Development of Prediction Model for Intensive Care Unit Admission Based on Heart Rate Variability: A Case–Control Matched Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Statement
2.2. Study Design and Setting
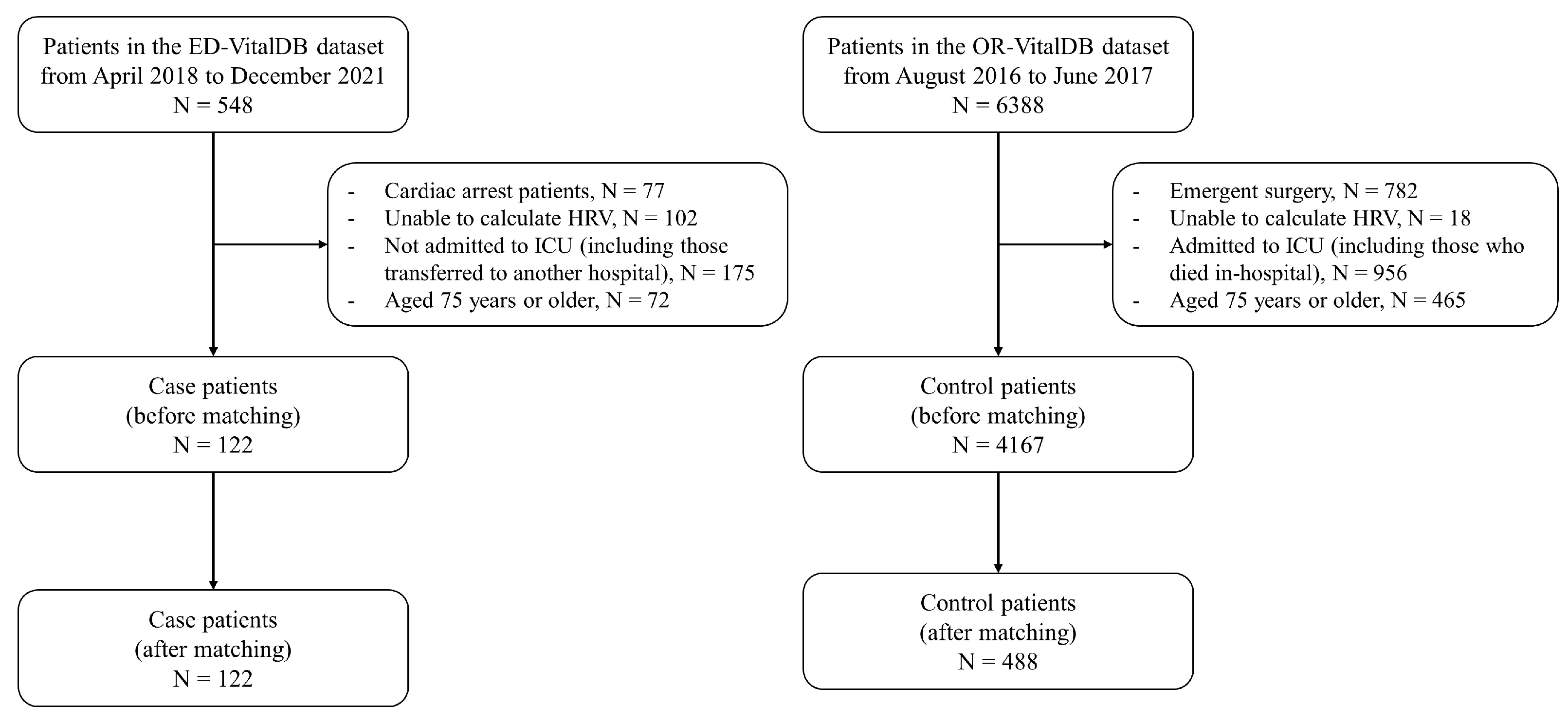
2.3. Study Population
2.4. Variables and Measurements
2.5. Model Development
2.6. Outcomes
2.7. Statistical Analysis
3. Results
4. Discussion
Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
- Male reference
- 2.
- Female reference
- 3.
