Characteristics of Patients with Laryngomalacia: A Tertiary Referral Center Experience of 106 Cases
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. General
3.2. Surgical Treatment
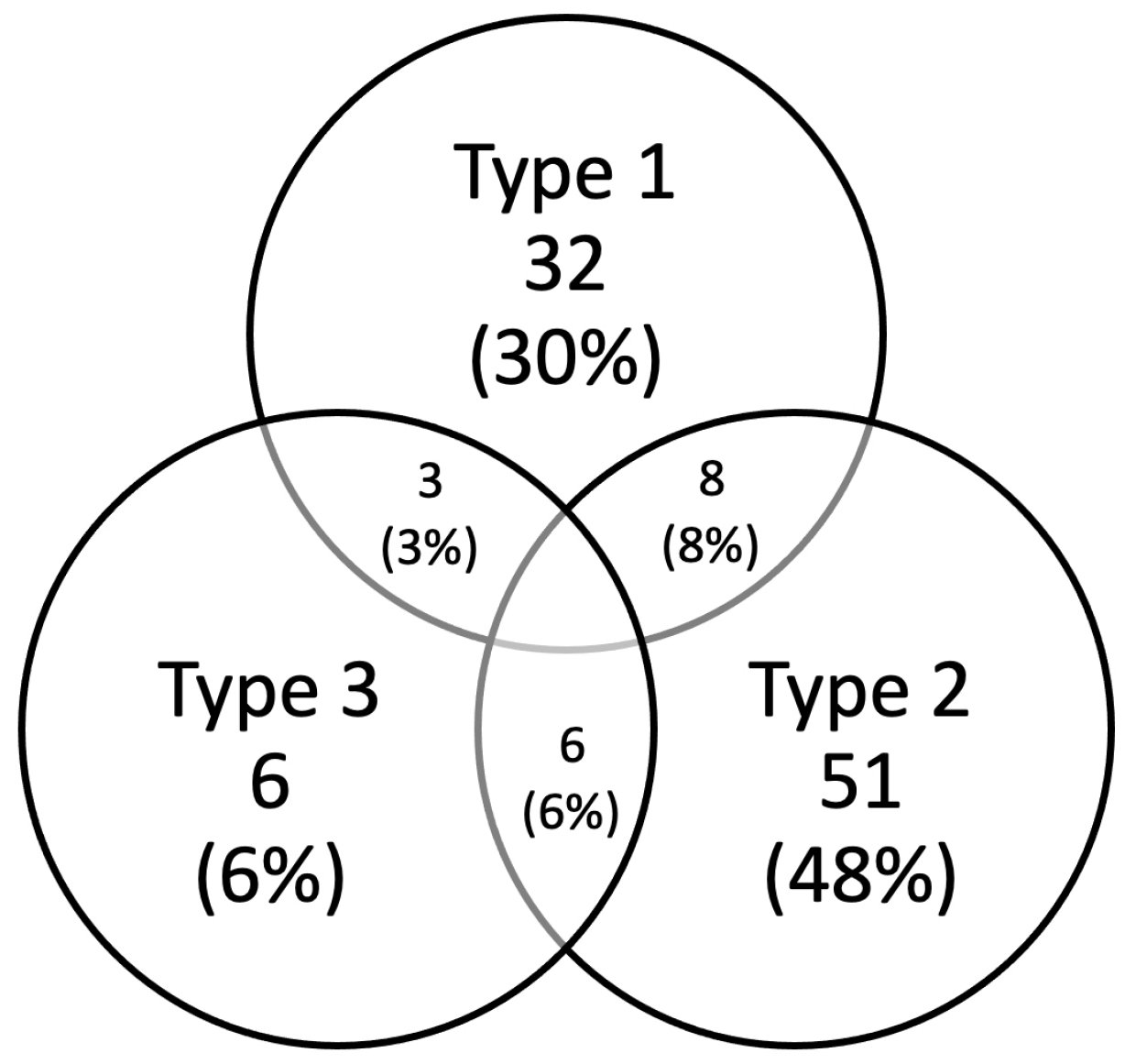
3.3. Type of LM
3.4. Comorbidities
4. Discussion
5. Conclusions
- Type 1 LM is significantly more characteristic for premature children.
- Children with LM type 2 significantly more often require surgical treatment.
- There is a significant interrelation between LM type 2 and feeding difficulty.
