Biocompatible Preparation of Beta-Lactoglobulin/Chondroitin Sulfate Carrier Nanoparticles and Modification of Their Colloidal and Hydropathic Properties by Tween 80
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Sample Preparation
2.2. Surface Charge, Hydropathy and Secondary Structure Calculations Based on the Crystal Structure of β-LG
2.3. Static and Dynamic Light Scattering
2.4. Electrophoretic Light Scattering
2.5. Fourier-Transform Infrared Spectroscopy
2.6. Fluorescence Spectroscopy
2.7. Circular Dichroism
2.8. Transmission Electron Microscopy
3. Results and Discussion
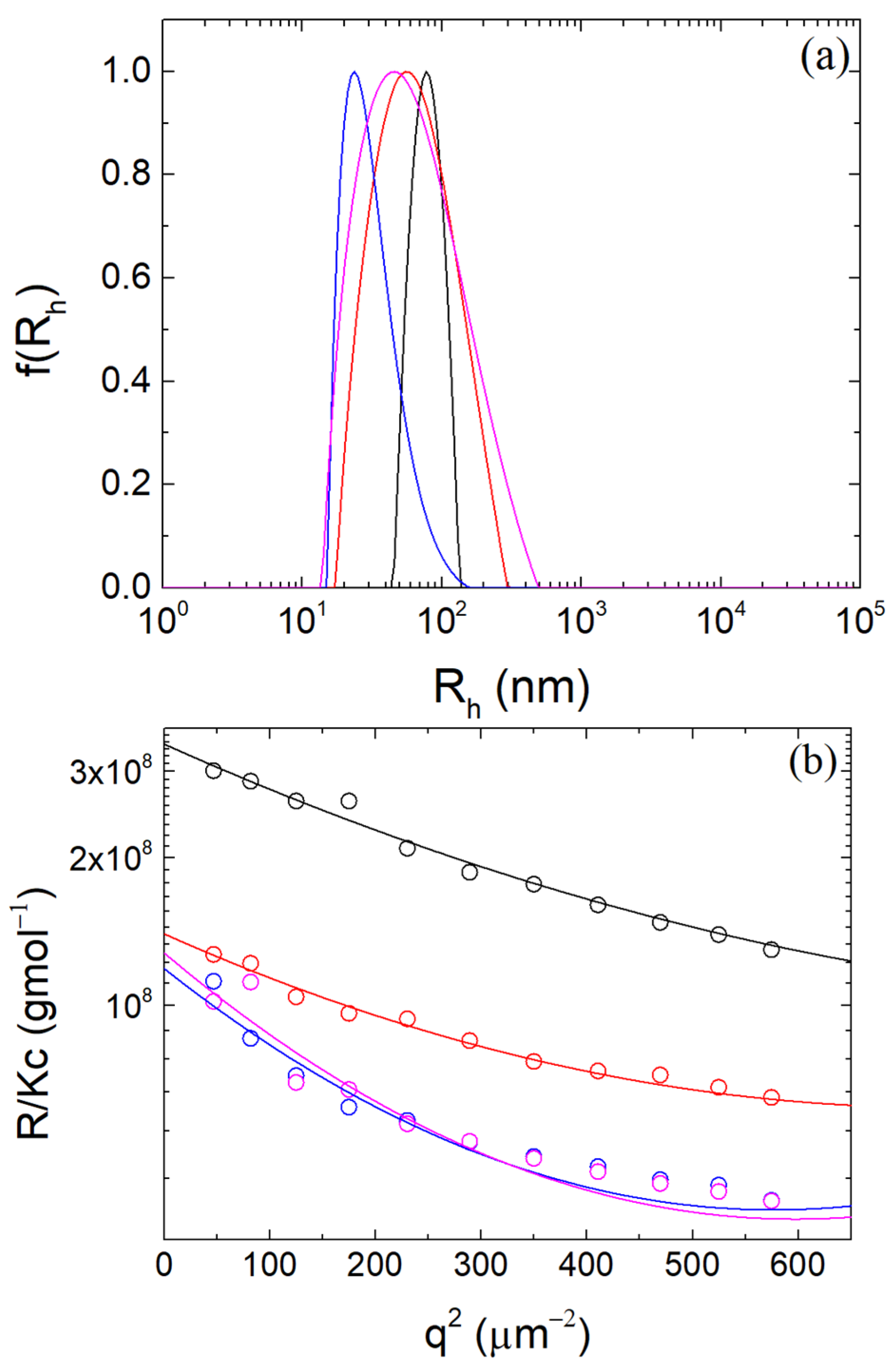
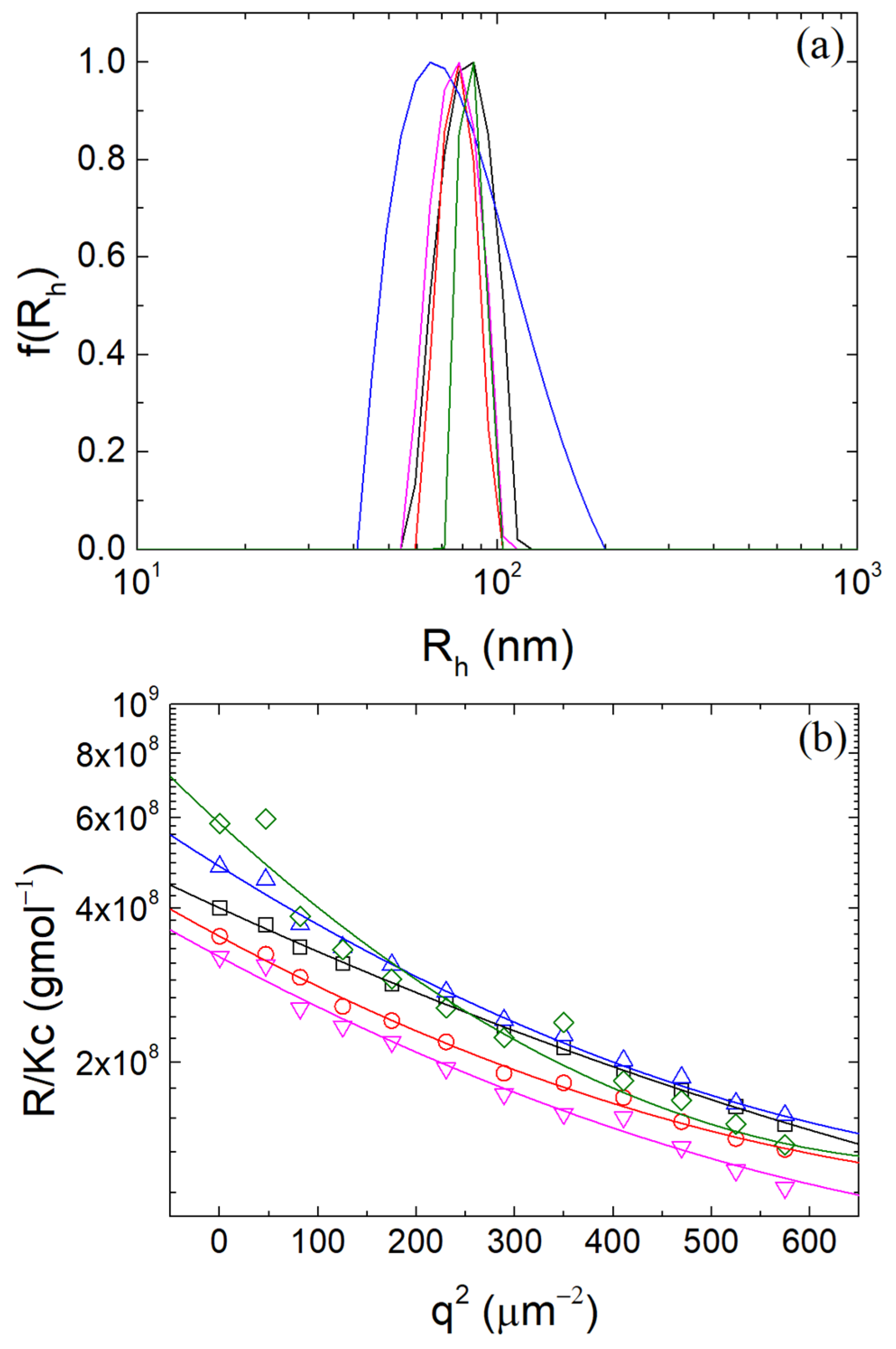
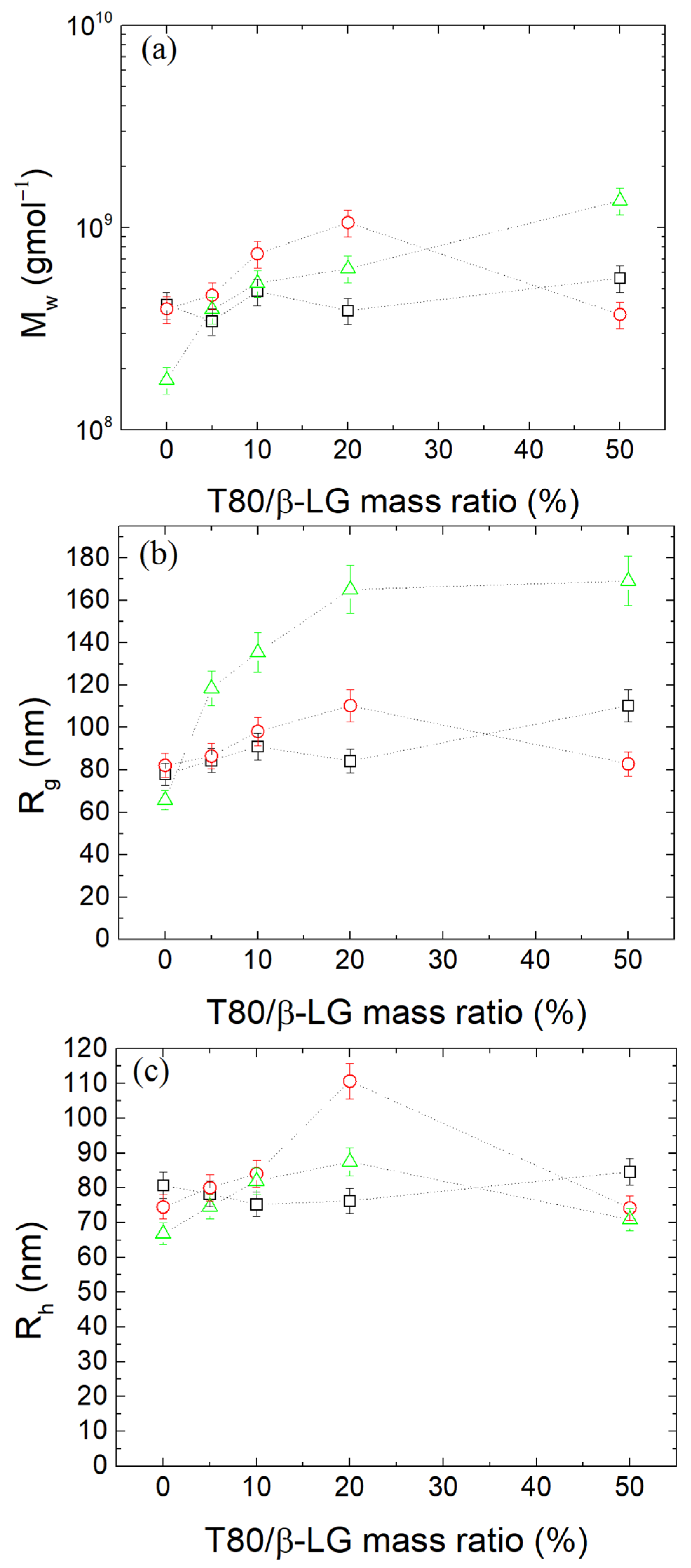
3.1. Formation of β-LG/CS Complexes and Effect of T80
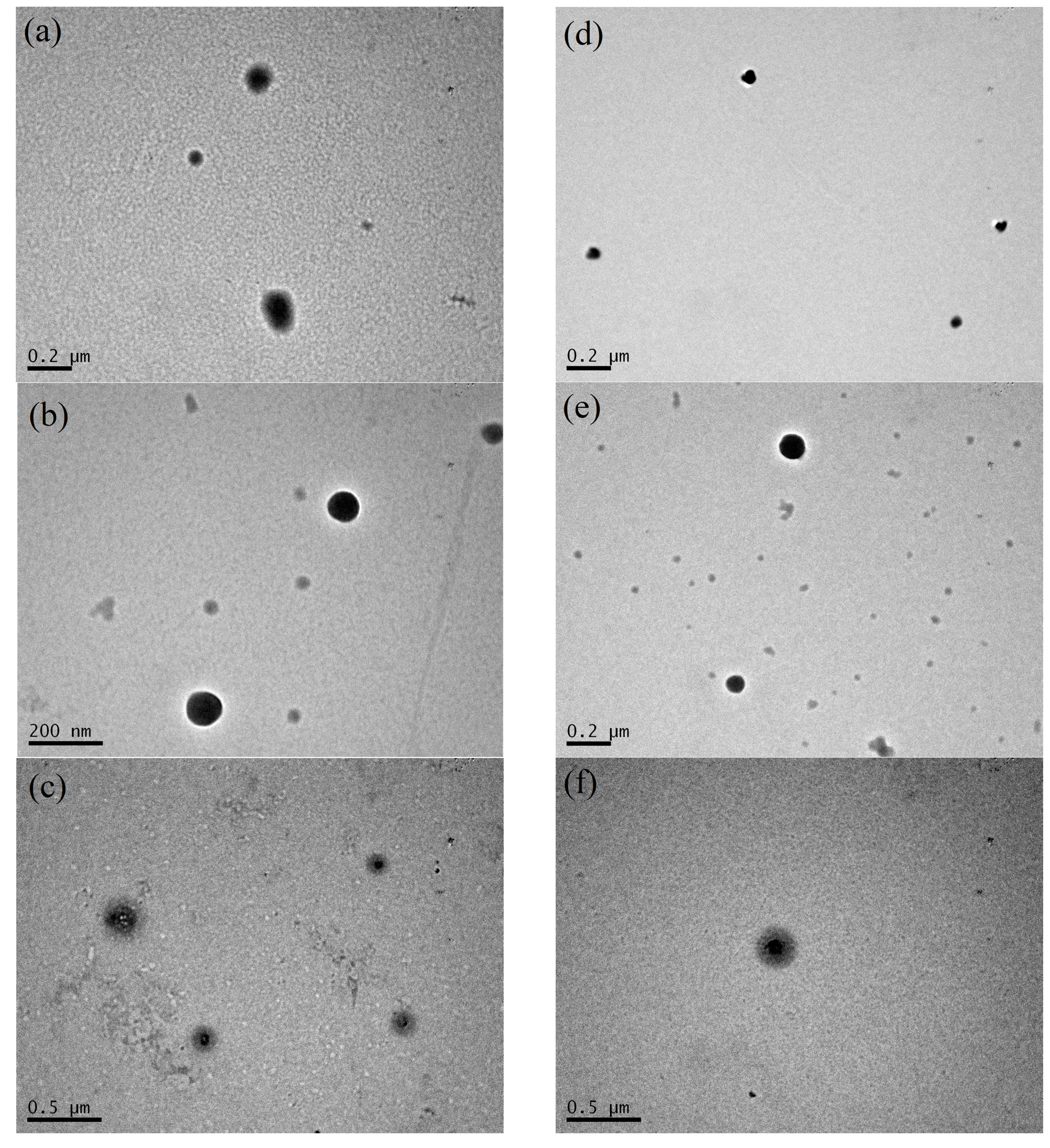
3.2. Thermal Stabilization and Morphology of β-LG/CS and β-LG/CS/T80 NPs
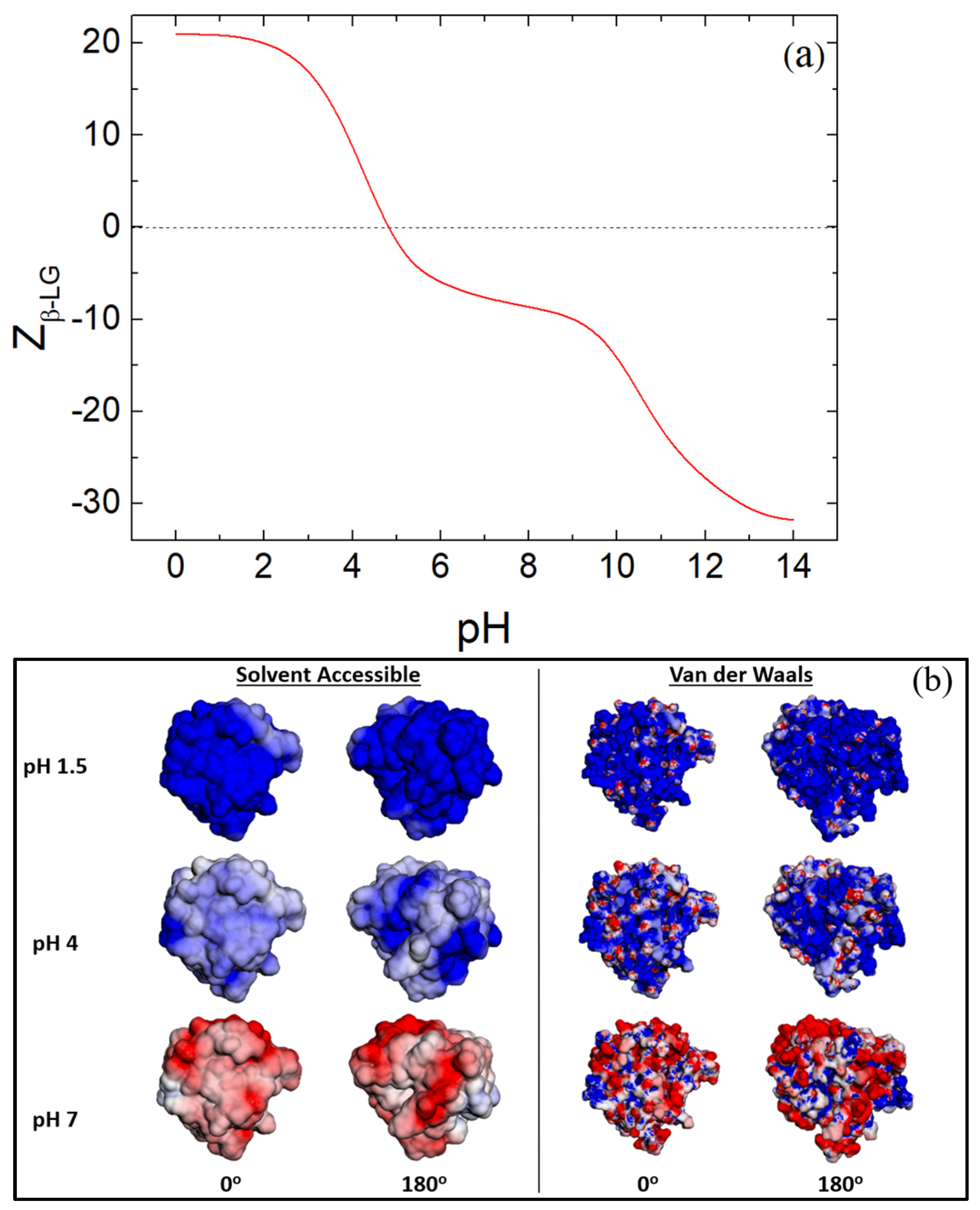
3.3. Zeta Potential of β-LG/CS and β-LG/CS/T80 NPs
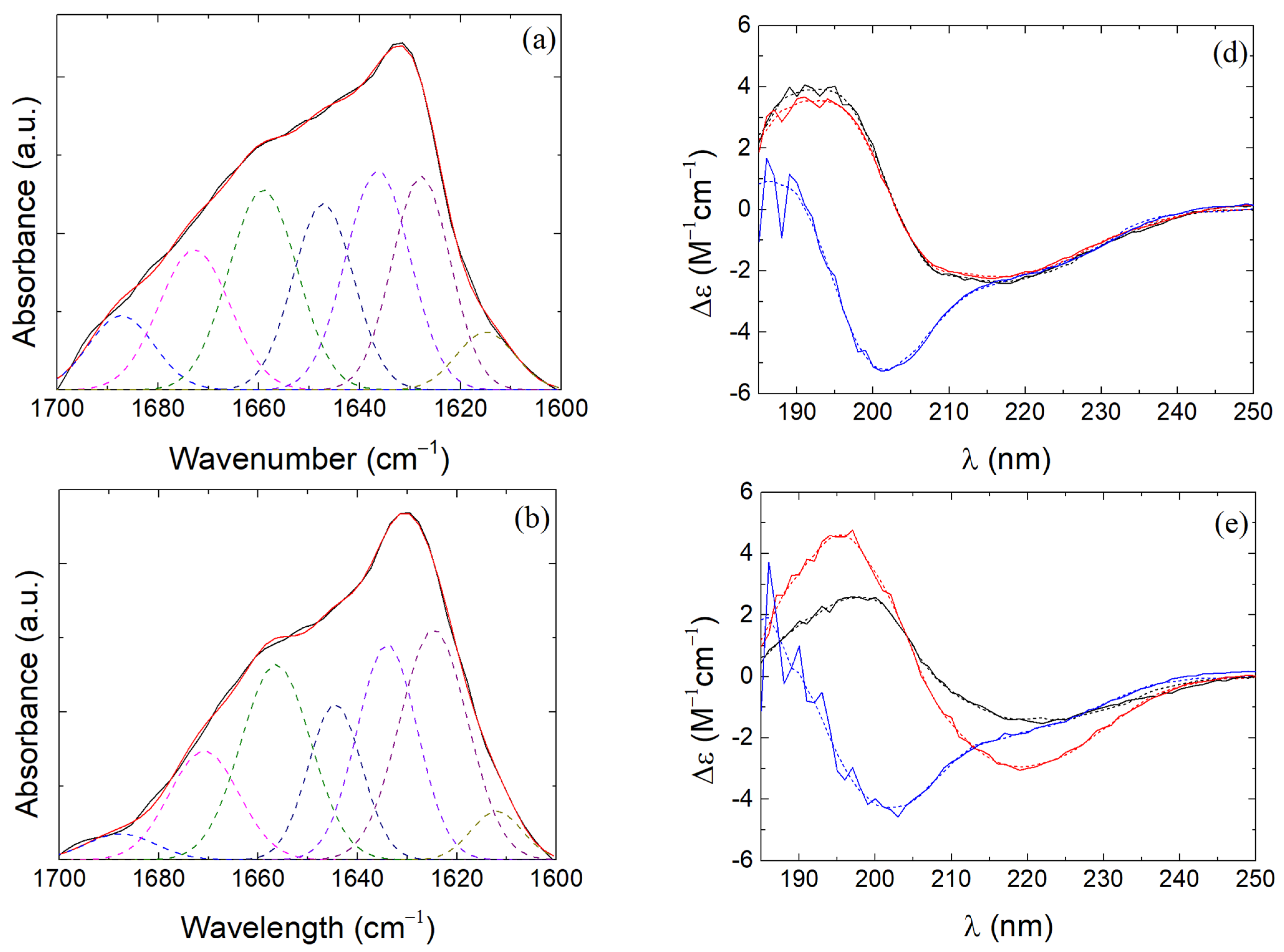
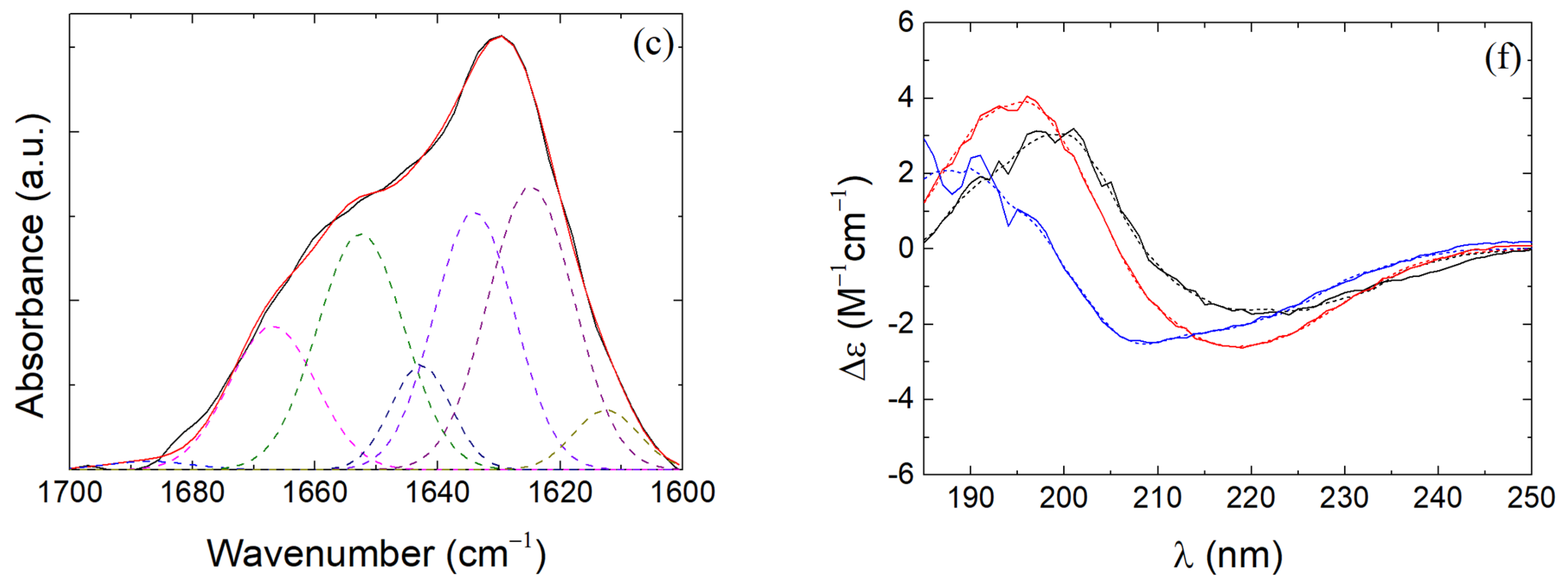
3.4. β-LG Conformation in β-LG/CS and β-LG/CS/T80 NPs
3.5. Tryptophan Endogenous Fluorescence of Pure β-LG and in Its Complexes upon Titration of CS and T80
3.6. Hydrophobicity of β-LG/CS and β-LG/CS/T80 NPs
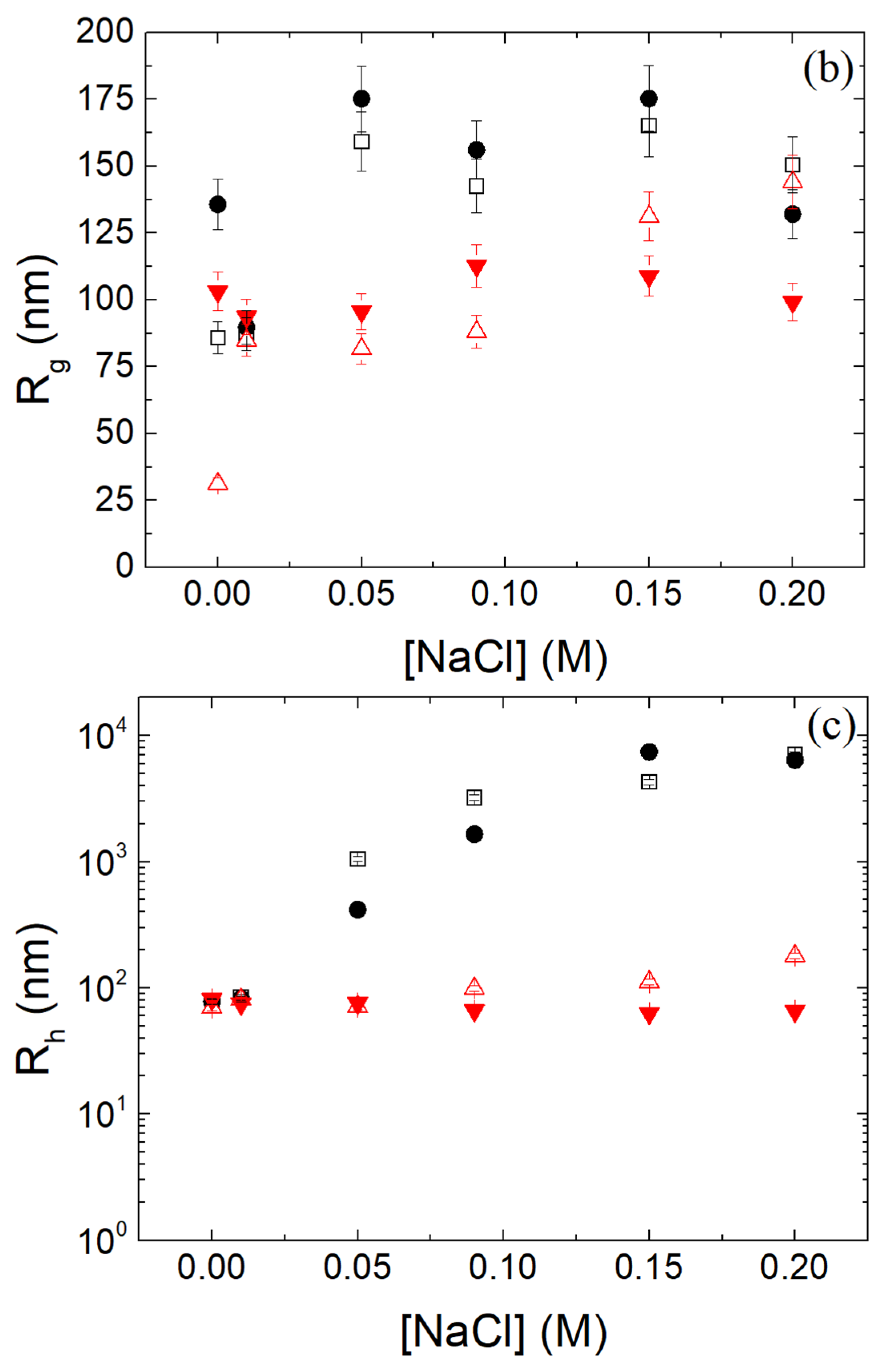
3.7. Stability of β-LG/CS and β-LG/CS/T80 NPs in Salt and Biological Fluids and over Time
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jawahar, N.; Meyyanathan, S. Polymeric nanoparticles for drug delivery and targeting: A comprehensive review. Int. J. Health Allied Sci. 2012, 1, 217–223. [Google Scholar] [CrossRef]
- Karabasz, A.; Bzowska, M.; Szczepanowicz, K. Biomedical Applications of Multifunctional Polymeric Nanocarriers: A Review of Current Literature. Int. J. Nanomed. 2020, 15, 8673–8696. [Google Scholar] [CrossRef] [PubMed]
- Zielińska, A.; Carreiró, F.; Oliveira, A.M.; Neves, A.; Pires, B.; Venkatesh, D.N.; Durazzo, A.; Lucarini, M.; Eder, P.; Silva, A.M.; et al. Polymeric Nanoparticles: Production, Characterization, Toxicology and Ecotoxicology. Molecules 2020, 25, 3731. [Google Scholar] [CrossRef] [PubMed]
- Elezaby, R.S.; Gad, H.A.; Metwally, A.A.; Geneidi, A.S.; Awad, G.A. Self-assembled amphiphilic core-shell nanocarriers in line with the modern strategies for brain delivery. J. Controlled Release 2017, 261, 43–61. [Google Scholar] [CrossRef] [PubMed]
- Papagiannopoulos, A.; Meristoudi, A.; Pispas, S.; Radulescu, A. Micelles from HOOC-PnBA-b-PAA-C12 H15 Diblock Amphiphilic Polyelectrolytes as Protein Nanocarriers. Biomacromolecules 2016, 17, 3816–3827. [Google Scholar] [CrossRef]
- Blocher, W.C.; Perry, S.L. Complex coacervate-based materials for biomedicine. WIREs Nanomed. Nanobiotechnol. 2017, 9, e1442. [Google Scholar] [CrossRef] [PubMed]
