Abstract
Interest in the consumption of the fruits of pepper (Capsicum spp.) is not only due to its organoleptic characteristics, but also due to its bioactive compounds content, which are reported to provide essential benefits to human health. However, the amount and diversity of these compounds in each fruit specimen depend on its genotype and on a number of environmental factors. This work describes the quantitative ultra-high-performance liquid chromatography coupled to photodiode-array (UHPLC-PDA) analysis of the capsinoids content in four varieties of pepper (‘Habanero’, ‘Habanero Roxo’, ‘Bode’, and ‘Malagueta’) grown until different development stages in a greenhouse under controlled conditions. In all the varieties analyzed, capsiate was the only capsinoid found. The accumulation of capsiate, in all the pepper varieties, started from the 10th to the 20th day post-anthesis (dpa), and increased during the first days (between the 20th and the 27th dpa). From that moment a drastic reduction took place until the end of the ripening stage, except for ‘Bode’ peppers, where the capsiate content increased from the first harvest point on the 20th dpa up to the 76th dpa. The capsiate accumulation patterns over the development of the fruit has been related to the capsaicionoids accumulation patterns in the same samples of the four varieties of pepper. According to our results, the content evolution of both families of compounds will vary depending on each fruit’s genotype, as well as on environmental conditions. No clear trends have been established and, therefore, an in-depth analysis under controlled conditions should be carried out.
1. Introduction
Pepper belongs to the genus Capsicum and the Solanaceae family, original from tropical areas in America. From the 35 described, only five species have been domesticated: Capsicum chinense Jacq., C. frutescens L., C. annuum L., C. baccatum L., and C. pubescens Ruiz & Pav., with significant economic and social impact worldwide []. Capsicum fruits vary in size (thick or thin), shape (round, elongated, etc.), color (green, purple, chocolate, yellow, orange, or red, depending on pepper variety and maturation stage.), flavor, and pungency (from the non-pungent varieties to the hottest species) []. Due to the vast quantity and the diverse varieties consumed, pepper is among the most valued and commonly cultivated produce because of their color, flavor, and taste sensory attributes. The food industry is the principal user of pepper fruits. It is often used as a coloring and flavoring agent in sauce, soup, processed meat, snacks, candies, soft drinks, and alcoholic beverages []. In addition to their sensory features, oleoresin is extracted from pepper fruits and used as an ingredient in numerous commercial products such as insect repellent or even self-defense sprays; and peppers can be also employed in medicinal applications, since they are an important source of the kind of bioactive compounds that provide health benefits to consumers []. Among such bioactive compounds two families should be noted: capsaicinoids and capsinoids, exclusive to the genus Capsicum and responsible for pepper pungency []. Both are widely known for their pharmacological properties, such as anti-inflammatory, anti-carcinogenic, neurological, antimicrobial, and antioxidant. They also contribute to weight loss treatments, relieve pain, and provide gastrointestinal and cardiovascular benefits when ingested regularly [,,,,].
Capsaicinoid and capsinoid biosynthesis takes place in the placenta between the 10th and the 20th days post anthesis (dpa), but they can also be detected in some of the fruit tissues, such as seeds or pericarp, due to the fact that they are eventually excreted []. Capsaicinoids, and also probably capsinoids, are ultimately produced by capsaicin synthase through the condensation of an aromatic moiety, derived from vanillin, with fatty acid branched-chains of 9–11 carbon atoms []. In addition, their fundamental chemical structures are rather similar, with the exception of their central bond. Thus, while capsinoids have an ester group, capsaicinoids have an amide group. This difference in their structure seems to be responsible for the lower pungency of capsinoids, roughly determined as 1000 times lower compared to that of capsaicinoids. Therefore, the employment of capsinoids is particularly attractive as a food additive or medicinal product, since they do not present the side effects of capsaicinoids such as irritation or burning sensations [,].
Capsinoids were first reported by Yazawa et al., in a non-pungent pepper cultivar CH-19 Sweet []. To date, three capsinoids, capsiate, dihydrocapsiate, and nordihydrocapsiate, have been described in pepper fruits, capsiate being the major one. Later on, these compounds were detected in other varieties of non-pungent and low pungent peppers, as well as in hot and super-hot pepper cultivars, although in considerably lower concentrations than capsaicinoids. In pungent peppers, vanillin is converted to both vanillylamine and vanillyl alcohol, which in turns gives place to the production of capsaicinoids and capsinoids, respectively []. The putative aminotransferase (p-AMT) gene encodes the aminotransferase enzyme (p-AMT) that catalyzes the formation of vanillylamine from vanillin in the capsaicinoid biosynthetic pathway. Different mutations in the p-AMT gene have been described as always leading to a loss-of-function with the subsequent increment in the production of vanillyl alcohol. Consequently, pepper genotypes carrying a pamt allele are non or low-pungent due to the high production of capsinoids over that of capsaicinoids [,]. Therefore p-AMT allele could be considered as a useful gene to control the content of capsaicinoids and capsinoids in pepper breeding programs [,].
The content of capsaicinoid and capsinoid compounds in peppers can be affected by different factors, including water availability (there is a significant reduction in fruit yield when a reduced amount of water is applied during the periods of vegetative growth, flowering, and fruiting) [], light (it regulates morphological characteristics and acts as a source of energy for the primary metabolism and photosynthetic processes) [], temperature, climatic conditions, genotype, cultivation techniques, mineral supply, growing conditions, and maturity stage (during the fruit ripening stage, several biochemical, physiological, and structural changes take place, and those changes govern the characteristics of the final fruit) [,]. Sampling and storage conditions need to be closely controlled to produce high-quality plant material for its characterization and further use [].
The present study has focused on four of the main pepper varieties consumed in Brazil: ‘Habanero’, ‘Habanero Roxo’, ‘Bode’, and ‘Malagueta’. ‘Habanero’ peppers are from the C. chinense family []. This intensely aromatic fruit is claimed to be one of the hottest varieties in the world, with pungency values between 100.000 and 300.000 Scoville Heat Units (SHUs). This chili pepper is dark green changing to orange, orange-red, red, or even chocolate (‘Habanero Roxo’) when fully ripe. Pod size normally varies from 2.9 to 6.0 cm in length, 2.5 to 4.6 cm in width, and 7 to 12 g in weight, and it is mainly used in sauces, chutneys, and marinades for seafood or pickles [,]. Its unique aroma, pungency and color are its most attractive properties and a quality reference for consumers. Brazil is considered a center of diversity for some Capsicum species (domesticated and wild) []. However, ‘Habanero’ pepper holds an enormous social and commercial relevance in other American countries, such as Mexico. The main production zones of ‘Habanero’ chili pepper in México are located in the states of Yucatan, Campeche, and Quintana Roo []. There is a current great interest in exporting this crop as a whole dehydrated product to the USA and Europe, where it is becoming an important source of extractable oleoresin. Pepper fruits and its derivatives are also commercialized worldwide as condiments, additives, and as the lachrymatory agent in pepper sprays; as well as a fungicidal and cytotoxic agent [].
