Silver-Nanowire-Based Elastic Conductors: Preparation Processes and Substrate Adhesion
Abstract
:1. Introduction
2. Fabrication of Elastic Bodies Containing Silver Nanowires
2.1. Prestretching/Geometric Topological Matrix
2.2. Conductive Fibers
2.3. Aerogel Composites

2.4. Mixed Seepage Dopant
3. Adhesion Principle of Silver Nanowires to Substrate
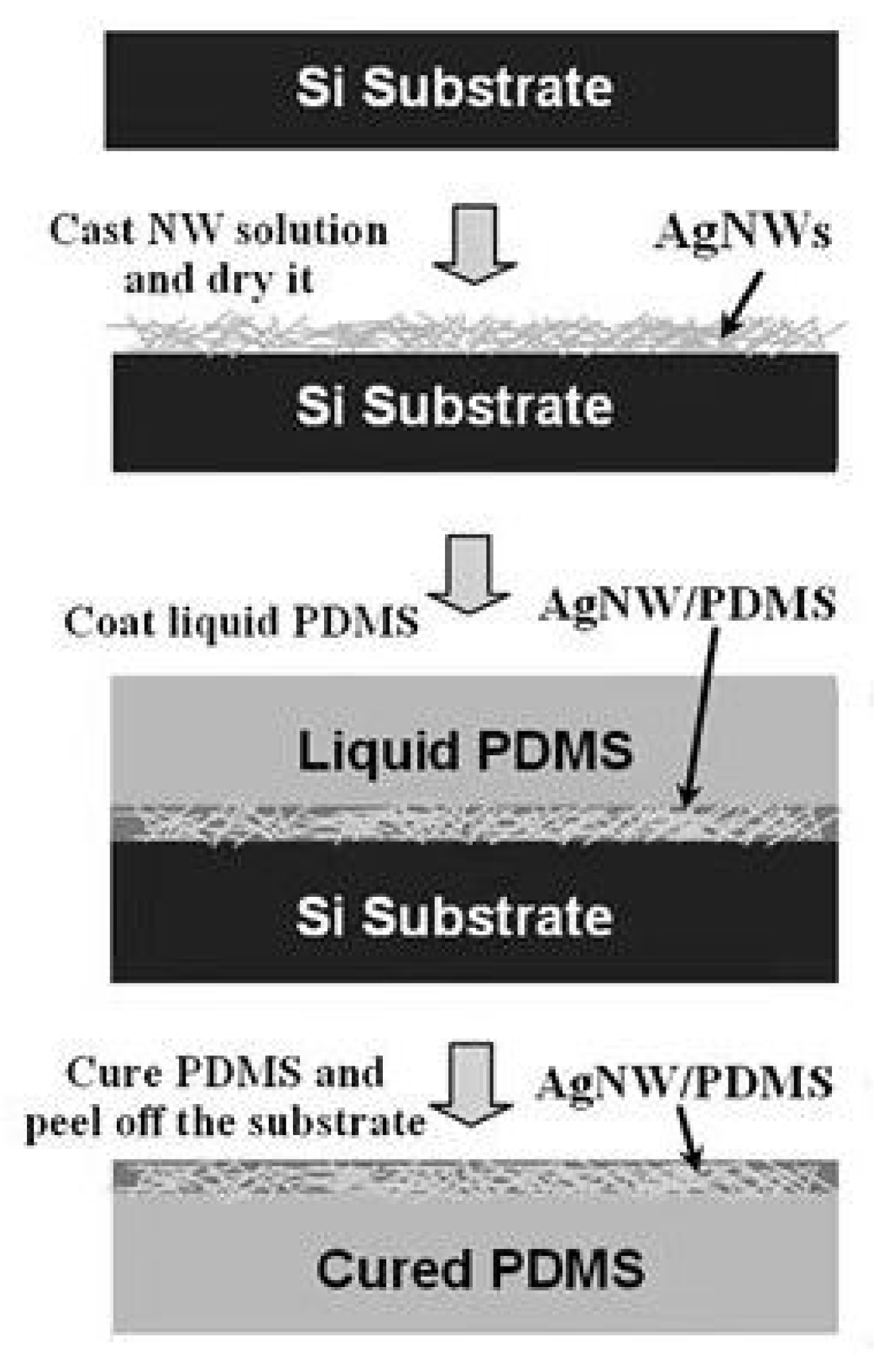
3.1. Coating Method
3.2. Embedding Method
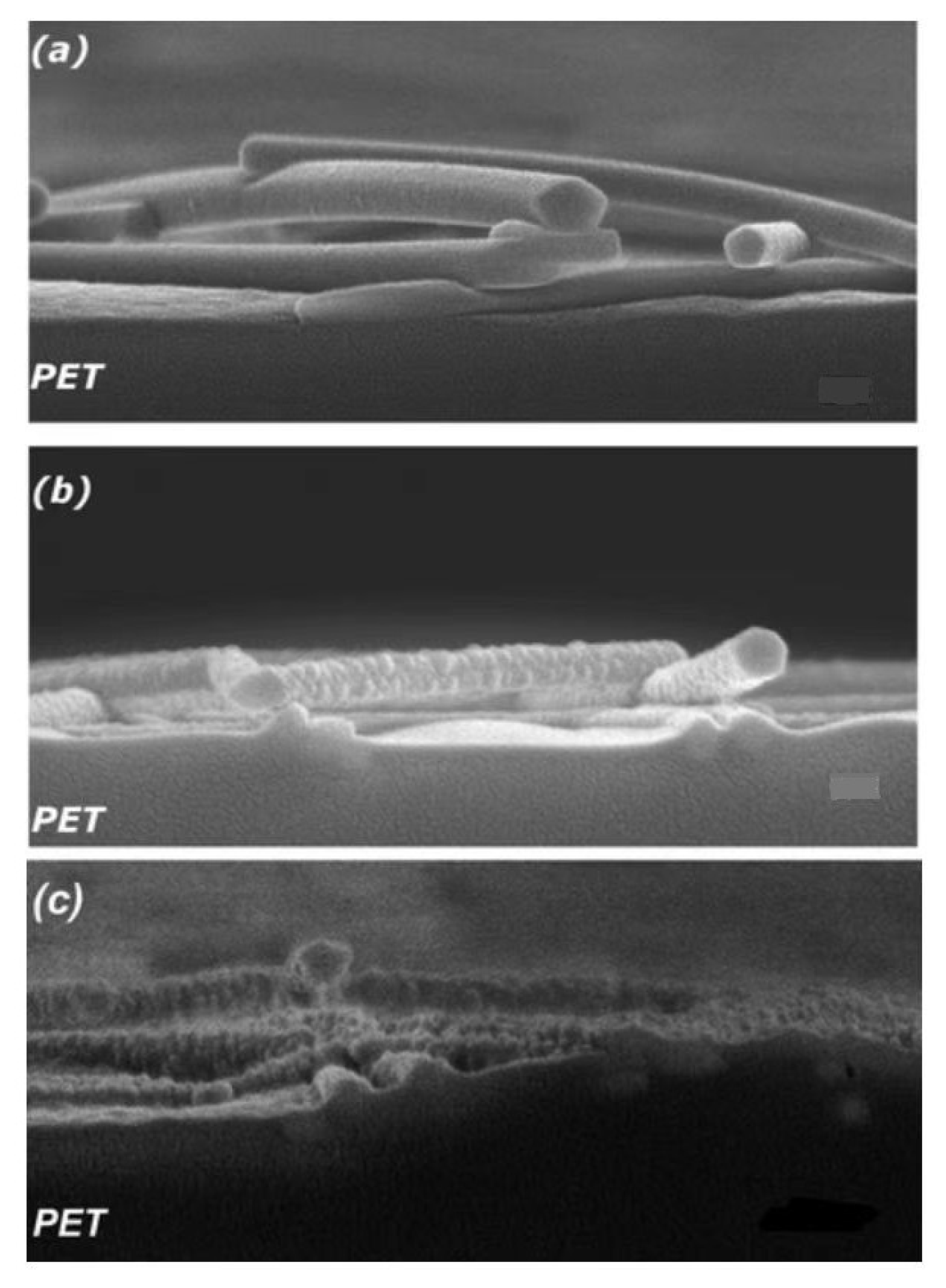
3.2.1. Reverse-Layer Processing
3.2.2. Sintering Method
3.3. Changing the Surface Energy
3.4. Chemical Bonds and Forces
3.4.1. Chemical Bonds
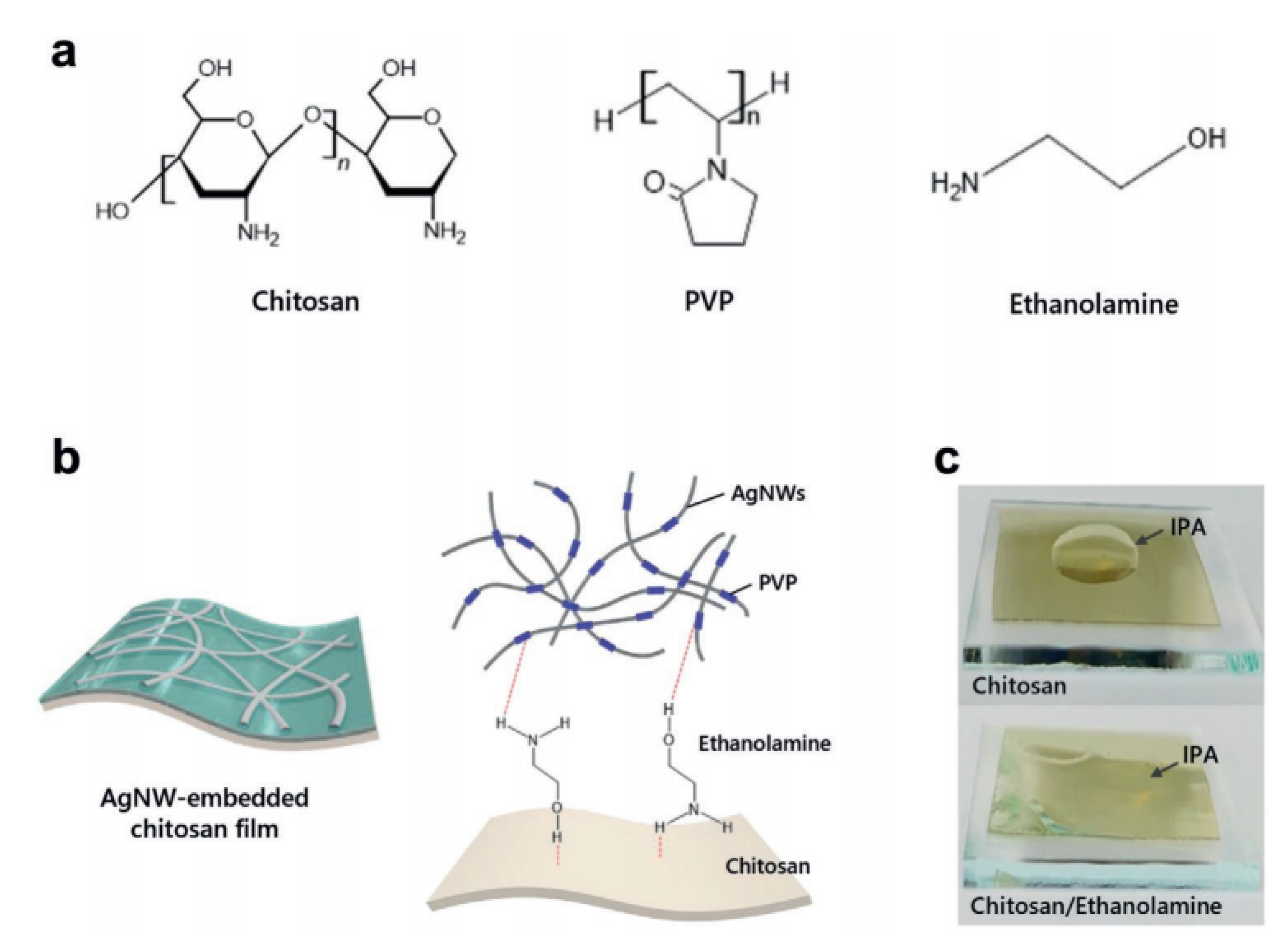
3.4.2. Hydrogen Bonding
3.5. Adjusting Tension and Diffusion
4. Applications in 3D Printing and Soft Robotics
5. Summary and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Ahn, B.Y.; Duoss, E.B.; Motala, M.J.; Guo, X.; Park, S.I.; Xiong, Y.; Yoon, J.; Nuzzo, R.G.; Rogers, J.A.; Lewis, J.A. Omnidirectional Printing of Flexible, Stretchable, and Spanning Silver Microelectrodes. Science 2009, 323, 1590. [Google Scholar] [CrossRef] [Green Version]
- Kang, B.J.; Lee, C.K.; Oh, J.H. All-inkjet-printed electrical components and circuit fabrication on a plastic substrate. Microelectron. Eng. 2012, 97, 251. [Google Scholar] [CrossRef]
- Mckerricher, G.; Perez, J.G.; Shamim, A. Fully inkjet printed RF Inductors and capacitors using polymer dielectric and silver conductive ink with through vias. IEEE Trans. Electron Devices 2015, 62, 1002. [Google Scholar] [CrossRef]
- Mckerricher, G.; Titterington, D.; Shamim, A. A fully inkjet printed 3D honeycomb inspired patch antenna. IEEE Antenn. Wirel. PR 2016, 15, 544. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.J.; Maleki, T.; Wei, P.; Ziaie, B.J. A Biaxial Stretchable Interconnect With Liquid-Alloy-Covered Joints on Elastomeric Substrate. Microelectromech. Syst. 2009, 18, 138. [Google Scholar]
- Ozutemiz, K.B.; Wissman, J.; Ozdoganlar, O.B.; Majidi, C. EGaIn-Metal Interfacing for Liquid Metal Circuitry and Microelectronics Integration. Adv. Mater. Interfaces 2018, 5, 1701596. [Google Scholar] [CrossRef]
- Thrasher, C.J.; Farrell, Z.J.; Morris, N.J.; Willey, C.L.; Tabor, C.E. Mechanoresponsive Polymerized Liquid Metal Networks. Adv. Mater. 2019, 31, 1903864. [Google Scholar] [CrossRef]
- Liu, S.Z.; Shah, D.S.; Kramer-Bottiglio, R. Highly stretchable multilayer electronic circuits using biphasic gallium-indium. Nat. Mater. 2021, 20, 851. [Google Scholar] [CrossRef]
- Li, G.Y.; Wu, X.; Lee, D.W. A galinstan-based inkjet printing system for highly stretchable electronics with self-healing capability. Lab Chip 2016, 16, 1366. [Google Scholar] [CrossRef]
- Lipomi, D.J.; Vosgueritchian, M.; Tee, B.C.-K.; Hellstrom, S.L.; Lee, J.A.; Fox, C.H.; Bao, Z.N. Skin-like pressure and strain sensors based on transparent elastic films of carbon nanotubes. Nat. Nanotechnol. 2011, 6, 788. [Google Scholar] [CrossRef]
- Yu, M.; Wang, C.; Yang, C.C.; Yu, Z. Synthesis of Stretchable Gold Films with Nanocracks: Stretched up to 120% Strain while Maintaining Conductivity. In Proceedings of the International Conference on Mechanical Engineering and Materials, Chengdu, China, 9–11 October 2017; Volume 265, p. 012009. [Google Scholar]
- Tybrandt, K.; Khodagholy, D.; Dielacher, B.; Stauffer, F.; Renz, A.F.; Buzsáki, G.; Vörös, J. High-Density Stretchable Electrode Grids for Chronic Neural Recording. Adv. Mater. 2018, 30, 1706520. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Lee, J.S. Flexible Organic Transistor Memory Devices. Nano Lett. 2010, 10, 2884. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.J.; Kang, B.; Cho, S.; Choi, M.; Baik, S. Extraordinarily High Conductivity of Stretchable Fibers of Polyurethane and Silver Nanoflowers. ACS Nano 2015, 9, 10876. [Google Scholar] [CrossRef] [PubMed]
- Hur, J.; Im, K.; Kim, S.W.; Kim, J.; Chung, D.Y.; Kim, T.H.; Jo, K.H.; Hahn, J.H.; Bao, Z.N.; Hwang, S.; et al. Polypyrrole, Agarose-Based Electronically Conductive and Reversibly Restorable Hydrogel. ACS Nano 2014, 8, 10066. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.B.; Wang, A.C.; Yang, W.F.; Zhang, M.Y.; Hou, C.Y.; Zhang, Q.H.; Li, Y.G.; Wang, H.Z. Hydrogel-based Hierarchically Wrinkled Stretchable Nanofibrous Membrane for High Performance Wearable Triboelectric Nanogenerator. Nano Energy 2019, 67, 104206. [Google Scholar] [CrossRef]
