Wind Tunnel Evaluation of Plant Protection Products Drift Using an Integrated Chemical–Physical Approach
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
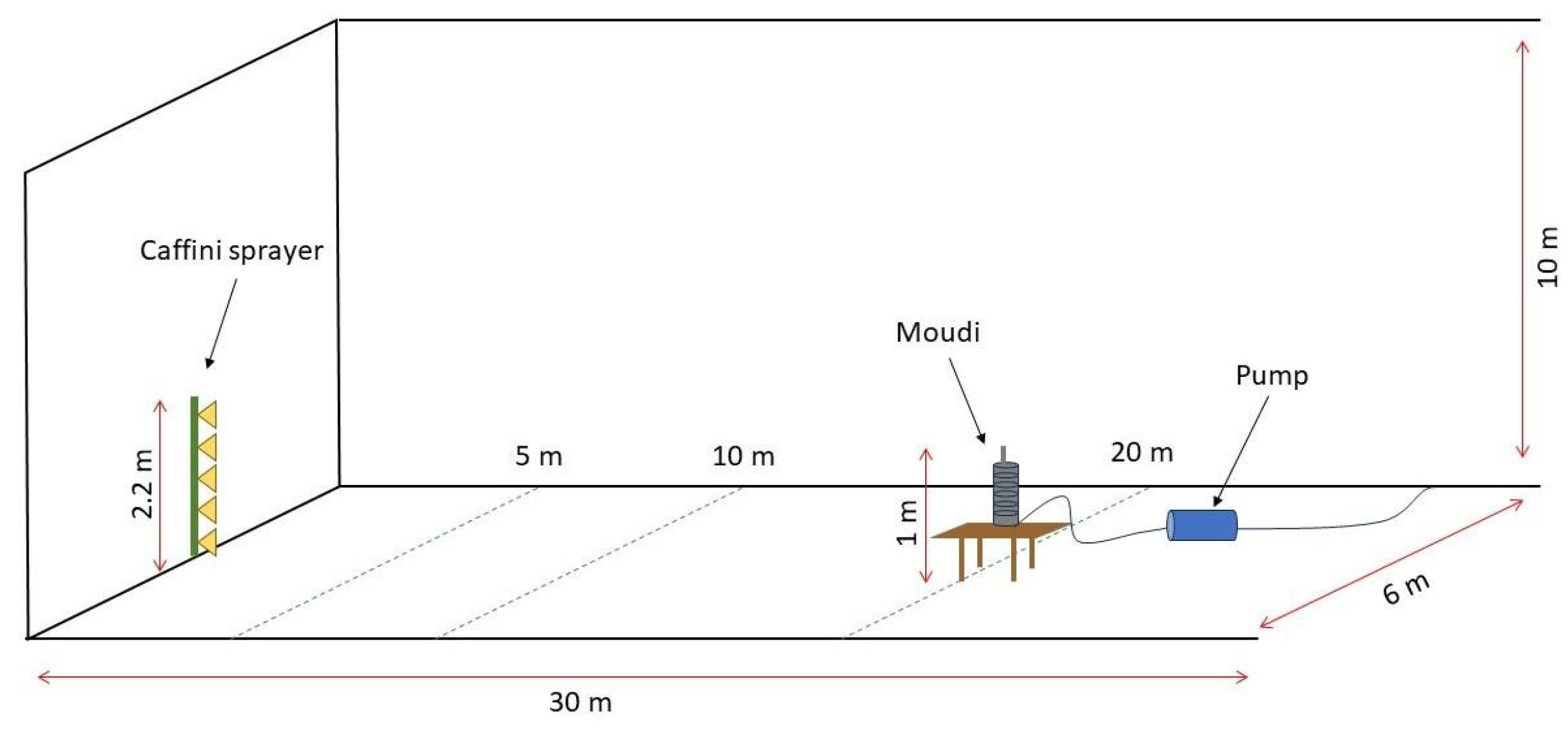
2.2. Experimental Set-Up
2.3. Nozzle Characterization
2.4. Aerosol Chemical Characterization
2.4.1. Preanalytical Treatment and Instrumental Analysis
2.4.2. Method Validation (AQ/CQ)
3. Results and Discussion
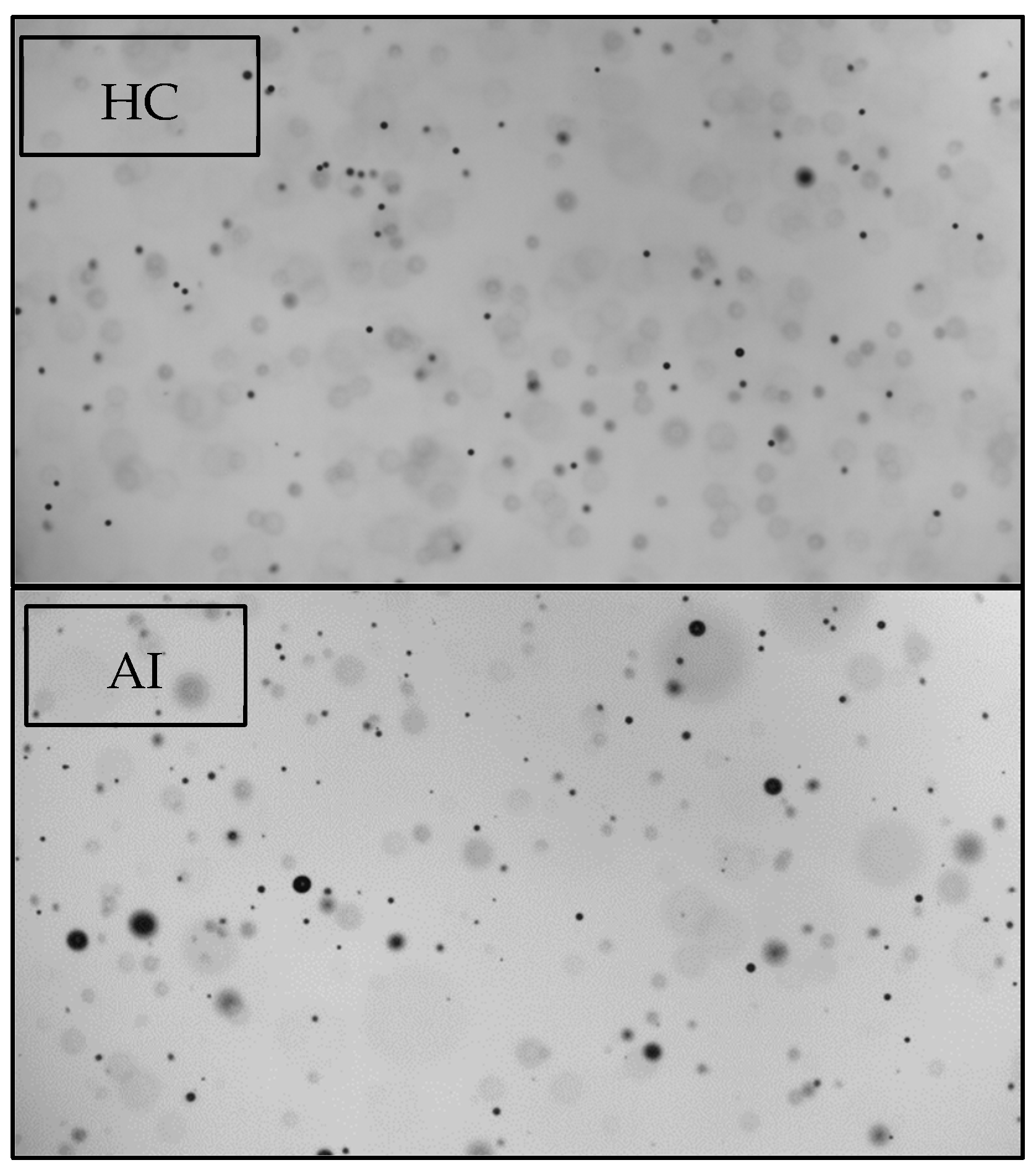
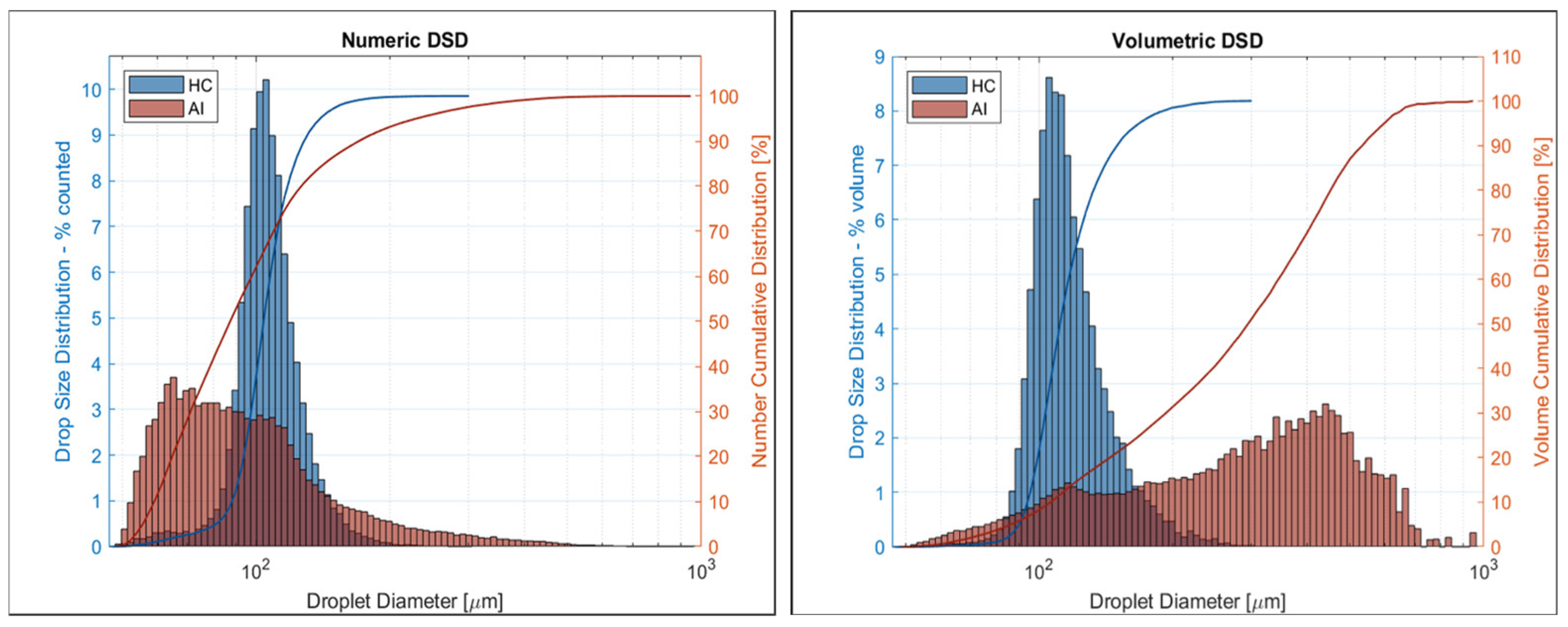
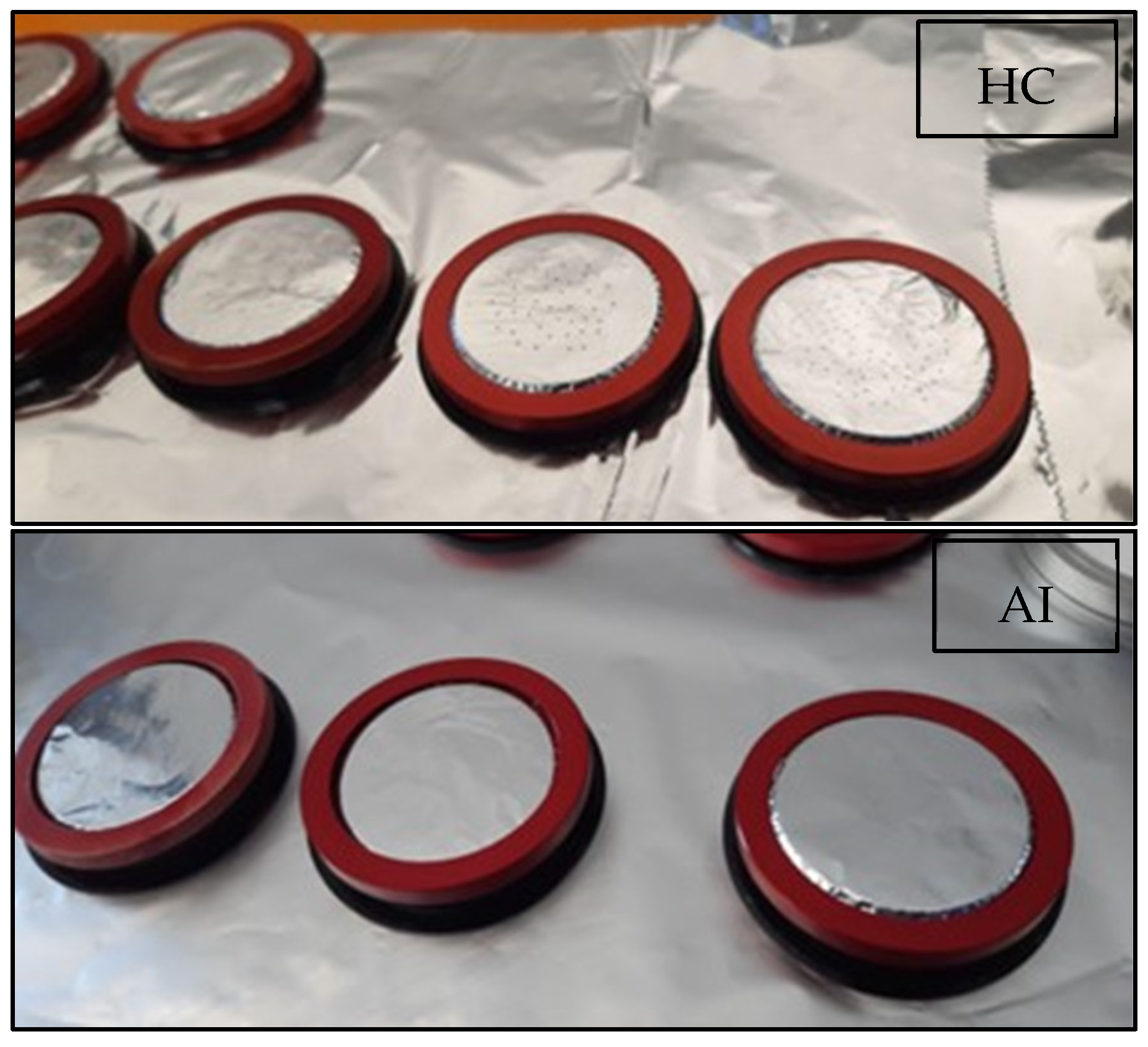
3.1. Nozzle Optical Analyses (Particles Larger Than 40 µm)
3.2. Chemical Analyses (Particle Range 0.056 to 40 µm)
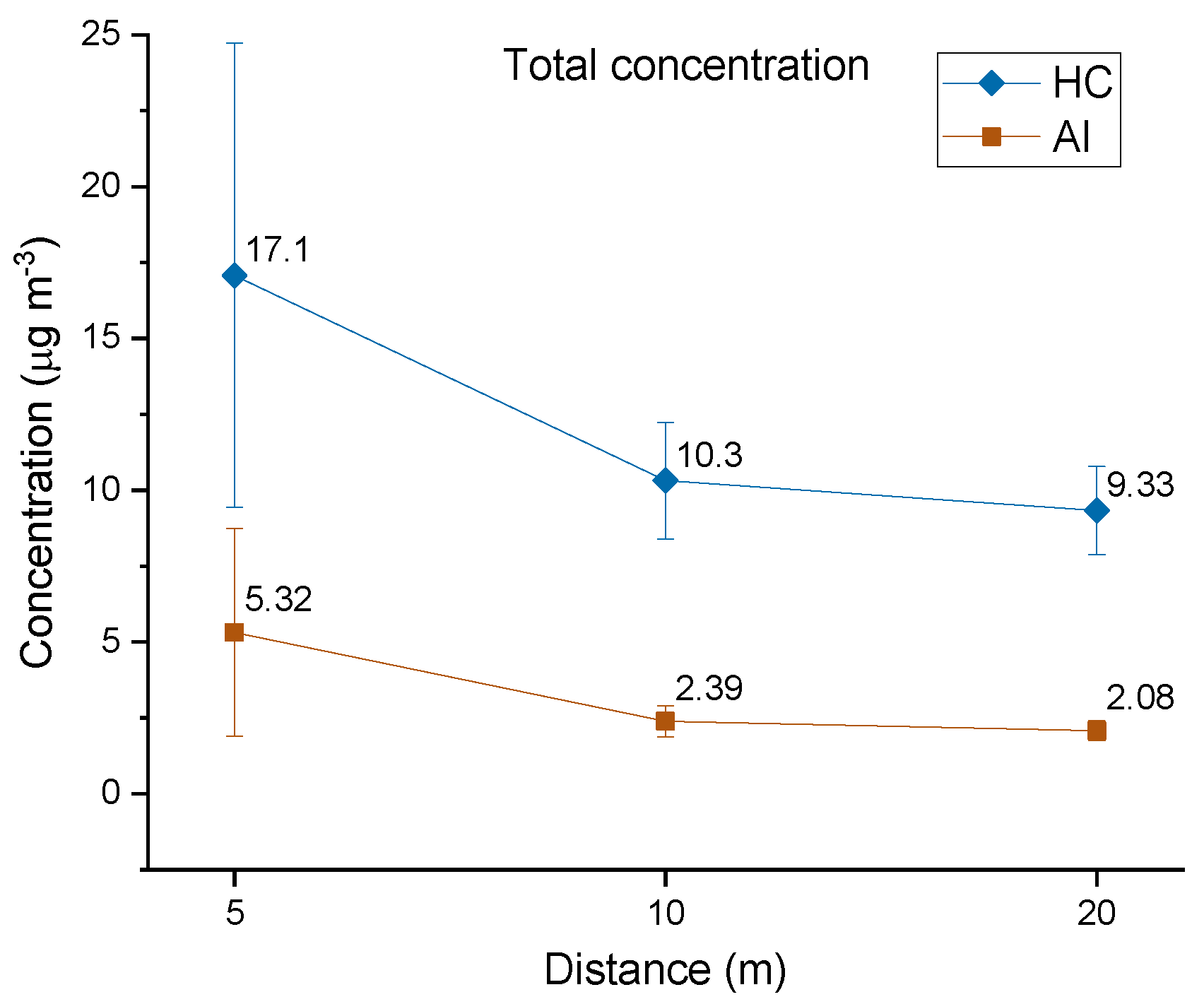
3.2.1. Total Concentration Trend and Stages
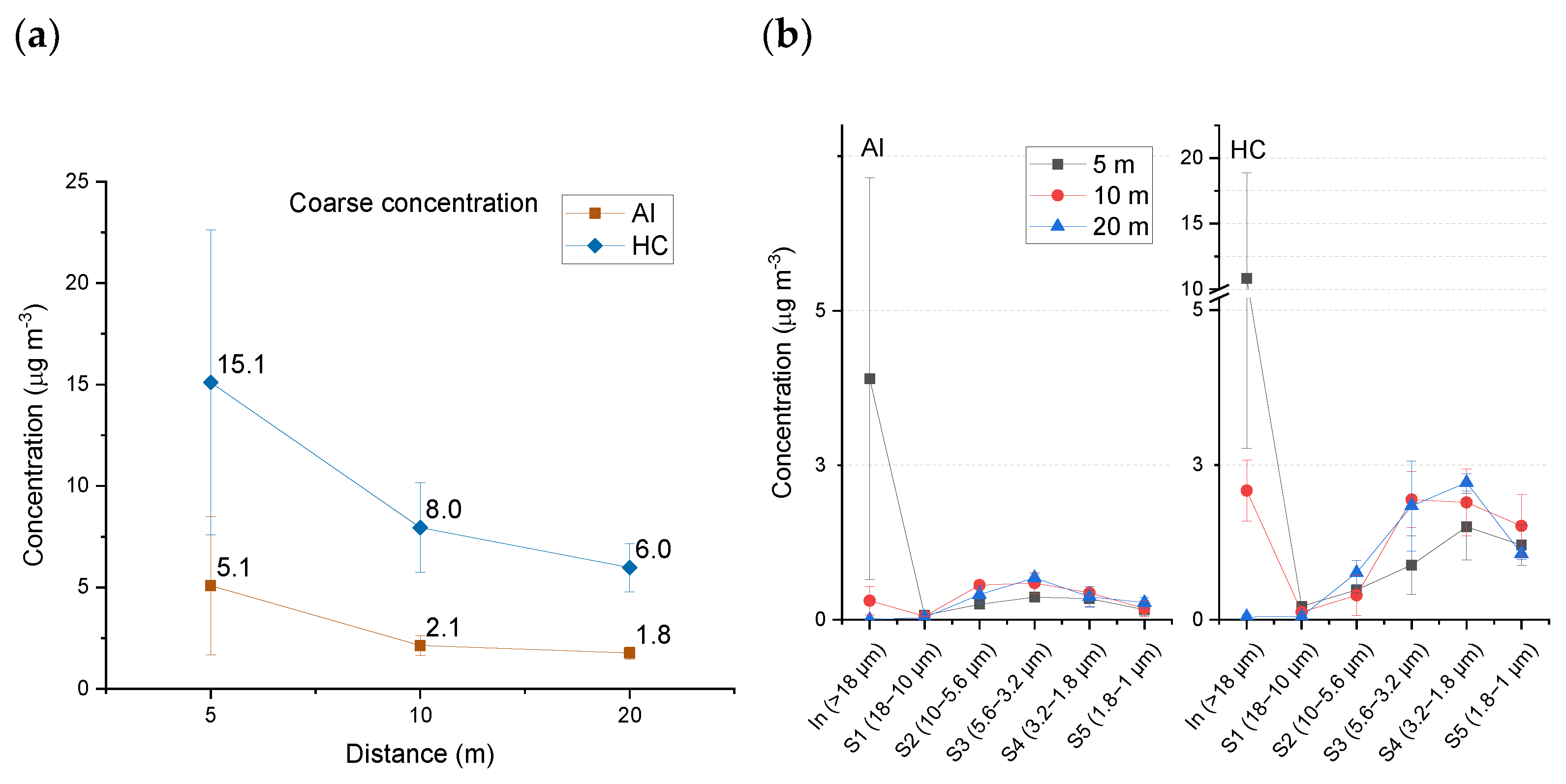
3.2.2. Coarse Fractions (Stages S Inlet–S5)
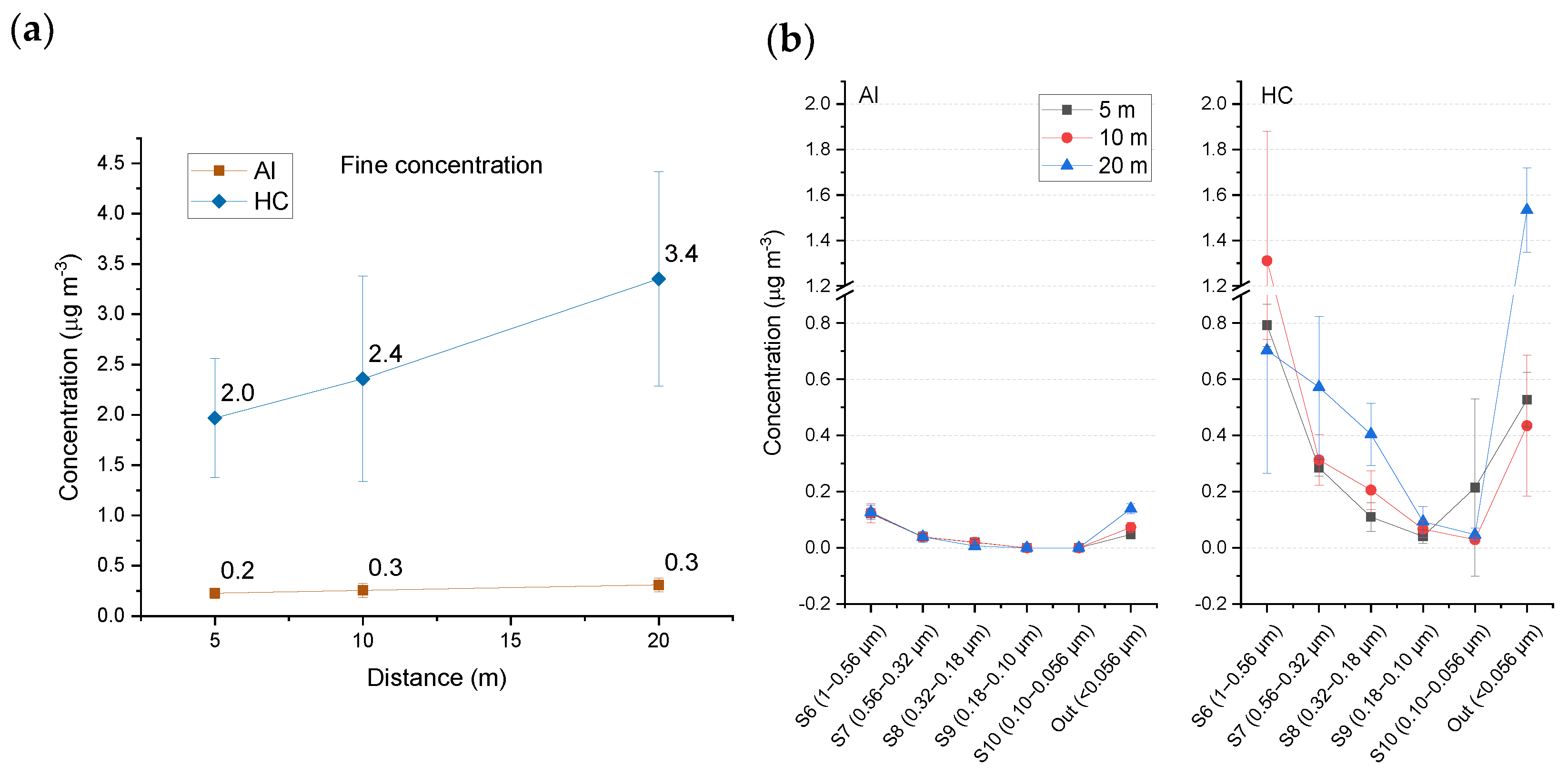
3.2.3. Fine Fractions (Stages S6-Out)
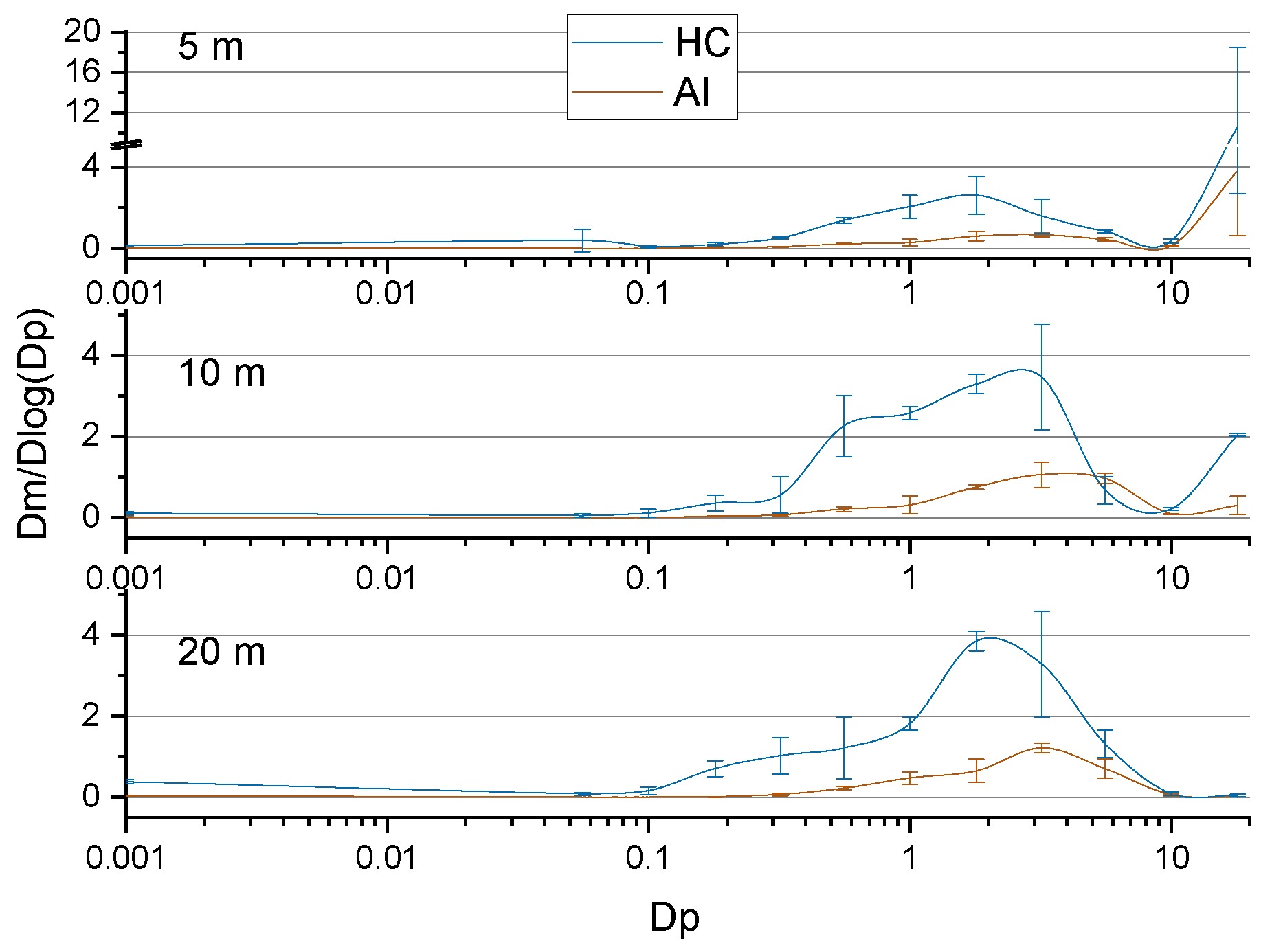
3.2.4. Modes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pogacean, M.; Gavrilescu, M. Plant Protection Products and Their Sustainable and Environmentally Friendly Use. Environ. Eng. Manag. J. 2009, 8, 607–627. [Google Scholar] [CrossRef]
- Betarbet, R.; Sherer, T.B.; MacKenzie, G.; Garcia-Osuna, M.; Panov, A.V.; Greenamyre, J.T. Chronic Systemic Pesticide Exposure Reproduces Features of Parkinson’s Disease. Nat. Neurosci. 2000, 3, 1301–1306. [Google Scholar] [CrossRef] [PubMed]
- Contini, D.; Cesari, D.; Genga, A.; Siciliano, M.; Ielpo, P.; Guascito, M.R.; Conte, M. Source Apportionment of Size-Segregated Atmospheric Particles Based on the Major Water-Soluble Components in Lecce (Italy). Sci. Total Environ. 2014, 472, 248–261. [Google Scholar] [CrossRef] [PubMed]
