y. Influence of the ultrasonic treatment on the properties of polybutylene adipate terephthalate, modified by antimicrobial additive. Polymers 2020, 12, 2412. [Google Scholar] [CrossRef]
- Iuliano, A.; Fabiszewska, A.; Kozik, K.; Rzepna, M.; Ostrowska, J.; Dębowski, M.; Plichta, A. Effect of electron-beam radiation and other sterilization techniques on structural, mechanical and microbiological properties of thermoplastic starch blend. J. Polym. Environ. 2020, 29, 1489–1504. [Google Scholar] [CrossRef]
- Fazeli, M.; Florez, J.P.; Simão, R.A. Improvement in adhesion of cellulose fibers to the thermoplastic starch matrix by plasma treatment modification. Compos. Part B Eng. 2019, 163, 207–216. [Google Scholar] [CrossRef]
- de Farias, J.G.G.; Cavalcante, R.C.; Canabarro, B.R.; Viana, H.M.; Scholz, S.; Simão, R.A. Surface lignin removal on coir fibers by plasma treatment for improved adhesion in thermoplastic starch composites. Carbohydr. Polym. 2017, 165, 429–436. [Google Scholar] [CrossRef]
- Florez, J.P.; Fazeli, M.; Simão, R.A. Preparation and characterization of thermoplastic starch composite reinforced by plasma-treated poly (hydroxybutyrate) PHB. Int. J. Biol. Macromol. 2019, 123, 609–621. [Google Scholar] [CrossRef]
- Silvestre, C.; Cimmino, S.; Stoleru, E.; Vasile, C. Application of radiation technology to food packaging. In Applications of Ionizing Radiation in Materials Processing; Sun, Y., Chmielewski, A.G., Eds.; Institute of Nuclear Chemistry and Technology: Warszawa, Poland, 2017; pp. 461–484. [Google Scholar]
- Chutamas, M.; Jackapon, S.; Sriroth, K.R. Evaluation of gamma radiation on NR/PHBV blends. Appl. Mech. Mater. 2013, 300–301, 1325–1329. [Google Scholar] [CrossRef]
- Zembouai, I.; Kaci, M.; Bruzaud, S.; Pillin, I.; Audic, J.-L.; Shayanfar, S.; Pillai, S.D. Electron beam radiation effects on properties and ecotoxicity of PHBV/PLA blends in presence of organo-modified montmorillonite. Polym. Degrad. Stab. 2016, 132, 117–126. [Google Scholar] [CrossRef]
- Gonçalves, S.M.; Chávez, D.W.H.; Oliveira, L.M.d.; Sarantópoulos, C.I.G.d.L.; Carvalho, C.W.P.d.; Melo, N.R.d.; Rosenthal, A. Effects of high hydrostatic pressure processing on structure and functional properties of biodegradable film. Heliyon 2020, 6, e05213. [Google Scholar] [CrossRef] [PubMed]
- El-Ashhab, F.; Sheha, L.; Abdalkhalek, M.; Khalaf, H.A. The influence of gamma irradiation on the intrinsic properties of cellulose acetate polymers. J. Assoc. Arab. Univ. Basic Appl. Sci. 2013, 14, 46–50. [Google Scholar] [CrossRef] [Green Version]
- El-Rehim, H.A.; Kamal, H.; Hegazy, E.-S.A.; Soliman, E.-S.; Sayed, A. Use of gamma rays to improve the mechanical and barrier properties of biodegradable cellulose acetate nanocomposite films. Radiat. Phys. Chem. 2018, 153, 180–187. [Google Scholar] [CrossRef]
- Hossain, M.A.; Shourove, J.H. Prospects and probabilities of irradiated cellulose and carrageenan in food and agricultural industries. In Radiation-Processed Polysaccharides: Emerging Roles in Agriculture; Elseavier: Amsterdam, The Netherlands, 2021; pp. 155–1771. [Google Scholar]
- Ortiz, R.; Basnett, P.; Roy, I.; Quintana, I. Picosecond Laser Ablation of Polyhydroxyalkanoates (PHAs): Comparative Study of Neat and Blended Material Response. Polymers 2020, 12, 127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dash, K.K.; Deka, P.; Bangar, S.P.; Chaudhary, V.; Trif, M.; Rusu, A. Applications of inorganic nanoparticles in food packaging: A comprehensive review. Polymers 2022, 14, 521. [Google Scholar] [CrossRef]
- Pirozzi, A.; Pataro, G.; Donsi, F.; Ferrari, G. Edible Coating and Pulsed Light to Increase the Shelf Life of Food Products. Food Eng. Rev. 2021, 13, 544–569. [Google Scholar] [CrossRef]
- Maherani, B.; Khlifi, M.A.; Salmieri, S.; Lacroix, M. Microemulsion-based biopreservatives and γ-irradiation as combined treatments to provide healthy and safe orange juice. J. Food Process. Preserv. 2019, 43, 13909. [Google Scholar] [CrossRef]
- Ben-Fadhel, Y.; Saltaji, S.; Khlifi, M.A.; Salmieri, S.; Dang Vu, K.; Lacroix, M. Active edible coating and gamma-irradiation as cold combined treatments to assure the safety of broccoli florets (Brassica oleracea L.). Int. J. Food Microbiol. 2017, 241, 30–38. [Google Scholar] [CrossRef]
- Ben-Fadhel, Y.; Cingolani, M.C.; Li, L.; Chazot, G.; Salmieri, S.; Horak, C.; Lacroix, M. Effect of γ-irradiation and the use of combined treatments with edible bioactive coating on carrot preservation. Food Packag. Shelf Life 2021, 28, 100635. [Google Scholar] [CrossRef]
- Morehouse, K.M.; Komolprasert, V. Irradiation of food and packaging: An overview. In Irradiation of Food and Packaging; ACS Symposium Series; ACS Publications: Washington, DC, USA, 2004; pp. 1–11. [Google Scholar]