- Choi, J.H.; Lee, K.Y.; Kim, S.W. Ultra-bendable and durable Graphene-Urethane composite/silver nanowire film for flexible transparent electrodes and electromagnetic-interference shielding. Compos. Part B 2019, 177, 107406. [Google Scholar] [CrossRef]
- Zhao, Y.; Fitzgerald, M.L.; Tao, Y.; Pan, Z.L.; Sauti, G.; Xu, D.Y.; Xu, Y.Q.; Li, D.Y. Electrical and Thermal Transport through Silver Nanowires and Their Contacts—Effects of Elastic Stiffening. Nano Lett. 2020, 20, 7389–7396. [Google Scholar] [CrossRef]
- Park, J.; Kim, G.; Lee, B.; Lee, S.; Won, P.; Yoon, H.; Cho, H.; Ko, S.H.; Hong, Y. Silver Nanowire Patterning: Highly Customizable Transparent Silver Nanowire Patterning via Inkjet-Printed Conductive Polymer Templates Formed on Various Surfaces. Adv. Mater. Technol. 2020, 5, 2000042. [Google Scholar] [CrossRef]
- Park, K.; Seo, D.; Lee, J. Conductivity of silver paste prepared from nanoparticles. Colloids Surf. A 2018, 313, 351. [Google Scholar] [CrossRef]
- Ding, S.; Zhang, L.X.; Su, W.T.; Huang, X.W. Facile fabrication of highly conductive tracks using long silver nanowires and graphene composite. RSC Adv. 2018, 8, 17739. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.; An, K.; Won, P.; Ka, Y.; Hwang, H.; Moon, H.; Kwon, Y.; Hong, S.; Kim, C.; Lee, C.; et al. A dual-scale metal nanowire network transparent conductor for highly efficient and flexible organic light emitting diodes. Nanoscale 2017, 9, 1978. [Google Scholar] [CrossRef] [PubMed]
- Ahmadzadeh, M.; Kashi, M.A.; Noormohammadi, M.; Ramazani, A. Small-Diameter Magnetic and Metallic Nanowire Arrays Grown in Anodic Porous Alumina Templates Anodized in Selenic Acid. Appl. Phys. A Mater. Sci. Process 2021, 127, 449. [Google Scholar] [CrossRef]
- Xu, Q.; Lan, T.; Wang, Z.J.; Sun, C.X.; Peng, Q.; Sun, H.Y. Preparation and growth mechanism of a gold-coloured@composite film with no angular dependence. Appl. Surf. Sci. 2021, 553, 149592. [Google Scholar] [CrossRef]
- Braun, E.; Eichen, Y.; Sivan, U.; Ben-Yoseph, G. DNA-templated assembly and electrode attachment of a conducting silver wire. Nature 1998, 391, 775. [Google Scholar] [CrossRef]
- Martin, C.R. Nanomaterials: A Membrane-Based Synthetic Approach. Science 1995, 266, 1961. [Google Scholar] [CrossRef]
- Zhong, S.; Koch, T.; Walheim, S.; Rösner, H.; Nold, E.; Kobler, A.; Scherer, T.; Wang, D.; Kübel, C.; Wang, M.; et al. Self-organization of mesoscopic silver wires by electrochemical deposition. Nanotechnology 2014, 5, 1285. [Google Scholar] [CrossRef] [Green Version]
- Caswell, K.K.; Bender, C.M.; Murphy, C.J. Seedless, Surfactantless Wet Chemical Synthesis of Silver Nanowires. Nano Lett. 2003, 3, 667. [Google Scholar] [CrossRef]
- Nguyen, N.T.; Liu, J.H. Wet Chemical Synthesis of Silver Nanowires Based on a Soft Template of Cholesteryl Pyridine Carbamate Organogel. Sci. Adv. Mater. 2015, 7, 1282. [Google Scholar] [CrossRef]
- Sun, Y.G.; Gates, B.; Mayers, B.; Xia, Y.N. Crystalline Silver Nanowires by Soft Solution Processing. Nano Lett. 2002, 2, 165. [Google Scholar] [CrossRef]
- Trung, T.N.; Arepalli, V.K.; Gudala, R.; Kim, E.T. Polyol synthesis of ultrathin and high-aspect-ratio Ag nanowires for transparent conductive films. Mater. Lett. 2017, 194, 66. [Google Scholar] [CrossRef]
- Junaidi; Triyana, K.; Suharyadi, E.; Harsojo; Wu, L.Y.L. The Roles of Polyvinyl Alcohol (PVA) as the Capping Agent on the Polyol Method for Synthesizing Silver Nanowires. Nano Res. 2017, 49, 174. [Google Scholar] [CrossRef]
- Chen, D.P.; Qiao, X.L.; Qiu, X.L.; Chen, J.G.; Jiang, R.Z.J. Convenient synthesis of silver nanowires with adjustable diameters via a solvothermal method. Colloid Interface Sci. 2010, 344, 286. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.H.; Yi, Z.; Tan, X.L.; Chen, J.F.; Wu, J.; Yi, Y.G.; Tang, Y.J. Effect of Na2S concentration on synthesis of Silver nanowires by solvothermal method. J. Funct. Mater. 2014, 45, 03075. [Google Scholar]
- Ren, X.L.; Zheng, M.L.; Jin, F.; Zhao, Y.Y.; Dong, X.Z.; Liu, J.; Yu, H.; Duan, X.M.; Zhao, Z.S. Laser Direct Writing of Silver Nanowire with Amino Acids-Assisted Multiphoton Photoreduction. J. Phys. Chem. C 2016, 120, 26532. [Google Scholar] [CrossRef]