- Comert, F.; Malanowski, A.J.; Azarikia, F.; Dubin, P.L. Coacervation and precipitation in polysaccharide–protein systems. Soft Matter 2016, 12, 4154–4161. [Google Scholar] [CrossRef] [PubMed]
- Cooper, C.L.; Dubin, P.L.; Kayitmazer, A.B.; Turksen, S. Polyelectrolyte–protein complexes. Curr. Opin. Colloid Interface Sci. 2005, 10, 52–78. [Google Scholar] [CrossRef]
- Zheng, J.; Van Der Meeren, P.; Sun, W. New insights into protein–polysaccharide complex coacervation: Dynamics, molecular parameters, and applications. Aggregate 2024, 5, e449. [Google Scholar] [CrossRef]
- Jones, O.G.; Decker, E.A.; McClements, D.J. Comparison of protein–polysaccharide nanoparticle fabrication methods: Impact of biopolymer complexation before or after particle formation. J. Colloid Interface Sci. 2010, 344, 21–29. [Google Scholar] [CrossRef]
- Jones, O.G.; McClements, D.J. Biopolymer Nanoparticles from Heat-Treated Electrostatic Protein–Polysaccharide Complexes: Factors Affecting Particle Characteristics. J. Food Sci. 2010, 75, N36–N43. [Google Scholar] [CrossRef] [PubMed]
- McKee, T.; McKee, J.R. Biochemistry: The Molecular Basis of Life, 7th ed.; Oxford University Press: New York, NY, USA, 2020. [Google Scholar]
- Cortés-Morales, E.A.; Mendez-Montealvo, G.; Velazquez, G. Interactions of the molecular assembly of polysaccharide-protein systems as encapsulation materials. A review. Adv. Colloid Interface Sci. 2021, 295, 102398. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Huang, Q. Assembly of Protein–Polysaccharide Complexes for Delivery of Bioactive Ingredients: A Perspective Paper. J. Agric. Food Chem. 2019, 67, 1344–1352. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Jiao, Y.; Wang, Y.; Zhou, C.; Zhang, Z. Polysaccharides-based nanoparticles as drug delivery systems. Adv. Drug Deliv. Rev. 2008, 60, 1650–1662. [Google Scholar] [CrossRef] [PubMed]
- Miyake, M.; Yamashita, Y. Molecular Structure and Phase Behavior of Surfactants. In Cosmetic Science and Technology; Elsevier: New York, NY, USA, 2017; pp. 389–414. [Google Scholar] [CrossRef]
- Nakama, Y. Surfactants. In Cosmetic Science and Technology; Elsevier: New York, NY, USA, 2017; pp. 231–244. [Google Scholar] [CrossRef]
- Le Maux, S.; Bouhallab, S.; Giblin, L.; Brodkorb, A.; Croguennec, T. Bovine β-lactoglobulin/fatty acid complexes: Binding, structural, and biological properties. Dairy Sci. Technol. 2014, 94, 409–426. [Google Scholar] [CrossRef] [PubMed]
- Roth-Walter, F.; Pacios, L.F.; Gomez-Casado, C.; Hofstetter, G.; Roth, G.A.; Singer, J.; Diaz-Perales, A.; Jensen-Jarolim, E. The Major Cow Milk Allergen Bos d 5 Manipulates T-Helper Cells Depending on Its Load with Siderophore-Bound Iron. PLoS ONE 2014, 9, e104803. [Google Scholar] [CrossRef]
- Van Den Akker, C.C.; Schleeger, M.; Bonn, M.; Koenderink, G.H. Structural Basis for the Polymorphism of β-Lactoglobulin Amyloid-Like Fibrils. In Bio-Nanoimaging; Elsevier: New York, NY, USA, 2014; pp. 333–343. [Google Scholar] [CrossRef]