- Damalas, C.A.; Eleftherohorinos, I.G. Pesticide Exposure, Safety Issues, and Risk Assessment Indicators. Int. J. Environ. Res. Public Health 2011, 8, 1402–1419. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Chen, S.; Wang, G.; Lan, Y. Drift Evaluation of a Quadrotor Unmanned Aerial Vehicle (UAV) Sprayer: Effect of Liquid Pressure and Wind Speed on Drift Potential Based on Wind Tunnel Test. Appl. Sci. 2021, 11, 7258. [Google Scholar] [CrossRef]
- Mostafalou, S.; Abdollahi, M. Pesticides and Human Chronic Diseases: Evidences, Mechanisms, and Perspectives. Toxicol. Appl. Pharmacol. 2013, 268, 157–177. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, J.P.; Silva-Pavez, E.; Carrillo-Beltrán, D.; Calaf, G.M. Occurrence and Exposure Assessment of Glyphosate in the Environment and Its Impact on Human Beings. Environ. Res. 2023, 231, 116201. [Google Scholar] [CrossRef] [PubMed]
- Directive 2009/128/EC of the European Parliament and of the Council of 21 October 2009 Establishing a Framework for Community Action to Achieve the Sustainable Use of Pesticides Text with EEA Relevance. 2009. Available online: http://data.europa.eu/eli/dir/2009/128/2009-11-25 (accessed on 28 May 2024).
- Matthews, G.A. Application of Pesticides to Crops; World Scientific Publishing Company: Singapore, 1999; ISBN 978-1-911298-12-0. [Google Scholar]
- Wang, G.; Han, Y.; Li, X.; Andaloro, J.; Chen, P.; Hoffmann, W.C.; Han, X.; Chen, S.; Lan, Y. Field Evaluation of Spray Drift and Environmental Impact Using an Agricultural Unmanned Aerial Vehicle (UAV) Sprayer. Sci. Total Environ. 2020, 737, 139793. [Google Scholar] [CrossRef]
- Singh, N.K.; Sanghvi, G.; Yadav, M.; Padhiyar, H.; Christian, J.; Singh, V. Fate of Pesticides in Agricultural Runoff Treatment Systems: Occurrence, Impacts and Technological Progress. Environ. Res. 2023, 237, 117100. [Google Scholar] [CrossRef] [PubMed]