- Liu, S.L.; Wu, H.B.; Wang, X.M.; Wang, Y.; Chen, J.J. Effect of anion controller on microstructure of silver nanowires. J. Electr. Comp. Mater. 2018, 37, 79. [Google Scholar]
- Sugiyama, S.; Yokoyama, S.; Huaman, J.L.C.; Ida, S.; Matsumoto, T.; Kodama, D.; Sato, K.; Miyamura, H.; Hirokawa, Y.; Balach, J. Design of monoalcohol—Copolymer system for high quality silver nanowires. J. Colloid Interface Sci. 2018, 527, 315. [Google Scholar] [CrossRef]
- Lee, J.; Lee, P.; Lee, H.; Lee, D.; Lee, S.S.; Ko, S.H. Very long Ag nanowire synthesis and its application in a highly transparent, conductive and flexible metal electrode touch panel. Nanoscale 2012, 4, 6408. [Google Scholar] [CrossRef]
- Mou, Y.; Cheng, H.; Wang, H.; Sun, Q.L.; Liu, J.X.; Peng, Y.; Chen, M.X. Facile Preparation of Stable Reactive Silver Ink for Highly Conductive and Flexible Electrodes. Appl. Surf. Sci. 2019, 475, 75. [Google Scholar] [CrossRef]
- Ye, N.; Liang, T.; Zhan, L.L.; Kong, Y.H.; Xie, S.; Ma, X.Y.; Chen, H.Z.; Su, H.X.; Xu, M.S. High-Performance Bendable Organic Solar Cells With Silver Nanowire-Graphene Hybrid Electrode. IEEE J. Photovolt. 2019, 9, 214. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Guo, X.; Wang, W.; Chen, L.; Liu, L.; Liu, H.; He, Y. Highly Sensitive, Low Hysteretic and Flexible Strain Sensor Based on Ecoflex-AgNWs-MWCNTs Flexible Composite Materials. IEEE Sens. J. 2020, 20, 14118. [Google Scholar] [CrossRef]
- Shi, R.L.; Lou, Z.; Chen, S.; Shen, G.Z. Flexible and transparent capacitive pressure sensor with patterned microstructured composite rubber dielectric for wearable touch keyboard application. Sci. China Mater. 2018, 61, 1587. [Google Scholar] [CrossRef] [Green Version]
- Ding, C.; Zhao, B.; Qi, N. Electronic Textiles Based on Silver Nanowire Conductive Network. Prog. Chem. 2017, 29, 892. [Google Scholar]
- Zhu, J.M.; Qian, J.H.; Sun, L.Y.; Li, Z.P.; Peng, H.M. Properties of functionalized composite polyester fabric prepared from silver nanowires of high aspect ratio. J. Text. Res. 2019, 40, 113. [Google Scholar]
- Rai, T.; Dantes, P.; Bahreyni, B.; Kim, W.S. A Stretchable RF Antenna With Silver Nanowires. IEEE Electron Device Lett. 2013, 34, 544. [Google Scholar] [CrossRef]
- Castro, H.F.; Correia, V.; Sowade, E.; Mitra, K.Y.; Rocha, J.G.; Baumann, R.R.; Lanceros-Méndez, S. All-inkjet-printed low-pass filters with adjustable cutoff frequency consisting of resistors, inductors and transistors for sensor applications. Org. Electron. 2016, 38, 205. [Google Scholar] [CrossRef]
- Guo, H.; Ren, F.; Ren, P.G. Preparation and Electromagnetic Shielding Properties of Hydrophobic Silver Nanowire/Cellulose Composite Paper. Polym. Mater. Sci. Eng. 2020, 36, 81. [Google Scholar]
- Zhu, L.Y.; Chen, S.J.; Zhou, H.Y.; Peng, S.; Zhang, Q.Q.; Zhang, X.C.; Guo, X.J. Silver Nanowire Mesh-Based Fuse Type Write-Once-Read-Many Memory. IEEE Electron Device Lett. 2018, 39, 347. [Google Scholar] [CrossRef]
- Amjadi, M.; Pichitpajongkit, A.; Lee, S.; Ryu, S.; Park, I. Highly Stretchable and Sensitive Strain Sensor Based on Silver Nanowire-Elastomer Nanocomposite. ACS Nano 2014, 8, 5154. [Google Scholar] [CrossRef]
- Wu, B.; Heidelberg, A.; Boland, J.J. Microstructure-hardened silver nanowires. Nano Lett. 2006, 6, 468. [Google Scholar] [CrossRef]
- Wang, Y.; Gong, S.; Wang, S.J.; Yang, X.Y.; Ling, Y.Z.; Yap, L.W.; Dong, D.S.; Simon, G.P.; Cheng, W.L. Standing Enokitake-like Nanowire Films for Highly Stretchable Elastronics. ACS Nano 2018, 12, 9742. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.H.; Jeong, D.W.; Jang, N.S.; Haa, S.H.; Kim, J.M. Extremely stretchable conductors based on hierarchically-structured metal nanowire network. RSC Adv. 2016, 6, 56896. [Google Scholar] [CrossRef]
- Yuan, W.; Lin, J.; Gu, W.B.; Liang, Z.W.; Cui, Z. Preparation of flexible and stretchable circuit via printing method based on silver nanowires. Sci. Sin. Phys. Mech. Astron. 2016, 46, 044611. [Google Scholar] [CrossRef]
- Park, J.S.; Kim, W.S. Sustained Percolation in Stretched Silver Nanowire Networks for Stretchable Inter-Connection Applications. Mater. Res. Soc. Symp. Proc. 2014, 1685, 905. [Google Scholar] [CrossRef]