- Sawyer, L.; Kontopidis, G. The core lipocalin, bovine β-lactoglobulin. Biochim. Biophys. Acta BBA—Protein Struct. Mol. Enzymol. 2000, 1482, 136–148. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Harris, P.; Kaur, A.; Pastrana, L.; Jauregi, P. Characterisation of β-lactoglobulin nanoparticles and their binding to caffeine. Food Hydrocoll. 2017, 71, 85–93. [Google Scholar] [CrossRef]
- Li, L.; Chunta, S.; Zheng, X.; He, H.; Wu, W.; Lu, Y. β-Lactoglobulin stabilized lipid nanoparticles enhance oral absorption of insulin by slowing down lipolysis. Chin. Chem. Lett. 2024, 35, 108662. [Google Scholar] [CrossRef]
- Rubinstein, A.; Nakar, D.; Sintov, A. Chondroitin sulfate: A potential biodegradable carrier for colon-specific drug delivery. Int. J. Pharm. 1992, 84, 141–150. [Google Scholar] [CrossRef]
- McAtee, C.O.; Barycki, J.J.; Simpson, M.A. Emerging Roles for Hyaluronidase in Cancer Metastasis and Therapy. In Advances in Cancer Research; Elsevier: New York, NY, USA, 2014; Volume 123, pp. 1–34. [Google Scholar] [CrossRef]
- Schieber, A.; Lopes-Lutz, D. Analytical Methods—Functional Foods and Dietary Supplements. In Comprehensive Biotechnology; Elsevier: New York, NY, USA, 2011; pp. 487–499. [Google Scholar] [CrossRef]
- Pigman, W.; Horton, D. , The Carbohydrates. 1B, 2nd ed.; Academic Press: New York, NY, USA, 1980. [Google Scholar]
- Henrotin, Y.; Mathy, M.; Sanchez, C.; Lambert, C. Chondroitin sulfate in the treatment of osteoarthritis: From in vitro studies to clinical recommendations. Ther. Adv. Musculoskelet. Dis. 2010, 2, 335–348. [Google Scholar] [CrossRef]
- Klecker, C.; Nair, L.S. Matrix Chemistry Controlling Stem Cell Behavior. In Biology and Engineering of Stem Cell Niches; Elsevier: New York, NY, USA, 2017; pp. 195–213. [Google Scholar] [CrossRef]
- Yamakoshi, Y. Dental and Oral Biology, Biochemistry. In Reference Module in Biomedical Sciences; Elsevier: New York, NY, USA, 2014; p. B9780128012383000374. [Google Scholar] [CrossRef]
- Mehmood, T.; Ahmed, A. Tween 80 and Soya-Lecithin-Based Food-Grade Nanoemulsions for the Effective Delivery of Vitamin D. Langmuir 2020, 36, 2886–2892. [Google Scholar] [CrossRef]
- Ravichandran, V.; Lee, M.; Nguyen Cao, T.G.; Shim, M.S. Polysorbate-Based Drug Formulations for Brain-Targeted Drug Delivery and Anticancer Therapy. Appl. Sci. 2021, 11, 9336. [Google Scholar] [CrossRef]
- Ponphaiboon, J.; Limmatvapirat, S.; Limmatvapirat, C. Development and Evaluation of a Stable Oil-in-Water Emulsion with High Ostrich Oil Concentration for Skincare Applications. Molecules 2024, 29, 982. [Google Scholar] [CrossRef]
- Liu, H.; Jin, Y.; Menon, R.; Laskowich, E.; Bareford, L.; De Vilmorin, P.; Kolwyck, D.; Yeung, B.; Yi, L. Characterization of Polysorbate 80 by Liquid Chromatography-Mass Spectrometry to Understand Its Susceptibility to Degradation and Its Oxidative Degradation Pathway. J. Pharm. Sci. 2022, 111, 323–334. [Google Scholar] [CrossRef]
