Insights into the Neutrophil-to-Lymphocyte Ratio and the Platelet-to-Lymphocyte Ratio as Predictors for the Length of Stay and Readmission in Chronic Heart Failure Patients
Abstract
1. Introduction
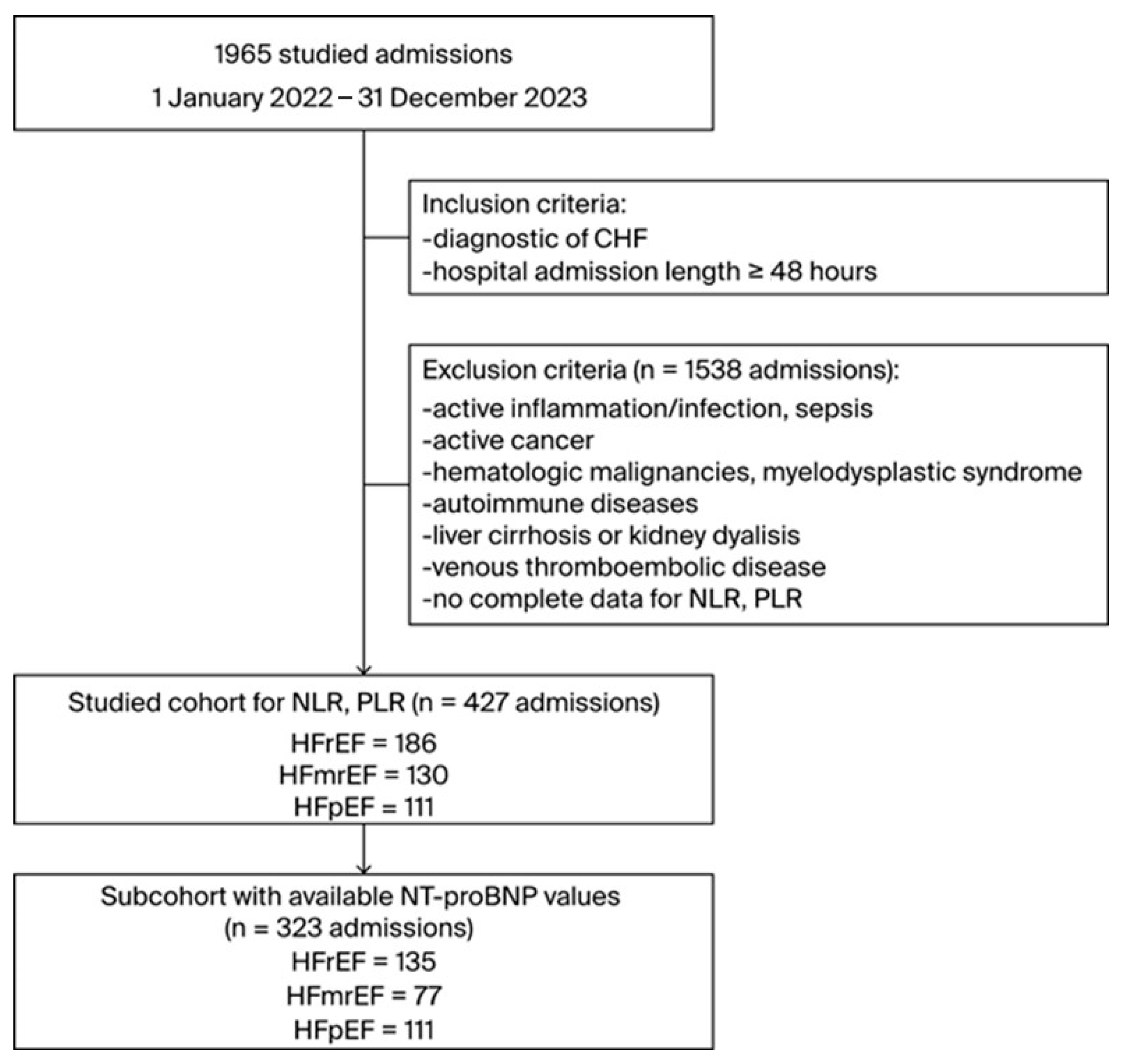
2. Materials and Methods
3. Results
3.1. Cohort Characteristics
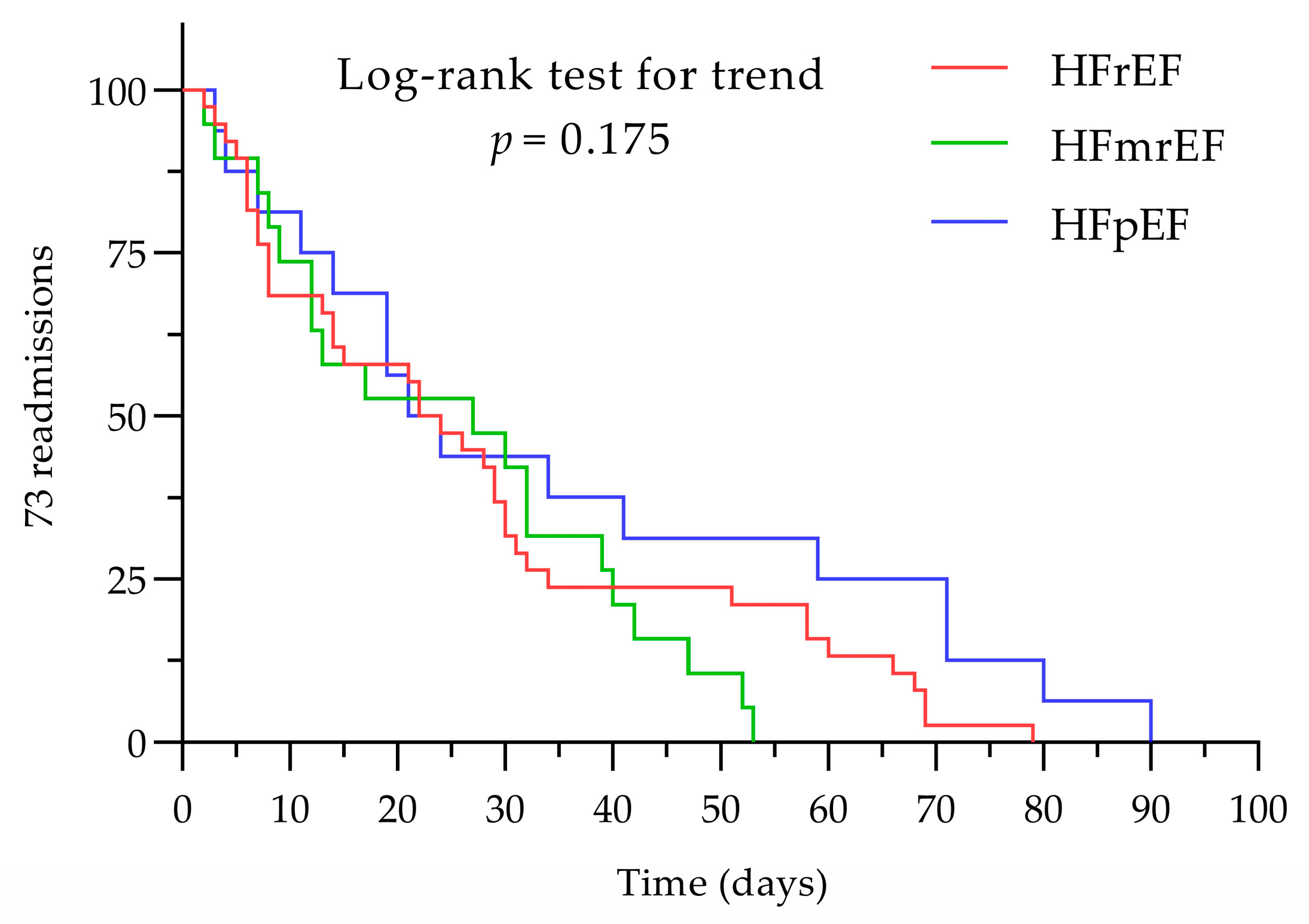
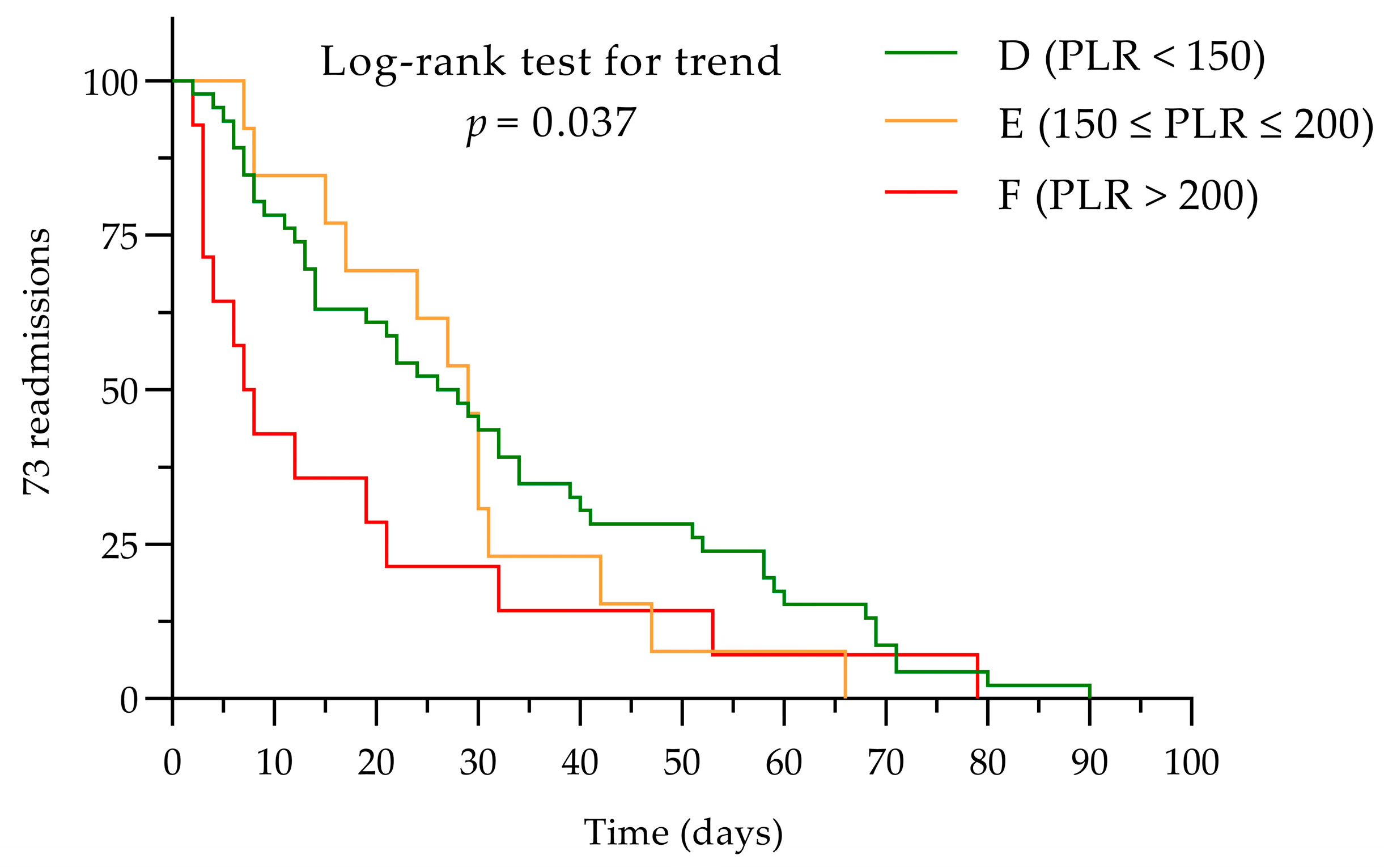
3.2. Admission and Readmission
3.3. NLR and PLR among the Entire Cohort
3.4. NLR and PLR Impact for the First Visit and Any Number of Readmissions < 90 Days
3.5. Cut-Off Values for NLR and PLR as Markers of Prognosis for Earlier Readmission (<30 Days)
3.6. Reduced Cohort Size Data Comparison with the NT-proBNP
3.7. Predictors for NLR and PLR in the NT-proBNP Subcohort
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef] [PubMed]
- Bui, A.L.; Horwich, T.B.; Fonarow, G.C. Epidemiology and risk profile of heart failure. Nat. Rev. Cardiol. 2011, 8, 30–41. [Google Scholar] [CrossRef] [PubMed]
- Mann, D.L. Innate immunity and the failing heart: The cytokine hypothesis revisited. Circ. Res. 2015, 116, 1254–1268. [Google Scholar] [CrossRef]
- Balta, S.; Celik, T.; Mikhailidis, D.P.; Ozturk, C.; Demirkol, S.; Aparci, M.; Iyisoy, A. The Relation Between Atherosclerosis and the Neutrophil-Lymphocyte Ratio. Clin. Appl. Thromb. Hemost. 2016, 22, 405–411. [Google Scholar] [CrossRef] [PubMed]
- Bhat, T.; Teli, S.; Rijal, J.; Bhat, H.; Raza, M.; Khoueiry, G.; Meghani, M.; Akhtar, M.; Costantino, T. Neutrophil to lymphocyte ratio and cardiovascular diseases: A review. Expert Rev. Cardiovasc. Ther. 2013, 11, 55–59. [Google Scholar] [CrossRef]
- Curran, F.M.; Bhalraam, U.; Mohan, M.; Singh, J.S.; Anker, S.D.; Dickstein, K.; Doney, A.S.; Filippatos, G.; George, J.; Metra, M.; et al. Neutrophil-to-lymphocyte ratio and outcomes in patients with new-onset or worsening heart failure with reduced and preserved ejection fraction. ESC Heart Fail. 2021, 8, 3168–3179. [Google Scholar] [CrossRef]