- Choi, S.; Park, J.; Hyun, W.; Kim, J.; Kim, J.; Lee, Y.B.; Song, C.; Hwang, H.J.; Kim, J.H.; Hyeon, T.; et al. Stretchable Heater Using Ligand-Exchanged Silver Nanowire Nanocomposite for Wearable Articular Thermotherapy. ACS Nano 2015, 9, 6626. [Google Scholar] [CrossRef]
- Ding, S.; Ying, J.J.; Chen, F.; Fu, L.; Lv, Y.F.; Zhao, S.C.; Ji, G.Q. Highly stretchable conductors comprising composites of silver nanowires and silver flakes. J. Nanopart. Res. 2021, 23, 111. [Google Scholar] [CrossRef]
- Lee, S.J.; Kim, J.W.; Park, J.H.; Porte, Y.; Kim, J.H.; Park, J.W.; Kim, S.; Myoung, J.M. SWCNT–Ag nanowire composite for transparent stretchable film heater with enhanced electrical stability. J. Mater. Sci. 2018, 53, 12284. [Google Scholar] [CrossRef]
- Jiang, Y.; Chen, Y.X.; Wang, W.; Yu, D. A wearable strain sensor based on polyurethane nanofiber membrane with silver nanowires/polyaniline electrically conductive dual-network. Colloids Surf. A 2021, 629, 127477. [Google Scholar] [CrossRef]
- Kim, A.; Ahn, J.; Hwang, H.; Lee, E.; Moon, J. Pre-strain strategy for developing a highly stretchable and foldable one-dimensional conductive cord based on a Ag nanowire network. Nanoscale 2017, 9, 5773–5778. [Google Scholar] [CrossRef]
- Lu, Y.; Jiang, J.W.; Yoon, S.; Kim, K.S.; Kim, J.H.; Park, S.; Kim, S.H.; Piao, L.H. High-Performance Stretchable Conductive Composite Fibers from Surface-Modified Silver Nanowires and Thermoplastic Polyurethane by Wet Spinning. ACS Appl. Mater. Interfaces 2018, 10, 2093. [Google Scholar] [CrossRef]
- Lee, S.; Shin, S.; Lee, S.; Seo, J.; Lee, J.; Son, S.; Cho, H.J.; Algadi, H.; Sayari, S.A.; Kim, D.E.; et al. Ag Nanowire Reinforced Highly Stretchable Conductive Fibers for Wearable Electronics. Adv. Funct. Mater. 2015, 25, 3114. [Google Scholar] [CrossRef]
- Cheng, Y.; Wang, R.R.; Sun, J.; Gao, L. Highly conductive and ultrastretchable electric circuits from covered yarns and silver nanowires. ACS Nano 2015, 9, 3887. [Google Scholar] [CrossRef]
- Ge, J.; Sun, L.; Zhang, F.R.; Zhang, Y.; Shi, L.A.; Zhao, H.Y.; Zhu, H.W.; Jiang, H.L.; Yu, S.H. Stretchable Electronics: A Stretchable Electronic Fabric Artificial Skin with Pressure-, Lateral Strain-, and Flexion-Sensitive Properties. Adv. Mater. 2016, 28, 722. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.M.; Jung, H.Y.; Dresselhaus, M.S.; Jung, Y.J.; Kong, J. A facile route for 3D aerogels from nanostructured 1D and 2D materials. Sci. Rep. 2012, 2, 849. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, S.; Feng, C.; Mayes, E.L.H.; Yao, B.C.; He, Z.J.; Asadi, S.; Alanc, T.; Yang, J. In situ synthesis of silver nanowire gel and its super-elastic composite foams. Nanoscale 2020, 12, 19861–19869. [Google Scholar] [CrossRef] [PubMed]
- Tian, Z.L.; Zhao, Y.; Wang, S.G.; Zhou, G.D.; Zhao, N.; Wong, C.P. A highly stretchable and conductive composite based on an emulsion-templated silver nanowire aerogel. J. Mater. Chem. A 2020, 8, 1724–1730. [Google Scholar] [CrossRef]
- Qian, F.; Lan, P.C.; Freyman, M.; Chen, W.; Kou, T.Y.; Olson, T.Y.; Zhu, C.; Worsley, M.A.; Duoss, E.B.; Spadaccini, C.M.; et al. Ultralight Conductive Silver Nanowire Aerogels. Nano Lett. 2017, 17, 7171–7176. [Google Scholar] [CrossRef]
- Gong, J.P.; Katsuyama, Y.; Kurokawa, T.; Osada, Y. Double-Network Hydrogels with Extremely High Mechanical Strength. Adv. Mater. 2003, 15, 1155. [Google Scholar] [CrossRef]
- Song, P.; Qin, H.L.; Gao, H.L.; Cong, H.P.; Yu, S.H. Self-healing and superstretchable conductors from hierarchical nanowire assemblies. Nat. Commun. 2018, 9, 2786. [Google Scholar] [CrossRef] [Green Version]
- Zhu, H.W.; Gao, H.L.; Zhao, H.Y.; Ge, J.; Hu, B.C.; Huang, J.; Yu, S.H. Printable elastic silver nanowire-based conductor for washable electronic textiles. Nano Res. 2020, 13, 2879. [Google Scholar] [CrossRef]
- Hwang, B.U.; Lee, J.H.; Trung, T.Q.; Roh, E.; Kim, D.I.; Kim, S.W.; Lee, N.E. Transparent Stretchable Self-Powered Patchable Sensor Platform with Ultrasensitive Recognition of Human Activities. ACS Nano 2015, 9, 8801. [Google Scholar] [CrossRef]
- Mao, Y.Y.; Wang, C.; Yang, H.W. A highly stretchable AgNWs@VPDMS-PMHS conductor exhibiting a stretchability of 800%. Mater. Lett. 2015, 150, 101. [Google Scholar] [CrossRef]