- Among different comorbidities, SALs are suspected of modification of the course and severity of LM. This study did not reveal a significant effect of SAL on the incidence of supraglottoplasty or feeding difficulty.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Thompson, D.M. Abnormal Sensorimotor Integrative Function of the Larynx in Congenital Laryngomalacia: A New Theory of Etiology. Laryngoscope 2007, 117, 1–33. [Google Scholar] [CrossRef]
- Thompson, D.M. Laryngomalacia: Factors that influence disease severity and outcomes of management. Curr. Opin. Otolaryngol. Head Neck Surg. 2010, 18, 564–570. [Google Scholar] [CrossRef] [PubMed]
- Landry, A.M.; Thompson, D.M. Laryngomalacia: Disease Presentation, Spectrum, and Management. Int. J. Pediatr. 2012, 2012, 753526. [Google Scholar] [CrossRef] [PubMed]
- Olney, D.R.; Greinwald, J.H.; Smith, R.J.H.; Bauman, N.M.; Greinwald, J.H., Jr. Laryngomalacia and Its Treatment. Laryngoscope 1999, 109, 1770–1775. [Google Scholar] [CrossRef] [PubMed]
- Kay, D.J.; Goldsmith, A.J. Laryngomalacia: A Classification System and Surgical Treatment Strategy. Ear Nose Throat J. 2006, 85, 328–336. [Google Scholar] [CrossRef] [PubMed]
- Roger, G.; Garabedian, E.; Denoyelle, F.; Triglia, J. Severe laryngomalacia: Surgical indications and results in 115 patients. Laryngoscope 1995, 105, 1111–1117. [Google Scholar] [CrossRef]
- Shah, U.K.; Wetmore, R.F. Laryngomalacia: A proposed classification form. Int. J. Pediatr. Otorhinolaryngol. 1998, 46, 21–26. [Google Scholar] [CrossRef]
- Holinger, L.D.; Konior, R.J. Surgical Management of Severe Laryngomalacia. Laryngoscope 1989, 99, 136–142. [Google Scholar] [CrossRef]
- Ayari, S.; Aubertin, G.; Girschig, H.; Abbeele, T.V.D.; Denoyelle, F.; Couloignier, V.; Mondain, M. Management of laryngomalacia. Eur. Ann. Otorhinolaryngol. Head Neck Dis. 2013, 130, 15–21. [Google Scholar] [CrossRef]
- Vijayasekaran, D.; Gowrishankar, N.C.; Kalpana, S.; Vivekanandan, V.E.; Balakrishnan, M.S.; Suresh, S. Lower airway anomalies in infants with laryngomalacia. Indian J. Pediatr. 2010, 77, 403–406. [Google Scholar] [CrossRef]
- Masters, I.; Chang, A.; Patterson, L.; Wainwright, C.; Buntain, H.; Dean, B.; Francis, P. Series of laryngomalacia, tracheomalacia, and bronchomalacia disorders and their associations with other conditions in children. Pediatr. Pulmonol. 2002, 34, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Dickson, J.M.; Richter, G.T.; Meinzen-Derr, J.; Rutter, M.J.; Thompson, D.M. Secondary Airway Lesions in Infants with Laryngomalacia. Ann. Otol. Rhinol. Laryngol. 2009, 118, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Krashin, E.; Ben-Ari, J.; Springer, C.; De Rowe, A.; Avital, A.; Sivan, Y. Synchronous airway lesions in laryngomalacia. Int. J. Pediatr. Otorhinolaryngol. 2008, 72, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Rifai, H.A.; Benoit, M.; El-Hakim, H. Secondary Airway Lesions in Laryngomalacia. Otolaryngol. Neck Surg. 2010, 144, 268–273. [Google Scholar] [CrossRef]
- Schroeder, J.W.; Bhandarkar, N.D.; Holinger, L.D. Synchronous Airway Lesions and Outcomes in Infants with Severe Laryngomalacia Requiring Supraglottoplasty. Arch. Otolaryngol. Neck Surg. 2009, 135, 647–651. [Google Scholar] [CrossRef]