- Mayadas, T.N.; Cullere, X.; Lowell, C.A. The multifaceted functions of neutrophils. Annu. Rev. Pathol. 2014, 9, 181–218. [Google Scholar] [CrossRef]
- Uthamalingam, S.; Patvardhan, E.A.; Subramanian, S.; Ahmed, W.; Martin, W.; Daley, M.; Capodilupo, R. Utility of the neutrophil to lymphocyte ratio in predicting long-term outcomes in acute decompensated heart failure. Am. J. Cardiol. 2011, 107, 433–438. [Google Scholar] [CrossRef]
- Horne, B.D.; Anderson, J.L.; John, J.M.; Weaver, A.; Bair, T.L.; Jensen, K.R.; Renlund, D.G.; Muhlestein, J.B.; Intermountain Heart Collaborative Study Group. Which white blood cell subtypes predict increased cardiovascular risk? J. Am. Coll. Cardiol. 2005, 45, 1638–1643. [Google Scholar] [CrossRef]
- García-Escobar, A.; Vera-Vera, S.; Tébar-Márquez, D.; Rivero-Santana, B.; Jurado-Román, A.; Jiménez-Valero, S.; Galeote, G.; Cabrera, J.Á.; Moreno, R. Neutrophil-to-lymphocyte ratio an inflammatory biomarker, and prognostic marker in heart failure, cardiovascular disease and chronic inflammatory diseases: New insights for a potential predictor of anti-cytokine therapy responsiveness. Microvasc. Res. 2023, 150, 104598. [Google Scholar] [CrossRef]
- Mann, D.L. Inflammatory mediators and the failing heart: Past, present, and the foreseeable future. Circ. Res. 2002, 91, 988–998. [Google Scholar] [CrossRef] [PubMed]
- Van Linthout, S.; Tschöpe, C. Inflammation—Cause or Consequence of Heart Failure or Both? Curr. Heart Fail. Rep. 2017, 14, 251–265. [Google Scholar] [CrossRef] [PubMed]
- Durmus, E.; Kivrak, T.; Gerin, F.; Sunbul, M.; Sari, I.; Erdogan, O. Neutrophil-to-Lymphocyte Ratio and Platelet-to-Lymphocyte Ratio are Predictors of Heart Failure. Arq. Bras. Cardiol. 2015, 105, 606–613. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Fan, X.; Ji, S.; Ma, A.; Wang, T. Prognostic value of neutrophil to lymphocyte ratio in heart failure patients. Clin. Chim. Acta 2018, 85, 44–49. [Google Scholar] [CrossRef]