- Choi, S.; Han, S.I.; Jung, D.; Hwang, H.J.; Lim, C.; Bae, S.; Park, O.K.; Tschabrunn, C.M.; Lee, M.; Bae, S.Y.; et al. Highly conductive, stretchable and biocompatible Ag–Au core–sheath nanowire composite for wearable and implantable bioelectronics. Nat. Nanotechnol. 2018, 13, 1048. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.G.; Kim, J.; Jung, S.B.; Kimc, Y.S.; Kim, J.W. Electrically and mechanically enhanced Ag nanowires-colorless polyimide composite electrode for flexible capacitive sensor. Appl. Surf. Sci. 2016, 380, 223. [Google Scholar] [CrossRef]
- Lee, J.C.; Lee, J.S.; Won, P.; Park, J.J.; Choi, S.H.; Ko, S.H.; Kim, B.J.; Lee, S.Y.; Joo, Y.C. Operation Range-Optimized Silver Nanowire Through Junction Treatment. Electron. Mater. Lett. 2020, 16, 491. [Google Scholar] [CrossRef]
- Catenacci, M.J.; Reyes, C.; Cruz, M.A.; Wiley, B.J. Stretchable Conductive Composites from Cu-Ag Nanowire Felt. ACS Nano 2018, 12, 3689. [Google Scholar] [CrossRef]
- Jeong, C.K.; Lee, J.; Han, S.; Ryu, J.; Hwang, G.T.; Park, D.Y.; Park, J.H.; Lee, S.S.; Byun, M.; Ko, S.H.; et al. A Hyper-Stretchable Elastic-Composite Energy Harvester. Adv. Mater. 2015, 27, 2866. [Google Scholar] [CrossRef]
- Chen, Y.X.; Lan, W.; Wang, J.; Zhu, R.R.; Yang, Z.W.; Ding, D.L.; Tang, G.M.; Wang, K.R.; Su, Q.; Xie, E.Q. Highly flexible, transparent, conductive and antibacterial films made of spin-coated silver nanowires and a protective ZnO layer. Physica E 2016, 76, 88. [Google Scholar] [CrossRef]
- Zhang, L.W.; Ji, Y.; Qiu, Y.J.; Xu, C.W.; Liu, Z.G.; Guo, Q.Q. Highly thermal-stable and transparent silver nanowire conductive films via magnetic assisted electrodeposition of Ni. J. Mater. Chem. C 2018, 6, 4887. [Google Scholar] [CrossRef]
- Zhang, X.; Zhong, Y.L.; Yan, Y. Electrical, Mechanical, and Electromagnetic Shielding Properties of Silver Nanowire-Based Transparent Conductive Films. Phys. Status Solidi 2018, 215, 1800014. [Google Scholar] [CrossRef]
- Xu, F.; Zhu, Y. Highly Conductive and Stretchable Silver Nanowire Conductors. Adv. Mater. 2012, 24, 5117. [Google Scholar] [CrossRef]
- Huang, G.W.; Xiao, H.M.; Fu, S.Y. Wearable Electronics of Silver-Nanowire/Poly(dimethylsiloxane) Nanocomposite for Smart Clothing. Sci. Rep. 2015, 5, 13971. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kisannagar, R.R.; Jha, P.; Navalkar, A.; Maji, S.K.; Gupta, D. Fabrication of Silver Nanowire/Polydimethylsiloxane Dry Electrodes by a Vacuum Filtration Method for Electrophysiological Signal Monitoring. ACS Omega 2020, 5, 10260. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Shin, D.; Park, J. Fabrication of silver nanowire-based stretchable electrodes using spray coating. Thin Solid Film. 2016, 608, 34. [Google Scholar] [CrossRef]
- Yan, C.Y.; Wang, X.; Cui, M.Q.; Wang, J.X.; Kang, W.B.; Foo, C.Y.; Lee, P.S. Stretchable Silver-Zinc Batteries Based on Embedded Nanowire Elastic Conductors. Adv. Energy Mater. 2014, 4, 1301396. [Google Scholar] [CrossRef]
- Zhang, L.L.; Wang, Y.; Gui, J.Z.; Wang, X.N.; Li, R.; Liu, W.; Sun, C.L.; Zhao, X.Z.; Guo, S.S. Efficient Welding of Silver Nanowires embedded in a Poly(vinylidene fluoride) Film for Robust Wearable Electronics. Adv. Mater. Technol. 2018, 4, 1800438. [Google Scholar] [CrossRef]
- Zhou, B.; Su, M.J.; Yang, D.Z.; Han, G.J.; Feng, Y.Z.; Wang, B.; Ma, J.L.; Ma, J.M.; Liu, C.T.; Shen, C.Y. Flexible MXene/Silver Nanowire-Based Transparent Conductive Film with Electromagnetic Interference Shielding and Electro-Photo-Thermal Performance. ACS Appl. Mater. Interfaces 2020, 12, 40859. [Google Scholar] [CrossRef] [PubMed]
- Jiu, J.T.; Sugahara, T.; Nogi, M.; Araki, T.; Suganuma, K.; Uchida, H.; Shinozaki, K. High-intensity pulse light sintering of silver nanowire transparent films on polymer substrates: The effect of the thermal properties of substrates on the performance of silver films. Nanoscale 2013, 5, 11820. [Google Scholar] [CrossRef] [PubMed]
- Jiu, J.T.; Nogi, M.; Sugahara, T.; Tokuno, T.; Araki, T.; Komoda, N.; Suganuma, K.; Uchidab, H.; Shinozaki, K. Strongly adhesive and flexible transparent silver nanowire conductive films fabricated with a high-intensity pulsed light technique. J. Mater. Chem. 2012, 22, 23561. [Google Scholar] [CrossRef]
- Song, C.H.; Han, C.J.; Ju, B.K.; Kim, J.W. Photoenhanced Patterning of Metal Nanowire Networks for Fabrication of Ultraflexible Transparent Devices. ACS Appl. Mater. Interface 2015, 8, 480. [Google Scholar] [CrossRef]
