Assessment of Ground Water Quality of Lucknow City under GIS Framework Using Water Quality Index (WQI)
Abstract
:1. Introduction
2. Study Area
3. Materials and Methods
3.1. Ground Water Quality Parameters
3.1.1. Hydrogen Ion Concentration (pH)
3.1.2. Turbidity
3.1.3. Total Dissolved Solids (TDS)
3.1.4. Chloride ()
3.1.5. Total Alkalinity
3.1.6. Total Hardness
3.1.7. Sulphate ()
3.1.8. Nitrate ()
3.1.9. Fluoride ()
3.1.10. Iron (Fe)
3.1.11. Arsenic (As)
3.1.12. Magnesium ()
3.1.13. Calcium ()
3.2. Water Quality Index (WQI)
3.2.1. Weightage Factor )
3.2.2. Calculation of Sub-Index ()/Quality Rating
3.2.3. Calculation of WQI
4. Result
4.1. Correlations Matrix and Statistical Assessment
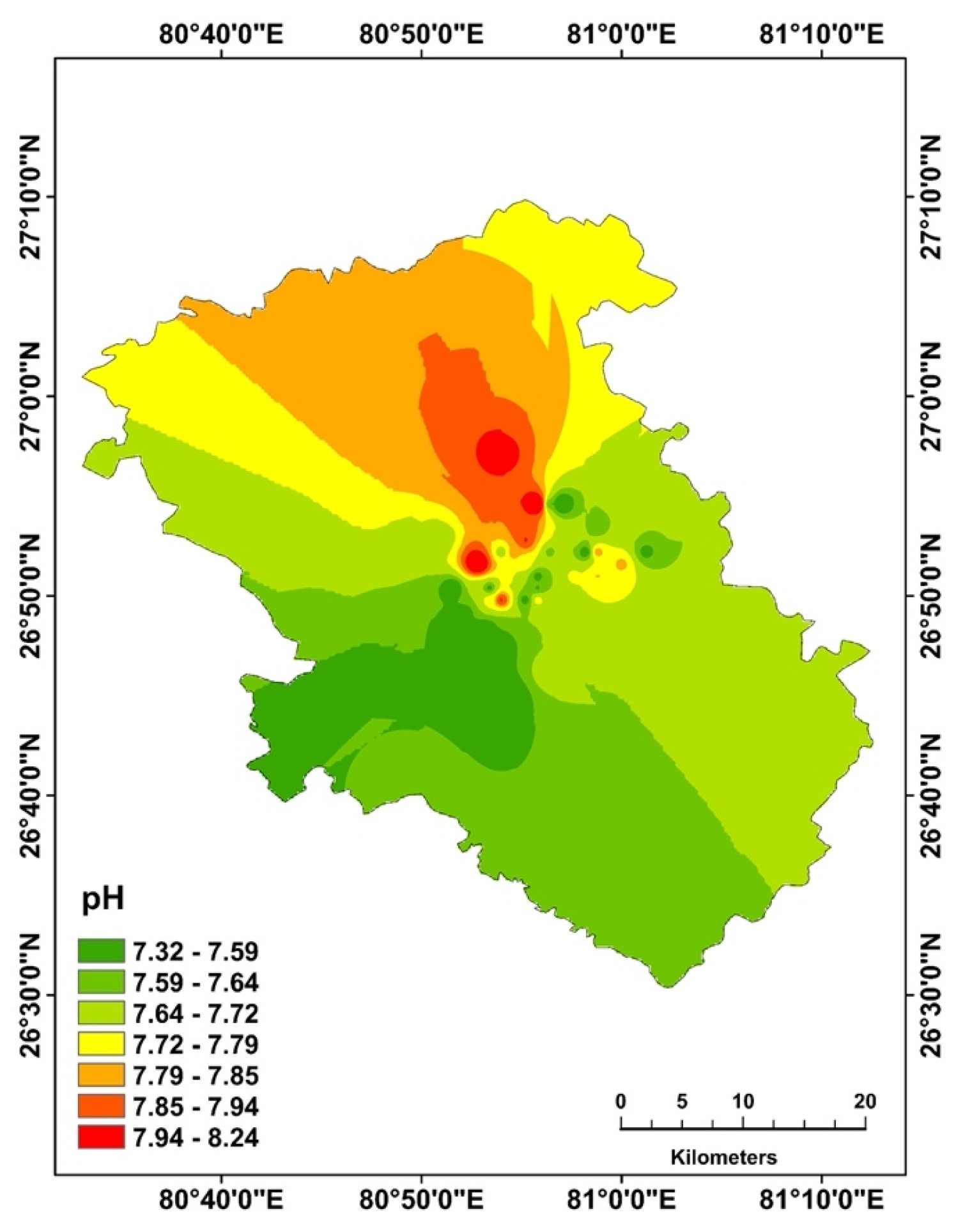
4.2. Spatial Distribution Pattern
4.3. Water Quality Index
5. Discussion
6. Conclusions
7. Recommendations
- Regular Monitoring: Establishing an ongoing groundwater quality monitoring system is imperative for promptly identifying changes or potential sources of contamination that may emerge over time.
- Focused Investigation: A comprehensive investigation is advised to pinpoint the precise origins of contamination in the area. This in-depth analysis will facilitate the development of targeted solutions.
- Treatment Implementation: Immediate measures should be taken to apply suitable treatment techniques in the Kukrail region to bring the water quality up to acceptable standards.