- Di Rosa, M.; Sabbatinelli, J.; Soraci, L.; Corsonello, A.; Bonfigli, A.R.; Cherubini, A.; Sarzani, R.; Antonicelli, R.; Pelliccioni, G.; Galeazzi, R.; et al. Neutrophil-to-lymphocyte ratio (NLR) predicts mortality in hospitalized geriatric patients independent of the admission diagnosis: A multicenter prospective cohort study. J. Transl. Med. 2023, 21, 835. [Google Scholar] [CrossRef]
- Fest, J.; Ruiter, T.R.; Groot Koerkamp, B.; Rizopoulos, D.; Ikram, M.A.; van Eijck, C.H.J.; Stricker, B.H. The neutrophil-to-lymphocyte ratio is associated with mortality in the general population: The Rotterdam Study. Eur. J. Epidemiol. 2019, 34, 463–470. [Google Scholar] [CrossRef]
- Pang, J.; Qian, L.Y.; Lv, P.; Che, X.R. Application of neutrophil-lymphocyte ratio and red blood cell distribution width in diabetes mellitus complicated with heart failure. World J. Diabetes 2024, 15, 1226–1233. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Cheang, I.; Xu, F.; Gao, R.; Liao, S.; Yao, W.; Zhou, Y.; Zhang, H.; Li, X. Long-term prognostic value of inflammatory biomarkers for patients with acute heart failure: Construction of an inflammatory prognostic scoring system. Front. Immunol. 2022, 13, 1005697. [Google Scholar] [CrossRef]
- Ang, S.P.; Chia, J.E.; Jaiswal, V.; Hanif, M.; Iglesias, J. Prognostic Value of Neutrophil-to-Lymphocyte Ratio in Patients with Acute Decompensated Heart Failure: A Meta-Analysis. J. Clin. Med. 2024, 13, 1212. [Google Scholar] [CrossRef]
- Delcea, C.; Buzea, C.A.; Dan, G.A. The neutrophil to lymphocyte ratio in heart failure: A comprehensive review. Rom. J. Intern. Med. 2019, 57, 296–314. [Google Scholar] [CrossRef]
- Zahorec, R. Neutrophil-to-lymphocyte ratio, past, present and future perspectives. Bratisl. Lek. Listy 2021, 122, 474–488. [Google Scholar] [CrossRef] [PubMed]
- Bhat, G.; Yost, G.L.; Ibrahim, K.; Pappas, P.; Tatooles, A. Risk stratification with longitudinal neutrophil to lymphocyte ratio assessment after left ventricular assist device implantation. Int. J. Artif. Organs 2018, 41, 445–451. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Feng, L.; Zhu, Z.; He, Y.; Li, X. Association between blood inflammatory indices and heart failure: A cross-sectional study of NHANES 2009–2018. Acta Cardiol. 2024, 79, 473–485. [Google Scholar] [CrossRef]