- Caillet, S.; Millette, M.; Turgis, M.; Salmieri, S.; Lacroix, M. Influence of antimicrobial compounds and modified atmosphere packaging on radiation sensitivity of Listeria monocytogenes present in ready-to-use carrots (Daucus carota). J. Food Prot. 2006, 69, 221–227. [Google Scholar] [CrossRef] [PubMed]
- El-Dein, A.E.; Khozemy, E.E.; Farag, S.A.; Abd El-Hamed, N.; Dosoukey, I.M. Effect of edible co-polymers coatings using γ-irradiation on Hyani date fruit behavior during marketing. Int. J. Biol. Macromol. 2018, 117, 851–857. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Yu, Y.; Xiao, C.; Wang, X.; Lei, Y. Effect of ultraviolet irradiation combined with chitosan coating on preservation of jujube under ambient temperature. LWT-Food Sci. Technol. 2014, 57, 749–754. [Google Scholar] [CrossRef]
- Vu, K.D.; Hollingsworth, R.G.; Salmieri, S.; Takala, P.N.; Lacroix, M. Development of bioactive coatings based on γ-irradiated proteins to preserve strawberries. Radiat. Phys. Chem. 2012, 81, 1211–1214. [Google Scholar] [CrossRef]
- Zúñiga, G.E.; Junqueira-Gonçalves, M.P.; Pizarro, M.; Contreras, R.; Tapia, A.; Silva, S. Effect of ionizing energy on extracts of Quillaja saponaria to be used as an antimicrobial agent on irradiated edible coating for fresh strawberries. Radiat. Phys. Chem. 2012, 81, 64–69. [Google Scholar] [CrossRef]
- Hussain, P.R.; Dar, M.A.; Wani, A.M. Effect of edible coating and gamma irradiation on inhibition of mould growth and quality retention of strawberry during refrigerated storage. Int. J. Food Sci. Technol. 2012, 47, 2318–2324. [Google Scholar] [CrossRef]
- Senna, M.M.H.; Al-Shamrani, K.M.; Al-Arifi, A.S. Edible coating for shelf-life extension of fresh banana fruit based on gamma irradiated plasticized poly(vinyl alcohol)/carboxymethyl cellulose/tannin composites. Mater. Sci. Appl. 2014, 5, 395–415. [Google Scholar] [CrossRef] [Green Version]
- Shankar, S.; Karboune, S.; Salmieri, S.; Lacroix, M. Development of antimicrobial formulation based on essential oils and gamma irradiation to increase the shelf life of boneless chicken thighs. Radiat. Phys. Chem. 2022, 192, 109893. [Google Scholar] [CrossRef]
- Moreira, M.R.; Tomadoni, B.; Martín-Belloso, O.; Soliva-Fortuny, R. Preservation of fresh-cut apple quality attributes by pulsed light in combination with gellan gum-based prebiotic edible coatings. LWT-Food Sci. Technol. 2015, 64, 1130–1137. [Google Scholar] [CrossRef] [Green Version]
- Moreira, M.R.; Álvarez, M.V.; Martín-Belloso, O.; Soliva-Fortuny, R. Effects of pulsed light treatments and pectin edible coatings on the quality of fresh-cut apples: A hurdle technology approach. J. Sci. Food Agric. 2017, 97, 261–268. [Google Scholar] [CrossRef] [Green Version]
- Salinas-Roca, B.; Soliva-Fortuny, R.; Welti-Chanes, J.; Martín-Belloso, O. Effect of pulsed light, edible coating, and dipping on the phenolic profile and antioxidant potential of fresh-cut mango. J. Food Process. Preserv. 2018, 42, 13591. [Google Scholar] [CrossRef]
- Koh, P.C.; Noranizan, M.A.; Nur Hanani, Z.A.; Karim, R.; Rosli, S.Z. Application of edible coatings and repetitive pulsed light for shelf life extension of fresh-cut cantaloupe (Cucumis melo L. reticulatus cv. Glamour). Postharvest Biol. Technol. 2017, 129, 64–78. [Google Scholar] [CrossRef]
- Koh, P.C.; Noranizan, M.A.; Karim, R.; Nur Hanani, Z.A.; Rosli, S.Z.; Hambali, N.H. Enzymatic activity of alginate coated and pulsed light treated fresh-cut cantaloupes (Cucumis melo L. var. reticulatus cv. Glamour) during chilled storage. Int. Food Res. J. 2019, 26, 547–556. [Google Scholar]
- Koh, P.C.; Noranizan, M.A.; Karim, R.; Nur Hanani, Z.A.; Lasik-Kurdyś, M. Combination of alginate coating and repetitive pulsed light for shelf life extension of fresh-cut cantaloupe (Cucumis melo L. reticulatus cv. Glamour). J. Food Process. Preserv. 2018, 42, 13786. [Google Scholar] [CrossRef]
- Donsì, F.; Marchese, E.; Maresca, P.; Pataro, G.; Vu, K.D.; Salmieri, S.; Lacroix, M.; Ferrari, G. Green beans preservation by combination of a modified chitosan based-coating containing nanoemulsion of mandarin essential oil with high pressure or pulsed light processing. Postharvest Biol. Technol. 2015, 106, 21–32. [Google Scholar] [CrossRef]
- Wang, B.; Wei, W.; Aputexiakere, J.; Li, Y.; Ma, H. Surface decontamination of whole eggs using pulsed light technology and shelf life study of combined pulsed light and vaseline coating during room temperature storage. Food Control 2022, 137, 108411. [Google Scholar] [CrossRef]
- Pankaj, S.K.; Bueno-Ferrer, C.; Misra, N.N.; O’Neill, L.; Tiwari, B.K.; Bourke, P.; Cullen, P.J. Physicochemical characterization of plasma-treated sodium caseinate film. Food Res. Int. 2014, 66, 438–444. [Google Scholar] [CrossRef]