The ‘Bode’ pepper variety also belongs to the C. chinense family and is native to Recife. It is widely cultivated throughout the northern and northwestern regions in Brazil []. Its fruits, of an intermediate pungency (15.000–30.000 SHU), are round and small, and their coloration varies between yellow, orange, and red when fully mature. This variety is highly valued in the kitchen for its smoky and fruity flavor and for its aroma. It is mostly consumed as pickles [].
‘Malagueta’ pepper is a variety of the C. frutescens species; mostly cultivated and consumed in Brazil, and particularly in the states of Minas Gerais, São Paulo, Bahia, and Goiás. It is widely used in the production of sauces and also in preserves, jams, and pastes []. Its color changes rapidly from green (unripe fruit) into red (ripe fruit) and, in some cases, it may present a light red color intermediate stage. As for the size of the fruit, it varies from 1 to 3 cm long and 0.4 to 0.5 cm wide, and they are conical with very thin walls [].
Capsaicinoid accumulation patterns for the four species above mentioned have been previously studied [,]. However, no assessment of the capsinoid accumulation patterns over the different fruit development stages, as well as a description of the correlation between capsinoid and capsaicinoid contents throughout those fruit development stages have been reported. For the purposes of this study, ultrasound-assisted extraction techniques will be used. Thus, high frequency ultrasonic waves, capable of causing cavitation due to the expansion and contraction cycles that the material goes through, will be applied. Such expansion and contraction cycles disrupt the cell walls in the vegetable matrix to favor the penetration of a solvent and, in turn, the mass transfer, which results in increasing extraction rates and yields [].
This paper intends to cast some light on two aspects that have been scarcely studied in relation to pepper cultivation: capsinoids accumulation at the different ripening stages of pepper fruits; and the potential correlation between capsinoid and capsaicinoid accumulation patterns in the varieties studied over their fruit development. The conclusions that may be reached with regards to these two aspects should help pepper breeders to determine the optimum harvesting moment that allow them to obtain the maximum added value from their crops.
2. Materials and Methods
2.1. Plant Material
Chili pepper seeds of the var. ‘Habanero’ (C. chinense), ‘Habanero Roxo’ (C. chinense), ‘Bode’ (C. chinense), and ‘Malagueta’ (C. frutescens) were supplied by the Vegetable Germplasm Bank in Zaragoza at the CITA of Aragón (Zaragoza, Spain). The seeds of the four varieties were germinated in Petri dishes, and then 10 plants per genotype were grown in a random distribution inside an acclimatized greenhouse in 17 cm diameter black plastic pots (one plant per pot), filled with a substrate mixture formed by peat, sand, and clay-loam soil as well as Humin Substrat (Klasman-Deilmann, Geeste, Germany) (1:1:1:1, v/v). Two grams of a slow-release fertilizer (Osmocote 16N-4P-9K, Scotts, Tarragona, Spain) were used as a topdressing for each pot. The plants were also watered daily by a drip irrigation system to maintain their optimum humidity levels for growth. Temperature levels were controlled of the whole process with values between 12–24 °C in the spring and 20–28 °C in the summer.
The flowers were labeled at the onset of their anthesis, so as to allow the fruit stage of development to be determined and hence each pepper’s age at the time of harvesting. The peppers were harvested during the last week of September, since the plants stopped producing new peppers (around 6-month-old plants). A total pepper weight varying between 232 and 346 g was harvested from all the plants at different stages of development in order to avoid particular effects from individual pepper plants. The maturation stages of the peppers at the time of harvest varied between immature green and senescent. Once the samples were harvested, all the fruits from all the plants of each variety were grouped together according to their dpa. The stem and seeds of the peppers were discarded before their analysis, while their pericarp and placenta were ground together in an Ultra-Turrax blender (IKA, Staufen, Germany) to produce a fully homogeneous sample that was then frozen at −20 °C until analysis.
2.2. Chemicals and Reagents
The analytical standards of the two major capsinoids, capsiate (CTE) (4-hydroxy-3-methoxybenzyl (E)-8-methyl-6-nonenoate) and dihydrocapsiate (DHCTE) (4-hydroxy-3-methoxybenzyl 8-methylnonanoate), were synthesized in the Department of Organic Chemistry at the University of Cadiz by Barbero et al. []. All of the samples were prepared in a mixture of HPLC grade methanol and ethyl acetate (99.9%) from Panreac Química S.L.U. (Castellar del Vallés, Barcelona, Spain), and Milli-Q water provided by a deionization system (Millipore, Bedford, MA, USA). For the chromatographic separation, HPLC grade acetonitrile (99.99%) from Panreac Química S.L.U. (Castellar del Vallés, Barcelona, Spain), glacial acetic acid (99%) from Merck (Darmstadt, Germany), and Milli-Q water were employed.
2.3. Fresh Pepper Extraction Procedure
The capsinoid extraction process from peppers at the different maturation stages was performed following a method previously developed by our research team []. The ultrasounds were applied by means of a Sonoplus probe (BANDELIN ELECTRONIC, Heinrichstraβe, Berlín, Germany) coupled to a 7 L refrigerated circulator for temperature control (PolyScience, Niles, IL, USA). The sample was immersed into a temperature-insulated double-walled vessel. Approximately 0.5 g of chili pepper from each different ripening stages were placed in a 50 mL plastic holder, followed by the addition of 15 mL of extraction solvent (which was composed by 42% methanol + 58% ethyl acetate). The sample was sonicated for 2 min at 5.5 °C, under 80% of the maximum allowed power (70 W) and applying duty cycles of 0.5 s. The extraction process was carried out in duplicate for each group of homogeneous samples. The average of the two values obtained would be considered as the final results. The extracts were centrifuged twice for 5 min at 7500 rpm (orbital radius 9.5 cm) and the supernatants were transferred to a 25 mL volumetric flask, which was made up to the mark with the same extraction solvent. The samples were filtered using a 0.22 µm nylon syringe filter (Membrane Solution, Dallas, TX, USA) and analyzed by means of a ultra-high-performance liquid chromatography coupled to photodiode-array (UHPLC-PDA) to confirm the presence of capsinoids.