- Felsot, A.S.; Unsworth, J.B.; Linders, J.B.H.J.; Roberts, G.; Rautman, D.; Harris, C.; Carazo, E. Agrochemical Spray Drift; Assessment and Mitigation—A Review. J. Environ. Sci. Health Part B 2010, 46, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Samsonov, Y.N.; Makarov, V.I.; Koutsenogii, K.P.; Raputa, V.F. Wind Drifts of Pesticide Aerosols after Various Methods of Pesticide Application. J. Aerosol Sci. 1998, 29, S177–S178. [Google Scholar] [CrossRef]
- Holterman, H.J.; van de Zande, J.C.; Porskamp, H.A.J.; Huijsmans, J.F.M. Modelling Spray Drift from Boom Sprayers. Comput. Electron. Agric. 1997, 19, 1–22. [Google Scholar] [CrossRef]
- Kira, O.; Linker, R.; Dubowski, Y. Estimating Drift of Airborne Pesticides during Orchard Spraying Using Active Open Path FTIR. Atmos. Environ. 2016, 142, 264–270. [Google Scholar] [CrossRef]
- Miller, P. The Measurement of Spray Drift. Pest. Outlook 2003, 14, 205. [Google Scholar] [CrossRef]
- Precipito, L.M.B.; Ferreira, L.A.I.; Paduan, N.A.; Lima, J.S.d.S.; de Oliveira, R.B. Use of the Test Bench for Spray Drift Assessment under Subtropical Climate Conditions. Rev. Bras. Eng. Agrícola E Ambient. 2022, 27, 223–229. [Google Scholar] [CrossRef]
- Butler Ellis, M.C.; Lane, A.G.; O’Sullivan, C.M.; Jones, S. Wind Tunnel Investigation of the Ability of Drift-Reducing Nozzles to Provide Mitigation Measures for Bystander Exposure to Pesticides. Biosyst. Eng. 2021, 202, 152–164. [Google Scholar] [CrossRef]
- Grant, S.; Perine, J.; Abi-Akar, F.; Lane, T.; Kent, B.; Mohler, C.; Scott, C.; Ritter, A. A Wind-Tunnel Assessment of Parameters That May Impact Spray Drift during UAV Pesticide Application. Drones 2022, 6, 204. [Google Scholar] [CrossRef]
- Nuyttens, D.; Zwertvaegher, I.K.A.; Dekeyser, D. Spray Drift Assessment of Different Application Techniques Using a Drift Test Bench and Comparison with Other Assessment Methods. Biosyst. Eng. 2017, 154, 14–24. [Google Scholar] [CrossRef]