- Liu, J.; Yao, L.; Xiang, P.; Lin, P.; Xiong, H.; Li, C.; Zhang, M.; Tan, J.; Xu, Y.; Song, R.; et al. Neutrophil-to-Lymphocyte Ratio Predicts Critical Illness Patients with 2019 Coronavirus Disease in the Early Stage. J. Transl. Med. 2020, 18, 206. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.L.; Zhang, H.F.; Zhang, Q.; Zhu, X.Y.; Liu, C.H.; Wang, N.; Zhang, J.B.; Chen, H.; Chen, Y.; Li, W.Q.; et al. Neutrophil-to-Lymphocyte Ratio in Adult Community-Acquired Pneumonia Patients Correlates with Unfavorable Clinical Outcomes. Clin. Lab. 2019, 65, 839. [Google Scholar] [CrossRef] [PubMed]
- Cupp, M.A.; Margarita, C.; Tzoulaki, I.; Aune, D.; Evangelou, E.; Berlanga-Taylor, A.J. Neutrophil to Lymphocyte Ratio and Cancer Prognosis: An Umbrella Review of Systematic Reviews and Meta-Analyses of Observational Studies. BMC Med. 2020, 18, 360. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wei, R.; Wang, X.; Zhang, W.; Li, M.; Ni, T.; Weng, W.; Li, Q. The neutrophil-to-lymphocyte ratio is associated with all-cause and cardiovascular mortality among individuals with hypertension. Cardiovasc. Diabetol. 2024, 23, 117. [Google Scholar] [CrossRef]
- Franco, A.T.; Corken, A.; Ware, J. Platelets at the interface of thrombosis, inflammation, and cancer. Blood 2015, 126, 582–588. [Google Scholar] [CrossRef]
- Mongirdienė, A.; Laukaitienė, J.; Skipskis, V.; Kuršvietienė, L.; Liobikas, J. Platelet Activity and Its Correlation with Inflammation and Cell Count Readings in Chronic Heart Failure Patients with Reduced Ejection Fraction. Medicina 2021, 57, 176. [Google Scholar] [CrossRef]
- Wu, C.C.; Wu, C.H.; Lee, C.H.; Cheng, C.I. Association between neutrophil percentage-to-albumin ratio (NPAR), neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR) and long-term mortality in community-dwelling adults with heart failure: Evidence from US NHANES 2005–2016. BMC Cardiovasc. Disord. 2023, 23, 312. [Google Scholar] [CrossRef]