- Public Awareness: Public awareness campaigns will empower residents with knowledge about their groundwater quality. Information about the overall water quality, the areas with unfit water, and potential health risks can help residents make informed decisions about water use.
- Localized Solutions: Since most of the area falls under very good water quality categories, it is essential to focus on localized solutions to address emerging water quality issues. This might involve promoting best practices for agricultural and industrial activities that could impact groundwater quality.
- Collaboration with Authorities: Close collaboration with local authorities and environmental agencies is pivotal in influencing policy formulation and regulations concerning groundwater management.
- Regular Updates: Keep the public and local authorities informed about the progress of groundwater quality improvements. Regular updates and transparent communication can build trust and foster cooperation in managing this valuable resource.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ouyang, T.; Zhu, Z.; Kuang, Y. Assessing impact of urbanization on river water quality in the Pearl River Delta Economic Zone, China. Environ. Monit. Assess. 2006, 120, 313–325. [Google Scholar]
- Saha, D.; Sahu, S. A decade of investigations on groundwater arsenic contamination in Middle Ganga Plain, India. Environ. Geochem. Health 2015, 38, 315–337. [Google Scholar]
- Brindha, K.; Elango, L.; RNV, N. Spatial and temporal variation of uranium in a shallow weathered rock aquifer in southern India. J. Earth Syst. Sci. 2011, 120, 911–920. [Google Scholar]
- Meraj, G. Assessing the Impacts of Climate Change on Ecosystem Service Provisioning in Kashmir Valley India. Ph.D. Thesis, Suresh Gyan Vihar University, Jaipur, India, 2021. [Google Scholar]
- Alban, K. Simulink application on dynamic modeling of biological waste water treatment for aerator tank case. Int. J. Sci. Technol. Res. 2014, 3, 69–72. [Google Scholar]
- Alban, K.; Irma, K.; Elisabeta, P. Simulink programming for dynamic modelling of activated sludge process: Aerator and settler tank case. Fresenius Environ. Bull. 2016, 25, 2891–2899. [Google Scholar]
- Mangukiya, R.; Bhattacharya, T.; Chakraborty, S. Quality characterization of groundwater using water quality index in Surat city, Gujarat, India. Int. Res. J. Environ. Sci. 2012, 1, 4–23. [Google Scholar]
- Kavitha, M.T.; Divahar, R.; Meenambal, T.; Shankar, K.; Vijay Singh, R.; Haile, T.D.; Gadafa, C. Dataset on the assessment of water quality of surface water in Kalingarayan Canal for heavy metal pollution, Tamil Nadu. Data Brief 2019, 22, 878–884. [Google Scholar]
- Kavitha, M.T.; Shankar, K.; Divahar, R.; Meenambal, T.; Saravanan, R. Impact of industrial wastewater disposal on surface water bodies in Kalingarayan canal, Erode district, Tamil Nadu, India. Arch. Agric. Environ. Sci. 2019, 4, 379–387. [Google Scholar]
- Sinha, K.K.; Gupta, M.K.; Banerjee, M.K.; Meraj, G.; Singh, S.K.; Kanga, S.; Farooq, M.; Kumar, P.; Sahu, N. Neural Network-Based Modeling of Water Quality in Jodhpur, India. Hydrology 2022, 9, 92. [Google Scholar]
- Panigrahi, T.; Das, K.K.; Dey, B.S.; Panda, R.B. Assessment of Water Quality of river Sono, Balasore. Int. J. Environ. Sci. 2012, 3, 49–56. [Google Scholar]
- Gebrehiwot, A.B.; Tadesse, N.; Jigar, E. Application of water quality index to assess suitability of groundwater quality for drinking purposes in Hantebet watershed, Tigray, Northern Ethiopia. ISABB J. Food Agric. Sci. 2011, 1, 22–30. [Google Scholar]
- WHO. Guideline for Drinking Water Quality, 4th ed.; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- Diersing, N.; Nancy, F. Water Quality: Frequently Asked Questions; Florida Brooks National Marine Sanctuary: Key West, FL, USA, 2009. [Google Scholar]
- Panneerselvam, B.; Paramasivam, S.K.; Karuppannan, S.; Selvaraj, P. A GIS-based evaluation of hydro chemical characterization of groundwater in hard rock region, South Tamil Nadu. India Arab. J. Geosci. 2020, 13, 837. [Google Scholar] [CrossRef]
- Smith, D.G. A better water quality indexing system for rivers and streams. Water Res. 1990, 24, 1237–1244. [Google Scholar] [CrossRef]
- Debnath, J.; Sahariah, D.; Saikia, A.; Meraj, G.; Nath, N.; Lahon, D.; Annayat, W.; Kumar, P.; Chand, K.; Singh, S.K.; et al. Shifting Sands: Assessing Bankline Shift Using an Automated Approach in the Jia Bharali River, India. Land 2023, 12, 703. [Google Scholar] [CrossRef]
- Stambuk Giljanvoic, N. Water quality evaluation by index in Dalmatia. Water Res. 1999, 33, 3423–3440. [Google Scholar] [CrossRef]
- Pesce, S.F.; Wunderlin, D.A. Use of water quality indices to verify the impact of Cordoba city (Argentina) on Suquiariver. Water Res. 2000, 34, 2915–2936. [Google Scholar] [CrossRef]