- Chen, S.; Lan, Y.; Zhou, Z.; Ouyang, F.; Wang, G.; Huang, X.; Deng, X.; Cheng, S. Effect of Droplet Size Parameters on Droplet Deposition and Drift of Aerial Spraying by Using Plant Protection UAV. Agronomy 2020, 10, 195. [Google Scholar] [CrossRef]
- Kira, O.; Dubowski, Y.; Linker, R. In-Situ Open Path FTIR Measurements of the Vertical Profile of Spray Drift from Air-Assisted Sprayers. Biosyst. Eng. 2018, 169, 32–41. [Google Scholar] [CrossRef]
- Ling, W.; Du, C.; Ze, Y.; xindong, N.; Shumao, W. Research on the Prediction Model and Its Influencing Factors of Droplet Deposition Area in the Wind Tunnel Environment Based on UAV Spraying. IFAC-PapersOnLine 2018, 51, 274–279. [Google Scholar] [CrossRef]
- Krogh, K.A.; Halling-Sørensen, B.; Mogensen, B.B.; Vejrup, K.V. Environmental Properties and Effects of Nonionic Surfactant Adjuvants in Pesticides: A Review. Chemosphere 2003, 50, 871–901. [Google Scholar] [CrossRef] [PubMed]
- Mesnage, R.; Benbrook, C.; Antoniou, M.N. Insight into the Confusion over Surfactant Co-Formulants in Glyphosate-Based Herbicides. Food Chem. Toxicol. 2019, 128, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Castro, M.J.L.; Ojeda, C.; Cirelli, A.F. Advances in Surfactants for Agrochemicals. Environ. Chem. Lett. 2014, 12, 85–95. [Google Scholar] [CrossRef]
- Cornacchia, I.; Tomas, S.; Douzals, J.-P.; Courault, D. Assessment of Airborne Transport of Potential Contaminants in a Wind Tunnel. J. Irrig. Drain. Eng. 2020, 146, 04019031. [Google Scholar] [CrossRef]
- Grella, M.; Miranda-Fuentes, A.; Marucco, P.; Balsari, P. Field Assessment of a Newly-Designed Pneumatic Spout to Contain Spray Drift in Vineyards: Evaluation of Canopy Distribution and off-Target Losses. Pest Manag. Sci. 2020, 76, 4173–4191. [Google Scholar] [CrossRef]