- Zinellu, A.; Mangoni, A.A. Neutrophil-to-lymphocyte and platelet-to-lymphocyte ratio and disease activity in rheumatoid arthritis: A systematic review and meta-analysis. Eur. J. Clin. Investig. 2023, 53, e13877. [Google Scholar] [CrossRef] [PubMed]
- Arfsten, H.; Cho, A.; Prausmüller, S.; Spinka, G.; Novak, J.; Goliasch, G.; Bartko, P.E.; Raderer, M.; Gisslinger, H.; Kornek, G.; et al. Inflammation-Based Scores as a Common Tool for Prognostic Assessment in Heart Failure or Cancer. Front. Cardiovasc. Med. 2021, 8, 725903. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. Standards of Medical Care in Diabetes-2021 Abridged for Primary Care Providers. Clin. Diabetes 2021, 39, 14–43. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. Standards of Medical Care in Diabetes-2022 Abridged for Primary Care Providers. Clin. Diabetes 2022, 40, 10–38. [Google Scholar] [CrossRef]
- Ilich, J.Z.; Kelly, O.J.; Kim, Y.; Spicer, M.T. Low-grade chronic inflammation perpetuated by modern diet as a promoter of obesity and osteoporosis. Arh. Hig. Rada Toksikol. 2014, 65, 139–148. [Google Scholar] [CrossRef]
- de Heredia, F.P.; Gómez-Martínez, S.; Marcos, A. Obesity, inflammation and the immune system. Proc. Nutr. Soc. 2012, 71, 332–338. [Google Scholar] [CrossRef]
- Colluoglu, T.; Akın, Y. The Value of Neutrophil-to-Lymphocyte Ratio and Epicardial Adipose Tissue Thickness in Heart Failure with Preserved Ejection Fraction. Cureus 2023, 15, e42846. [Google Scholar] [CrossRef]
- Vakhshoori, M.; Nemati, S.; Sabouhi, S.; Yavari, B.; Shakarami, M.; Bondariyan, N.; Emami, S.A.; Shafie, D. Neutrophil to lymphocyte ratio (NLR) prognostic effects on heart failure; a systematic review and meta-analysis. BMC Cardiovasc. Disord. 2023, 23, 555. [Google Scholar] [CrossRef]
- Delcea, C.; Buzea, C.A.; Vijan, A.; Draghici, A.; Stoichitoiu, L.E.; Dan, G.A. Comparative role of hematological indices for the assessment of in-hospital outcome of heart failure patients. Scand. Cardiovasc. J. 2021, 55, 227–236. [Google Scholar] [CrossRef]