- Yang, Y.; Ding, S.; Araki, T.; Jiu, J.T.; Sugahara, T.; Wang, J.; Vanfleteren, J.; Sekitani, T.; Suganuma, K. Facile fabrication of stretchable Ag nanowire/polyurethane electrodes using high intensity pulsed light. Nano Res. 2016, 9, 401. [Google Scholar] [CrossRef] [Green Version]
- Kaikanov, M.; Kemelbay, A.; Amanzhulov, B.; Demeuova, G.; Akhtanova, G.; Bozheyev, F.; Tikhonov, A. Electrical conductivity enhancement of transparent silver nanowire films on temperature-sensitive flexible substrates using intense pulsed ion beam. Nanotechnology 2021, 32, 145706. [Google Scholar] [CrossRef]
- Dai, S.; Li, Q.; Liu, G.; Yang, H.; Yang, Y.; Zhao, D.; Wang, W.; Qiu, M. Laser-induced single point nanowelding of silver nanowires. Appl. Phys. Lett. 2016, 108, 121103. [Google Scholar] [CrossRef] [Green Version]
- Raveendran, R.; Namboothiry, M.A.G. Surface-Treated Poly(dimethylsiloxane) as a Gate Dielectric in Solution-Processed Organic Field-Effect Transistors. ACS Omega 2018, 3, 1127. [Google Scholar] [CrossRef] [PubMed]
- Pandey, P.; Vongphachanh, S.; Yoon, J.; Kim, B.; Choi, C.J.; Sohn, J.I.; Hong, W.K. Silver nanowire-network-film-coated soft substrates with wrinkled surfaces for use as stretchable surface enhanced Raman scattering sensors. J. Alloy. Compd. 2020, 859, 157862. [Google Scholar] [CrossRef]
- Lin, T.Y.; Pfeiffer, T.T.; Lillehoj, P.B. Stability of UV/ozone-treated thermoplastics under different storage conditions for microfluidic analytical devices. RSC Adv. 2017, 7, 37374. [Google Scholar] [CrossRef] [Green Version]
- Shen, G.Z.; Zhang, C.; Liang, T.L.; Xin, Y.; Liang, J.H.; Zhong, Y.; He, J.; He, X.; He, X. Microstructure Engineering of Stretchable Resistive Strain Sensors with Discrimination Capabilities in Transverse and Longitudinal Directions. Macromol. Mater. Eng. 2021, 306, 2100283. [Google Scholar] [CrossRef]
- Ho, X.; Tey, J.N.; Cheng, C.K.; Wei, J. Highly Flexible Transparent Conductors Based on 2D Silver Nanowire Network. In Proceedings of the IEEE 65th Electronic Components and Technology Conference (ECTC), San Diego, CA, USA, 26–29 May 2015; p. 1749. [Google Scholar]
- Park, J.H.; Hwang, G.T.; Kim, S.; Seo, J.; Park, H.J.; Yu, K.; Kim, T.S.; Lee, K.J. Flash-Induced Self-Limited Plasmonic Welding of Silver Nanowire Network for Transparent Flexible Energy Harvester. Adv. Mater. 2017, 29, 1603473. [Google Scholar] [CrossRef]
- Li, L.M.; Zhu, C.H.; Wu, Y.P.; Wang, J.H.; Zhang, T.L.; Liu, Y. A conductive ternary network of a highly stretchable AgNWs/AgNPs conductor based on a polydopamine-modified polyurethane sponge. RSC Adv. 2015, 5, 62905. [Google Scholar] [CrossRef]
- Li, L.M.; Zhang, T.L.; Liu, Y.; Zhu, C.H. Flexible conductor fabrication via silver nanowire deposition on a polydopamine-modified pre-strained substrate. J. Mater. Sci. Mater. Electron. 2016, 27, 3193. [Google Scholar] [CrossRef]
- Kim, S.M.; Lee, J.; In, I. Formulation of Silver Nanowire-Polyaniline Hybrid Transparent Electrodes by Using Catechol-enriched Polyaniline. Chem. Lett. 2014, 43, 1453. [Google Scholar] [CrossRef]
- Kim, S.H.; Jung, S.; Yoon, I.S.; Lee, C.; Oh, Y.; Hong, J.M. Ultrastretchable Conductor Fabricated on Skin-Like Hydrogel–Elastomer Hybrid Substrates for Skin Electronics. Adv. Mater. 2018, 30, 1800109. [Google Scholar] [CrossRef]
- Jin, Y.X.; Deng, D.Y.; Cheng, Y.R.; Kong, L.Q.; Xiao, F. Annealing-free and strongly adhesive silver nanowire networks with long-term reliability by introduction of a nonconductive and biocompatible polymer binder. Nanoscale 2014, 6, 4812. [Google Scholar] [CrossRef] [PubMed]
- Ko, Y.; Kim, D.; Kwon, G.; You, J. High-Performance Resistive Pressure Sensor Based on Elastic Composite Hydrogel of Silver Nanowires and Poly(ethylene glycol). Micromachines 2018, 9, 438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.P.; Qi, S.H.; Liang, J.J.; Li, L.; Xiong, Y.; Hu, W.; Pei, Q.B. Synthesizing a Healable Stretchable Transparent Conductor. ACS Appl. Mater. Interfaces 2015, 7, 14140. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.L.; Niu, X.F.; Li, L.; Yun, S.; Yu, Z.B.; Pei, Q.B. Intrinsically stretchable transparent electrodes based on silver-nanowire-crosslinked-polyacrylate composites. Nanotechnology 2012, 23, 344002. [Google Scholar] [CrossRef]