- Ron, N.; Zimet, P.; Bargarum, J.; Livney, Y.D. Beta-lactoglobulin–polysaccharide complexes as nanovehicles for hydrophobic nutraceuticals in non-fat foods and clear beverages. Int. Dairy J. 2010, 20, 686–693. [Google Scholar] [CrossRef]
- Dai, L.; Wei, Y.; Sun, C.; Mao, L.; McClements, D.J.; Gao, Y. Development of protein-polysaccharide-surfactant ternary complex particles as delivery vehicles for curcumin. Food Hydrocoll. 2018, 85, 75–85. [Google Scholar] [CrossRef]
- Fuenzalida, J.P.; Xiong, T.; Moerschbacher, B.M.; Ostermeier, M.; Goycoolea, F.M. Protein-surfactant-polysaccharide nanoparticles increase the catalytic activity of an engineered β-lactamase maltose-activated switch enzyme. bioRxiv 2019. [Google Scholar] [CrossRef]
- Berman, H.M. The Protein Data Bank. Nucleic Acids Res. 2000, 28, 235–242. [Google Scholar] [CrossRef]
- Dolinsky, T.J.; Nielsen, J.E.; McCammon, J.A.; Baker, N.A. PDB2PQR: An automated pipeline for the setup of Poisson-Boltzmann electrostatics calculations. Nucleic Acids Res. 2004, 32, W665–W667. [Google Scholar] [CrossRef]
- Jurrus, E.; Engel, D.; Star, K.; Monson, K.; Brandi, J.; Felberg, L.E.; Brookes, D.H.; Wilson, L.; Chen, J.; Liles, K.; et al. Improvements to the APBS biomolecular solvation software suite. Protein Sci. 2018, 27, 112–128. [Google Scholar] [CrossRef] [PubMed]
- Hebditch, M.; Warwicker, J. Web-based display of protein surface and pH-dependent properties for assessing the developability of biotherapeutics. Sci. Rep. 2019, 9, 1969. [Google Scholar] [CrossRef] [PubMed]
- Klose, D.P.; Wallace, B.A.; Janes, R.W. 2Struc: The secondary structure server. Bioinformatics 2010, 26, 2624–2625. [Google Scholar] [CrossRef] [PubMed]
- Kabsch, W.; Sander, C. Dictionary of protein secondary structure: Pattern recognition of hydrogen-bonded and geometrical features. Biopolymers 1983, 22, 2577–2637. [Google Scholar] [CrossRef] [PubMed]
- Chu, B. Laser Light Scattering: Basic Principles and Practise; Courier Corporation: New York, NY, USA, 2007. [Google Scholar]
- Minton, A.P. Recent applications of light scattering measurement in the biological and biopharmaceutical sciences. Anal. Biochem. 2016, 501, 4–22. [Google Scholar] [CrossRef] [PubMed]
- Vlassi, E.; Papagiannopoulos, A. Nanoformulation of fibrinogen by thermal stabilization of its electrostatic complexes with hyaluronic acid. Int. J. Biol. Macromol. 2020, 158, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Tian, Z.; Jiang, F.; Cong, H.; Zhu, S. Molecular weight determination of low molecular weight hyaluronic acid and chondroitin sulfate by gel permeation chromatography. Carbohydr. Polym. 2023, 311, 120488. [Google Scholar] [CrossRef] [PubMed]
- Borsali, R.; Pecora, R. Soft-Matter Characterization; Springer: New York, NY, USA, 2008. [Google Scholar]