- Toynton, S.C.; Saunders, M.W.; Bailey, C.M. Aryepiglottoplasty for laryngomalacia: 100 consecutive cases. J. Laryngol. Otol. 2001, 115, 35–38. [Google Scholar] [CrossRef]
- van der Heijden, M.; Dikkers, F.G.; Halmos, G.B. Treatment outcome of supraglottoplasty vs. wait-and-see policy in patients with laryngomalacia. Eur. Arch. Oto-Rhino-Laryngol. 2016, 273, 1507–1513. [Google Scholar] [CrossRef]
- Monnier, P. Pediatric Airway Surgery; Springer: Berlin/Heidelberg, Germany, 2011; pp. 99–106. [Google Scholar]
- Erickson, B.; Cooper, T.; El-Hakim, H. Factors Associated with the Morphological Type of Laryngomalacia and Prognostic Value for Surgical Outcomes. JAMA Otolaryngol. Neck Surg. 2014, 140, 927–933. [Google Scholar] [CrossRef]
- Wright, C.T.; Goudy, S.L. Congenital Laryngomalacia: Symptom Duration and Need for Surgical Intervention. Ann. Otol. Rhinol. Laryngol. 2012, 121, 57–60. [Google Scholar] [CrossRef]
- Simons, J.P.; Greenberg, L.L.; Mehta, D.K.; Fabio, A.; Maguire, R.C.; Mandell, D.L. Laryngomalacia and swallowing function in children. Laryngoscope 2015, 126, 478–484. [Google Scholar] [CrossRef]
- Holinger, L.D.; Lusk, R.P.; Green, C.G. Pediatric Laryngology and Bronchoesophagology; Lippincott-Rav: Philadelphia, PA, USA, 1997; pp. 137–164. [Google Scholar]
- Richter, G.T.; Thompson, D.M. The Surgical Management of Laryngomalacia. Otolaryngol. Clin. N. Am. 2008, 41, 837–864. [Google Scholar] [CrossRef] [PubMed]
- Glibbery, N.; Bance, R.R.; Jonas, N.; Bewick, J. Synchronous airway lesions in children with severe, progressive and atypical laryngomalacia—Experience of a UK tertiary referral centre. Int. J. Pediatr. Otorhinolaryngol. 2021, 152, 110984. [Google Scholar] [CrossRef] [PubMed]
- Kusak, B.; Cichocka-Jarosz, E.; Jedynak-Wasowicz, U.; Lis, G. Types of laryngomalacia in children: Interrelationship between clinical course and comorbid conditions. Eur. Arch. Oto-Rhino-Laryngol. 2016, 274, 1577–1583. [Google Scholar] [CrossRef] [PubMed]
- Ayari, S.; Aubertin, G.; Girschig, H.; Abbeele, T.V.D.; Mondain, M. Pathophysiology and diagnostic approach to laryngomalacia in infants. Eur. Ann. Otorhinolaryngol. Head Neck Dis. 2012, 129, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Scott, B.L.; Lam, D.; MacArthur, C. Laryngomalacia and Swallow Dysfunction. Ear Nose Throat J. 2019, 98, 613–616. [Google Scholar] [CrossRef]
- Bluestone, C.D.; Healy, G.B.; Cotton, R.T. Diagnosis of Laryngomalacia Is Not Enough! Arch. Otolaryngol. Neck Surg. 1996, 122, 1417. [Google Scholar] [CrossRef]
- Nussbaum, E.; Maggi, J.C. Laryngomalacia in Children. Chest 1990, 98, 942–944. [Google Scholar] [CrossRef]
- Gonzalez, C.; Reilly, J.S.; Bluestone, C.D. Synchronous Airway Lesions in Infancy. Ann. Otol. Rhinol. Laryngol. 1987, 96, 77–80. [Google Scholar] [CrossRef]
- Yuen, H.-W.; Tan, H.K.-K.; Balakrishnan, A. Synchronous airway lesions and associated anomalies in children with laryngomalacia evaluated with rigid endoscopy. Int. J. Pediatr. Otorhinolaryngol. 2006, 70, 1779–1784. [Google Scholar] [CrossRef]
- Mancuso, R.F.; Choi, S.S.; Zalzal, G.H.; Grundfast, K.M. Laryngomalacia: The Search for the Second Lesion. Arch. Otolaryngol. Neck Surg. 1996, 122, 302–306. [Google Scholar] [CrossRef]
