Abstract
In this presented case, a 77-year-old woman with an implanted prosthesis and ongoing knee pain underwent a bone scan using 99mTc-hydroxydiphosphonate (HDP) in suspicion for bone infection. An incidental finding from this scan revealed diffuse cardiac uptake, necessitating further diagnostic procedures to exclude the possibility of cardiac amyloidosis. In the subsequent 99mTc-3,3-diphosphono-1,2-propanodicarboxylic acid (DPD) scan and SPECT images, no perceptible cardiac uptake was observed at all. Upon retrospective review of the patient’s medical records, she received 1000 mg of ferric carboxymaltose for iron-deficient anemia the day before the 99mTc-HDP bone scan. Therefore, it was assumed that the diffuse and temporary cardiac activity was due to the transient iron overload. We present and share these bone scan images in order to avoid possible future misinterpretation of cardiac amyloidosis.
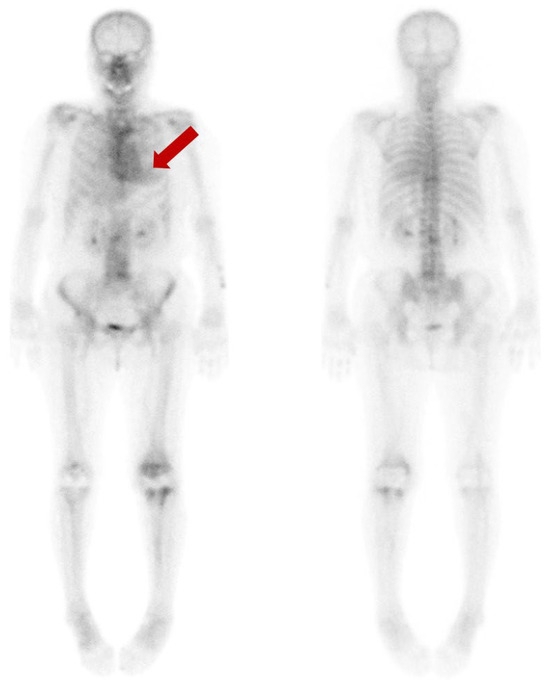
Figure 1.
We present a 77-year-old woman who suffered ongoing left knee pain after the prosthesis insertion. To evaluate the extent and severity of the periprosthetic bone infection, a bone scan using 99mTc-hydroxydiphosphonate (HDP) was performed. Beyond the expected uptake in the left knee’s periprosthetic region, an incidental finding indicated a diffuse and moderate cardiac uptake (arrow). This finding was classified as grade 2, based on the visual grading system ranging from 0 to 3, proposed by Perugini for the diagnosis of cardiac amyloidosis in bone scan [1].
Figure 1.
We present a 77-year-old woman who suffered ongoing left knee pain after the prosthesis insertion. To evaluate the extent and severity of the periprosthetic bone infection, a bone scan using 99mTc-hydroxydiphosphonate (HDP) was performed. Beyond the expected uptake in the left knee’s periprosthetic region, an incidental finding indicated a diffuse and moderate cardiac uptake (arrow). This finding was classified as grade 2, based on the visual grading system ranging from 0 to 3, proposed by Perugini for the diagnosis of cardiac amyloidosis in bone scan [1].