- Kim, M.J.; Kwon, S.S.; Ji, Y.S.; Lee, M.Y.; Kim, K.H.; Lee, N.; Park, S.K.; Won, J.H.; Yoon, S.Y. Neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio as new possible minor criteria for diagnosis of polycythemia vera. Int. J. Lab. Hematol. 2023, 45, 853–859. [Google Scholar] [CrossRef]
- Ren, L.; Xu, J.; Li, J.; Xu, T.; Yang, Y.; Wang, W.; Ren, Y.; Gu, S.; Chen, C.; Wei, Z.; et al. A prognostic model incorporating inflammatory cells and cytokines for newly diagnosed multiple myeloma patients. Clin. Exp. Med. 2023, 23, 2583–2591. [Google Scholar] [CrossRef] [PubMed]
- Delcea, C.; Buzea, C.A.; Vîjan, A.E.; Bădilă, E.; Dan, G.A. The platelet to lymphocyte ratio in heart failure: A comprehensive review. Rom. J. Intern. Med. 2023, 61, 84–97. [Google Scholar] [CrossRef] [PubMed]
- Turcato, G.; Sanchis-Gomar, F.; Cervellin, G.; Zorzi, E.; Sivero, V.; Salvagno, G.L.; Tenci, A.; Lippi, G. Evaluation of Neutrophil-lymphocyte and Platelet-lymphocyte Ratios as Predictors of 30-day Mortality in Patients Hospitalized for an Episode of Acute Decompensated Heart Failure. J. Med. Biochem. 2019, 38, 452–460. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhou, X. A nomogram based on systemic inflammation markers can predict adverse outcomes in patients with heart failure. Nan Fang Yi Ke Da Xue Xue Bao 2022, 42, 1149–1158. (In Chinese) [Google Scholar] [CrossRef]



| Parameter | Entire Cohort | HFrEF | HFmrEF | HFpEF | Intergroup Variation (p-Value) |
|---|---|---|---|---|---|
| Admissions (n) | 427 | 186 | 130 | 111 | - |
| Male | 247 | 126 | 76 | 45 | - |
| Urban | 298 | 134 | 93 | 71 | - |
| Age (years, mean ± SD) | 68.48 ± 11.53 | 66.48 ± 11.53 | 69.14 ± 11.97 | 70.97 ± 9.52 | 0.002 |
| BMI (kg/m2, mean ± SD) | 29.78 ± 6.15 | 29.43 ± 5.77 | 29.48 ± 6.22 | 30.7 ± 6.63 | 0.254 |
| HR (bpm, mean ± SD) | 81.06 ± 22.24 | 85.83 ± 23.61 | 79.77 ± 22.62 | 75.07 ± 17.48 | <0.001 |