- Wang, C.; Wongsuk, S.; Huang, Z.; Yu, C.; Han, L.; Zhang, J.; Sun, W.; Zeng, A.; He, X. Comparison between Drift Test Bench and Other Techniques in Spray Drift Evaluation of an Eight-Rotor Unmanned Aerial Spraying System: The Influence of Meteorological Parameters and Nozzle Types. Agronomy 2023, 13, 270. [Google Scholar] [CrossRef]
- Kjær, C.; Bruus, M.; Bossi, R.; Løfstrøm, P.; Andersen, H.V.; Nuyttens, D.; Larsen, S.E. Pesticide Drift Deposition in Hedgerows from Multiple Spray Swaths. J. Pestic. Sci. 2014, 39, 14–21. [Google Scholar] [CrossRef]
- Makhnenko, I.; Alonzi, E.R.; Fredericks, S.A.; Colby, C.M.; Dutcher, C.S. A Review of Liquid Sheet Breakup: Perspectives from Agricultural Sprays. J. Aerosol Sci. 2021, 157, 105805. [Google Scholar] [CrossRef]
- ISO 10625; Equipment for Crop Protection—Sprayer Nozzles—Colour Coding for Identification. International Standards Organisation (ISO): Geneva, Switzerland, 2018.
- Carvalho, T.C.; Peters, J.I.; Williams, R.O. Influence of Particle Size on Regional Lung Deposition—What Evidence Is There? Int. J. Pharm. 2011, 406, 1–10. [Google Scholar] [CrossRef]
- National Research Council. Breathing, Deposition, and Clearance. In Comparative Dosimetry of Radon in Mines and Homes; National Academies Press (US): Washington, DC, USA, 1991. [Google Scholar]
- Hatch, T.F. Distribution and Deposition of Inhaled Particles in Respiratory Tract. Bacteriol. Rev. 1961, 25, 237–240. [Google Scholar] [CrossRef] [PubMed]
- Jabbal, S.; Poli, G.; Lipworth, B. Does Size Really Matter?: Relationship of Particle Size to Lung Deposition and Exhaled Fraction. J. Allergy Clin. Immunol. 2017, 139, 2013–2014.e1. [Google Scholar] [CrossRef]