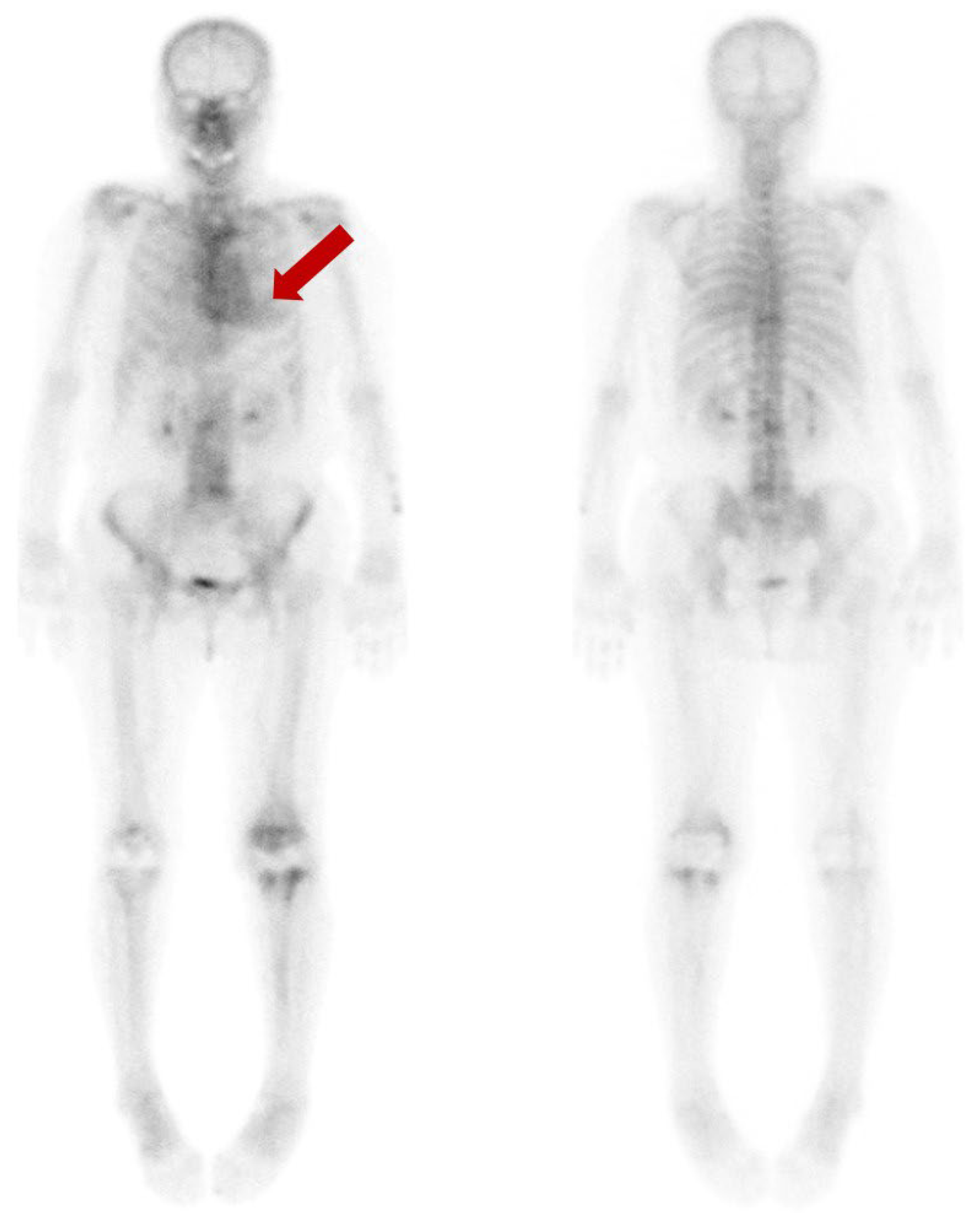
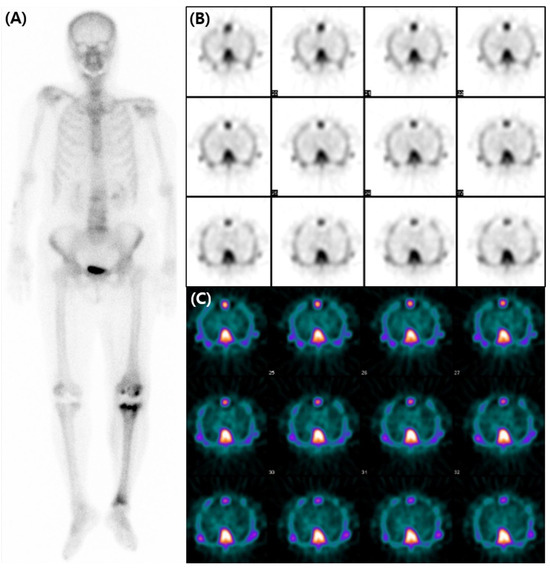
Figure 2.