- Fan, J.-M.; Ma, W.; Liu, G.-Q.; Yin, S.-W.; Tang, C.-H.; Yang, X.-Q. Preparation and characterization of kidney bean protein isolate (KPI)–chitosan (CH) composite films prepared by ultrasonic pretreatment. Food Hydrocoll. 2014, 36, 60–69. [Google Scholar] [CrossRef]
- Shahbazi, M.; Majzoobi, M.; Farahnaky, A. Physical modification of starch by high-pressure homogenization for improving functional properties of κ-carrageenan/starch blend film. Food Hydrocoll. 2018, 85, 204–214. [Google Scholar] [CrossRef]
- Molinaro, S.; Cruz-Romero, M.; Sensidoni, A.; Morris, M.; Lagazio, C.; Kerry, J.P. Combination of high-pressure treatment, mild heating and holding time effects as a means of improving the barrier properties of gelatin-based packaging films using response surface modeling. Innov. Food Sci. Emerg. Technol. 2015, 30, 15–23. [Google Scholar] [CrossRef]
- Benbettaïeb, N.; Chambin, O.; Karbowiak, T.; Debeaufort, F. Release behavior of quercetin from chitosan-fish gelatin edible films influenced by electron beam irradiation. Food Control 2016, 66, 315–319. [Google Scholar] [CrossRef]
- Sifuentes-Nieves, I.; Neira-Velázquez, G.; Hernández-Hernández, E.; Barriga-Castro, E.; Gallardo-Vega, C.; Velazquez, G.; Mendez-Montealvo, G. Influence of gelatinization process and HMDSO plasma treatment on the chemical changes and water vapor permeability of corn starch films. Int. J. Biol. Macromol. 2019, 135, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Sifuentes-Nieves, I.; Hernández-Hernández, E.; Neira-Velázquez, G.; Morales-Sánchez, E.; Mendez-Montealvo, G.; Velazquez, G. Hexamethyldisiloxane cold plasma treatment and amylose content determine the structural, barrier and mechanical properties of starch-based films. Int. J. Biol. Macromol. 2019, 124, 651–658. [Google Scholar] [CrossRef]
- Marcuzzo, E.; Peressini, D.; Debeaufort, F.; Sensidoni, A. Effect of ultrasound treatment on properties of gluten-based film. Innov. Food Sci. Emerg. Technol. 2010, 11, 451–457. [Google Scholar] [CrossRef]
- Sorrentino, A.; Gorrasi, G.; Vittoria, V. Potential perspectives of bio-nanocomposites for food packaging applications. Trends Food Sci. Technol. 2007, 18, 84–95. [Google Scholar] [CrossRef]
- Yadav, S.; Mehrotra, G.K.; Dutta, P.K. Chitosan based ZnO nanoparticles loaded gallic-acid films for active food packaging. Food Chem. 2021, 334, 127605. [Google Scholar] [CrossRef] [PubMed]
- Duncan, T.V. Applications of nanotechnology in food packaging and food safety: Barrier materials, antimicrobials and sensors. J. Colloid Interface Sci. 2011, 363, 1–24. [Google Scholar] [CrossRef]
- Pinto, R.J.B.; Daina, S.; Sadocco, P.; Neto, C.P.; Trindade, T. Antibacterial activity of nanocomposites of copper and cellulose. BioMed Res. Int. 2013, 2013, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Mihindukulasuriya, S.D.F.; Lim, L.T. Nanotechnology development in food packaging: A review. Trends Food Sci. Technol. 2014, 40, 149–167. [Google Scholar] [CrossRef]
- Ghanbarzadeh, B.; Oleyaei, S.A.; Almasi, H. Nanostructured materials utilized in biopolymer-based plastics for food packaging applications. Crit. Rev. Food Sci. Nutr. 2014, 55, 1699–1723. [Google Scholar] [CrossRef]
- Singh, T.; Shukla, S.; Kumar, P.; Wahla, V.; Bajpai, V.K.; Rather, I.A. Application of nanotechnology in food science: Perception and overview. Front. Microbiol. 2017, 8, 1501. [Google Scholar] [CrossRef] [Green Version]
- Aswathanarayan, J.B.; Vittal, R.R. Nanoemulsions and their potential applications in food industry. Front. Sustain. Food Syst. 2019, 3, 95. [Google Scholar] [CrossRef] [Green Version]
- Sothornvit, R. Nanostructured materials for food packaging systems: New functional properties. Curr. Opin. Food Sci. 2019, 25, 82–87. [Google Scholar] [CrossRef]
- Wilson, M.D.; Stanley, R.A.; Eyles, A.; Ross, T. Innovative processes and technologies for modified atmosphere packaging of fresh and fresh-cut fruits and vegetables. Crit. Rev. Food Sci. Nutr. 2019, 59, 411–422. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, F.; Andreescu, S. Nanotechnology-based approaches for food sensing and packaging applications. RSC Adv. 2020, 10, 19309–19336. [Google Scholar] [CrossRef]
- Velásquez, E.; Patiño Vidal, C.; Rojas, A.; Guarda, A.; Galotto, M.J.; López de Dicastillo, C. Natural antimicrobials and antioxidants added to polylactic acid packaging films. Part I: Polymer processing techniques. Compr. Rev. Food Sci. Food Saf. 2021, 20, 3388–3403. [Google Scholar] [CrossRef]
- Othman, S.H.; Abd Salam, N.R.; Zainal, N.; Kadir Basha, R.; Talib, R.A. Antimicrobial activity of TiO2 nanoparticle-coated film for potential food packaging applications. Int. J. Photoenergy 2014, 2014, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Rhim, J.-W.; Ng, P.K.W. Natural biopolymer-based nanocomposite films for packaging applications. Crit. Rev. Food Sci. Nutr. 2007, 47, 411–433. [Google Scholar] [CrossRef]