- Sargaonkar, A.; Deshpande, V. Development of an overall index of pollution for surface water based on general classification scheme in Indian context. Environ. Monit. Assess. 2003, 89, 43–67. [Google Scholar] [CrossRef]
- Saeedi, M.; Abessi, O.; Sharifi, F.; Meraji, H. Development of Ground Water Quality Index. Environ. Monit. Assess. 2010, 163, 327–335. [Google Scholar] [CrossRef]
- Alexakis, D.E. Meta-Evaluation of Water Quality Indices. Application into Groundwater Resources. Water 2020, 12, 1890. [Google Scholar] [CrossRef]
- Yisa, J.; Jimoh, T. Analytical Studies on Water Quality Index of river Landzu. Am. J. Appl. Sci. 2010, 7, 453–458. [Google Scholar] [CrossRef]
- Finotti, A.R.; Finkler, R.; Susin, N.; Schneider, V.E. Use of water quality index as a tool for Urban Water Resource Management. Int. J. Sustain. Dev. Plann. 2015, 10, 781–794. [Google Scholar] [CrossRef]
- Kannel, P.R.; Lee, S.; Lee, Y.S.; Kanel, S.R.; Khan, S.P. Application of water quality indices and dissolved oxygen as indicators of river water classification and urban impact assessment. Environ. Monit. Assess. 2007, 132, 93–110. [Google Scholar] [CrossRef]
- Tomar, P.; Singh, S.K.; Kanga, S.; Meraj, G.; Kranjčić, N.; Đurin, B.; Pattanaik, A. GIS-Based Urban Flood Risk Assessment and Management—A Case Study of Delhi National Capital Territory (NCT), India. Sustainability 2021, 13, 12850. [Google Scholar] [CrossRef]
- Singh, P.; Nath, S.; Prasad, S.C.; Nema, A.K. Selection of suitable aggregation function for estimation of aggregate pollution for river Ganges in India. J. Environ. Eng. 2008, 134, 689–701. [Google Scholar] [CrossRef]
- Aravindan, S.; Shankar, K.; Ganesh, B.P.; Rajan, K.D. Hydro-geochemical mapping of in the hard rock area of Gadilam River basin, using GIS technique. Tamil Nadu Indian J. Appl. Geochem. 2010, 12, 209–216. [Google Scholar]
- Shankar, K.; Aravindan, S.; Rajendran, S. GIS based groundwater quality mapping in Paravanar River Sub-Basin, Tamil Nadu, India. Int. J. Geomat. Geosci. 2010, 1, 282–296. [Google Scholar]
- Venkateswaran, S.; Karuppannan, S.; Shankar, K. Groundwater quality in Pambar sub basin, Tamil Nadu, India using GIS. Int. J. Recent. Sci. Res. 2012, 3, 782–787. [Google Scholar]
- Magesh, N.S.; Elango, L. Spatio-temporal variations of fluoride in the groundwater of Dindigul District, Tamil Nadu, India: A comparative assessment using two interpolation techniques. In GIS and Geostatistical Techniques for Groundwater Science; Elsevier: Amsterdam, The Netherlands, 2019; pp. 283–296. [Google Scholar]
- Balamurugan, P.; Kumar, P.S.; Shankar, K.; Nagavinothini, R.; Vijayasurya, K. Non-carcinogenic risk assessment of groundwater in Southern Part of Salem District in Tamilnadu, India. J. Chil. Chem. Soc. 2020, 65, 4697–4707. [Google Scholar] [CrossRef]
- Kanga, S.; Singh, S.K.; Meraj, G.; Kumar, A.; Parveen, R.; Kranjčić, N.; Đurin, B. Assessment of the Impact of Urbanization on Geoenvironmental Settings Using Geospatial Techniques: A Study of Panchkula District, Haryana. Geographies 2022, 2, 1–10. [Google Scholar] [CrossRef]
- Horton, R.K. An index number system for rating water quality. J. Water Pollut. Control Fed. 1965, 373, 303–306. [Google Scholar]
- Choudhury, U.; Singh, S.K.; Kumar, A.; Meraj, G.; Kumar, P.; Kanga, S. Assessing Land Use/Land Cover Changes and Urban Heat Island Intensification: A Case Study of Kamrup Metropolitan District, Northeast India (2000–2032). Earth 2023, 4, 503–521. [Google Scholar] [CrossRef]
- Meraj, G.; Kanga, S.; Kranjčić, N.; Đurin, B.; Singh, S.K. Role of Natural Capital Economics for Sustainable Management of Earth Resources. Earth 2021, 2, 622–634. [Google Scholar] [CrossRef]
- Alobaidy, A.M.; Abid, H.S.; Maulood, B.K. Application of water quality index for assessment of Dokan lake ecosystem, Kurdistan region, Iraqi. J. Water Res. Prot. 2010, 2, 792–798. [Google Scholar] [CrossRef]
- Shankar, K.; Kawo, N.S. Groundwater quality assessment using geospatial techniques and WQI in North East of Adama Town, Oromia Region. Ethiopia Hydrospatial Anal. 2019, 3, 22–36. [Google Scholar]
- Rather, M.A.; Meraj, G.; Farooq, M.; Shiekh, B.A.; Kumar, P.; Kanga, S.; Singh, S.K.; Sahu, N.; Tiwari, S.P. Identifying the Potential Dam Sites to Avert the Risk of Catastrophic Floods in the Jhelum Basin, Kashmir, NW Himalaya, India. Remote Sens. 2022, 14, 1538. [Google Scholar] [CrossRef]
- Jagadeeswari, P.B.; Ramesh, K. Deciphering fresh and saline groundwater interface in south Chennai coastal aquifer, Tamil Nadu, India. Int. J. Res. Chem. Environ. 2012, 2, 123–132. [Google Scholar]
- Kalavathy, S.; Sharma, T.R.; Suresh, K.P. Water quality index of river Cauvery in Tiruchirappalli district, Tamilnadu. Arch. Environ. Sci. 2011, 5, 55–61. [Google Scholar]
- Census of India. 2011. Available online: http://censusindia.gov.in/ (accessed on 20 May 2023).