- Suarez-Lopez, J.R.; Nazeeh, N.; Kayser, G.; Suárez-Torres, J.; Checkoway, H.; López-Paredes, D.; Jacobs, D.R.; de la Cruz, F. Residential Proximity to Greenhouse Crops and Pesticide Exposure (via Acetylcholinesterase Activity) Assessed from Childhood through Adolescence. Environ. Res. 2020, 188, 109728. [Google Scholar] [CrossRef] [PubMed]
- Pascuzzi, S.; Manetto, G.; Santoro, F.; Cerruto, E. A Brief Review of Nozzle Spray Drop Size Measurement Techniques. In Proceedings of the 2021 IEEE International Workshop on Metrology for Agriculture and Forestry (MetroAgriFor), Trento-Bolzano, Italy, 3–5 November 2021; pp. 351–355. [Google Scholar]
- Grella, M.; Maffia, J.; Dinuccio, E.; Balsari, P.; Miranda-Fuentes, A.; Marucco, P.; Gioelli, F. Assessment of Fine Droplets (<10 Μm) in Primary Airborne Spray Drift: A New Methodological Approach. J. Aerosol Sci. 2023, 169, 106138. [Google Scholar] [CrossRef]
- Kashdan, J.T.; Shrimpton, J.S.; Whybrew, A. Two-Phase Flow Characterization by Automated Digital Image Analysis. Part 2: Application of PDIA for Sizing Sprays. Part. Part. Syst. Charact. 2004, 21, 15–23. [Google Scholar] [CrossRef]
- Kashdan, J.T.; Shrimpton, J.S.; Whybrew, A. Two-Phase Flow Characterization by Automated Digital Image Analysis. Part 1: Fundamental Principles and Calibration of the Technique. Part. Part. Syst. Charact. 2003, 20, 387–397. [Google Scholar] [CrossRef]
- Cooper, J.F.; Smith, D.N.; Dobson, H.M. An Evaluation of Two Field Samplers for Monitoring Spray Drift. Crop Prot. 1996, 15, 249–257. [Google Scholar] [CrossRef]
- Coscollà, C.; Muñoz, A.; Borrás, E.; Vera, T.; Ródenas, M.; Yusà, V. Particle Size Distributions of Currently Used Pesticides in Ambient Air of an Agricultural Mediterranean Area. Atmos. Environ. 2014, 95, 29–35. [Google Scholar] [CrossRef]