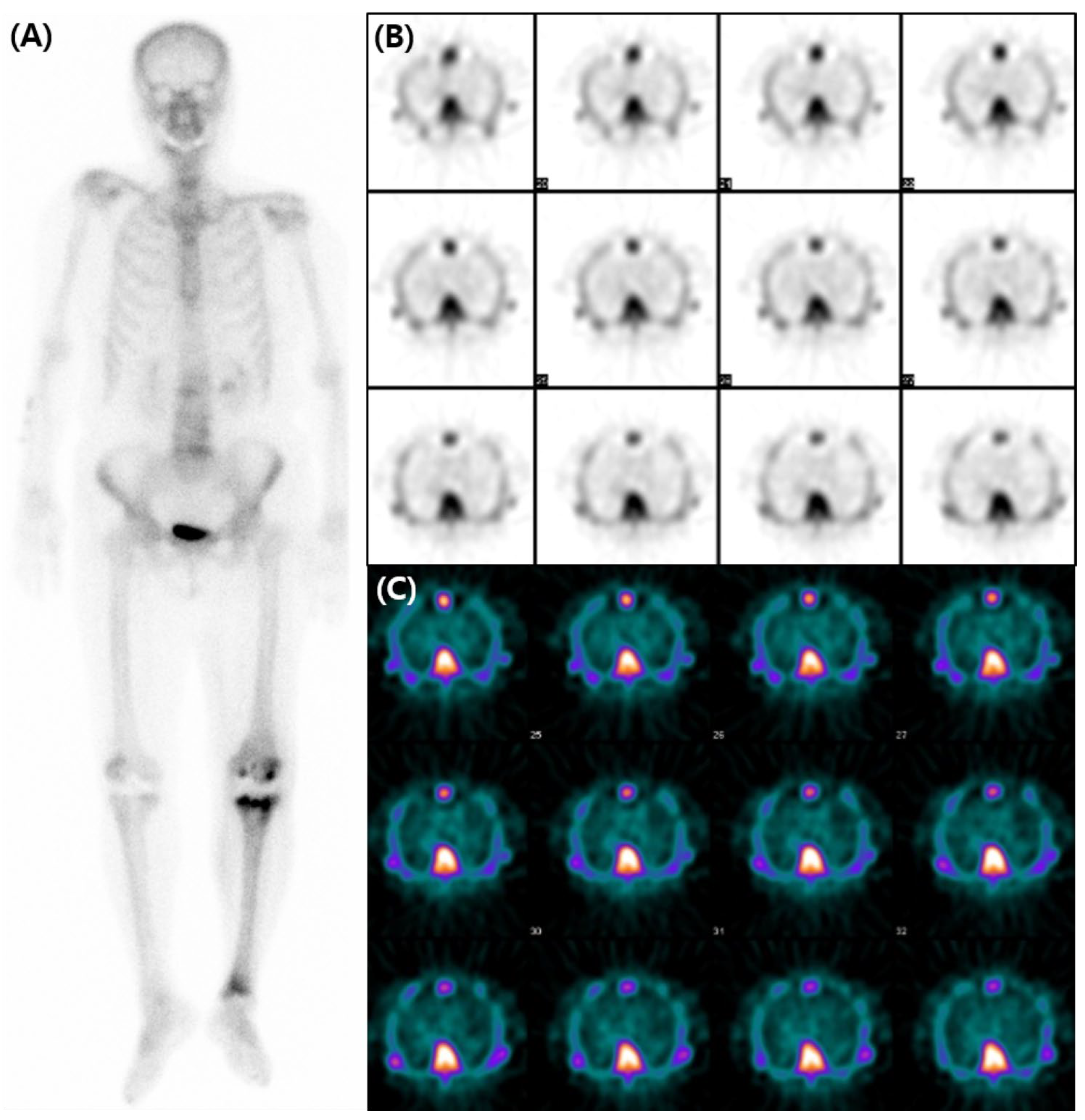
A subsequent bone scan using 99mTc-3,3-diphosphono-1,2-propanodicarboxylic acid (DPD) was obtained three weeks later including SPECT images for assessment of transthyretin-type cardiac amyloidosis (CA). The decision was influenced by the accumulating evidence that favored the use of 99mTc-DPD over 99mTc-HDP for detecting CA [2,3,4], and by the necessity of SPECT images. However, no perceptible radiotracer accumulation was observed in the subsequent 99mTc-DPD scan (A). Additionally, no perceptible radiotracer uptake was noted in the myocardium on the axial SPECT images (B,C). An echocardiogram revealed preserved ejection fraction of 60% without any abnormal wall thickness or abnormal wall motion. No abnormality was found in serum free light chain, serum and urine protein electrophoresis/immunofixation data. Upon thorough review of her medical history, her hemoglobin level was 8.7 g/dL, and she was diagnosed with iron-deficient anemia. We also observed that a total of 1000mg of ferric carboxymaltose had been intravenously administered the day before the 99mTc-HDP bone scan. Hence, it is assumed that the diffuse and temporary cardiac activity was a result of transient iron overload. Bone scan using 99mTc-DPD or 99mTc-PYP is known for its several advantages in the diagnosis in CA. They not only have high sensitivity and specificity but also provide a non-invasive, whole-body evaluation and are readily accessible [5,6]. Currently, multidisciplinary experts in cardiovascular imaging and cardiac amyloidosis recommend including bone scans for diagnosis of CA [7]. However, caution should be exercised in the interpretation of myocardial uptake in a bone scan, as altered biodistribution of radiotracer can occur in various medical conditions including iron overload [8,9,10,11,12]. As bone scans are widely used in the diagnosis of CA, we present and share this image in order to avoid possible future misinterpretation.
Figure 2.