- Dutta, V.; Sharma, U.; Iqbal, K.; Adeeba; Kumar, R.; Pathak, A.K. Impact of river channelization and riverfront development on fluvial habitat: Evidence from Gomti River, a tributary of Ganges, India. Environ. Sustain. 2018, 1, 167–184. [Google Scholar] [CrossRef]
- Groundwater Scenario in Major Cities of India. Ministry of Water Resources; Government of India: Lucknow, India, 2011. Available online: www.cgwb.gov.in (accessed on 10 June 2023).
- CWC. Gomti River Basin Water Year Book; Upper Ganga Basin Organization, Ministry of Water Resources, Government of India: Lucknow, India, 2012.
- SGWB. Ground Water Year Book; Uttar Pradesh, Central Ground Water Board, Ministry of water resources, River Development and Ganga Rejuvenation, Government of India: Lucknow, India, 2015.
- Status of Groundwater Quality in India Part-1. Available online: https://www.yumpu.com/en/document/view/32360586/status-of-groundwater-quality-in-india-central-pollution- (accessed on 20 May 2023).
- American Public Health Association. Standard Methods for the Examination for Water and Wastewater, 19th ed.; Byrd Prepess Springfield: Washington, DC, USA, 1995. [Google Scholar]
- Alexakis, D.E.; Gotsis, D.; Giakoumakis, S. Assessment of Drainage Water Quality in Pre and Post- Irrigation Seasons for Supplemental Irrigation Use. Environ. Monit. Assess. 2012, 184, 5051–5063. [Google Scholar] [CrossRef]
- Magesh, N.S.; Krishnakumar, S.; Chandrasekar, N.; Soundranayagam, J.P. Groundwater Quality Assessment using WQI and GIS Techniques, Dindigul district, Tamil Nadu, India. Arab. J. Geosci. 2013, 6, 4179–4189. [Google Scholar] [CrossRef]
- Alexakis, D.E.; Bathrellos, D.G.; Skilodimou, H.D.; Gamvroula, D.E. Spatial Distribution and Evaluation of Arsenic and Zinc Content in the Soil of a Karst Landscape. Sustainibility 2021, 13, 6976. [Google Scholar] [CrossRef]
- Ranga, V.; Pani, P.; Kanga, S.; Meraj, G.; Farooq, M.; Nathawat, M.S.; Singh, S.K. National health-GIS Portal-A conceptual framework for effective epidemic management and control in India. Preprints 2020, 2020060325. [Google Scholar] [CrossRef]
- Boyd, C.E. Water Quality an Introduction; Kluwer Academic Publishers: Boston, MA, USA, 2000; p. 330. [Google Scholar]
- Shyam, M.; Meraj, G.; Kanga, S.; Sudhanshu; Farooq, M.; Singh, S.K.; Sahu, N.; Kumar, P. Assessing the Groundwater Reserves of the Udaipur District, Aravalli Range, India, Using Geospatial Techniques. Water 2022, 14, 648. [Google Scholar] [CrossRef]
- Sud, A.; Kanga, R.; Singh, S.K.; Meraj, G.; Kanga, S.; Kumar, P.; Ramanathan, A.; Sudhanshu; Bhardwaj, V. Simulating Groundwater Potential Zones in Mountainous Indian Himalayas—A Case Study of Himachal Pradesh. Hydrology 2023, 10, 65. [Google Scholar] [CrossRef]
- Egereonu, U.U.; Nwachukwu, U.L. Evaluation of the surface and groundwater resources of Efuru River Catchment, Mbano, South Eastern. Nigeria J. Assoc. Adv. Model. Simulat. Tech. Enterpr. 2005, 66, 53–71. [Google Scholar]
- Majumdar, D.; Gupta, N. Nitrate pollution of groundwater and associated human health disorders. Indian. J. Environ. Health 2000, 42, 28–39. [Google Scholar]
- Haddad, B.O.; Delpasand, M.; Loáiciga, A.H. Economic, Political and Social Issues in Water Resources; Elsevier: Amsterdam, The Netherlands, 2021; pp. 217–257. [Google Scholar]
- Anderson, T.W.; Neri, L.C.; Schreiber, G.B.; Talbot, F.D.; Zdrojewski, A. Letter: Ischemic heart disease, water hardness and myocardial magnesium. Can. Med. Assoc. J. 1975, 113, 199–203. [Google Scholar]
- IS 10500; Drinking Water Specification (Second Revision). BIS (Bureau of Indian Standard): New Delhi, India, 2015; pp. 2–6.