- Calculation of SDRR and RMSSD values adjusted for age and sex
Appendix B
| Total | Derivation Set | Validation Set | p-Value | |
|---|---|---|---|---|
| Number of patients | 610 | 457 | 153 | |
| Number of data points | 10,189 | 7661 | 2528 | |
| Age, years | 63 (56–69) | 63 (56–69) | 63 (56–68) | 0.44 |
| Sex, male | 400 (65.6) | 299 (65.4) | 101 (66.0) | 0.92 |
| Hypertension | 198 (32.5) | 156 (34.1) | 42 (27.5) | 0.12 |
| Diabetes mellitus | 75 (12.3) | 54 (11.8) | 21 (13.7) | 0.54 |
| HR, beats/min * | 74.2 (65.2–87.3) | 74.4 (64.9–88.2) | 73.0 (66.0–85.4) | 0.40 |
| SDRR, ms * | 64.2 (40.5–98.7) | 63.1 (39.4–97.6) | 68.9 (46.8–103.3) | 0.16 |
| Adjusted SDRR, ms * | 24.4 (−1.5–57.2) | 23.6 (−1.9–54.4) | 27.6 (2.7–64.5) | 0.29 |
| RMSSD, ms * | 51.9 (26.5–98.6) | 48.9 (26.8–97.6) | 59.8 (25.0–103.1) | 0.23 |
| Adjusted RMSSD, ms * | 23.4 (−1.9–70.4) | 20.8 (−1.1–68.8) | 31.6 (−2.9–78.3) | 0.35 |
| Normalized LF power, % * | 22.2 (15.9–29.6) | 22.3 (16.3–29.4) | 21.7 (14.9–30.4) | 0.93 |
| Normalized HF power, % * | 40.7 (25.3–51.6) | 40.2 (25.1–51.5) | 41.9 (25.6–52.5) | 0.55 |
| LF/HF ratio | 0.62 (0.37–1.30) | 0.63 (0.37–1.34) | 0.60 (0.37–1.24) | 0.68 |
| ICU admission | 122 (20.0) | 91 (19.9) | 31 (20.3) | 0.93 |
Appendix C
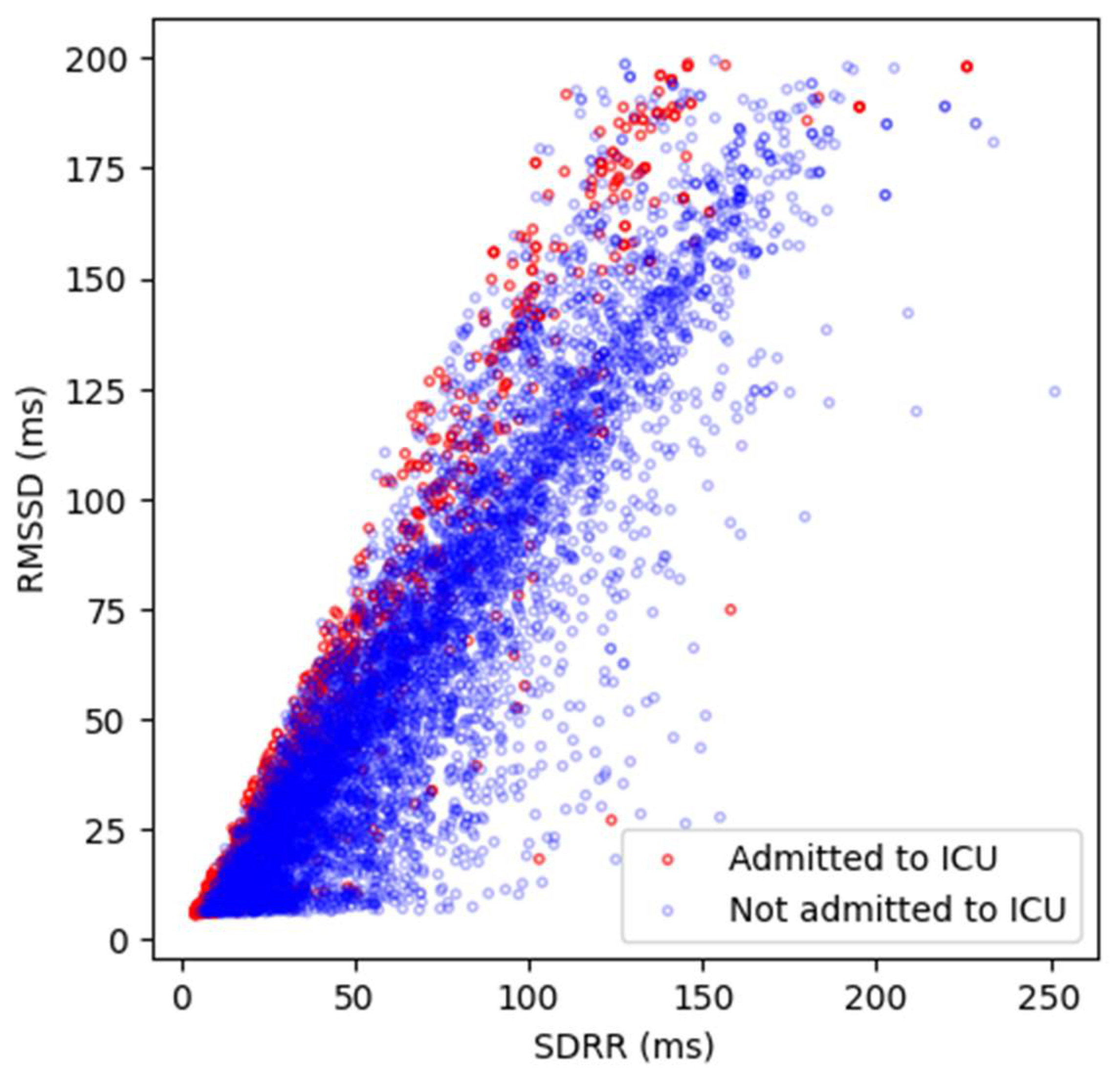
Appendix D
| Total | Case Group | Control Group | p-Value | |
|---|---|---|---|---|
| Number of patients | 375 | 29 | 346 | |
| Number of data points | 6736 | 494 | 6242 | |
| Age, years | 63 (56–69) | 63 (56–68) | 63 (56–69) | 0.73 |
| Sex, male | 242 (64.5) | 21 (72.4) | 221 (63.9) | 0.36 |
| Hypertension | 130 (34.7) | 11 (37.9) | 119 (34.4) | 0.70 |
| Diabetes mellitus | 46 (12.3) | 7 (24.1) | 39 (11.3) | 0.04 |
| HR, beats/min * | 69.9 (63.8–78.7) | 106.5 (93.3–120.2) | 69.1 (63.4–76.9) | <0.01 |
| SDRR, ms * | 63.7 (42.6–90.3) | 41.2 (25.1–53.5) | 66.2 (45.0–90.6) | <0.01 |
| Adjusted SDRR, ms * | 22.9 (0.9–50.7) | −3.5 (−15.5–16.7) | 24.4 (3.8–52.0) | <0.01 |
| RMSSD, ms * | 60.2 (33.3–89.8) | 44.1 (25.6–67.2) | 60.8 (35.1–89.8) | 0.08 |