- Øgendal, L. Light Scattering a Brief Introduction; University of Copenhagen: Copenhagen, Denmark, 2019. [Google Scholar]
- Papagiannopoulos, A.; Vlassi, E. Stimuli-responsive nanoparticles by thermal treatment of bovine serum albumin inside its complexes with chondroitin sulfate. Food Hydrocoll. 2019, 87, 602–610. [Google Scholar] [CrossRef]
- Berne, B.J.; Pecora, R. Dynamic Light Scattering: With Applications to Chemistry, Biology, and Physics; Dover Publications Inc.: New York, NY, USA, 2000. [Google Scholar]
- Alexander, M.; Dalgleish, D.G. Dynamic Light Scattering Techniques and Their Applications in Food Science. Food Biophys. 2006, 1, 2–13. [Google Scholar] [CrossRef]
- Hunter, R.J. Zeta Potential in Colloid Science: Principles and Applications, New paperback ed.; Colloid science; Academic Press: London, UK; San Diego, CA, USA, 1988. [Google Scholar]
- Ohshima, H. Electrophoretic Mobility of a Spherical Colloidal Particle in a Salt-Free Medium. J. Colloid Interface Sci. 2002, 248, 499–503. [Google Scholar] [CrossRef]
- Papagiannopoulos, A.; Sklapani, A. Xanthan-based polysaccharide/protein nanoparticles: Preparation, characterization, encapsulation and stabilization of curcumin. Carbohydr. Polym. Technol. Appl. 2021, 2, 100075. [Google Scholar] [CrossRef]
- Micsonai, A.; Wien, F.; Kernya, L.; Lee, Y.-H.; Goto, Y.; Réfrégiers, M.; Kardos, J. Accurate secondary structure prediction and fold recognition for circular dichroism spectroscopy. Proc. Natl. Acad. Sci. USA 2015, 112, E3095–E3103. [Google Scholar] [CrossRef] [PubMed]
- Matsunami, H.; Kikuchi, R.; Ogawa, K.; Kokufuta, E. Light scattering study of complex formation between protein and polyelectrolyte at various ionic strengths. Colloids Surf. B Biointerfaces 2007, 56, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.C.; Chen, W.L.; Mao, S.J.T. Antioxidant nature of bovine milk beta-lactoglobulin. J. Dairy Sci. 2007, 90, 547–555. [Google Scholar] [CrossRef]
- Nunes, C.S.; Rufato, K.B.; Souza, P.R.; De Almeida, E.A.M.S.; Da Silva, M.J.V.; Scariot, D.B.; Nakamura, C.V.; Rosa, F.A.; Martins, A.F.; Muniz, E.C. Chitosan/chondroitin sulfate hydrogels prepared in [Hmim][HSO4] ionic liquid. Carbohydr. Polym. 2017, 170, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Hassan, M.; Islam, A.; Ahmad, F. A Review of Methods Available to Estimate Solvent-Accessible Surface Areas of Soluble Proteins in the Folded and Unfolded States. Curr. Protein Pept. Sci. 2014, 15, 456–476. [Google Scholar] [CrossRef] [PubMed]
- Müller, A.; Burchard, W. Structure formation of surfactants in concentrated sulphuric acid: A light scattering study. Colloid Polym. Sci. 1995, 273, 866–875. [Google Scholar] [CrossRef]
- Schmitz, K.S.; Wang, B.; Kokufuta, E. Mechanism of Microgel Formation via Cross-Linking of Polymers in Their Dilute Solutions: Mathematical Explanation with Computer Simulations. Macromolecules 2001, 34, 8370–8377. [Google Scholar] [CrossRef]