2.4. UHPLC-Q-ToF-MS Identification of Capsinoids
In order to identify the capsinoids present in the pepper samples, a UHPLC system (Waters Corporation, Milford, MA, USA) with a 2.1 × 100 mm, 1.7 µm particle size rp-C18 analytical column (Acquity UPLC BEH C-18, Waters, MA, USA) was used. The UHPLC system was coupled to a quadrupole time-of-flight mass spectrometer (Q-ToF-MS) equipped with an electrospray ionization source (ESI) interface (Xevo G2 QToF, Waters Corporation, Milford, MA, USA) operating in positive ion mode. For the control of the equipment, its integration, and the subsequent data analysis, Masslynx software version 4.1 was employed. The UHPLC variables, as well as the operating conditions of the mass spectrometer were performed according to the method described by Vázquez-Espinosa et al. []. Spectra were acquired in the full-scan mode (m/z = 100–600). The molecular ions [M + H]+ and [M + Na]+ monitored for their identification were: CTE (m/z 307 and m/z 329), and DCHTE (m/z 309 and m/z 330), respectively. However, CTE was the only capsinoid detected and, therefore, quantified in the different varieties of pepper analyzed. Its chemical structure is shown in Figure 1. Furthermore, the mass spectrum showing the characteristic fragments that allow their identification can be found in supplementary material (Figure S1).
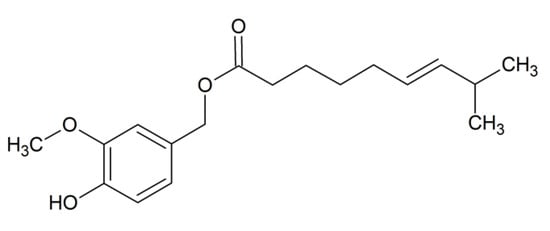
Figure 1.
Chemical structure of capsiate (CTE).
2.5. UHPLC-PDA Analysis of Capsinoids
After identifying the only capsinoid present in these pepper samples (CTE), the extracts were subjected to UHPLC-PDA using an Acquity Ultra Performance LC Class system (Waters Corporation, Milford, MA, USA) equipped with an autosampler operated at 15 °C, a Quaternary Pump System, and a Photodiode Array Detector (PDA) set to a wavelength of 280 nm for the detection and subsequent quantification of the compound present in the different pepper varieties. A Waters ACQUITY UPLC BEH rp-C18 100 x 2.1 mm column with 1.7 µm particle size, maintained at 50 °C, was used as the analytical column. Empower 3 software (Waters Corp., Milford, MA, USA) was used for the data treatment and equipment control. The UHPLC equipment variables were the same as in the method previously described by Vazquez-Espinosa et al. []. CTE, the major capsinoid, was the only one found in all the four pepper varieties that were analyzed. A calibration curve (y = 1682.50x + 164.74) was used for CTE quantification. Its regression equation and correlation coefficients (R2 = 0.9997), limit of detection (LOD = 3.60 ng g−1 of fresh weight (FW)) and quantification (LOQ = 12.00 ng g−1 of FW) were all determined. The quantitative data were obtained based on the integration of the UHPLC peak areas corresponding to three injections of the CTE analytical standard. A chromatogram of each variety obtained by UHPLC-PDA (280 nm), at the time of maximum capsiate concentration for each variety, has been included in supplementary material (Figure S2).
2.6. Statistical Analysis
A one-way analysis of variance (ANOVA), followed by a Tukey’s test, were performed to determine any significant differences (p-value < 0.05) in CTE contents depending on ripening stage. The results were expressed as the mean ± standard deviation (SD) for duplicate analysis. All of the data obtained from the analyses were dealt with by means of Statgraphic Centurion Version XVII (Statgraphics Technologies, Inc., The Plains, VA, USA).
3. Results and Discussion
3.1. Evolution of the Total Capsinoids Content
As mentioned above, capsinoids have excellent pharmacological effects on human health. In addition, they are considerably less spicy than capsaicinoids, which makes them more attractive and favorable for a regular daily intake, so that they provide all their benefits without the pungency side-effects. This is what makes any study on the correlation between fruit development stage and capsinoids content so interesting, so that harvesting can take place when highest capsinoid concentration is to be expected.
The number of reports that can be found in the literature on the evolution of capsinoids content in the different varieties of peppers is very low. Fayos et al., analyzed capsinoids content in the varieties ‘Chiltepín’, ‘Tampiqueño 74′, and ‘Bhut Jolokia’, but only at four specific moments over the fruit ripening period, specifically on the 10th, 20th, 40th, and 60th dpa []. According to their study, similar trends could be observed in the three genotypes, with the accumulation of capsinoids beginning between the 10th and the 20th dpa and then increasing up to their maximum concentration on the 40th dpa (with values reaching 276.39 µg g−1 of FW, 69.31 µg g−1 of FW, and 122.62 µg g−1 of FW, respectively). Finally, the content gradually decreased over the last stages of development []. Jang et al., also studied the evolution of these compounds at four different moments during the ripening process of four different varieties of pepper that ranged between spicy and slightly spicy ones. Similarly, they registered the largest content of capsinoids at the intermediate stages of the fruit development, i.e., between the 30th and the 40th dpa (with values around 603.66 µg g−1 of DW for ‘Habanero’ and around 300–400 µg g−1 for the other varieties) []. Finally, Jarret et al. carried out the same study on the variety C. annuum ‘509-45-1′. Immature green, mature green, turning, and mature red stages were considered in that occasion. As expected, capsinoid concentrations in the fruits increased rapidly on the 10th dpa and reached their maximum value over the mature green stage (at 1013 µg g−1 of DW), followed by a fall in capsinoid concentration levels [].
The aim of the present work was to complete a much more detailed monitoring process by increasing the number of time lapses analyzed throughout the ripening of the fruit, so that a deeper knowledge of and more detailed information about pepper’s beneficial compounds was attained, since previous studies had not reached such a thorough understanding of the process for any of the pepper varieties of interest. The ultimate objective is to precisely determine the moment of largest level of capsinoid concentration, and therefore, their optimal harvesting time. ‘Habanero’, ‘Habanero Roxo’, ‘Bode’, and ‘Malagueta’ are the pepper varieties selected for the study, and their content has been controlled at 10 different fruit developing stages (specifically on the 13th, 20th, 27th, 34th, 41st, 48th, 55th, 62nd, 69th, and 76th dpa). The visual appearance after the harvesting of the peppers analyzed at each one of the developing stages is shown in Figure 2. The samples were crushed before carrying out the extraction in order to increase the contact surface and facilitate the penetration of the solvent to favor a larger recovery [].

Figure 2.
Pepper fruits assayed for their capsiate accumulation patterns during development and maturation. Fruits of ‘Habanero’ (A), ‘Habanero Roxo’ (B), ‘Bode’ (C), and ‘Malagueta (D) at 13, 20, 27, 34, 41, 48, 55, 62, 69, and 76 dpa from left-to-right.