- Yusof, N.L.; Mutalib, N.-A.A.; Nazatul, U.K.; Nadrah, A.H.; Aziman, N.; Fouad, H.; Jawaid, M.; Ali, A.; Kian, L.K.; Sain, M. Efficacy of biopolymer/starch based antimicrobial packaging for chicken breast fillets. Foods 2021, 10, 2379. [Google Scholar] [CrossRef]
- Vlasveld, D.P.N.; Vaidya, S.G.; Bersee, H.E.N.; Picken, S.J. A comparison of the temperature dependence of the modulus, yield stress and ductility of nanocomposites based on high and low MW PA6 and PA66. Polymer 2005, 46, 3452–3461. [Google Scholar] [CrossRef]
- Halim, L.; Pascall, M.A.; Lee, J.; Finnigan, B. Effect of pasteurization, high-pressure processing, and retorting on the barrier properties of nylon 6, nylon 6/ethylene vinyl alcohol, and nylon 6/nanocomposites films. J. Food Sci. 2009, 74, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Yang, X.; Abdel-Samie, M.A.; Lin, L. Cold plasma treated phlorotannin/Momordica charantia polysaccharide nanofiber for active food packaging. Carbohydr. Polym. 2020, 239, 116214. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Liao, X.; Cui, H. Cold plasma treated thyme essential oil/silk fibroin nanofibers against Salmonella Typhimurium in poultry meat. Food Packag. Shelf Life 2019, 21, 100337. [Google Scholar] [CrossRef]
- Galotto, M.J.; Ulloa, P.; Escobar, R.; Guarda, A.; Gavara, R.; Miltz, J. Effect of high-pressure food processing on the mass transfer properties of selected packaging materials. Packag. Technol. Sci. 2010, 23, 253–266. [Google Scholar] [CrossRef]
- Pascall, M.A. Packaging for high-pressure processing, irradiation, and pulsed electric field. In Packaging for Nonthermal Processing of Food; John Wiley & Sons, Ltd.: Chichester, UK, 2018; pp. 95–120. [Google Scholar]
- Fan, C.; Cui, R.; Lu, W.; Chen, H.; Yuan, M.; Qin, Y. Effect of high pressure treatment on properties and nano–Ag migration of PLA-based food packaging film. Polym. Test. 2019, 76, 73–81. [Google Scholar] [CrossRef]
- Chi, H.; Xue, J.; Zhang, C.; Chen, H.; Li, L.; Qin, Y. High pressure treatment for improving water vapour barrier properties of poly(lactic acid)/Ag nanocomposite Films. Polymers 2018, 10, 1011. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, J. Use of graphene/graphene oxide in food packaging materials: Thermomechanical, structural and barrier properties. In Reference Module in Food Science; Elsevier: Amsterdam, The Netherlands, 2019; pp. 1–22. [Google Scholar]
- Lian, Z.; Zhang, Y.; Zhao, Y. Nano-TiO 2 particles and high hydrostatic pressure treatment for improving functionality of polyvinyl alcohol and chitosan composite films and nano-TiO 2 migration from film matrix in food simulants. Innov. Food Sci. Emerg. Technol. 2016, 33, 145–153. [Google Scholar] [CrossRef]
- Savadekar, N.R.; Karande, V.S.; Vigneshwaran, N.; Kadam, P.G.; Mhaske, S.T. Preparation of cotton linter nanowhiskers by high-pressure homogenization process and its application in thermoplastic starch. Appl. Nanosci. 2014, 5, 281–290. [Google Scholar] [CrossRef] [Green Version]
- Barra, G.; Guadagno, L.; Vertuccio, L.; Simonet, B.; Santos, B.; Zarrelli, M.; Arena, M.; Viscardi, M. Different methods of dispersing carbon nanotubes in epoxy resin and initial evaluation of the obtained nanocomposite as a matrix of carbon fiber reinforced laminate in terms of vibroacoustic performance and flammability. Materials 2019, 12, 2998. [Google Scholar] [CrossRef] [Green Version]
- Barra, A.; Santos, J.D.C.; Silva, M.R.F.; Nunes, C.; Ruiz-Hitzky, E.; Gonçalves, I.; Yildirim, S.; Ferreira, P.; Marques, P.A.A.P. Graphene derivatives in biopolymer-based composites for food packaging applications. Nanomaterials 2020, 10, 2077. [Google Scholar] [CrossRef]
- Jäger, H.; Knorr, D. Pulsed electric fields treatment in food technology: Challenges and opportunities. In Handbook of Electroporation; Springer: Berlin/Heidelberg, Germany, 2017; pp. 1–24. [Google Scholar]
- Novickij, V.; Stanevičienė, R.; Staigvila, G.; Gruškienė, R.; Sereikaitė, J.; Girkontaitė, I.; Novickij, J.; Servienė, E. Effects of pulsed electric fields and mild thermal treatment on antimicrobial efficacy of nisin-loaded pectin nanoparticles for food preservation. LWT 2020, 120, 108915. [Google Scholar] [CrossRef]
- Mergen, Ö.B.; Arda, E.; Evingür, G.A. Electrical, optical and mechanical properties of chitosan biocomposites. J. Compos. Mater. 2019, 54, 1497–1510. [Google Scholar] [CrossRef]
- Alakrach, A.M.; Zulkepli, N.N.; Al-Rashdi, A.A.; Ting, S.S.; Hamzah, R.; Dahham, O.S. Modification of polylactic acid/ halloysite bionanocomposites using electron beam radiation: Physical, barrier and thermal properties. Mater. Sci. Forum 2020, 1002, 57–65. [Google Scholar] [CrossRef]