| SBP (mmHg, mean ± SD) | 131.25 ± 23.61 | 127.24 ± 24.17 | 131.65 ± 21.33 | 137.51 ± 23.98 | <0.001 |
| DBP (mmHg, mean ± SD) | 78.63 ± 13.5 | 78.57 ± 12.28 | 77.58 ± 12.94 | 79.95 ± 15.89 | 0.596 |
| NYHA functional class (n) | - | ||||
| I | 11 | 1 | 3 | 7 | - |
| II | 123 | 37 | 44 | 42 | - |
| III | 244 | 110 | 78 | 56 | - |
| IV | 49 | 38 | 5 | 6 | - |
| Coronary artery disease (n) | 71 | 35 | 20 | 16 | - |
| Atrial fibrillation/flutter (n) | 180 | 75 | 53 | 52 | - |
| Valvular heart disease ≥ moderate (n) | 160 | 67 | 48 | 45 | - |
| Hypertension (n) | 346 | 138 | 113 | 95 | - |
| T2DM (n) | 166 | 67 | 49 | 50 | - |
| ESR (mm/h, mean ± SD) | 17.85 ± 17.06 | 14.44 ± 12.7 | 17.16 ± 17.88 | 23.09 ± 19.88 | 0.001 |
| Fibrinogen (g/L, mean ± SD) | 4.08 ± 1.07 | 4.01 ± 1.08 | 3.99 ± 1.1 | 4.33 ± 1.01 | 0.031 |
| Neutrophils (×103/µL, mean ± SD) | 5.58 ± 2.33 | 5.78 ± 2.63 | 5.37 ± 2.07 | 5.47 ± 2.03 | 0.622 |
| Lymphocytes (×103/µL, mean ± SD) | 1.90 ± 1.15 | 1.88 ± 1.42 | 1.90 ± 0.89 | 1.95 ± 0.88 | 0.035 |
| Platelets (×103/µL, mean ± SD) | 224.34 ± 71.67 | 216.12 ± 71.86 | 221.75 ± 62.98 | 241.15 ± 78.43 | 0.011 |
| NLR (mean ± SD) | 3.79 ± 3.32 | 4.05 ± 3.71 | 3.59 ± 3.06 | 3.61 ± 2.91 | 0.025 |
| PLR (mean ± SD) | 144.84 ± 83.08 | 144.08 ± 79.27 | 138.13 ± 73.42 | 153.97 ± 98.52 | 0.711 |
| Admission length (days, mean ± SD) | 8.33 ± 5.26 | 9.15 ± 5.79 | 7.88 ± 5.30 | 7.5 ± 4.00 | <0.001 |
| Extended LOS (n, >7 days) | 192 | 97 | 52 | 43 | 0.542 |
| Number of readmissions ≤ 90 days (n = 73 *) | HF phenotypes at readmission (n = 56 unique patients) | - | |||
| HFrEF | HFmrEF | HFpEF | - | ||
| 1 | 22 | 9 | 12 | - | |
| 2 | 6 | 5 | 0 | - | |
| 3 | 0 | 0 | 0 | - | |
| 4 | 1 | 0 | 1 | - | |
| Parameters | Entire Cohort | HFrEF | HFmrEF | HFpEF | Intergroup Variation (p-Value) |
|---|---|---|---|---|---|
| Patients (n) | 323 | 135 | 77 | 111 | - |
| NT-proBNP (pg/mL, mean ± SD) | 4695.80 ± 7561.20 | 7197.80 ± 8957.43 | 3023.26 ± 4302.50 | 2813.10 ± 6603.80 | <0.001 |
| LOS (days, mean ± SD) | 8.28 ± 5.32 | 8.87 ± 5.78 | 8.40 ± 6.02 | 7.50 ± 4.00 | 0.21 |