| Adjusted RMSSD, ms * | 32.3 (6.7–64.4) | 13.2 (−5.7–40.8) | 33.3 (8.1–64.5) | 0.08 |
| Normalized LF power, % * | 23.6 (19.0–26.6) | 22.7 (14.2–26.2) | 23.6 (19.4–26.6) | 0.16 |
| Normalized HF power, % * | 40.8 (32.6–47.8) | 37.8 (33.5–48.3) | 40.8 (32.6–47.8) | 0.97 |
| LF/HF ratio | 0.82 (0.52–1.28) | 0.59 (0.35–0.98) | 0.83 (0.54–1.32) | 0.02 |
Appendix E
| OR (95% CI) | |
|---|---|
| Model 1 | |
| Diabetes mellitus, yes vs. no | 2.03 (0.27–15.10) |
| HR (for every 10 increase) | 5.35 (3.39–8.44) |
| SDRR (for every 10 increase) | 0.73 (0.51–1.05) |
| RMSSD (for every 10 increase) | 1.19 (0.90–1.59) |
| Model 2 | |
| Diabetes mellitus, yes vs. no | 2.42 (0.34–17.25) |
| HR (for every 10 increase) | 5.23 (3.34–8.19) |
| Adjusted SDRR (for every 10 increase) | 0.75 (0.53–1.06) |
| Adjusted RMSSD (for every 10 increase) | 1.20 (0.91–1.59) |
References
- Cairns, C.; Ashman, J.J.; Kang, K. Emergency department visit rates by selected characteristics: United States, 2019. NCHS Data Brief 2022, 434, 1–8. [Google Scholar]
- Lane, B.H.; Mallow, P.J.; Hooker, M.B.; Hooker, E. Trends in United States emergency department visits and associated charges from 2010 to 2016. Am. J. Emerg. Med. 2020, 38, 1576–1581. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Yeo, Y.; Ji, Y.; Kim, B.; Han, K.; Cha, W.; Shin, D. Factors associated with emergency department visits and consequent hospitalization and death in Korea using a population-based national health database. Healthcare 2022, 10, 1324. [Google Scholar] [CrossRef] [PubMed]
- Morley, C.; Unwin, M.; Peterson, G.M.; Stankovich, J.; Kinsman, L. Emergency department crowding: A systematic review of causes, consequences and solutions. PLoS ONE 2018, 13, e0203316. [Google Scholar] [CrossRef] [PubMed]
- Munroe, B.; Curtis, K.; Balzer, S.; Royston, K.; Fetchet, W.; Tucker, S.; Considine, J. Translation of evidence into policy to improve clinical practice: The development of an emergency department rapid response system. Australas Emerg. Care 2021, 24, 197–209. [Google Scholar] [CrossRef] [PubMed]