A subsequent bone scan using 99mTc-3,3-diphosphono-1,2-propanodicarboxylic acid (DPD) was obtained three weeks later including SPECT images for assessment of transthyretin-type cardiac amyloidosis (CA). The decision was influenced by the accumulating evidence that favored the use of 99mTc-DPD over 99mTc-HDP for detecting CA [2,3,4], and by the necessity of SPECT images. However, no perceptible radiotracer accumulation was observed in the subsequent 99mTc-DPD scan (A). Additionally, no perceptible radiotracer uptake was noted in the myocardium on the axial SPECT images (B,C). An echocardiogram revealed preserved ejection fraction of 60% without any abnormal wall thickness or abnormal wall motion. No abnormality was found in serum free light chain, serum and urine protein electrophoresis/immunofixation data. Upon thorough review of her medical history, her hemoglobin level was 8.7 g/dL, and she was diagnosed with iron-deficient anemia. We also observed that a total of 1000mg of ferric carboxymaltose had been intravenously administered the day before the 99mTc-HDP bone scan. Hence, it is assumed that the diffuse and temporary cardiac activity was a result of transient iron overload. Bone scan using 99mTc-DPD or 99mTc-PYP is known for its several advantages in the diagnosis in CA. They not only have high sensitivity and specificity but also provide a non-invasive, whole-body evaluation and are readily accessible [5,6]. Currently, multidisciplinary experts in cardiovascular imaging and cardiac amyloidosis recommend including bone scans for diagnosis of CA [7]. However, caution should be exercised in the interpretation of myocardial uptake in a bone scan, as altered biodistribution of radiotracer can occur in various medical conditions including iron overload [8,9,10,11,12]. As bone scans are widely used in the diagnosis of CA, we present and share this image in order to avoid possible future misinterpretation.
Author Contributions
Conceptualization, Y.L.; S.J.N.; methodology, Y.L.; J.J.; S.J.N.; writing—original draft preparation, Y.L.; writing—review and editing, Y.L.; J.J.; S.J.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki. The need for ethical review and approval was waived because of the retrospective nature of this study.