- Coscollà, C.; Yahyaoui, A.; Colin, P.; Robin, C.; Martinon, L.; Val, S.; Baeza-Squiban, A.; Mellouki, A.; Yusà, V. Particle Size Distributions of Currently Used Pesticides in a Rural Atmosphere of France. Atmos. Environ. 2013, 81, 32–38. [Google Scholar] [CrossRef]
- Radoman, N.; Christiansen, S.; Johansson, J.H.; Hawkes, J.A.; Bilde, M.; Cousins, I.T.; Salter, M.E. Probing the Impact of a Phytoplankton Bloom on the Chemistry of Nascent Sea Spray Aerosol Using High-Resolution Mass Spectrometry. Environ. Sci. Atmos. 2022, 2, 1152–1169. [Google Scholar] [CrossRef]
- Ware, G.W.; Estesen, B.J.; Cahill, W.P.; Gerhardt, P.D.; Frost, K.R. Pesticide Drift. I. High-Clearance vs. Aerial Application of Sprays. J. Econ. Entomol. 1969, 62, 840–843. [Google Scholar] [CrossRef]
- Becce, L.; Carabin, G.; Mazzetto, F. Agroforestry Innovations Lab Activities on Sprayer Performance and Certification. In Proceedings of the AIIA 2022: Biosystems Engineering Towards the Green Deal, Palermo, Italy, 19–22 September 2022; pp. 305–313. [Google Scholar]
- Becce, L.; Mazzi, G.; Ali, A.; Bortolini, M.; Gambaro, A.; Mazzetto, F. Nozzle Characterisation to Support Aerosol Spray Drift Measurement in a Semi-Controlled Environment. In Proceedings of the 2023 IEEE International Workshop on Metrology for Agriculture and Forestry (MetroAgriFor), Pisa, Italy, 6 November 2023; pp. 646–651. [Google Scholar]
- Becce, L.; Amin, S.; Carabin, G.; Mazzetto, F. Preliminary Spray Nozzle Characterization Activities through Shadowgraphy at the AgroForestry Innovation Lab (AFI-Lab). In Proceedings of the 2022 IEEE Workshop on Metrology for Agriculture and Forestry (MetroAgriFor), Perugia, Italy, 3 November 2022; pp. 136–140. [Google Scholar]
- Lefebvre, A.H.; McDonell, V.G. Atomization and Sprays, 2nd ed.; CRC Press; Taylor & Francis Group: Boca Raton, FL, USA, 2017; ISBN 978-1-4987-3625-1. [Google Scholar]