- Shaffer, F.; Ginsberg, J.P. An overview of heart rate variability metrics and norms. Front. Public Health 2017, 5, 258. [Google Scholar] [CrossRef] [PubMed]
- Arbo, J.E.; Lessing, J.K.; Ford, W.J.; Clark, S.; Finkelsztein, E.; Schenck, E.J.; Heerdt, P.M. Heart rate variability measures for prediction of severity of illness and poor outcome in ED patients with sepsis. Am. J. Emerg. Med. 2020, 38, 2607–2613. [Google Scholar] [CrossRef]
- Buccelletti, F.; Gilardi EM, A.N.; Scaini, E.; Galiuto, L.E.O.N.; Persiani, R.O.B.E.; Biondi, A.L.B.E.; Silveri, N.G. Heart rate variability and myocardial infarction: Systematic literature review and metanalysis. Eur. Rev. Med. Pharmacol. Sci. 2009, 13, 299–307. [Google Scholar]
- de Castilho, F.M.; Ribeiro, A.L.P.; da Silva, J.L.P.; Nobre, V.; de Sousa, M.R. Heart rate variability as predictor of mortality in sepsis: A prospective cohort study. PLoS ONE 2017, 12, e0180060. [Google Scholar] [CrossRef]
- Lee, H.; Yang, H.L.; Ryu, H.G.; Jung, C.W.; Cho, Y.J.; Yoon, S.B.; Lee, H.C. Real-time machine learning model to predict in-hospital cardiac arrest using heart rate variability in ICU. Npj Digit. Med. 2023, 6, 215. [Google Scholar] [CrossRef]
- Lee, J.H.; Park, Y.S.; Park, I.C.; Lee, H.S.; Kim, J.H.; Park, J.M.; Kim, M.J. Over-triage occurs when considering the patient’s pain in Korean Triage and Acuity Scale (KTAS). PLoS ONE 2019, 14, e0216519. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.C.; Jung, C.W. Vital Recorder-a free research tool for automatic recording of high-resolution time-synchronised physiological data from multiple anaesthesia devices. Sci. Rep. 2018, 8, 1527. [Google Scholar] [CrossRef]
- Lee, H.C.; Park, Y.; Yoon, S.B.; Yang, S.M.; Park, D.; Jung, C.W. VitalDB, a high-fidelity multi-parameter vital signs database in surgical patients. Sci. Data 2022, 9, 279. [Google Scholar] [CrossRef] [PubMed]