- Moura, E.A.B. The potential of micro- and nano-sized fillers extracted from agroindustry residues as reinforcements of thermoplastic-based biocomposites—A Review. In Green Materials Engineering; The Minerals, Metals & Materials Series; Springer: Berlin/Heidelberg, Germany, 2019; pp. 89–100. [Google Scholar]
- Cardoso, R.V.C.; Carocho, M.; Fernandes, Â.; Barreira, J.C.M.; Cabo Verde, S.; Santos, P.M.P.; Antonio, A.L.; Gonzaléz-Paramás, A.M.; Barros, L.; Ferreira, I.C.F.R. Combined effects of irradiation and storage time on the nutritional and chemical parameters of dried Agaricus bisporus Portobello mushroom flour. J. Food Sci. 2021, 86, 2276–2287. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, N.; Wang, H.Y.; Sui, S.Y.; Sun, X.X.; Ma, Z.S. The effects of ultrasonic/microwave assisted treatment on the properties of soy protein isolate/titanium dioxide films. LWT-Food Sci. Technol. 2014, 57, 548–555. [Google Scholar] [CrossRef]
- da Silva, N.M.C.; Correia, P.R.C.; Druzian, J.I.; Fakhouri, F.M.; Fialho, R.L.L.; de Albuquerque, E.C.M.C. PBAT/THERMOPLASTIC STARCH Composite Films Reinforced with Starch Nanoparticles Produced by Ultrasound. Int. J. Polym. Sci. 2017, 2017, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Targino de Souza Pedrosa, G.; Pimentel, T.C.; Gavahian, M.; Lucena de Medeiros, L.; Pagán, R.; Magnani, M. The combined effect of essential oils and emerging technologies on food safety and quality. LWT-Food Sci. Technol. 2021, 147, 111593. [Google Scholar] [CrossRef]
- Keenan, D.F.; Rößle, C.; Gormley, R.; Butler, F.; Brunton, N.P. Effect of high hydrostatic pressure and thermal processing on the nutritional quality and enzyme activity of fruit smoothies. LWT-Food Sci. Technol. 2012, 45, 50–57. [Google Scholar] [CrossRef]
- Bolumar, T.; Andersen, M.L.; Orlien, V. Antioxidant active packaging for chicken meat processed by high pressure treatment. Food Chem. 2011, 129, 1406–1412. [Google Scholar] [CrossRef]
- Martillanes, S.; Rocha-Pimienta, J.; Ramírez, R.; García-Parra, J.; Delgado-Adámez, J. Effect of an active packaging with rice bran extract and high-pressure processing on the preservation of sliced dry-cured ham from Iberian pigs. LWT-Food Sci. Technol. 2021, 151, 112128. [Google Scholar] [CrossRef]
- Martillanes, S.; Rocha-Pimienta, J.; Llera-Oyola, J.; Gil, M.V.; Ayuso-Yuste, M.C.; García-Parra, J.; Delgado-Adámez, J. Control of Listeria monocytogenes in sliced dry-cured Iberian ham by high pressure processing in combination with an eco-friendly packaging based on chitosan, nisin and phytochemicals from rice bran. Food Control 2021, 124, 107933. [Google Scholar] [CrossRef]
- Amaro-Blanco, G.; Delgado-Adámez, J.; Martín, M.J.; Ramírez, R. Active packaging using an olive leaf extract and high pressure processing for the preservation of sliced dry-cured shoulders from Iberian pigs. Innov. Food Sci. Emerg. Technol. 2018, 45, 1–9. [Google Scholar] [CrossRef]
- Pavli, F.; Argyri, A.A.; Skandamis, P.; Nychas, G.-J.; Tassou, C.; Chorianopoulos, N. Antimicrobial activity of oregano essential oil incorporated in sodium alginate edible films: Control of Listeria monocytogenes and spoilage in ham slices treated with high pressure processing. Materials 2019, 12, 3726. [Google Scholar] [CrossRef] [PubMed]
- Benbettaïeb, N.; Assifaoui, A.; Karbowiak, T.; Debeaufort, F.; Chambin, O. Controlled release of tyrosol and ferulic acid encapsulated in chitosan–gelatin films after electron beam irradiation. Radiat. Phys. Chem. 2016, 118, 81–86. [Google Scholar] [CrossRef]
- Shankar, S.; Danneels, F.; Lacroix, M. Coating with alginate containing a mixture of essential oils and citrus extract in combination with ozonation or gamma irradiation increased the shelf life of Merluccius sp. fillets. Food Packag. Shelf Life 2019, 22, 100434. [Google Scholar] [CrossRef]
- Fallah, A.A.; Sarmast, E.; Habibian Dehkordi, S.; Isvand, A.; Dini, H.; Jafari, T.; Soleimani, M.; Mousavi Khaneghah, A. Low-dose gamma irradiation and pectin biodegradable nanocomposite coating containing curcumin nanoparticles and ajowan (Carum copticum) essential oil nanoemulsion for storage of chilled lamb loins. Meat Sci. 2022, 184, 108700. [Google Scholar] [CrossRef]
- Shankar, S.; Khodaei, D.; Lacroix, M. Effect of chitosan/essential oils/silver nanoparticles composite films packaging and gamma irradiation on shelf life of strawberries. Food Hydrocoll. 2021, 117, 106750. [Google Scholar] [CrossRef]
- Dini, H.; Fallah, A.A.; Bonyadian, M.; Abbasvali, M.; Soleimani, M. Effect of edible composite film based on chitosan and cumin essential oil-loaded nanoemulsion combined with low-dose gamma irradiation on microbiological safety and quality of beef loins during refrigerated storage. Int. J. Biol. Macromol. 2020, 164, 1501–1509. [Google Scholar] [CrossRef]
- Bertuzzi, M.; Slavutsky, A. Standard and new processing techniques used in the preparation of films and coatings at the lab level and scale-up. In Edible Films and Coatings; CRC Press: Boca Raton, FL, USA, 2016; pp. 24–46. [Google Scholar]