- WHO. International Standards for Drinking Water; World Health Organization: Geneva, Switzerland, 1992. [Google Scholar]
- ICMR. Manual of Standards of Quality for Drinking Water Supplies; ICMR: New Delhi, India, 1975. [Google Scholar]
- Ramakrishnaiah, C.R.; Sadashivaiah, C.; Ranganna, C. Assessment of water quality index for the groundwater in Tumkur Taluk, Karnataka State India. J. Chem. 2009, 6, 523–530. [Google Scholar] [CrossRef]
- Pandey, H.K.; Tiwari, V.; Srivastava, S.K. Groundwater quality assessment of Allahabad smart city using GIS and water quality index. Sustain. Water Resour. Manag. 2020, 6, 28. [Google Scholar] [CrossRef]
- Burnt, R.; Vasak, L.; Griffieon, J. Fluoride in Groundwater: Probability of Occurrence of Excessive Concentration on Global Scale; Report nr. SP 2004-2; IGRAC: Delft, The Netherlands, 2004. [Google Scholar]
- Ram, A.; Tiwari, S.K.; Pandey, H.K.; Singh, Y.V. Groundwater Quality Assessment Using Water Quality Index (WQI) under GIS Framework. Appl. Water Sci. 2021, 11, 46. [Google Scholar]
- Nath, N.; Sahariah, D.; Meraj, G.; Debnath, J.; Kumar, P.; Lahon, D.; Chand, K.; Farooq, M.; Chandan, P.; Singh, S.K.; et al. Land Use and Land Cover Change Monitoring and Prediction of a UNESCO World Heritage Site: Kaziranga Eco-Sensitive Zone Using Cellular Automata-Markov Model. Land 2023, 12, 151. [Google Scholar]
- Ding, X.; Song, L.; Xu, Z. Effects of Fe3+ on Acute Toxicity and Regeneration of Planarian (Dugesia japonica) at Different Temperatures. Hindawi BioMed Res. Int. 2019, 2019, 8591631. [Google Scholar] [CrossRef] [PubMed]
- Chaurasia, A.K.; Pandey, H.K.; Tiwari, S.K.; Prakash, R.; Pandey, P.; Ram, A. Groundwater Quality Assessment Using Water Quality Index (WQI) in parts of Varanasi district, Uttar Pradesh, India. J. Geol. Soc. India 2018, 92, 76–82. [Google Scholar] [CrossRef]
- Keshavarzi, B.; Moore, F.; Esmaeili, A.; Rastmanesh, F. The Source of Fluoride Toxicity in Muteh area, Isfahan. Iran. Environ. Earth Sci. 2010, 61, 777–786. [Google Scholar] [CrossRef]
- Rafique, T.; Naseem, S.; Usmani, T.H.; Bashir, E.; Khan, F.A.; Bhanger, M.I. Geochemical Factors Controlling the Occurrence of High Fluoride Groundwater in the Nagar Parkar area, Sindh, Pakistan. J. Hazard. Mater. 2009, 171, 424–430. [Google Scholar] [CrossRef] [PubMed]
- Saxena, V.; Ahmed, S. Inferring the Chemical Parameters for the Dissolution of Fluoride in Groundwater. Environ. Geol. 2003, 43, 731–736. [Google Scholar] [CrossRef]
- Rao, N.S. Fluoride in groundwater, Varaha River Basin, Visakhapatnam District, Andhra Pradesh, India. Environ. Monit. Assess. 2009, 152, 47–60. [Google Scholar] [CrossRef]
- Cardenas, L.M.; Bhogal, A.; Chadwick, D.R.; Calvet, S. Nitrogen use efficiency and nitrous oxide emissions from five UK fertilised grasslands. Natl. Libr. Med. 2019, 661, 696–710. [Google Scholar] [CrossRef]
- Mehta, M. Status of groundwater and policy issues for its sustainable development in India. In Groundwater Research and Management: Integrating Science into Management and Decisions; Sharma, B.R., Villholth, K.G., Sharma, K.D., Eds.; International Water Management Institute: Colombo, Sri Lanka, 2006; pp. 62–74. [Google Scholar]
- Apambire, W.B.; Boyle, D.R.; Michel, F.A. Geochemistry, Genesis, and Health Implications of Fluoriferous Groundwater in the Upper Regions of Ghana. Environ. Geol. 1997, 33, 13–22. [Google Scholar] [CrossRef]
- Chapman, D. Water Quality Assessment—A Guide to Use of Biota, Sediments and Water Environmental Monitoring, 2nd ed.; EPFN Spon: London, UK, 1996; p. 626. [Google Scholar]
- Bera, A.; Meraj, G.; Kanga, S.; Farooq, M.; Singh, S.K.; Sahu, N.; Kumar, P. Vulnerability and Risk Assessment to Climate Change in Sagar Island, India. Water 2022, 14, 823. [Google Scholar] [CrossRef]
- World Health Organization Working Group. Health impact of acidic deposition. Sci. Total Environ. 1986, 52, 157–187. [Google Scholar]
- Fewell, J.K.; Lund, U.; Mintz, B. pH in Drinking-water. In Guidelines for Drinking-Water Quality. Vol. 2, Health Criteria and Other Sup-Porting Information; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- Verma, A.; Yadav, B.K.; Singh, N.B. Hydrochemical exploration and assessment of groundwater quality in part of the Ganga-Gomti fluvial plain in northern India. Ground. Sust. Dev. 2021, 13, 100560. [Google Scholar]
- Mann, A.G.; Tam, C.C.; Higgins, C.D.; Rodrigues, L.C. The association between drinking water turbidity and gastrointestinal illness: A systematic review. BMC Publ. Health 2007, 7, 256. [Google Scholar]
- Health Canada. Guidelines for Canadian Drinking Water Quality: Guideline Technical Document—Turbidity. Ottawa, Ontario: Water, Air and Climate Change Bureau, Healthy Environments and Consumer Safety Branch, Health Canada. 2012. Available online: www.hc-sc.gc.ca/ewh-semt/pubs/water-eau/turbidity/index-eng.php (accessed on 25 June 2023).