Capsiate, the major capsinoid, was the olnly one to be found in all of the pepper varieties analyzed. The evolution with regards to capsiate content (µg g−1 of FW) over the ripening process of the fruits is represented in Figure 3.
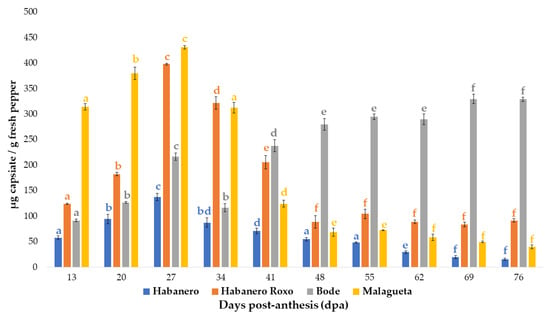
Figure 3.
Evolution of total capsiate content (µg g−1 of FW) during the development of pepper fruits (n = 2). According to the Tuckey’s test, results with a p-value less than 0.05 were considered to be statistically different. Taking this into account, the use of different letters in this figure indicates that there is a significant difference between results depending on Tuckey’s test. In turn, the letters of each color refer to their respective variety, that would be from left-to-right, blue for ‘Habanero’, orange for ‘Habanero Roxo’, grey for ‘Bode’, and yellow for ‘Malagueta’.
Similar behavior was observed in the ‘Habanero’, ‘Habanero Roxo’, and ‘Malagueta’ peppers, where the maximum CTE content was registered on the 27th dpa (with 137.84 µg g−1 of FW, 398.28 µg g−1 of FW, and 431.10 µg g−1 of FW, respectively). After that, ‘Habanero Roxo’ and ‘Malagueta’ presented drastic reductions in CTE content between the 27th and 48th days, corresponding to 77.66% and 83.96% of their maximum registered concentration, respectively. After the 48th day, their CTE concentration remained practically stable until the end of the ripening process. In the case of ‘Habanero’, after reaching its maximum level of CTE content, there was a substantial reduction (88.47% decrease) over this last period until the day 76th. All of these results are in agreement with those obtained by Jarret et al., for C. annuum ‘509-45-1′ pepper, as above explained. This evolution has also been reported by other researchers in relation to other Capsicum spp. such as ‘Chiltepín’, ‘Tampiqueño 74′ or ‘Bhut Jolokia’, although in their case, they reached their maximum concentration a few days later; this difference could be attributed to growing conditions or genotype reasons [].
Generally, the short number of studies that have been conducted on the accumulation of capsinoids in Capsicum fruits have shown that the concentration of these compounds increase during the early stages of the fruit development, and this trend goes on during the first stages of the ripening process until a maximum value is reached, usually between the 20th and 40th dpa [,,]. After that time, there is an inversion of the trend and a marked reduction in capsinoids content is observed. Such reduction in capsinoids content over the last stages of the fruit development, could be associated with a reduction in the biosynthesis of capsinoids inside the pepper according to the specific cultivation conditions in the greenhouse [] or, alternatively, to the effect of the peroxidases that can be found in peppers. Hot pepper peroxidases, especially peroxidase isoenzyme 6, oxidizes the phenolic precursors involved in capsaicin biosynthesis. Basic peroxidase isoenzyme 6 is located in the placental epidermal cells and in the vacuoles, where capsaicinoids are synthesized, and their capacity to degrade these compounds is due to the strong capsaicin-oxidizing activity of this isoenzyme [,]. Cell walls and vacuoles are also the places where capsiate is accumulated. Based on this fact, Lema et al., have suggested that the same chili peroxidases that oxidize capsaicinoids vanillyl residues were also capable of oxidizing the vanillyl residues from capsinoids. The use of different inhibitors allowed to confirm that this peroxidase actually has the capacity to perform such oxidation. These results strongly support the assumption that the basic peroxidases that can be found in C. annuum could be responsible for the oxidation of their own CTE content [].
Conversely, it can be seen from Figure 3 that, in a global context, the total capsiate content in ‘Bode’ peppers raised from the first point of harvest on the 13th dpa until the 76th dpa, at which point there was a concentration of 329.46 µg g−1 of FW, which corresponds to 350% increment compared to the initial content. And this was so, despite two perceptible CTE content falls that were registered during the maturation period: specifically, a decrease of 46.15% between the 27th dpa and the 34th dpa, and a slight reduction by just 1.63% between the 55th and the 62nd dpa. These falls in CTE content could be attributed to the action of the peroxidases in the peppers and to the subsequent reduction in the synthesis of their capsinoid content. In contrast to what is generally reported, a significant increase in the content of CTE was observed over the first ripening days, specifically from the first point of harvest on the 13th dpa (91.96 µg g−1 of FW) up to the 27th dpa, where it reached a concentration of 217.03 µg g-1 of FW. This was followed by an increment in CTE content between the 34th and 55th dpa. The final increase in the total content of CTE that took place in the last stage of the maturation process could be due to the loss of water suffered by overripen peppers. Nevertheless, these facts will not be considered for the object of this study, since such overripe peppers are not suitable for commercialization due to inadequate organoleptic attributes.
Most of the studies conducted on the different pepper varieties have reported that capsinoid content decreased rapidly as the fruits matured and changed color. This substantial reduction in capsinoid content as pepper ripens supports the need to perform sampling of the fruit at the appropriate development stages. In addition to all the factors that affect capsinoid content and that been already mentioned, including the decreased gene expression or peroxidase action, the instability of these compounds in different solvents should also be taken into account. Capsinoids are esters of fatty acid and vanillyl alcohol, so they are stable in non-polar solvents such as ethyl acetate, but they decompose easily in polar solvents such as water, methanol, and so on []. This is why, if the highest concentration of these compounds of interest and their health benefits are to be attained, green peppers should be eaten raw. Since these compounds are unstable in water, and also when subjected to high temperatures, cooking should be avoided in order to keep the largest possible content of capsinoids. Another factor to keep in mind is that as these fruits mature and turn from green into red color, their present a smaller capsinoid content [].
Although it has been seen that, in general, the accumulation pattern of CTE content in pepper fruit during its ripening stages followed similar trends, it is also true that, depending on the pepper’s genotype, as well as on the growing conditions or environmental factors, such content may vary and result differently accordingly. In this sense, it would be necessary to monitor every detail of each crop’s cultivation conditions, including all the possible environmental factors, since they may greatly influence the final product and its composition [,]. For this reason, it would be necessary to complete deeper studies where a greater number of varieties and under a wider range of different conditions would be analyzed. The present work intends to be a preliminary study that can be used as a starting point to demonstrate that there is no fixed pattern with regards to the variations in capsinoid content during the fruit ripening, so that each variety reaches a maximum concentration of these beneficial compounds at different stages of development.