- Bide, Y.; Fashapoyeh, M.A.; Shokrollahzadeh, S. Structural investigation and application of Tween 80-choline chloride self-assemblies as osmotic agent for water desalination. Sci. Rep. 2021, 11, 17068. [Google Scholar] [CrossRef]
- Brändén, C.-I.; Tooze, J. Introduction to Protein Structure, 2nd ed.; Garland Pub: New York, NY, USA, 1999. [Google Scholar]
- Whitford, D. Proteins: Structure and Function; John Wiley & Sons: Hoboken, NJ, USA, 2005. [Google Scholar]
- Loveday, S.M. β-Lactoglobulin heat denaturation: A critical assessment of kinetic modelling. Int. Dairy J. 2016, 52, 92–100. [Google Scholar] [CrossRef]
- Iurciuc-Tincu, C.-E.; Cretan, M.S.; Purcar, V.; Popa, M.; Daraba, O.M.; Atanase, L.I.; Ochiuz, L. Drug Delivery System Based on pH-Sensitive Biocompatible Poly(2-vinyl pyridine)-b-poly(ethylene oxide) Nanomicelles Loaded with Curcumin and 5-Fluorouracil. Polymers 2020, 12, 1450. [Google Scholar] [CrossRef] [PubMed]
- Rohman, N.; Ahmed, K.; Skelton, A.A.; Mohiuddin, T.; Khan, I.; Selvaraj, R.; Yamin, M. Theoretical insights and implications of pH-dependent drug delivery systems using silica and carbon nanotube. J. Mol. Graph. Model. 2023, 125, 108609. [Google Scholar] [CrossRef] [PubMed]
- McSwiney, M.; Singh, H.; Campanella, O.H. Thermal aggregation and gelation of bovine β-lactoglobulin. Food Hydrocoll. 1994, 8, 441–453. [Google Scholar] [CrossRef]
- Xiong, Y.L.; Dawson, K.A.; Wan, L. Thermal Aggregation of β-Lactoglobulin: Effect of pH, Ionic Environment, and Thiol Reagent. J. Dairy Sci. 1993, 76, 70–77. [Google Scholar] [CrossRef]
- Lapanje, S.; Poklar, N. Calorimetric and circular dichroic studies of the thermal denaturation of β-lactoglobulin. Biophys. Chem. 1989, 34, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, L.; Sarangi, J.; Misra, P.K. Organization of Amphiphiles, Part 1: Evidence in Favor of Pre-micellar Aggregates through Fluorescence Spectroscopy. Bull. Chem. Soc. Jpn. 2002, 75, 859–865. [Google Scholar] [CrossRef]
- Lombardo, D.; Munaò, G.; Calandra, P.; Pasqua, L.; Caccamo, M.T. Evidence of pre-micellar aggregates in aqueous solution of amphiphilic PDMS–PEO block copolymer. Phys. Chem. Chem. Phys. 2019, 21, 11983–11991. [Google Scholar] [CrossRef] [PubMed]
- Adabi, M.; Naghibzadeh, M.; Adabi, M.; Zarrinfard, M.A.; Esnaashari, S.S.; Seifalian, A.M.; Faridi-Majidi, R.; Tanimowo Aiyelabegan, H.; Ghanbari, H. Biocompatibility and nanostructured materials: Applications in nanomedicine. Artif. Cells Nanomedicine Biotechnol. 2017, 45, 833–842. [Google Scholar] [CrossRef] [PubMed]
- Tatur, S.; Maccarini, M.; Barker, R.; Nelson, A.; Fragneto, G. Effect of Functionalized Gold Nanoparticles on Floating Lipid Bilayers. Langmuir 2013, 29, 6606–6614. [Google Scholar] [CrossRef]