- Torrent, X.; Gregorio, E.; Douzals, J.-P.; Tinet, C.; Rosell-Polo, J.R.; Planas, S. Assessment of Spray Drift Potential Reduction for Hollow-Cone Nozzles: Part 1. Classification Using Indirect Methods. Sci. Total Environ. 2019, 692, 1322–1333. [Google Scholar] [CrossRef] [PubMed]
- Arvidsson, T.; Bergström, L.; Kreuger, J. Spray Drift as Influenced by Meteorological and Technical Factors: Spray Drift as Influenced by Meteorological and Technical Factors. Pest Manag. Sci. 2011, 67, 586–598. [Google Scholar] [CrossRef] [PubMed]




| HC | AI | |||
|---|---|---|---|---|
| Avg ± std | CV (%) | Avg ± std | CV (%) | |
| dV10 [µm] | 98 ± 5 | 6 | 109 ± 4 | 4 |
| dV50 [µm] | 114 ± 6 | 5 | 286 ± 11 | 4 |
| dV90 [µm] | 150 ± 12 | 8 | 530 ± 16 | 3 |
| RS | 0.46 ± 0.08 | 11 | 1.47 ± 0.05 | 5 |
| V100 [%] | 12 ± 12 | 99 | 8 ± 1 | 13 |
| V200 [%] | 99 ± 1 | 1 | 33 ± 2 | 7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Becce, L.; Mazzi, G.; Ali, A.; Bortolini, M.; Gregoris, E.; Feltracco, M.; Barbaro, E.; Contini, D.; Mazzetto, F.; Gambaro, A. Wind Tunnel Evaluation of Plant Protection Products Drift Using an Integrated Chemical–Physical Approach. Atmosphere 2024, 15, 656. https://doi.org/10.3390/atmos15060656
Becce L, Mazzi G, Ali A, Bortolini M, Gregoris E, Feltracco M, Barbaro E, Contini D, Mazzetto F, Gambaro A. Wind Tunnel Evaluation of Plant Protection Products Drift Using an Integrated Chemical–Physical Approach. Atmosphere. 2024; 15(6):656. https://doi.org/10.3390/atmos15060656
Chicago/Turabian StyleBecce, Lorenzo, Giovanna Mazzi, Ayesha Ali, Mara Bortolini, Elena Gregoris, Matteo Feltracco, Elena Barbaro, Daniele Contini, Fabrizio Mazzetto, and Andrea Gambaro. 2024. "Wind Tunnel Evaluation of Plant Protection Products Drift Using an Integrated Chemical–Physical Approach" Atmosphere 15, no. 6: 656. https://doi.org/10.3390/atmos15060656