| NLR (mean ± SD) | 3.89 ± 3.46 | 4.15 ± 4.16 | 3.84 ± 2.78 | 3.61 ± 2.91 | 0.13 |
| PLR (mean ± SD) | 149.62 ± 89.49 | 149.03 ± 85.65 | 144.39 ± 83.03 | 153.97 ± 98.52 | 0.82 |
| Parameters | NT-proBNP | |||||
|---|---|---|---|---|---|---|
| HFrEF (n = 135) | HFmrEF (n = 77) | HFpEF (n = 111) | ||||
| r * | p | r | p | r | p | |
| LOS | 0.19 | 0.03 | 0.56 | <0.001 | −0.01 | 0.88 |
| NLR | 0.21 | 0.017 | 0.4 | <0.001 | 0.24 | 0.013 |
| PLR | 0.17 | 0.048 | 0.25 | 0.028 | 0.13 | 0.182 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cristescu, L.; Tilea, I.; Iancu, D.-G.; Stoica, F.; Moldovan, D.-A.; Capriglione, V.; Varga, A. Insights into the Neutrophil-to-Lymphocyte Ratio and the Platelet-to-Lymphocyte Ratio as Predictors for the Length of Stay and Readmission in Chronic Heart Failure Patients. Diagnostics 2024, 14, 2102. https://doi.org/10.3390/diagnostics14182102
Cristescu L, Tilea I, Iancu D-G, Stoica F, Moldovan D-A, Capriglione V, Varga A. Insights into the Neutrophil-to-Lymphocyte Ratio and the Platelet-to-Lymphocyte Ratio as Predictors for the Length of Stay and Readmission in Chronic Heart Failure Patients. Diagnostics. 2024; 14(18):2102. https://doi.org/10.3390/diagnostics14182102
Chicago/Turabian StyleCristescu, Liviu, Ioan Tilea, Dragos-Gabriel Iancu, Florin Stoica, Diana-Andreea Moldovan, Vincenzo Capriglione, and Andreea Varga. 2024. "Insights into the Neutrophil-to-Lymphocyte Ratio and the Platelet-to-Lymphocyte Ratio as Predictors for the Length of Stay and Readmission in Chronic Heart Failure Patients" Diagnostics 14, no. 18: 2102. https://doi.org/10.3390/diagnostics14182102
APA StyleCristescu, L., Tilea, I., Iancu, D.-G., Stoica, F., Moldovan, D.-A., Capriglione, V., & Varga, A. (2024). Insights into the Neutrophil-to-Lymphocyte Ratio and the Platelet-to-Lymphocyte Ratio as Predictors for the Length of Stay and Readmission in Chronic Heart Failure Patients. Diagnostics, 14(18), 2102. https://doi.org/10.3390/diagnostics14182102