3.2. Comparison of Capsiate and Capsaicinoids Contents
A comparison between the results obtained in this work with respect to the capsaicinoid accumulation patterns previously reported has been carried out in order to determine any similarities or differences in both compound families (capsinoids and capsaicinoids), both with similar pharmacological properties but different pungent capacities. It should be noted that the fruits have been grown in acclimatized greenhouses and under the same conditions (they are the same samples as the ones used for our previous work on capsaicinoids [,]), which should allow a more reliable comparison. It should also be noted that both families of compounds share part of their biosynthetic pathway.
3.2.1. ‘Habanero’ pepper
The maximum capsaicinoid content in ‘Habanero’ peppers was obtained on the 33th dpa (≈1400 µg g−1 of DW) []. A slight reduction in their content (8.5%) was observed from the 34th until the 48th dpa, which was associated to the effect of the peroxidases. After that, the capsaicinoid content remained practically constant over the rest of the ripening process. The evolution of the CTE content followed a similar trend. However, it reached its maximum concentration (137.84 µg g−1 of FW) a few days earlier, specifically on the 27th dpa, possibly as a consequence of the greater degradability and instability of this compound []. Furthermore, it was notable that such reduction was substantially more drastic (88.47%) than that in capsaicinoids and continued decreasing until the end of the maturation process. This correlation between the accumulation patterns from each compound family seems to indicate that the environmental factors have had similar effects on both biosynthetic pathways.
3.2.2. ‘Habanero Roxo’ pepper
As can be seen in our previous work [], each family of compounds present distinctive evolution patterns, even when capsaicinoid and capsinoid contents have been determined for the same plant that had been grown under the same environmental conditions. It can then be said that the differences in content between the two families seem to be due to genetic factors inherent to this variety. The maximum capsaicinoid content was registered on the 41st dpa, which coincided, in this case, with a change of color in the peppers from green to violet. From that moment, there was a period over which the concentration remained practically stable until the 55th dpa. After that, the capsaicinoid concentration increased substantially until the over-ripening stage was reached. On the contrary, the maximum CTE content was registered on the 27th dpa, and then a drastic reduction by 77.66% took place between the 27th and 48th dpa. From then on, the CTE concentration remained practically stable until the end of the fruit’s ripening process.
3.2.3. ‘Bode’ pepper
In our previous studies on this pepper variety [], a similar behavior was observed with regards to the accumulation pattern of the two families of compounds. Both the total capsaicinoid and CTE content raised from the first point of harvest until the end of the ripening period. A substantial increase in the content of these compounds was observed in the early stages of the fruit development up to the 33rd dpa for capsaicinoids and to the 20th dpa for CTE. At the end of the fruit’s maturation, two more moderate increments were registered (the former took place between the 48th and the 69th dpa, and the latter between the 76th and 83rd dpa), which could be reasonably associated with genetic factors since the plant had been cultivated under the same environmental conditions and, therefore, we should assume that both families of compounds had been equally affected. The specific growing conditions that were controlled in the greenhouse were temperature, humidity, irrigation, and fertilization, and these controlled conditions resulted in some increment in the amount of both compounds. It was also observed that halfway through the development of the fruit, a decrease in the amount of these compounds took place as a result of the action of the peroxidase enzymes in the peppers [].
3.2.4. ‘Malagueta’ pepper
The fruit accumulation pattern for capsaicinoids during the ripening period of this variety followed the same trend as the ‘Bode’ variety []. Thus, in general, there was a concentration increment over the ripening, even if, as previously mentioned, there was a series of increases and decreases throughout the process. Nevertheless, CTE evolution followed a particular evolution pattern where the maximum concentration was reached on the 27th dpa, after which a drastic reduction in the content (83.96%) took place. This was followed by a content stability period until the end of the maturation process.
Firstly, it should be noted that for all of the pungent varieties that have been analyzed, capsaicinoids were found in peppers in substantially larger concentrations that capsinoids. The main capsaicinoids found in peppers are capsaicin and dihydrocapsaicin, both with considerably larger concentrations than that of CTE, the only capsinoid detected. Nevertheless, the concentration of CTE in the varieties that have been analyzed was slightly above the concentration levels registered for other capsaicinoids (nordihydrocapsaicin, homocapsaicin, and homodihydrocapsaicin). Moreover, no similar concentration pattern or trend has been encountered that could equally be applied to the different varieties under study. This indicates, as mentioned above, that the genetic factors that are inherent to each variety play a significant role with regards to accumulation patterns or compound contents. Furthermore, since environmental factors seem to have a considerable influence on such contents and patterns, every possible detail with regards to growing conditions should be closely monitored in order to determine their influence on the fruit final composition [,]. Thus, and considering the greater degradability of capsinoids, growing conditions should be carefully contemplated and implemented. Future studies that intend to deepen not only in the study of capsinoid accumulation patterns in a greater number of varieties, but also in their comparison with those of capsaicinoids in the same varieties should be covered.
4. Conclusions
The current work has demonstrated, for a number of pepper varieties, that the bioactive content of their fruits with regards to the bioactive compounds responsible for pepper pungency, capsaicinoids, and capsinoids, may vary widely depending on their genotype, the fruit developmental stage, and the specific growing conditions. Since drastic changes in CTE content have been observed over the ripening period, determining how maturity stages may affect the composition of the peppers with regards to such biologically interesting bioactive compounds is of the utmost interest. This study intends to determine the optimal harvesting moment based on the moment of the greatest CTE content, which would be on the 27th dpa for ‘Habanero’, ‘Habanero Roxo’, and ‘Malagueta’ peppers, and on the 55th dpa for ‘Bode’ peppers (the later increase has not been taken into account since they are overripe peppers that are not suitable for consumption due to their organoleptic properties). This study also has deepened knowledge of the accumulation patterns for CTE content over the fruit development and their correlation with pepper capsaicinoid content. For the four varieties under study, different accumulation patterns of the two families of compounds of interest have been determined. Since no definitely clear pattern has been established, an in-depth study with a greater number of varieties and fruit-development monitoring points would be necessary. The variations that our study has registered with regards to bioactive compound contents, and that can be attributed to genetic factors, constitute a practical foundation for the improvement of the nutritional qualities of pepper products.
Supplementary Materials
The following are available online at https://www.mdpi.com/2073-4395/10/9/1337/s1. Figure S1. Mass spectrum of capsiate obtained by UHPLC-QToF-MS. Figure S2. Representative chromatograms for the four varieties of peppers studied ((A) ‘Habanero’; (B) ‘Habanero Roxo’; (C) ‘Bode’; (D) ‘Malagueta’) obtained by UHPLC-PDA (280 nm) at the point of maximum capsiate content. (1) Nor-dihydrocapsaicin (n-DHC); (2) Capsaicin (C); (3) Dihydrocapsaicin (DHC); (4) Homo-capsaicin (h-C); (5) Homo-dihydrocapsaicin (h-DHC); (6) Capsiate (CTE).