- Makowski, D.; Pham, T.; Lau, Z.J.; Brammer, J.C.; Lespinasse, F.; Pham, H.; Chen, S.A. NeuroKit2: A Python toolbox for neurophysiological signal processing. Behav. Res. Methods 2021, 53, 1689–1696. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, A.; Pantelopoulos, A.; Emir-Farinas, H.; Natarajan, P. Heart rate variability with photoplethysmography in 8 million individuals: A cross-sectional study. Lancet Digit Health 2020, 2, e650–e657. [Google Scholar] [CrossRef] [PubMed]
- Papaioannou, V.; Pneumatikos, I.; Maglaveras, N. Association of heart rate variability and inflammatory response in patients with cardiovascular diseases: Current strengths and limitations. Front Physiol 2013, 4, 174. [Google Scholar] [CrossRef]
- Fernandes, M.; Mendes, R.; Vieira, S.M.; Leite, F.; Palos, C.; Johnson, A.; Celi, L.A. Predicting Intensive Care Unit admission among patients presenting to the emergency department using machine learning and natural language processing. PLoS ONE 2020, 15, e0229331. [Google Scholar] [CrossRef]
- Omerbegovic, M. Alterations of short-term heart rate variability in periinduction period of general anaesthesia with two intravenous anaesthetics. Med. Arch. 2013, 67, 233–236. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.S.; Kim, S.H.; Shin, S.; Pak, H.N.; Yang, S.J.; Oh, Y.J. Effects of Dexmedetomidine on Changes in Heart Rate Variability and Hemodynamics During Tracheal Intubation. Am. J. Ther. 2016, 23, e369–e376. [Google Scholar] [CrossRef]
- Covino, M.; Sandroni, C.; Della Polla, D.; De Matteis, G.; Piccioni, A.; De Vita, A.; Franceschi, F. Predicting ICU admission and death in the Emergency Department: A comparison of six early warning scores. Resuscitation 2023, 190, 109876. [Google Scholar] [CrossRef]
- Marin, O.M.M.; Garcia, P.Á.A.; Munoz, V.O.M.; Castellanos, R.J.C.; Cáceres, M.E.; Santacruz, P.D. Portable single-lead electrocardiogram device is accurate for QTc evaluation in hospitalized patients. Heart Rhythm O2 2021, 2, 382–387. [Google Scholar] [CrossRef] [PubMed]
- Henriksen, D.P.; Brabrand, M.; Lassen, A.T. Prognosis and risk factors for deterioration in patients admitted to a medical emergency department. PLoS ONE 2014, 9, e94649. [Google Scholar] [CrossRef] [PubMed]
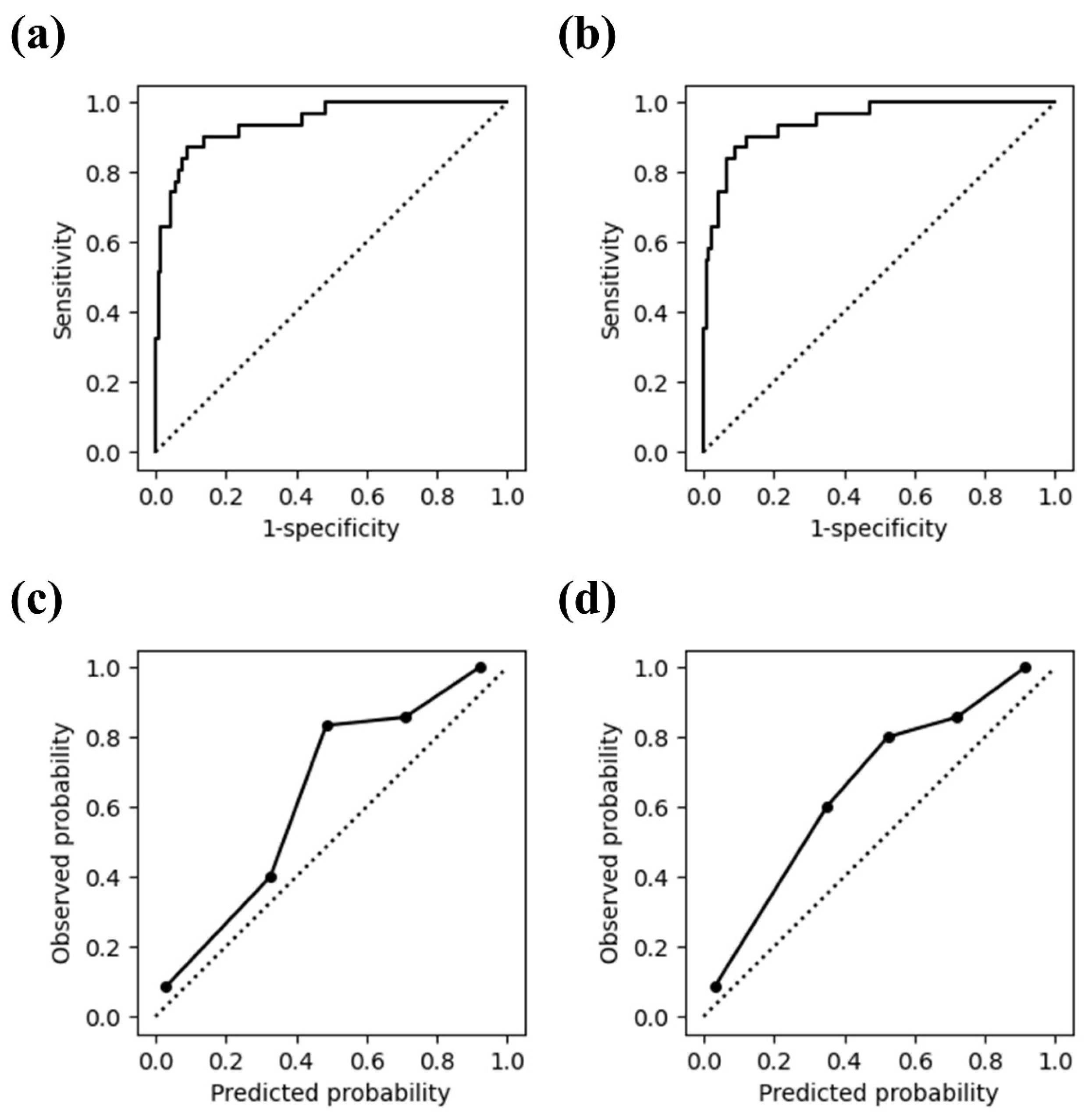