- Roach, P.; Farrar, D.; Perry, C.C. Interpretation of Protein Adsorption: Surface-Induced Conformational Changes. J. Am. Chem. Soc. 2005, 127, 8168–8173. [Google Scholar] [CrossRef]
- Tsutsumi, M.; Otaki, J.M. Parallel and Antiparallel β-Strands Differ in Amino Acid Composition and Availability of Short Constituent Sequences. J. Chem. Inf. Model. 2011, 51, 1457–1464. [Google Scholar] [CrossRef]
- Albani, J.-R. Sub-Structures Formed in the Excited State are Responsible for Tryptophan Residues Fluorescence in β-Lactoglobulin. J. Fluoresc. 2011, 21, 1683–1687. [Google Scholar] [CrossRef]
- Otteson, E.W.; Welch, W.H.; Kozel, T.R. Protein-polysaccharide interactions. A monoclonal antibody specific for the capsular polysaccharide of Cryptococcus neoformans. J. Biol. Chem. 1994, 269, 1858–1864. [Google Scholar] [CrossRef]
- Instrumentation for Fluorescence Spectroscopy. In Principles of Fluorescence Spectroscopy; Springer: Boston, MA, USA, 2006; pp. 27–61. [CrossRef]
- Mohammadi, F.; Bordbar, A.-K.; Divsalar, A.; Mohammadi, K.; Saboury, A.A. Analysis of Binding Interaction of Curcumin and Diacetylcurcumin with Human and Bovine Serum Albumin Using Fluorescence and Circular Dichroism Spectroscopy. Protein J. 2009, 28, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Barik, A.; Priyadarsini, K.I.; Mohan, H. Photophysical Studies on Binding of Curcumin to Bovine Serum Albumin. Photochem. Photobiol. 2003, 77, 597. [Google Scholar] [CrossRef]
- Rabe, M.; Kerth, A.; Blume, A.; Garidel, P. Albumin displacement at the air–water interface by Tween (Polysorbate) surfactants. Eur. Biophys. J. 2020, 49, 533–547. [Google Scholar] [CrossRef] [PubMed]
- Garidel, P.; Hoffmann, C.; Blume, A. A thermodynamic analysis of the binding interaction between polysorbate 20 and 80 with human serum albumins and immunoglobulins: A contribution to understand colloidal protein stabilisation. Biophys. Chem. 2009, 143, 70–78. [Google Scholar] [CrossRef]
- Piñeiro, L.; Novo, M.; Al-Soufi, W. Fluorescence emission of pyrene in surfactant solutions. Adv. Colloid Interface Sci. 2015, 215, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Dong, D.C.; Winnik, M.A. The P y Scale of Solvent Polarities. Solvent Effects on The Vibronic Fine Structure of Pyrene Fluorescence and Empirical Correlations with E T and Y VALUES. Photochem. Photobiol. 1982, 35, 17–21. [Google Scholar] [CrossRef]
- Matulis, D.; Baumann, C.G.; Bloomfield, V.A.; Lovrien, R.E. 1-Anilino-8-naphthalene sulfonate as a protein conformational tightening agent. Biopolymers 1999, 49, 451–458. [Google Scholar] [CrossRef]



| Composition of Secondary Structure Elements | Three State Composition (%) | Analysis vs. DSSP | DSSP |
|---|---|---|---|
| Helix | 16.04 | Similarity% | 100 |
| Sheet | 40.74 | Matthews CC | 1 |
| Other | 43.20 |
| Assignment | Β-Sheet/Β-Turn | A-Helix | Random Coil | Intramolecular β-Sheet | Others |
|---|---|---|---|---|---|
| Wavenumbers (cm−1) | 1685–1663 | 1655–1650 | 1648–1644 | 1632–1621 | 1639–1635 1616–1600 |