Author Contributions
Conceptualization, G.F.B. and A.G.-C.; methodology, M.V.-E.; software, M.F.-G.; formal analysis, M.V.-E., O.F., and A.V.G.-d.-P.; investigation, M.V.-E.; resources, M.P., and A.G.-C.; data curation, M.F.-G., O.F., and E.E.-B.; writing—original draft preparation, M.V.-E.; writing—review and editing, G.F.B., E.E.-B., and O.F.; supervision, G.F.B.; project administration, A.G.-C. and G.F.B.; funding acquisition, A.G.-C. and G.F.B. All authors have read and agreed to the published version of the manuscript.
Funding
This work is part of the RTA2015-00042-C02-01 project funded by the National institute for Agriculture and Food Research and Technology (INIA, Spain) and cofinanced by the European Fund for Regional Development (FEDER). It was also supported by A11-17R project and V. la Andaluza and University of Cadiz by the project OT2016/046. The authors thank the Spanish Ministry of Education, Culture, and Sport for the predoctoral contract (FPU17-02962) granted to Mercedes Vázquez-Espinosa.
Acknowledgments
The authors are grateful to the Instituto de Investigación Vitivinícola y Agroalimentaria (IVAGRO) for providing the necessary facilities to carry out the research. A special remark goes to Carmelo García Barroso (in memoriam) for his contribution to the scientific community in the area of phenolic compounds and oenology and his important inputs to this research.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Mo, H.-S.; Jang, K.-S.; Hwang, J.-E.; Jeon, S.-G.; Kim, B. Horticultural and chemical quality characterization of accessions selected from four species of Capsicum. Hortic. Environ. Biotechnol. 2015, 56, 54–66. [Google Scholar] [CrossRef]
- Baenas, N.; Belović, M.; Ilic, N.; Moreno, D.A.; García-Viguera, C. Industrial use of pepper (Capsicum annum L.) derived products: Technological benefits and biological advantages. Food Chem. 2018, 274, 872–885. [Google Scholar] [CrossRef] [PubMed]
- Pino, J.; Gonzalez, M.; Ceballos, L.; Centurionyah, A.; Trujilloaguirre, J.; Latourneriemoreno, L.; Sauriduch, E. Characterization of total capsaicinoids, colour and volatile compounds of Habanero chilli pepper (Capsicum chinense Jack.) cultivars grown in Yucatan. Food Chem. 2007, 104, 1682–1686. [Google Scholar] [CrossRef]
- Davis, C.B.; Markey, C.E.; Busch, M.A.; Busch, K.W. Determination of Capsaicinoids in Habanero Peppers by Chemometric Analysis of UV Spectral Data. J. Agric. Food Chem. 2007, 55, 5925–5933. [Google Scholar] [CrossRef]
- Cisneros-Pineda, O.; Torres-Tapia, L.W.; Gutiérrez-Pacheco, L.C.; Contreras-Martín, F.; González-Estrada, T.; Peraza-Sánchez, S.R. Capsaicinoids quantification in chili peppers cultivated in the state of Yucatan, Mexico. Food Chem. 2007, 104, 1755–1760. [Google Scholar] [CrossRef]
- Cordell, G.A.; Araujo, O.E. Capsaicin: Identification, Nomenclature, and Pharmacotherapy. Ann. Pharmacother. 1993, 27, 330–336. [Google Scholar] [CrossRef] [PubMed]
- Chinn, M.S.; Sharma-Shivappa, R.R.; Cotter, J.L. Solvent extraction and quantification of capsaicinoids from Capsicum chinense. Food Bioprod. Process. 2011, 89, 340–345. [Google Scholar] [CrossRef]
- Sasahara, I.; Furuhata, Y.; Iwasaki, Y.; Inoue, N.; Sato, H.; Watanabe, T.; Takahashi, M. Assessment of the Biological Similarity of Three Capsaicin Analogs (Capsinoids) Found in Non-Pungent Chili Pepper (CH-19 Sweet) Fruits. Biosci. Biotechnol. Biochem. 2010, 74, 274–278. [Google Scholar] [CrossRef] [PubMed]
- Macho, A.; Sancho, R.; Daddario, N.; Minassi, A.; Appendino, G. Non-pungent capsaicinoids from sweet pepper. Eur. J. Nutr. 2003, 42, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Snitker, S.; Fujishima, Y.; Shen, H.; Ott, S.; Pi-Sunyer, X.; Furuhata, Y.; Sato, H.; Takahashi, M. Effects of novel capsinoid treatment on fatness and energy metabolism in humans: Possible pharmacogenetic implications. Am. J. Clin. Nutr. 2008, 89, 45–50. [Google Scholar] [CrossRef]
- Aza-González, C.; Nuñez-Palenius, H.G.; Ochoa-Alejo, N. Molecular biology of capsaicinoid biosynthesis in chili pepper (Capsicum spp.). Plant Cell Rep. 2010, 30, 695–706. [Google Scholar] [CrossRef]
- Avellán, O.F.; Giménez, C.M.; Garcés-Claver, A. Evolución del conocimiento sobre la pungencia de la cebolla (Allium cepa L.) y del pimiento (Capsicum spp.): Desde sus orígenes hasta el potencial nutracéutico actual. Revisión bibliográfica. Inf. Tec. Econ. Agrar. 2018, 114, 99–118. [Google Scholar]
- Kobata, K.; Sugawara, M.; Mimura, M.; Yazawa, S.; Watanabe, T. Potent Production of Capsaicinoids and Capsinoids by Capsicum Peppers. J. Agric. Food Chem. 2013, 61, 11127–11132. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Sonoyama, T.; Muraga, Y.; Koeda, S.; Goto, T.; Yoshida, Y.; Yasuba, K. Multiple loss-of-function putative aminotransferase alleles contribute to low pungency and capsinoid biosynthesis in Capsicum chinense. Mol. Breed. 2015, 35, 142. [Google Scholar] [CrossRef]
- Kobata, K.; Todo, T.; Yazawa, S.; Iwai, K.; Watanabe, T. Novel Capsaicinoid-like Substances, Capsiate and Dihydrocapsiate, from the Fruits of a Nonpungent Cultivar, CH-19 Sweet, of Pepper (Capsicum annuum L.). J. Agric. Food Chem. 1998, 46, 1695–1697. [Google Scholar] [CrossRef]