- Beaudeau, P.; Schwartz, J.; Levin, R. Drinking water quality and hospital admissions of elderly people for gastrointestinal illness in Eastern Massachusetts, 1998–2008. Water Res. 2014, 52, 188–198. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, K.; Kumar, M.B.; Tilak, L.N.; Vashistha, S. Analysis of Groundwater Quality Using GIS-Based Water Quality Index in Noida, Gautam Buddh Nagar, Uttar Pradesh (UP), India. Appl. Artif. Intell. Mach. Learn. 2020, 778, 171–187. [Google Scholar]
- Haydar, S.; Haider, H.; Bari, A.J.; Faragh, A. Effect of Mehmood Booti Dumping Site in Lahore on Ground Water Quality. Pak. J. Eng. Appl. Sci. 2012, 10, 51–56. [Google Scholar]
- Selvakumar, S.; Ramkumar, K.; Chandrasekar, N.; Magesh, N.S.; Kaliraj, S. Groundwater quality and its suitability for drinking and irrigational use in the Southern Tiruchirappalli district, Tamil Nadu, India. Appl. Water Sci. 2017, 7, 411–420. [Google Scholar] [CrossRef]
- CPCB. Central Pollution Control Board; Ministry of Environment of Forests: New Delhi, India, 2008.
- Singh, S.; Hariteja, N.; Prasad, T.J.; Raju, N.J.; Ramakrishna, C. Impact assessment of faecal sludge on groundwater and river water quality in Lucknow environs, Uttar Pradesh, India. Groundw. Sust. Dev. 2020, 11, 100461. [Google Scholar]
- Sengupta, P. Potential health impact of hard water. Int. J. Prev. Med. 2021, 4, 866–875. [Google Scholar]
- Kumar, A.; Kumar, V.; Ojha, A.; Bisen, P.; Singh, A.; Markandeya. Consequences of environmental characteristic from livestock and domestic wastes in wetland disposal on ground water quality in Lucknow (India). Int. Res. J. Public Environ. Health 2016, 3, 112–119. [Google Scholar]
- Digesti, R.D.; Weeth, H.J. A defensible maximum for inorganic sulphate in the drinking water of cattle. J. Anim. Sci. 1976, 42, 1498–1502. [Google Scholar] [CrossRef] [PubMed]
- Kozisek, F. Health Risks from Drinking Demineralized Water; World Health Organization: Geneva, Switzerland, 2005; pp. 148–163. Available online: http://www.who.int/water_sanitation_health/dwq/nutrientschap12.pdf (accessed on 20 July 2023).
- Gomez, G.G.; Sandler, R.S.; Seal, E., Jr. High levels of inorganic sulphate cause diarrhea in neonatal piglets. J. Nutr. 1995, 125, 2325–2332. [Google Scholar] [PubMed]
- Fewtrell, L. Drinking-water nitrate, methemoglobinemia, and global burden of disease: A discussion. Environ. Health Perspect. 2004, 112, 1371–1374. [Google Scholar] [PubMed]
- Shukla, S.; Saxena, A. Global status of nitrate contamination in groundwater: Its occurrence, health impacts, and mitigation measures. In Handbook of Environmental Materials Management; Hussain, C.M., Ed.; Springer: Cham, Switzerland, 2018; pp. 869–888. [Google Scholar] [CrossRef]
- Gupta, A.B.; Gupta, S.K. Presence of fluoride and nitrates in drinking water and human health—A case study of Rajasthan. In Proceedings of the International Conference on Environmental Health and Technology, Athens, Greece, 15–17 March 2010; IIT, Kanpur: Kanpur, India, 2010. [Google Scholar]
- Sun, Y.; Fang, Q.; Dong, J.; Cheng, X.; Xu, J. Removal of fluoride from drinking water by natural stilbite zeolite modified with Fe (III). Desalination 2011, 277, 121–127. [Google Scholar]
- Underwood, E. Trace Elements in Human and Animal Nutrition; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Milman, N.; Pedersen, P.A.; á Steig, T.; Byg, K.E.; Graudal, N.; Fenger, K. Clinically overt hereditary hemochromatosis in Denmark 1948–1985: Epidemiology, factors of significance for long-term survival, and causes of death in 179 patients. Ann. Hematol. 2001, 80, 737–744. [Google Scholar]
- Ellervik, C.; Mandrup-Poulsen, T.; Nordestgaard, B.G.; Larsen, L.E.; Appleyard, M.; Frandsen, M.; Birgens, H. Prevalence of hereditary haemochromatosis in late-onset type 1 diabetes mellitus: A retrospective study. Lancet 2001, 358, 1405–1409. [Google Scholar] [PubMed]