- Almasi, H.; Jahanbakhsh Oskouie, M.; Saleh, A. A review on techniques utilized for design of controlled release food active packaging. Crit. Rev. Food Sci. Nutr. 2020, 61, 2601–2621. [Google Scholar] [CrossRef]
- Benbettaïeb, N.; Chambin, O.; Assifaoui, A.; Al-Assaf, S.; Karbowiak, T.; Debeaufort, F. Release of coumarin incorporated into chitosan-gelatin irradiated films. Food Hydrocoll. 2016, 56, 266–276. [Google Scholar] [CrossRef]
- Savolainen, K.; Pylkkänen, L.; Norppa, H.; Falck, G.; Lindberg, H.; Tuomi, T.; Vippola, M.; Alenius, H.; Hämeri, K.; Koivisto, J.; et al. Nanotechnologies, engineered nanomaterials and occupational health and safety – A review. Saf. Sci. 2010, 48, 957–963. [Google Scholar] [CrossRef]
- Marangoni Júnior, L.; Cristianini, M.; Padula, M.; Anjos, C.A.R. Effect of high-pressure processing on characteristics of flexible packaging for foods and beverages. Food Res. Int. 2019, 119, 920–930. [Google Scholar] [CrossRef] [PubMed]
- Lajarrige, A.; Gontard, N.; Gaucel, S.; Peyron, S. Evaluation of the food contact suitability of aged bio-nanocomposite materials dedicated to food packaging applications. Appl. Sci. 2020, 10, 877. [Google Scholar] [CrossRef]
- Lauriano Souza, V.G.; Rodrigues, P.F.; Duarte, M.P.; Fernando, A.L. Antioxidant migration studies in chitosan films incorporated with plant extracts. J. Renew. Mater. 2018, 6, 548–558. [Google Scholar] [CrossRef]
- Vasile, C.; Pamfil, D.; Zaharescu, T.; Dumitriu, R.-P.; Pricope, G.M.; Râpă, M.; Vasilievici, G. Effect of gamma irradiation on the PLA-based blends and biocomposites containing rosemary ethanolic extract and chitosan. Polymers 2022, 14, 1398. [Google Scholar] [CrossRef]
- Scarfato, P.; Di Maio, L.; Incarnato, L. Recent advances and migration issues in biodegradable polymers from renewable sources for food packaging. J. Appl. Polym. Sci. 2015, 132, 42597. [Google Scholar] [CrossRef]
- Magnuson, B.A.; Jonaitis, T.S.; Card, J.W. A brief review of the occurrence, use, and safety of food-related nanomaterials. J. Food Sci. 2011, 76, 126–133. [Google Scholar] [CrossRef]
- Kuorwel, K.K.; Cran, M.J.; Orbell, J.D.; Buddhadasa, S.; Bigger, S.W. Review of mechanical properties, migration, and potential applications in active food packaging systems containing nanoclays and nanosilver. Compr. Rev. Food Sci. Food Saf. 2015, 14, 411–430. [Google Scholar] [CrossRef] [Green Version]
- Riquet, A.M.; Breysse, C.; Dahbi, L.; Loriot, C.; Severin, I.; Chagnon, M.C. The consequences of physical post-treatments (microwave and electron-beam) on food/packaging interactions: A physicochemical and toxicological approach. Food Chem. 2016, 199, 59–69. [Google Scholar] [CrossRef] [Green Version]
- Jain, A.; Ranjan, S.; Dasgupta, N.; Ramalingam, C. Nanomaterials in food and agriculture: An overview on their safety concerns and regulatory issues. Crit. Rev. Food Sci. Nutr. 2017, 58, 297–317. [Google Scholar] [CrossRef]
- Toussaint, B.; Raffael, B.; Angers-Loustau, A.; Gilliland, D.; Kestens, V.; Petrillo, M.; Rio-Echevarria, I.M.; Van den Eede, G. Review of micro- and nanoplastic contamination in the food chain. Food Addit. Contam. Part A 2019, 36, 639–673. [Google Scholar] [CrossRef] [PubMed]
- Alfei, S.; Marengo, B.; Zuccari, G. Nanotechnology application in food packaging: A plethora of opportunities versus pending risks assessment and public concerns. Food Res. Int. 2020, 137, 109664. [Google Scholar] [CrossRef] [PubMed]
- Parliament, C.t.t.E. Communication from the Commission to the European Parliament, the Council and the European Economic and Social Committee-Regulatory Aspects of Nanomaterials [SEC(2008) 2036]; COMMISSION OF THE EUROPEAN COMMUNITIES: Brussels, Belgium, 2008. [Google Scholar]
- Ma, L.; Zhang, M.; Bhandari, B.; Gao, Z. Recent developments in novel shelf life extension technologies of fresh-cut fruits and vegetables. Trends Food Sci. Technol. 2017, 64, 23–38. [Google Scholar] [CrossRef]
- Huang, Y.; Mei, L.; Chen, X.; Wang, Q. Recent Developments in Food Packaging Based on Nanomaterials. Nanomaterials 2018, 8, 830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guillard, V.; Mauricio-Iglesias, M.; Gontard, N. Effect of novel food processing methods on packaging: Structure, composition, and migration properties. Crit. Rev. Food Sci. Nutr. 2010, 50, 969–988. [Google Scholar] [CrossRef]
- Paidari, S.; Tahergorabi, R.; Anari, E.S.; Nafchi, A.M.; Zamindar, N.; Goli, M. Migration of various nanoparticles into food samples: A review. Foods 2021, 10, 2114. [Google Scholar] [CrossRef] [PubMed]
- Souza, V.G.L.; Fernando, A.L. Nanoparticles in food packaging: Biodegradability and potential migration to food—A review. Food Packag. Shelf Life 2016, 8, 63–70. [Google Scholar] [CrossRef]