- Singh, S.; Jarret, R.; Russo, V.; Majetich, G.; Shimkus, J.; Bushway, R.; Perkins, B. Determination of Capsinoids by HPLC-DAD in Capsicum Species. J. Agric. Food Chem. 2009, 57, 3452–3457. [Google Scholar] [CrossRef]
- Lang, Y.; Kisaka, H.; Sugiyama, R.; Nomura, K.; Morita, A.; Watanabe, T.; Tanaka, Y.; Yazawa, S.; Miwa, T. Functional loss of pAMT results in biosynthesis of capsinoids, capsaicinoid analogs, in Capsicum annuumcv. CH-19 Sweet. Plant J. 2009, 59, 953–961. [Google Scholar] [CrossRef]
- Tanaka, Y.; Hosokawa, M.; Miwa, T.; Watanabe, T.; Yazawa, S. Newly Mutated putative-aminotransferase in Nonpungent Pepper (Capsicum annuum) Results in Biosynthesis of Capsinoids, Capsaicinoid Analogues. J. Agric. Food Chem. 2010, 58, 1761–1767. [Google Scholar] [CrossRef]
- Han, K.; Jeong, H.-J.; Sung, J.; Keum, Y.S.; Cho, M.-C.; Kim, J.-H.; Kwon, J.-K.; Kim, B.-D.; Kang, B.-C. Biosynthesis of capsinoid is controlled by the Pun1 locus in pepper. Mol. Breed. 2012, 31, 537–548. [Google Scholar] [CrossRef]
- Park, Y.-J.; Nishikawa, T.; Minami, M.; Nemoto, K.; Iwasaki, T.; Matsushima, K. A low-pungency S3212 genotype of Capsicum frutescens caused by a mutation in the putative aminotransferase (p-AMT) gene. Mol. Genet. Genom. 2015, 290, 2217–2224. [Google Scholar] [CrossRef]
- López-López, R.; Inzunza-Ibarra, M.A.; Cohen, I.S.; Fierro-Alvarez, A.; Sifuentes-Ibarra, E. Water use efficiency and productivity of habanero pepper (Capsicum chinense Jacq.) based on two transplanting dates. Water Sci. Technol. 2015, 71, 885–891. [Google Scholar] [CrossRef] [PubMed]
- Jeeatid, N.; Techawongstien, S.; Suriharn, B.; Bosland, P.W.; Techawongstien, S. Light intensity affects capsaicinoid accumulation in hot pepper (Capsicum chinense Jacq.) cultivars. Hortic. Environ. Biotechnol. 2017, 58, 103–110. [Google Scholar] [CrossRef]
- Menichini, F.; Tundis, R.; Bonesi, M.; Loizzo, M.; Conforti, F.; Statti, G.; De Cindio, B.; Houghton, P. The influence of fruit ripening on the phytochemical content and biological activity of Capsicum chinense Jacq. cv Habanero. Food Chem. 2009, 114, 553–560. [Google Scholar] [CrossRef]
- Barbero, G.F.; Ruiz, A.G.; Liazid, A.; Palma, M.; Vera, J.C.; Barroso, C.G.; Lovillo, M.P. Evolution of total and individual capsaicinoids in peppers during ripening of the Cayenne pepper plant (Capsicum annuum L.). Food Chem. 2014, 153, 200–206. [Google Scholar] [CrossRef]
- De Aguiar, A.C.; Coutinho, J.P.; Barbero, G.F.; Godoy, H.T.; Martínez, J. Comparative Study of Capsaicinoid Composition in Capsicum Peppers Grown in Brazil. Int. J. Food Prop. 2015, 19, 1292–1302. [Google Scholar] [CrossRef]
- The Food and Agriculture Organization (FAO). Global Forest Resources Assessment 2015; FAO: Rome, Italy, 2016. [Google Scholar]
- Soares, R.S.; Ribeiro, C.S.D.C.; Ragassi, C.F.; De Carvalho, S.I.C.; Maldonade, I.R.; Filho, J.G.D.S.; Braz, L.T.; Reifschneider, F.J.B. New Brazilian lines of Habanero pepper (Capsicum chinense): Morpho-agronomic and biochemical characterization in different environments. Sci. Hortic. 2020, 261, 108941. [Google Scholar] [CrossRef]
- Pino, J.; Sauri-Duch, E.; Marbot, R. Changes in volatile compounds of Habanero chile pepper (Capsicum chinense Jack. cv. Habanero) at two ripening stages. Food Chem. 2006, 94, 394–398. [Google Scholar] [CrossRef]
- Alvares-Bianchi, P.; Almeida da Silva, L.R.; da Silva Alencar, A.A.; Araújo Diniz Santos, P.H.; Pimenta, S.; Pombo Sudré, C.; Erpen-Dalla Corte, L.; Azeredo Gonçalves, L.S.; Rodrigues, R. Biomorphological Characterization of Brazilian Capsicum Chinense Jacq. Germplasm. Agronomy 2020, 10, 447. [Google Scholar] [CrossRef]
- Sosa-Moguel, O.; Pino, J.A.; Ayora-Talavera, G.; Sauri-Duch, E.; Cuevas-Glory, L. Biological activities of volatile extracts from two varieties of Habanero pepper (Capsicum chinense Jacq.). Int. J. Food Prop. 2017, 20, S3042–S3051. [Google Scholar] [CrossRef]
- Gonçalves, V.D.; Müller, D.H.; Fava, C.L.F.; Camili, E.C. Physiological ripeness of pepper ‘Bode Vermelha’ seeds. Rev. Caatinga 2015, 28, 137–146. [Google Scholar] [CrossRef][Green Version]
- Rossato, M.; Santiago, T.R.; Lopes, C.A. Reaction of Capsicum peppers commercialized in the Federal District to bacterial wilt. Hortic. Bras. 2018, 36, 173–177. [Google Scholar] [CrossRef]
- Dos Santos, P.; Aguiar, A.C.; Barbero, G.F.; A Rezende, C.; Martínez, J. Supercritical carbon dioxide extraction of capsaicinoids from malagueta pepper (Capsicum frutescens L.) assisted by ultrasound. Ultrason. Sonochem. 2015, 22, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, S.; Bianchetti, L.; Ragassi, C.F.; Ribeiro, C.; Reifschneider, F.; Buso, G.; Faleiro, F. Genetic variability of a Brazilian Capsicum frutescens germplasm collection using morphological characteristics and SSR markers. Genet. Mol. Res. 2017, 16, 16039689. [Google Scholar] [CrossRef] [PubMed]