- Parkkila, S.; Niemelä, O.; Savolainen, E.R.; Koistinen, P. HFE mutations do not account for transfusional iron overload in patients with acute myeloid leukemia. Transfusion 2001, 41, 828–831. [Google Scholar]
- Berg, D.; Gerlach, M.; Youdim, M.B.H.; Double, K.L.; Zecca, L.; Riederer, P. Brain iron pathways and their relevance to Parkinson’s disease. J. Neurochem. 2001, 79, 225–236. [Google Scholar]
- Saha, D. Arsenic groundwater contamination in parts of Middle Ganga Plain, Bihar. Curr. Sci. 2009, 97, 753–755. [Google Scholar]
- Martinez, D.V.; Vucic, A.E.; Santos, D.D.; Gil, L.; Lam, L.W. Arsenic exposure and the induction of human cancers. National library of medicine. J. Toxicol. 2011, 2011, 431287. [Google Scholar] [CrossRef]
- Saha, J.C.; Dikshit, A.K.; Bandyopadhyay, M. A Review of Arsenic Poisoning and its Effects on Human Health. Crit. Rev. Environ. Sci. Technol. 1999, 29, 281–313. [Google Scholar] [CrossRef]
- Flanagan, S.; Johnston, R.; Zheng, Y. Arsenic in tube well water in Bangladesh: Health and economic impacts and implications for arsenic mitigation. Bull. World Health Organ. 2012, 90, 839–846. [Google Scholar] [CrossRef] [PubMed]
- Martin, K.J.; Gonzalez, E.A.; Slatopolsky, E. Clinical consequences and management of hypomagnesemia. J. Am. Soc. Nephrol. 2009, 20, 2291–2295. [Google Scholar] [PubMed]
- MediLexicon International Limited. Low Magnesium Linked to Heart Disease; MediLexicon International Limited: Bexhill-on-Sea, UK, 2013. [Google Scholar]
- Saris, N.E.L.; Mervaala, E.; Karppanen, H.; Khawaja, J.A.; Lewenstam, A. Magnesium: An update on physiological, clinical and analytical aspects. Clin. Chim. Acta 2000, 294, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Heaney, R.P.; Gallagher, J.C.; Johnston, C.C.; Neer, R.; Parfitt, A.M.; Whedon, G.D. Calcium nutrition and bone health in the elderly. Am. J. Clin. Nutr. 1982, 36, 986–1013. [Google Scholar]

















| Parameters | Abbreviation | Method Adopted | Instrument Used |
|---|---|---|---|
| pH | pH | Electrometric method | pH meter |
| Turbidity | Turb | Nephelometric method | Turbidity meter |
| Total dissolved solids | TDS | Gravimetric method | Electronic balance, hot air, oven |
| Chloride | Titrimetric method | – | |
| Total alkalinity | T-Alk | Titrimetric method | – |
| Total hardness | T-Hard | Titrimetric method | – |
| Sulphate | Turbidimetric method | Spectrophotometer | |
| Nitrate | UV screening method | Spectrophotometer | |
| Fluoride | Ion selective electrode method | Ion meter | |
| Iron | Fe | ICP-MS | ICP-MS |
| Arsenic | As | ICP-MS | ICP-MS |
| Magnesium | ICP-MS | ICP-MS | |
| Calcium | ICP-MS | ICP-MS |
| Parameters | Unit | Standards of Drinking Water | Statistical Analysis of Observed Values | |||||
|---|---|---|---|---|---|---|---|---|
| BIS (2012, 2015) | WHO (2017) | Min. | Max. | Mean | SD (σ) | Relative Weight (Wi) | ||
| pH | (On Scale) | 6.5–8.5 | 7–8 | 7.32 | 8.25 | 7.69 | 0.19 | 0.001123 |
| Turbidity | (NTU *) | 1.00–5.00 | – | 1.2 | 19.04 | 2.40 | 2.96 | 0.001909 |
| TDS | 500–2000 | 600–1000 | 300 | 1090 | 539.15 | 160.26 | 0.000019 | |
| 250–1000 | 250 | 14.68 | 112 | 50.94 | 30.74 | 0.000038 | ||
| Total alkalinity | 200–600 | – | 170 | 490 | 320.25 | 66.95 | 0.000047 | |
| Total hardness | 200–600 | 200 | 190 | 495 | 316.62 | 74.87 | 0.000031 | |
| 200–400 | 250 | 2 | 160 | 26.58 | 31.66 | 0.000047 | ||
| 45 | 50 | 0.43 | 100 | 23.26 | 27.01 | 0.000212 | ||
| () | 1–1.5 | 1.5 | 0.15 | 1.15 | 0.45 | 0.24 | 0.009547 | |
| (Fe) | 0.3–1.0 | 0.3 | 0.01 | 0.5 | 0.05 | 0.09 | 0.031825 | |
| (As) | 0.01–0.05 | – | 0.0002 | 0.017 | 0.0012 | 0.0026 | 0.954752 | |
| () | 30–100 | – | 17.9 | 122.79 | 48.39 | 26.11 | 0.000318 | |
| () | 75–200 | 100–300 | 8.1 | 75 | 20.30 | 11.77 | 0.000127 | |
| PH | Turbidity | TDS | Total Alkalinity | Total Hardness | Fe | As | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PH | 1 | ||||||||||||
| Turbidity | 0.63 | 1 | |||||||||||
| TDS | −0.23 | 0.02 | 1 | ||||||||||
| 0.59 | 0.92 | 0.15 | 1 | ||||||||||