- Nam, S.; Lee, S.M.; Kim, J.; Oh, I.H.; Chang, S.T. (3-Aminopropyl)Triethoxysilane-Modified Silver Nanowire Network with Strong Adhesion to Coating Substrates for Highly Transparent Electrodes. Coatings 2021, 11, 499. [Google Scholar] [CrossRef]
- Lee, Y.; Suh, M.; Kim, K.; Kim, H.; Kim, D.; Chang, H.; Lee, D.; Kim, Y.; Kim, S.W.; Jeon, D.Y. Conjugated polyelectrolyte-assisted vacuum-free transfer-printing of silver nanowire network for top electrode of polymer light-emitting diodes. Org. Electron. 2017, 43, 64. [Google Scholar] [CrossRef]
- Park, S.B.; Han, J.W.; Kim, J.H.; Wibowo, A.F.; Prameswati, A.; Park, J.; Lee, J.; Moon, M.W.; Kim, M.S.; Kim, Y.H. Multifunctional Stretchable Organic-Inorganic Hybrid Electronics with Transparent Conductive Silver Nanowire/Biopolymer Hybrid Films. Adv. Opt. Mater. 2021, 9, 2002041. [Google Scholar] [CrossRef]
- Jiang, Z.; Nayeem, M.O.G.; Fukuda, K.; Ding, S.; Jin, H.; Yokota, T.; Inoue, D.; Hashizume, D.; Someya, T. Highly Stretchable Metallic Nanowire Networks Reinforced by the Underlying Randomly Distributed Elastic Polymer Nanofibers via Interfacial Adhesion Improvement. Adv. Mater. 2019, 31, 1903446. [Google Scholar] [CrossRef]
- Kim, D.H.; Yu, K.C.; Kim, Y.; Kim, J.W. Highly Stretchable and Mechanically Stable Transparent Electrode Based on Composite of Silver Nanowires and Polyurethane-Urea. ACS Appl. Mater. Interfaces 2015, 7, 15214. [Google Scholar] [CrossRef]
- Guo, H.S.; Han, Y.; Zhao, W.Q.; Yang, J.; Zhang, L. Universally autonomous self-healing elastomer with high stretchability. Nat. Commun. 2020, 11, 2037. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Lee, P.; Lee, H.B.; Hong, S.; Lee, I.; Yeo, J.; Lee, S.S.; Kim, T.S.; Lee, D.; Ko, S.H. Room-Temperature Nanosoldering of a Very Long Metal Nanowire Network by Conducting-Polymer-Assisted Joining for a Flexible Touch-Panel Application. Adv. Funct. Mater. 2013, 23, 4165. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Zhang, L.; Wang, D.R. Ultrastretchable Hybrid Electrodes of Silver Nanowires and Multiwalled Carbon Nanotubes Realized by Capillary- Force-Induced Welding. Adv. Mater. Technol. 2019, 9, 1900721. [Google Scholar] [CrossRef]
- Wang, K.; Stark, J.P.W. Direct fabrication and morphology of metallic micropatterns by pulsed jet nanoelectrospraying of silver nano-ink. Soft Matter 2012, 9, 317. [Google Scholar] [CrossRef]
- Wu, J.T.; Hsu, L.C.; Tsai, M.H.; Liu, Y.F.; Hwang, W.S. Direct ink-jet printing of silver nitrate–silver nanowire hybrid inks to fabricate silver conductive lines. J. Mater. Chem. 2012, 22, 15599. [Google Scholar] [CrossRef]
- Liang, J.J.; Tong, K.; Pei, Q.B. A Water-Based Silver-Nanowire Screen-Print Ink for the Fabrication of Stretchable Conductors and Wearable Thin-Film Transistors. Adv. Mater. 2016, 28, 5986. [Google Scholar] [CrossRef]
- Williams, N.X.; Noyce, S.; Cardenas, J.A.; Catenacci, M.; Wiley, B.J.; Franklin, A.D. Silver Nanowire Inks for Direct-Write Electronic Tattoo Applications. Nanoscale 2019, 11, 14294. [Google Scholar] [CrossRef]
- Cai, L.; Zhang, S.M.; Zhang, Y.H.; Li, J.Q.; Miao, J.S.; Wang, Q.F.; Yu, Z.B.; Wang, C. Direct Printing for Additive Patterning of Silver Nanowires for Stretchable Sensor and Display Applications. Adv. Mater. Technol. 2017, 3, 1700232. [Google Scholar] [CrossRef]
- Maisch, P.; Tam, K.C.; Lucera, L.; Egelhaaf, H.J.; Scheiber, H.; Maier, E.; Brabec, C.J. Inkjet printed silver nanowire percolation networks as electrodes for highly efficient semitransparent organic solar cells. Org. Electron. 2016, 38, 139. [Google Scholar] [CrossRef]
- Wu, X.L.; Wang, S.Y.; Luo, Z.W.; Lu, J.X.; Lin, K.W.; Xie, H.; Wang, Y.H.; Li, J.Z. Inkjet Printing of Flexible Transparent Conductive Films with Silver Nanowires Ink. Nanomaterials 2022, 11, 1571. [Google Scholar] [CrossRef]
- Tan, K.Y.; Zheng, X.H.; Show, P.L.; Huang, N.M.; Hong, N.L.; Foo, C.Y. Single droplet 3D printing of electrically conductive resin using high aspect ratio silver nanowires. Nanomaterials 2021, 48, 102473. [Google Scholar] [CrossRef]