| Total | Case Group | Control Group | p-Value | |
|---|---|---|---|---|
| Number of patients | 610 | 122 | 488 | |
| Number of data points | 10,189 | 1594 | 8595 | |
| Age, years | 63 (56–69) | 63 (56–69) | 63 (56–69) | 0.78 |
| Sex, male | 400 (65.6) | 80 (65.6) | 320 (65.6) | 1.00 |
| Hypertension | 198 (32.5) | 37 (30.3) | 161 (33.0) | 0.57 |
| Diabetes mellitus | 75 (12.3) | 26 (21.3) | 49 (10.0) | <0.01 |
| HR, beats/min * | 72.1 (64.8–84.4) | 105.4 (91.5–116.7) | 69.5 (63.3–77.1) | <0.01 |
| SDRR, ms * | 61.3 (41.0–86.4) | 41.6 (24.3–61.8) | 66.2 (45.3–89.2) | <0.01 |
| Adjusted SDRR, ms * | 20.6 (−1.5–46.4) | 1.8 (−15.5–21.1) | 24.4 (3.4–49.2) | <0.01 |
| RMSSD, ms * | 59.5 (32.3–89.7) | 51.0 (25.6–81.0) | 60.3 (35.1–90.2) | 0.04 |
| Adjusted RMSSD, ms * | 31.2 (6.2–63.0) | 24.0 (−1.2–54.4) | 33.1 (7.9–65.0) | 0.04 |
| Normalized LF power, % * | 23.6 (19.0–27.6) | 22.4 (16.1–28.8) | 23.9 (19.4–27.3) | 0.13 |
| Normalized HF power, % * | 40.8 (31.9–48.1) | 41.3 (31.3–48.1) | 40.7 (32.0–48.2) | 0.98 |
| LF/HF ratio | 0.81 (0.51–1.31) | 0.62 (0.42–1.00) | 0.87 (0.53–1.39) | <0.01 |
| OR (95% CI) | |
|---|---|
| Model 1 | |
| Diabetes mellitus, yes vs. no | 3.33 (1.71–6.48) |
| HR (for every 10 increase) | 3.40 (2.97–3.90) |
| SDRR (for every 10 increase) | 0.68 (0.60–0.78) |
| RMSSD (for every 10 increase) | 1.36 (1.22–1.51) |
| Model 2 | |
| Diabetes mellitus, yes vs. no | 3.27 (1.69–6.36) |
| HR (for every 10 increase) | 3.44 (3.00–3.95) |
| Adjusted SDRR (for every 10 increase) | 0.72 (0.63–0.82) |
| Adjusted RMSSD (for every 10 increase) | 1.33 (1.20–1.48) |
| AUC (95% CI) | Sensitivity (95% CI) | Specificity (95% CI) | |
|---|---|---|---|
| Model 1 | |||
| Randomly sampled data point | 0.942 (0.897–0.987) | 0.871 (0.753–0.989) | 0.910 (0.859–0.961) |
| First data point | 0.921 (0.852–0.989) | 0.807 (0.667–0.946) | 0.943 (0.901–0.984) |
| Last data point | 0.883 (0.806–0.961) | 0.871 (0.753–0.989) | 0.812 (0.742–0.881) |
| Model 2 | |||
| Randomly sampled data point | 0.947 (0.906–0.987) | 0.871 (0.753–0.989) | 0.910 (0.859–0.961) |