| Pure β-LG | 21.3% | 19.6% | 16.2% | 17.8% | 25.1% |
| β-LG/CS (NoTT) | 15.4% | 22.0% | 13.9% | 20.3% | 28.5% |
| β-LG/CS (TT) | 15.1% | 21.8% | 13.3% | 25.0% | 24.7% |
| β-LG/CS/T80 (NoTT) | 18.5% | 22.2% | 14.1% | 19.7% | 25.5% |
| β-LG/CS/T80 (TT) | 14.8% | 23.0% | 7.1% | 27.3% | 27.8% |
| Pure β-LG | A-Helix | Β-Sheet | Β-Turn | Irregular/Others |
|---|---|---|---|---|
| Untreated/pH 4 | 14.6% | 35.0% | 10.3% | 40.1% |
| Treated/pH 4 | 13.1% | 35.4% | 10.9% | 40.7% |
| Treated/pH 7 | 13.2% | 22.3% | 14.6% | 49.8% |
| β-LG/CS NPs | α-helix | β-sheet | β-turn | Irregular/Others |
| Untreated/pH 4 | 3.1% | 36.4% | 15.1% | 45.3% |
| Treated/pH 4 | 9.8% | 30.0% | 10.7% | 49.6% |
| Treated/pH 7 | 9.2% | 28.0% | 17.5% | 45.3% |
| β-LG/CS/T80 NPs | α-helix | β-sheet | β-turn | Irregular/Others |
| Untreated/pH 4 | 5.9% | 37.4% | 13.5% | 43.2% |
| Treated/pH 4 | 10.4% | 33.6% | 12.4% | 43.4% |
| Treated/pH 7 | 16.1% | 26.7% | 13.5% | 43.7% |
| Parameter/Sample | Pure β-LG | β-LG/CS NPs |
|---|---|---|
| KSV (104 M−1) | 0.211 ± 0.032 | 0.383 ± 0.104 |
| KA (104 M−1) | 4.96 ± 0.64 | 0.451 ± 0.586 |
| n | 1.32 ± 0.20 | 1.00 ± 0.27 |
| State | Sample/Parameter | I1/I3 | So (105 CPS·mL/mg) |
|---|---|---|---|
| Before TT | Pure β-LG | 1.60 ± 0.08 | 144 ± 9 |
| Pure β-LG/T80 | 1.27 ± 0.07 | 454 ± 20 | |
| β-LG/CS NPs | 1.36 ± 0.07 | 28.0 ± 0.9 | |
| β-LG/CS/T80 NPs | 1.32 ± 0.07 | 127 ± 7 | |
| After TT | Pure β-LG | 1.54 ± 0.08 | 128 ± 19 |
| Pure β-LG/T80 | 1.34 ± 0.07 | 414 ± 40 | |
| β-LG/CS NPs | 1.30 ± 0.07 | 334 ± 9 | |
| β-LG/CS/T80 NPs | 1.31 ± 0.07 | 422 ± 33 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pispas, I.; Spiliopoulos, N.; Papagiannopoulos, A. Biocompatible Preparation of Beta-Lactoglobulin/Chondroitin Sulfate Carrier Nanoparticles and Modification of Their Colloidal and Hydropathic Properties by Tween 80. Polymers 2024, 16, 1995. https://doi.org/10.3390/polym16141995
Pispas I, Spiliopoulos N, Papagiannopoulos A. Biocompatible Preparation of Beta-Lactoglobulin/Chondroitin Sulfate Carrier Nanoparticles and Modification of Their Colloidal and Hydropathic Properties by Tween 80. Polymers. 2024; 16(14):1995. https://doi.org/10.3390/polym16141995
Chicago/Turabian StylePispas, Ioannis, Nikolaos Spiliopoulos, and Aristeidis Papagiannopoulos. 2024. "Biocompatible Preparation of Beta-Lactoglobulin/Chondroitin Sulfate Carrier Nanoparticles and Modification of Their Colloidal and Hydropathic Properties by Tween 80" Polymers 16, no. 14: 1995. https://doi.org/10.3390/polym16141995
APA StylePispas, I., Spiliopoulos, N., & Papagiannopoulos, A. (2024). Biocompatible Preparation of Beta-Lactoglobulin/Chondroitin Sulfate Carrier Nanoparticles and Modification of Their Colloidal and Hydropathic Properties by Tween 80. Polymers, 16(14), 1995. https://doi.org/10.3390/polym16141995