- Cui, Z.; Han, Y.W.; Huang, Q.J.; Dong, J.Y.; Zhu, Y. Electrohydrodynamic printing of silver nanowires for flexible and stretchable electronics. Nanoscale 2018, 10, 6086. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, S.; Iijima, M.; Tatami, J.; Fuke, T.; Hinotsu, T.; Sato, K. Polymer Ligand Design and Surface Modification of Ag Nanowires toward Color-Tone-Tunable Transparent Conductive Films. ACS Appl. Mater. Interfaces 2021, 13, 13705. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.Y.; Yuk, H.; Lin, S.T.; Jian, N.N.; Qu, K.; Xu, J.K.; Zhao, X.H. Pure PEDOT: PSS hydrogels. Nat. Commun. 2019, 10, 1043. [Google Scholar] [CrossRef] [Green Version]
- Ying, W.B.; Liu, H.X.; Gao, P.Y.; Kong, Z.Y.; Hu, H.; Wang, K.; Shen, A.; Jin, Z.J.; Zheng, L.; Guo, H.X.; et al. An anti-stress relaxation, anti-fatigue, mildew proof and self-healing poly(thiourethane-urethane)for durably stretchable electronics. Chem. Eng. J. 2020, 420, 127691. [Google Scholar] [CrossRef]
- Li, F.L.; Xu, Z.F.; Hu, H.; Kong, Z.Y.; Chen, C.; Tian, Y.; Zhang, W.W.; Ying, W.B.; Zhang, R.Y.; Zhu, J. A polyurethane integrating self-healing, anti-aging and controlled degradation for durable and eco-friendly E-skin. Chem. Eng. J. 2021, 410, 128363. [Google Scholar] [CrossRef]
- Zhang, M.Q.; Xie, Y.H.; Yao, T.G.; Cao, X.N.; Zhang, Z.; Li, G.R.; Ma, Z.P.; Mao, J.; Yang, T.; Luo, Y.W.; et al. Scar-Like Self-Reinforced and Failure-Tolerant Dielectric Elastomer Actuator With AgNWs Electrode. J. Appl. Mech. 2018, 85. [Google Scholar] [CrossRef]
- Wang, J.X.; Gao, D.; Lee, P.S. Recent Progress in Artificial Muscles for Interactive Soft Robotics. Adv. Mater. 2021, 33, 20030883. [Google Scholar] [CrossRef]
- Cho, S.; Kang, D.H.; Lee, H.; Kim, M.P.; Kang, S.; Shanker, R.; Ko, H. Highly Stretchable Sound-in-Display Electronics Based on Strain-Insensitive Metallic Nanonetworks. Adv. Sci. 2021, 8, 2001647. [Google Scholar] [CrossRef]
- Gogurla, N.; Roy, B.; Min, K.; Park, J.Y.; Kim, S. A Skin-Inspired, Interactive, and Flexible Optoelectronic Device with Hydrated Melanin Nanoparticles in a Protein Hydrogel-Elastomer Hybrid. Adv. Mater. Technol. 2020, 5, 1900936. [Google Scholar] [CrossRef]
- Jun, K.; Kim, J.; Oh, I. An Electroactive and Transparent Haptic Interface Utilizing Soft Elastomer Actuators with Silver Nanowire Electrode. Small 2018, 14, 1801603. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.R.; Kwon, H.; Lee, D.H.; Lee, B.Y. Highly flexible and transparent dielectric elastomer actuators using silver nanowire and carbon nanotube hybrid electrodes. Soft Matter 2017, 13, 6390. [Google Scholar] [CrossRef] [PubMed] [Green Version]








| Method | Maximum Tensile Rate under Conductive Condition or Maximum Conductivity | Ref. | Advantages | Disadvantages |
|---|---|---|---|---|
| Prestretching/topological matrix | 750% | [52] | Simple preparation process, low cost | Generally low tensile rate, estimate deformation direction |
| 100% | [55] | |||
| Conductive fiber | 32.09 S/m | [58] | High conductivity, good tensile properties, can be woven | Process is relatively complex, best to assist with pre-stretching |
| 500% | [59] | |||
| 200%, 1.4 × 10 S/m | [60] | |||
| 220% | [61] | |||
| 500%, 6.88 × 10 S/m | [62] | |||
| 100% | [63] | |||
| Aerogel composites | 3 × 10 S/m | [64] | Light weight, low density | Low tensile property, complex preparation process |
| 2.1 × 10 S/m | [65] | |||
| 130%, 6570 S/m | [66] | |||
| 5.1 × 10 S/m | [67] | |||
| Mixed seepage dopant | 200%, 3.668 × 10 S/m | [70] | High elongation, components are combined, arbitrary structure, good versatility | Complex material ratio, more procedures |
| 240% | [71] | |||
| 800% | [72] | |||
| 840%, 7.6 × 10 S/m | [73] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, K.; He, T. Silver-Nanowire-Based Elastic Conductors: Preparation Processes and Substrate Adhesion. Polymers 2023, 15, 1545. https://doi.org/10.3390/polym15061545
Yu K, He T. Silver-Nanowire-Based Elastic Conductors: Preparation Processes and Substrate Adhesion. Polymers. 2023; 15(6):1545. https://doi.org/10.3390/polym15061545
Chicago/Turabian StyleYu, Kai, and Tian He. 2023. "Silver-Nanowire-Based Elastic Conductors: Preparation Processes and Substrate Adhesion" Polymers 15, no. 6: 1545. https://doi.org/10.3390/polym15061545
APA StyleYu, K., & He, T. (2023). Silver-Nanowire-Based Elastic Conductors: Preparation Processes and Substrate Adhesion. Polymers, 15(6), 1545. https://doi.org/10.3390/polym15061545