| First data point | 0.923 (0.855–0.990) | 0.807 (0.667–0.946) | 0.943 (0.901–0.984) |
| Last data point | 0.886 (0.809–0.962) | 0.839 (0.709–0.968) | 0.861 (0.799–0.922) |
| AUC (95% CI) | Sensitivity (95% CI) | Specificity (95% CI) | |
|---|---|---|---|
| Model 1 | |||
| Randomly sampled data point | 0.928 (0.855–1.000) | 0.929 (0.794–1.000) | 0.824 (0.755–0.892) |
| First data point | 0.920 (0.828–1.000) | 0.929 (0.794–1.000) | 0.832 (0.765–0.899) |
| Last data point | 0.911 (0.838–0.984) | 0.786 (0.571–1.000) | 0.916 (0.866–0.966) |
| Model 2 | |||
| Randomly sampled data point | 0.926 (0.853–0.998) | 0.929 (0.794–1.000) | 0.807 (0.736–0.878) |
| First data point | 0.920 (0.830–1.000) | 0.857 (0.674–1.000) | 0.899 (0.845–0.953) |
| Last data point | 0.910 (0.835–0.985) | 0.786 (0.571–1.000) | 0.916 (0.866–0.966) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, D.H.; Lee, H.; Joo, H.; Kong, H.-J.; Lee, S.B.; Kim, S.; Shin, S.D.; Kim, K.H. Development of Prediction Model for Intensive Care Unit Admission Based on Heart Rate Variability: A Case–Control Matched Analysis. Diagnostics 2024, 14, 816. https://doi.org/10.3390/diagnostics14080816
Choi DH, Lee H, Joo H, Kong H-J, Lee SB, Kim S, Shin SD, Kim KH. Development of Prediction Model for Intensive Care Unit Admission Based on Heart Rate Variability: A Case–Control Matched Analysis. Diagnostics. 2024; 14(8):816. https://doi.org/10.3390/diagnostics14080816
Chicago/Turabian StyleChoi, Dong Hyun, Hyunju Lee, Hyunjin Joo, Hyoun-Joong Kong, Seung Bok Lee, Sungwan Kim, Sang Do Shin, and Ki Hong Kim. 2024. "Development of Prediction Model for Intensive Care Unit Admission Based on Heart Rate Variability: A Case–Control Matched Analysis" Diagnostics 14, no. 8: 816. https://doi.org/10.3390/diagnostics14080816
APA StyleChoi, D. H., Lee, H., Joo, H., Kong, H.-J., Lee, S. B., Kim, S., Shin, S. D., & Kim, K. H. (2024). Development of Prediction Model for Intensive Care Unit Admission Based on Heart Rate Variability: A Case–Control Matched Analysis. Diagnostics, 14(8), 816. https://doi.org/10.3390/diagnostics14080816