- Echegoyen, Y.; Rodríguez, S.; Nerín, C. Nanoclay migration from food packaging materials. Food Addit. Contam. Part A 2016, 33, 530–539. [Google Scholar] [CrossRef]
- Kumar, P.; Han, J.H. Packaging materials for non-thermal processing of food and beverages. In Emerging Food Packaging Technologies; Elsevier: Amsterdam, The Netherlands, 2012; pp. 323–334. [Google Scholar]
- Ashfaq, M.; Hashmi, M.Z.; Mumtaz, A.; Javed, D.; Ain, N.U.; Shifaqat, S.; Rehman, M.S.U. Environmental risk assessment of antibiotics and AMR/ARGs. In Antibiotics and Antimicrobial Resistance Genes in the Environment; Elsevier: Amsterdam, The Netherlands, 2020; pp. 331–349. [Google Scholar]
- Rapisarda, M.; Patanè, C.; Pellegrino, A.; Malvuccio, A.; Rizzo, V.; Muratore, G.; Rizzarelli, P. Compostable polylactide and cellulose based packaging for fresh-cut cherry tomatoes: Performance evaluation and influence of sterilization treatment. Materials 2020, 13, 3432. [Google Scholar] [CrossRef]
- Mauricio-Iglesias, M.; Jansana, S.; Peyron, S.; Gontard, N.; Guillard, V. Effect of high-pressure/temperature (HP/T) treatments of in-package food on additive migration from conventional and bio-sourced materials. Food Addit. Contam. Part A 2010, 27, 118–127. [Google Scholar] [CrossRef] [Green Version]
- Zhu, B.; Fan, C.; Cheng, C.; Lan, T.; Li, L.; Qin, Y. Migration kinetic of silver from polylactic acid nanocomposite film into acidic food simulant after different high-pressure food processing. J. Food Sci. 2021, 86, 2481–2490. [Google Scholar] [CrossRef] [PubMed]

| Packaging Material | Treatment | Effect of Process on Packaging Mterial | Reference |
|---|---|---|---|
| κ-carrageenan/starch blend film | HPP 14 MPa (2–5 passes) and 20 MPa (2 passes) | Increased water resistance and WVP; increased surface hydrophobicity and tensile strength | [98] |
| Gelatin-based films | HPP (600 MPa), 30 min at 20.5 °C | Decrease in OTR; significant increase in TS and Tm; Significant decrease in WVTR | [99] |
| Calcium caseinate- whey protein isolate-glycerol film | γ-Irradiation of 32 kGy | Increased puncture strength; no detrimental effect on WVP | [83] |
| Chitosan-gelatin films +5 wt % quercetin | Electron beam irradiation of 60 kGy | Decreased the release rate of quercetin; 42% increase in TS; 65% decrease in O2P; improvement of thermal stability | [100] |
| Chitosan (1.5%) coating on fresh jujube fruit | UV-irradiation 253.7 nm; 4 and 6 min | Reduced decay of jujube fruit | [82] |
| Starch-based film | HMDSO cold plasma, 70 w, 30 min | Increased film crystallinity; improved WVP and mechanical properties of films | [101,102] |
| Chitosan-based + zein coatings | Cold plasma 100 W (65 V, 1.5 A), d = 5 mm, 30 s | Improved surface wettability; slower drug release rate within 24 h from 72.8% to 49.3% | [38] |
| Gluten-based film | Ultrasound 600 W/cm2, 24 Hz, 3–12 min | Enhanced protein dispersion and the appearance of film | [103] |
| κ-carrageenan/starch blend film | HPP 14 MPa (2–5 passes) and 20 MPa (2 passes) | Increased water resistance and WVP; Increased surface hydrophobicity and tensile strength | [98] |
| Gelatin-based films | HPP (600 MPa), 30 min at 20.5 °C | Decrease in OTR; Significant increase in TS and Tm; Significant decrease in WVTR | [99] |
| Calcium caseinate- whey protein isolate-glycerol film | γ-Irradiation of 32 kGy | Increased puncture strength; no detrimental effect on WVP | [83] |
| Chitosan-gelatin films +5 wt % quercetin | Electron beam irradiation of 60 kGy | Decreased the release rate of quercetin;42% increase in TS; 65% decrease in O2P; improvement of thermal stability | [100] |
| Packaging Material | Treatment | Effect of Process on Packaging Material | Reference |
|---|---|---|---|
| PA/LDPE PA/nano/LDPE PA/EVOH/LDPE | Pasteurization 75 °C, 30 min | OTR > 13.3%; WVTR > 96.7% OTR > 75.9%; WVTR > 40.7% OTR < 44.5%; WVTR > 43.8% | [120] |
| PA/LDPE PA/nano/LDPE PA/EVOH/LDPE | HPP 70 °C, 800 MPa, 10 min | OTR > 16.9%; WVTR > 21% OTR > 39.7 %; WVTR > 21.2% OTR < 53.9%; WVTR > 48.9% | |
| PA/PP PA/nano/PP | 121 °C, 30 min | OTR > 63.3% OTR > 112.6% | |
| High and low molecular weight (MW) PA6 and PA66 silica nanocomposites; Commercial nanocomposites | Temperatures from 20 to 120 °C | Yield stress increases with the addition of layered silicate; Low MW PA6 and PA66 nanocomposites show very brittle fracture behaviour at room temperature; High MW PA6 nanocomposites are ductile; Commercial nanocomposites are brittle; With temperature increase all the nanocomposites become ductile at a certain temperature | [119] |
| Bioactive coating: 3% N-palmitoyl chitosan + mandarin EOs nanoemulsion | HPP 200–400 MPa, 25 °C, 5 min; pulsed light 3 × 104– 1.2 × 105 J/m2 | HPP caused disintegration of the coating layer; pulsed light treatment did not affect samples firmness during storage, nor coating integrity | [94] |