- Carter, J.; Rahbar, R.; Brigger, M.; Chan, K.; Cheng, A.; Daniel, S.J.; De Alarcon, A.; Garabedian, N.; Hart, C.; Hartnick, C.; et al. International Pediatric ORL Group (IPOG) laryngomalacia consensus recommendations. Int. J. Pediatr. Otorhinolaryngol. 2016, 86, 256–261. [Google Scholar] [CrossRef] [PubMed]
- Adil, E.; Rager, T.; Carr, M. Location of airway obstruction in term and preterm infants with laryngomalacia. Am. J. Otolaryngol. 2012, 33, 437–440. [Google Scholar] [CrossRef] [PubMed]
- Thorne, M.C.; Garetz, S.L. Laryngomalacia: Review and Summary of Current Clinical Practice in 2015. Paediatr. Respir. Rev. 2015, 17, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Parsons, D.S.; Stivers, F.E.; Giovanetto, D.R.; Phillips, S.E.; Major, U.S.E.P. Type I Posterior Laryngeal Clefts. Laryngoscope 1998, 108, 403–410. [Google Scholar] [CrossRef]
- Chien, W.; Ashland, J.; Haver, K.; Hardy, S.C.; Curren, P.; Hartnick, C.J. Type 1 laryngeal cleft: Establishing a functional diagnostic and management algorithm. Int. J. Pediatr. Otorhinolaryngol. 2006, 70, 2073–2079. [Google Scholar] [CrossRef]
- Benjamin, B.; Inglis, A. Minor Congenital Laryngeal Clefts: Diagnosis and Classification. Ann. Otol. Rhinol. Laryngol. 1989, 98, 417–420. [Google Scholar] [CrossRef]
- Irace, A.L.; Dombrowski, N.D.; Kawai, K.; Watters, K.; Choi, S.; Perez, J.; Dodrill, P.; Hernandez, K.; Davidson, K.; Rahbar, R. Evaluation of Aspiration in Infants with Laryngomalacia and Recurrent Respiratory and Feeding Difficulties. JAMA Otolaryngol. Neck Surg. 2019, 145, 146–151. [Google Scholar] [CrossRef]

| Prematurity | SAL | Feeding Difficulties | |
|---|---|---|---|
| All patients | 12 (11.3%) | 16 (15%) | 35 (33%) |
| Type 1 | 8 (25%) | 6 (18.8%) | 6 (18.8%) |
| Type 2 | 3 (5.9%) | 6 (11.8%) | 24 (47.1%) |
| Type 3 | 0 (0%) | 0 (0%) | 1 (16.7%) |
| Combined types of LM | 1 (5.9%) | 4 (23.5%) | 4 (23.5) |
| Prematurity | 2 (16.7%) | 2 (16.7%) | |
| SAL | 2 (12.5%) | 7 (43.7%) | |
| Feeding difficulties | 2 (5.7%) | 7 (20%) |
| Prematurity | SAL | Feeding Difficulties | Surgical Treatment | |
|---|---|---|---|---|
| LM type 1 | p = 0.0036 | p = 0.4914 | p = 0.0409 | p = 0.0023 |
| LM type 2 | p = 0.0903 | p = 0.3588 | p = 0.0032 | p = 0.0054 |
| LM type 3 | p = 0.3698 | p = 0.2899 | p = 0.3828 | p = 0.8635 |
| Combined forms of LM | p = 0.4421 | p = 0.2913 | p = 0.3662 | p = 0.9396 |
| Prematurity | - | p = 0.8723 | p = 0.2030 | p = 0.6790 |
| SAL | p = 0.8723 | - | p = 0.3242 | p = 0.6253 |
| Feeding difficulties | p = 0.2030 | p = 0.3242 | - | p = 0.5208 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bredun, S.; Kotowski, M.; Mezydlo, J.; Szydlowski, J. Characteristics of Patients with Laryngomalacia: A Tertiary Referral Center Experience of 106 Cases. Diagnostics 2023, 13, 3180. https://doi.org/10.3390/diagnostics13203180
Bredun S, Kotowski M, Mezydlo J, Szydlowski J. Characteristics of Patients with Laryngomalacia: A Tertiary Referral Center Experience of 106 Cases. Diagnostics. 2023; 13(20):3180. https://doi.org/10.3390/diagnostics13203180
Chicago/Turabian StyleBredun, Sergii, Michal Kotowski, Jakub Mezydlo, and Jaroslaw Szydlowski. 2023. "Characteristics of Patients with Laryngomalacia: A Tertiary Referral Center Experience of 106 Cases" Diagnostics 13, no. 20: 3180. https://doi.org/10.3390/diagnostics13203180