Informed Consent Statement
The IRB Board waived the need for patient consent for this retrospective single-case report.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Perugini, E.; Guidalotti, P.L.; Salvi, F.; Cooke, R.M.T.; Pettinato, C.; Riva, L.; Leone, O.; Farsad, M.; Ciliberti, P.; Bacchi-Reggiani, L.; et al. Noninvasive Etiologic Diagnosis of Cardiac Amyloidosis Using 99mTc-3,3-Diphosphono-1,2-Propanodicarboxylic Acid Scintigraphy. J. Am. Coll. Cardiol. 2005, 46, 1076–1084. [Google Scholar] [CrossRef] [PubMed]
- Treglia, G.; Glaudemans, A.; Bertagna, F.; Hazenberg, B.P.C.; Erba, P.A.; Giubbini, R.; Ceriani, L.; Prior, J.O.; Giovanella, L.; Slart, R. Diagnostic accuracy of bone scintigraphy in the assessment of cardiac transthyretin-related amyloidosis: A bivariate meta-analysis. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 1945–1955. [Google Scholar] [CrossRef] [PubMed]
- Brownrigg, J.; Lorenzini, M.; Lumley, M.; Elliott, P. Diagnostic performance of imaging investigations in detecting and differentiating cardiac amyloidosis: A systematic review and meta-analysis. ESC Heart Fail. 2019, 6, 1041–1051. [Google Scholar] [CrossRef] [PubMed]
- de Haro-del Moral, F.J.; Sánchez-Lajusticia, A.; Gómez-Bueno, M.; García-Pavía, P.; Salas-Antón, C.; Segovia-Cubero, J. Role of cardiac scintigraphy with ⁹⁹mTc-DPD in the differentiation of cardiac amyloidosis subtype. Rev. Esp. Cardiol. (Engl. Ed.) 2012, 65, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Bokhari, S.; Castaño, A.; Pozniakoff, T.; Deslisle, S.; Latif, F.; Maurer, M.S. (99m)Tc-pyrophosphate scintigraphy for differentiating light-chain cardiac amyloidosis from the transthyretin-related familial and senile cardiac amyloidoses. Circ. Cardiovasc. Imaging 2013, 6, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Rapezzi, C.; Quarta, C.C.; Guidalotti, P.L.; Pettinato, C.; Fanti, S.; Leone, O.; Ferlini, A.; Longhi, S.; Lorenzini, M.; Reggiani, L.B.; et al. Role of (99m)Tc-DPD scintigraphy in diagnosis and prognosis of hereditary transthyretin-related cardiac amyloidosis. JACC Cardiovasc. Imaging 2011, 4, 659–670. [Google Scholar] [CrossRef] [PubMed]
- Dorbala, S.; Ando, Y.; Bokhari, S.; Dispenzieri, A.; Falk, R.H.; Ferrari, V.A.; Fontana, M.; Gheysens, O.; Gillmore, J.D.; Glaudemans, A.; et al. ASNC/AHA/ASE/EANM/HFSA/ISA/SCMR/SNMMI Expert Consensus Recommendations for Multimodality Imaging in Cardiac Amyloidosis: Part 2 of 2-Diagnostic Criteria and Appropriate Utilization. J. Card. Fail. 2019, 25, 854–865. [Google Scholar] [CrossRef] [PubMed]
- Shin, E.; Oh, M.; Sung, C.; Kim, K.H.; Ryu, J.S. Altered Biodistribution of (99m)Tc-DPD on Bone Scan After Intravenous Iron Supplement. Nucl. Med. Mol. Imaging 2017, 51, 347–349. [Google Scholar] [CrossRef] [PubMed]
- Choy, D.; Murray, I.P.; Hoschl, R. The effect of iron on the biodistribution of bone scanning agents in humans. Radiology 1981, 140, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Byun, H.H.; Rodman, S.G.; Chung, K.E. Soft-tissue concentration of 99mTc-phosphates associated with injections of iron dextran complex. J. Nucl. Med. 1976, 17, 374–375. [Google Scholar] [PubMed]
- Parker, J.A.; Jones, A.G.; Davis, M.A.; Mcilmoyle, G.; Tow, D.E. Reduced uptake of bone-seeking radiopharmaceuticals related to iron excess. Clin. Nucl. Med. 1976, 1, 267–268. [Google Scholar] [CrossRef]
- Forauer, A.R.; Grossman, S.J.; Joyce, J.M. Altered biodistribution of Tc-99m HMDP on bone scintigraphy from recent intravenous iron therapy. Clin. Nucl. Med. 1994, 19, 817–818. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