| Thyme EOs/silk fibroin nanofibers | Cold plasma 400W, 4 min; N2 flow rate = 100 cm3/min | With silk fibroin increased, from 50% to 100%, moisture content increased from 11.87% to 15.77%; water solubility increased from 52.54% to 63.54%; WVP decreased from 1.58 to 0.77 g mm/m2 h kPa; TS decreased from 12.9 to 6.53 MPa; EAB increased from 17.06 to 21.39 | [122] |
| Phlorotannin (PT) encapsulated in Momordica charantia polysaccharide (MCP) nanofibers | cold plasma 30 s, 350 W, N2 flow rate = 100 cm3/min | Release efficiency of PT from the nanofibers was enhanced by 23.5% (4 °C) and 25% (25 °C); Antibacterial and anti-oxidant activities of PT/MCP nanofibers were markedly improved; moisture content and water solubility of the MCP nanofibers increased (from 4.28% and 10.42% to 8.91% and 18.94%, respectively); maximum TS was achieved when MCP:PT was 6:1; free radical scavenging capacity of PT/MCP increased to 91.74% | [121] |
| NTT | Conditions | Active Substance | Active Character | Food | Effect | Reference |
|---|---|---|---|---|---|---|
| HPP | 800 MPa, 10 min. at 5 °C | Rosemary extract 0.45 mg/cm2 on LDPE | AO | Chicken patties | HPP reduced the microbial growth and the rosemary suppressed the lipid oxidation | [142] |
| 600 MPa, 7 min, water at 10 °C | Rice bran extract on internal surface of vacuum package film | AM, AO | Dry-cured Iberian ham | HPP+AP does not improve activity of AP film | [143] | |
| 600 MPa, 8 min | Chitosan, nisin and phytochemicals from rice bran | AM | Sliced dry-cured Iberian ham | HPP+nisin or oryzanol chitosan based-films reduced the population of L. monocytogenes by 6 log CFU/g | [144] | |
| 600 MPa, 7 min | Olive leaf extract on internal surface of vacuum package film | AO, AM | Sliced dry-cured shoulders | AP not efficient to preserve the volatile compounds profile of the samples from the changes induced by HPP | [145] | |
| 500 MPa, 2 min at 20 °C | Oregano EOs +Na-alginate edible film | AM | Sliced ham | Reduction of Listeria counts below the detection limit | [146] | |
| EBI | 60 kGy | Ferulic acid and tyrosol incorporated into chitosan–gelatin edible films | AO | Food simulant (water) at 25 °C | Effective diffusivity of tyrosol was 40 times greater than that of ferulic acid. | [147] |
| 40 and 60 kGy | Quercetin incorporated into chitosan-gelatin edible film | AO | Ethanol 30% (v/v) at 25 °C | Irradiation induced a reduction of the quercetin release rate. Effective diffusion coefficient of quercetin was not significantly modified by the irradiation. | [100] | |
| OZ or γ irr | OZ = 10 ppm, 15 min; γ irr = 1 kGy | Alginate/EOs + citrus extract | AM | Merluccius sp. fillet | Increased shelf-life of fish fillets from 7 days (control) to 28 days for alginate/EOs/γ irr samples; and 21 days for alginate/EOs/OZ treatment | [148] |
| γ irr | Low dose γ irr | EOs: Thyme + Cannelle + Oregano | AM | Boneless chicken thigh samples | Shelf-life of the chicken sample increased by 3 days and 8 days when treated with Thyme + Cannelle + Oregano EOs and gamma irradiation, respectively. γ irr + EOs increased shelf-life by 14 days | [87] |
| 2 kGy | Pectin + curcumin NPs + ajowan EOs nanoemulsion | AM | Chilled lamb loins | Increased shelf-life of lamb loins from 5 days (control) to 25 days | [149] | |
| 1 kGy | Chitosan (film + EOs; Chitosan + Silver NPs (AgNPs); Chitosan + Eos + AgNPs | AM | Strawberry | Strong AM activity against Escherichia coli, Listeria monocytogenes, Salmonella Typhimurium, and Aspergillus niger. All composite films exhibited lower weight loss than control samples, and γ-irr reduce the firmness and decay during 12 days of storage | [150] | |
| 2.5 kGy | Chitosan + Cumin EO nanoemulsion | AM | Beef loins | Effective to control microbial population; Enhanced storage life (~ 14 days) of beef loins and slowed some physico-chemical changes | [151] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gabrić, D.; Kurek, M.; Ščetar, M.; Brnčić, M.; Galić, K. Effect of Non-Thermal Food Processing Techniques on Selected Packaging Materials. Polymers 2022, 14, 5069. https://doi.org/10.3390/polym14235069
Gabrić D, Kurek M, Ščetar M, Brnčić M, Galić K. Effect of Non-Thermal Food Processing Techniques on Selected Packaging Materials. Polymers. 2022; 14(23):5069. https://doi.org/10.3390/polym14235069
Chicago/Turabian StyleGabrić, Domagoj, Mia Kurek, Mario Ščetar, Mladen Brnčić, and Kata Galić. 2022. "Effect of Non-Thermal Food Processing Techniques on Selected Packaging Materials" Polymers 14, no. 23: 5069. https://doi.org/10.3390/polym14235069
APA StyleGabrić, D., Kurek, M., Ščetar, M., Brnčić, M., & Galić, K. (2022). Effect of Non-Thermal Food Processing Techniques on Selected Packaging Materials. Polymers, 14(23), 5069. https://doi.org/10.3390/polym14235069