- Olguín-Rojas, J.A.; Fayos, O.; Vázquez-León, L.A.; Palma, M.; Rodríguez-Jiménes, G.D.C.; Palma, M.; Garcés-Claver, A.; Barbero, G.F. Progression of the Total and Individual Capsaicinoids Content in the Fruits of Three Different Cultivars of Capsicum chinense Jacq. Agronomy 2019, 9, 141. [Google Scholar] [CrossRef]
- Fayos, O.; De Aguiar, A.C.; Jiménez-Cantizano, A.; Palma, M.; Garcés-Claver, A.; Martínez, J.; Mallor, C.; Ruiz-Rodríguez, A.; Palma, M.; Barroso, C.G.; et al. Ontogenetic Variation of Individual and Total Capsaicinoids in Malagueta Peppers (Capsicum frutescens) during Fruit Maturation. Molecules 2017, 22, 736. [Google Scholar] [CrossRef]
- Toma, M.; Vinatoru, M.; Paniwnyk, L.; Mason, T. Investigation of the effects of ultrasound on vegetal tissues during solvent extraction. Ultrason. Sonochem. 2001, 8, 137–142. [Google Scholar] [CrossRef]
- Barbero, G.F.; Molinillo, J.M.G.; Varela, R.M.; Palma, M.; Macías, F.A.; Barroso, C.G. Application of Hansch’s Model to Capsaicinoids and Capsinoids: A Study Using the Quantitative Structure−Activity Relationship. A Novel Method for the Synthesis of Capsinoids. J. Agric. Food Chem. 2010, 58, 3342–3349. [Google Scholar] [CrossRef]
- Vázquez-Espinosa, M.; González-De-Peredo, A.V.; Palma, M.; Barroso, C.G.; Palma, M.; Barbero, G.F.; Espada-Bellido, E.; González-De-Peredo, A.V. Optimizing and Comparing Ultrasound- and Microwave-Assisted Extraction Methods Applied to the Extraction of Antioxidant Capsinoids in Peppers. Agronomy 2019, 9, 633. [Google Scholar] [CrossRef]
- Vázquez-Espinosa, M.; Olguín-Rojas, J.A.; Fayos, O.; González-De-Peredo, A.V.; Espada-Bellido, E.; Palma, M.; Barroso, C.G.; Barbero, G.F.; Garcés-Claver, A.; Palma, M. Influence of Fruit Ripening on the Total and Individual Capsaicinoids and Capsiate Content in Naga Jolokia Peppers (Capsicum chinense Jacq.). Agronomy 2020, 10, 252. [Google Scholar] [CrossRef]
- Fayos, O.; Ochoa-Alejo, N.; De La Vega, O.M.; Savirón, M.; Orduna, J.; Mallor, C.; Barbero, G.F.; Garcés-Claver, A. Assessment of Capsaicinoid and Capsinoid Accumulation Patterns during Fruit Development in Three Chili Pepper Genotypes (Capsicum spp.) Carrying Pun1 and pAMT Alleles Related to Pungency. J. Agric. Food Chem. 2019, 67, 12219–12227. [Google Scholar] [CrossRef]
- Jang, S.; Han, K.; Jo, Y.D.; Jeong, H.-J.; Siddique, M.I.; Kang, B.-C. Substitution of a Dysfunctional pAMT Allele Results in Low-Pungency but High Levels of Capsinoid in Capsicum chinense ‘Habanero’. Plant Breed. Biotechnol. 2015, 3, 119–128. [Google Scholar] [CrossRef]
- Jarret, R.L.; Bolton, J.; Perkins, L.B. 509-45-1, a Capsicum annuum Pepper Germplasm Containing High Concentrations of Capsinoids. HortScience 2014, 49, 107–108. [Google Scholar] [CrossRef]
- Chan, C.-H.; Yusoff, R.; Ngoh, G.-C.; Kung, F.W.-L. Microwave-assisted extractions of active ingredients from plants. J. Chromatogr. A 2011, 1218, 6213–6225. [Google Scholar] [CrossRef] [PubMed]
- A Arce-Rodriguez, M.L.; Ochoa-Alejo, N. Silencing AT3 gene reduces the expression of pAmt, BCAT, Kas, and Acl genes involved in capsaicinoid biosynthesis in chili pepper fruits. Boil. Plant. 2015, 59, 477–484. [Google Scholar] [CrossRef]
- Bernal, M.A.; Calderón, A.A.; Ferrer, M.A.; De Cáceres, F.M.; Barceló, A.R. Oxidation of Capsaicin and Capsaicin Phenolic Precursors by the Basic Peroxidase Isoenzyme B6 from Hot Pepper. J. Agric. Food Chem. 1995, 43, 352–355. [Google Scholar] [CrossRef]
- Contreras-Padilla, M.; Yahia, E.M. Changes in Capsaicinoids during Development, Maturation, and Senescence of Chile Peppers and Relation with Peroxidase Activity. J. Agric. Food Chem. 1998, 46, 2075–2079. [Google Scholar] [CrossRef]
- Lema, A.; Martínez-Cortés, T.; Garcés, A.; Mallor Giménez, C.; Fayos, O.; Barbero, G.F.; Silvar, C.; Pomar, F. 5-5′ dicapsiate: Product of the oxidation of capsiate by cationic peroxidases from pepper (Capsicum annuum L.). In Proceedings of the XVIth EUCARPIA Capsicum and Eggplant Working Group Meeting, Kecskemét, Hungary, 12–14 September 2016; pp. 500–505, ISBN 978-615-5270-27-7. [Google Scholar] [CrossRef]
- Sutoh, K.; Kobata, K.; Watanabe, T. Stability of Capsinoid in Various Solvents. J. Agric. Food Chem. 2001, 49, 4026–4030. [Google Scholar] [CrossRef]
- Tanaka, Y.; Yoneda, H.; Hosokawa, M.; Miwa, T.; Yazawa, S. Application of marker-assisted selection in breeding of a new fresh pepper cultivar (Capsicum annuum) containing capsinoids, low-pungent capsaicinoid analogs. Sci. Hortic. 2014, 165, 242–245. [Google Scholar] [CrossRef]
- Harvell, K.P.; Bosland, P.W. The Environment Produces a Significant Effect on Pungency of Chiles. HortScience 1997, 32, 32. [Google Scholar] [CrossRef]
- Lima, M.; Carvalho, S.; Ragassi, C.F.; Bianchetti, L.; Faleiro, F.; Reifschneider, F. Characterization of a pepper collection (Capsicum frutescens L.) from Brazil. Genet. Mol. Res. 2017, 16, 16039704. [Google Scholar] [CrossRef]
- Ohyama, K.; Nogusa, Y.; Shinoda, K.; Suzuki, K.; Bannai, M.; Kajimura, S. A Synergistic Antiobesity Effect by a Combination of Capsinoids and Cold Temperature Through Promoting Beige Adipocyte Biogenesis. Diabetes 2016, 65, 1410–1423. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).