| Total alkalinity | −0.64 | –0.61 | 0.54 | −0.61 | 1 | ||||||||
| Total hardness | −0.19 | 0.16 | 0.35 | −0.02 | 0.19 | 1 | |||||||
| 0.68 | 0.83 | 0.16 | 0.94 | −0.67 | −0.09 | 1 | |||||||
| –0.22 | 0.35 | 0.62 | 0.5 | 0.01 | 0.42 | 0.41 | 1 | ||||||
| 0.5 | 0.62 | –0.37 | 0.56 | −0.66 | −0.22 | 0.55 | −0.01 | 1 | |||||
| Fe | 0.21 | 0.57 | −0.18 | 0.58 | −0.58 | 0.05 | 0.48 | 0.22 | 0.47 | 1 | |||
| As | 0.6 | 0.88 | −0.17 | 0.9 | −0.82 | −0.11 | 0.88 | 0.29 | 0.58 | 0.6 | 1 | ||
| −0.56 | −0.51 | 0.41 | −0.64 | 0.74 | 0.7 | −0.66 | 0.05 | −0.62 | −0.45 | −0.76 | 1 | ||
| 0.69 | 0.9 | −0.14 | 0.88 | −0.8 | 0.08 | 0.88 | 0.32 | 0.74 | 0.61 | 0.89 | −0.61 | 1 |
| WQI | Category |
|---|---|
| 0–34 | Very good |
| 35–44 | Good |
| 45–54 | Moderate |
| 55–64 | Poor |
| >65 | Unfit for drinking |
| Hydro-Station | Sample Code | Source of Sample | Value of WQI | Category |
|---|---|---|---|---|
| Kadam Rasool | T1 | Tube well | 10.39 | Very good |
| Triveni Nagar | T2 | Tube well | 10.51 | Very good |
| Madiyawa | T3 | Tube well | 39.21 | Good |
| Faizullahganj | T4 | Tube well | 10.35 | Very good |
| Motijheel | T5 | Tube well | 10.34 | Very good |
| Rajajipuram | T6 | Tube well | 13.16 | Very good |
| Balaganj | T7 | Tube well | 10.44 | Very good |
| Mawaiya | T8 | Tube well | 10.52 | Very good |
| Daliganj | T9 | Tube well | 10.24 | Very good |
| Aishbagh | H1 | Hand pump | 11.85 | Very good |
| High Court | H2 | Hand pump | 8.89 | Very good |
| Dubagga | H3 | Hand pump | 5.05 | Very good |
| Hanskhera | H4 | Hand pump | 6.86 | Very good |
| Sikandarpur | H5 | Hand pump | 5.72 | Very good |
| Amausi Airport | H6 | Hand pump | 6.68 | Very good |
| Pariwartan Chowk | W1 | Borehole | 5.53 | Very good |
| Alamnagar | W2 | Borehole | 7.50 | Very good |
| South City | W3 | Borehole | 5.79 | Very good |
| KMCL University | W4 | Borehole | 7.37 | Very good |
| Husainabad | W5 | Borehole | 8.45 | Very good |
| HAL | W6 | Borehole | 6.67 | Very good |
| Polytechnic | W7 | Borehole | 7.36 | Very good |
| Sikandarbagh | W8 | Borehole | 3.63 | Very good |
| Lalkuan | W9 | Borehole | 7.50 | Very good |
| Devpur Para | W10 | Borehole | 10.73 | Very good |
| Alambagh | W11 | Borehole | 7.73 | Very good |
| Khurram Nagar | W12 | Borehole | 8.31 | Very good |
| Kukrail | W13 | Borehole | 168.68 | Unfit for drinking |
| Naka | W14 | Borehole | 6.62 | Very good |
| Nishatganj | W15 | Borehole | 8.49 | Very good |
| Indira Nagar | W16 | Borehole | 7.83 | Very good |
| Naharia | W17 | Borehole | 10.34 | Very good |
| Lalbagh | W18 | Borehole | 6.81 | Very good |
| Jankipuram | W19 | Borehole | 21.38 | Very good |
| Charbagh | W20 | Borehole | 10.34 | Very good |
| Vrindawan | W21 | Borehole | 10.89 | Very good |
| Chinhat | W22 | Borehole | 2.64 | Very good |
| 1090 Chauraha | W23 | Borehole | 11.13 | Very good |
| RSAC | W24 | Borehole | 10.46 | Very good |
| Manak Nagar | W25 | Borehole | 10.57 | Very good |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saqib, N.; Rai, P.K.; Kanga, S.; Kumar, D.; Đurin, B.; Singh, S.K. Assessment of Ground Water Quality of Lucknow City under GIS Framework Using Water Quality Index (WQI). Water 2023, 15, 3048. https://doi.org/10.3390/w15173048
Saqib N, Rai PK, Kanga S, Kumar D, Đurin B, Singh SK. Assessment of Ground Water Quality of Lucknow City under GIS Framework Using Water Quality Index (WQI). Water. 2023; 15(17):3048. https://doi.org/10.3390/w15173048
Chicago/Turabian StyleSaqib, Nazmu, Praveen Kumar Rai, Shruti Kanga, Deepak Kumar, Bojan Đurin, and Suraj Kumar Singh. 2023. "Assessment of Ground Water Quality of Lucknow City under GIS Framework Using Water Quality Index (WQI)" Water 15, no. 17: 3048. https://doi.org/10.3390/w15173048
APA StyleSaqib, N., Rai, P. K., Kanga, S., Kumar, D., Đurin, B., & Singh, S. K. (2023). Assessment of Ground Water Quality of Lucknow City under GIS Framework Using Water Quality Index (WQI). Water, 15(17), 3048. https://doi.org/10.3390/w15173048










