Use of a Silicon Microneedle Chip-Based Device for the Extraction and Subsequent Analysis of Dermal Interstitial Fluid in Heart Failure Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling of Dermal Interstitial Fluid Using Microneedles
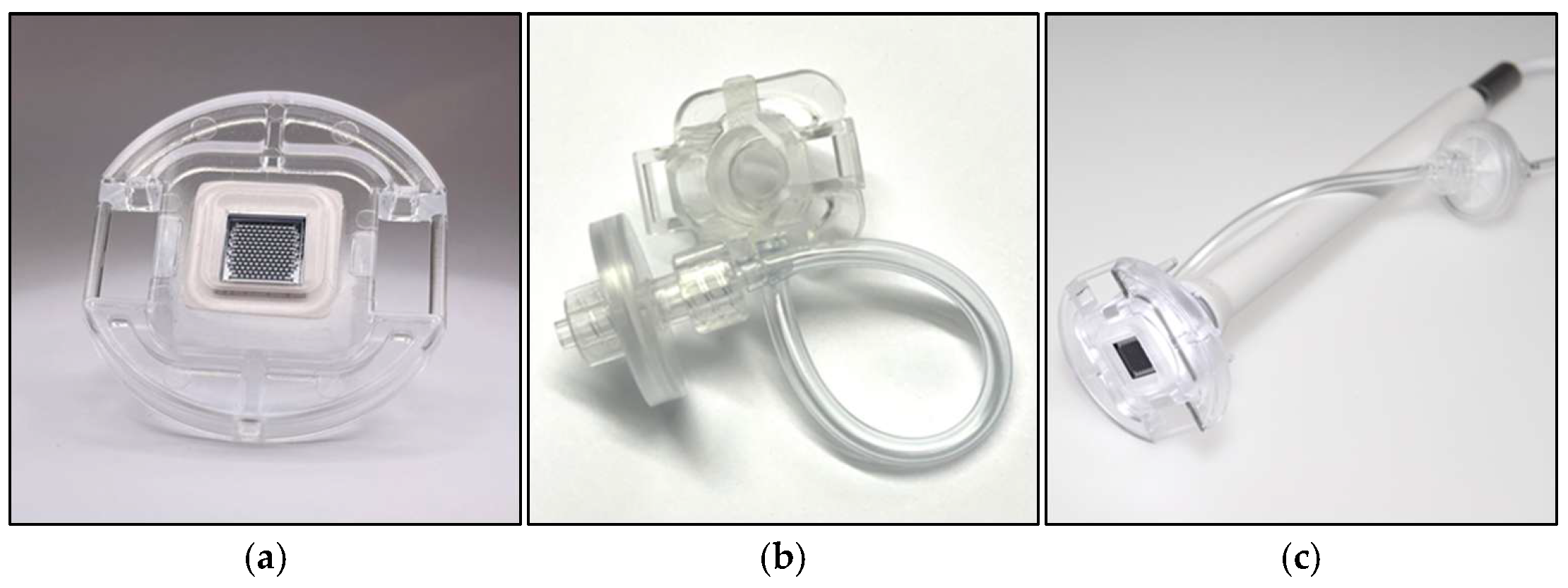
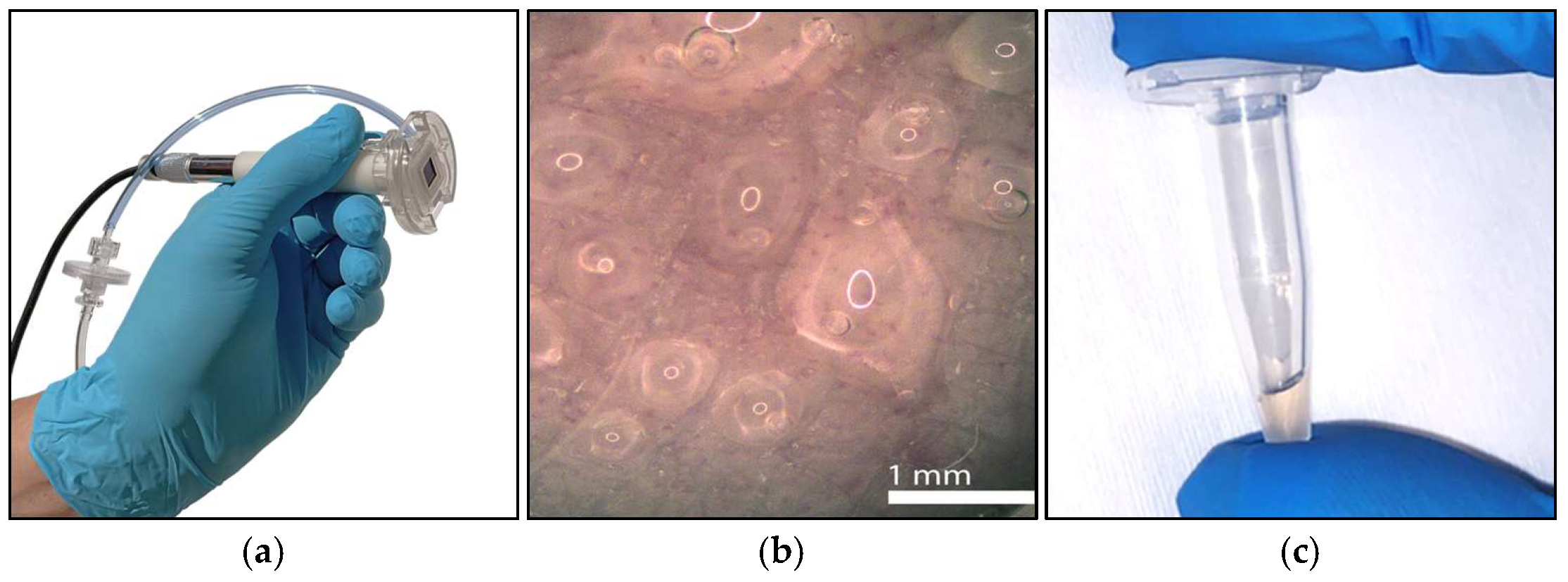
2.1.1. Silicon Microneedle Chip Technology
2.1.2. Interfaces and Usability
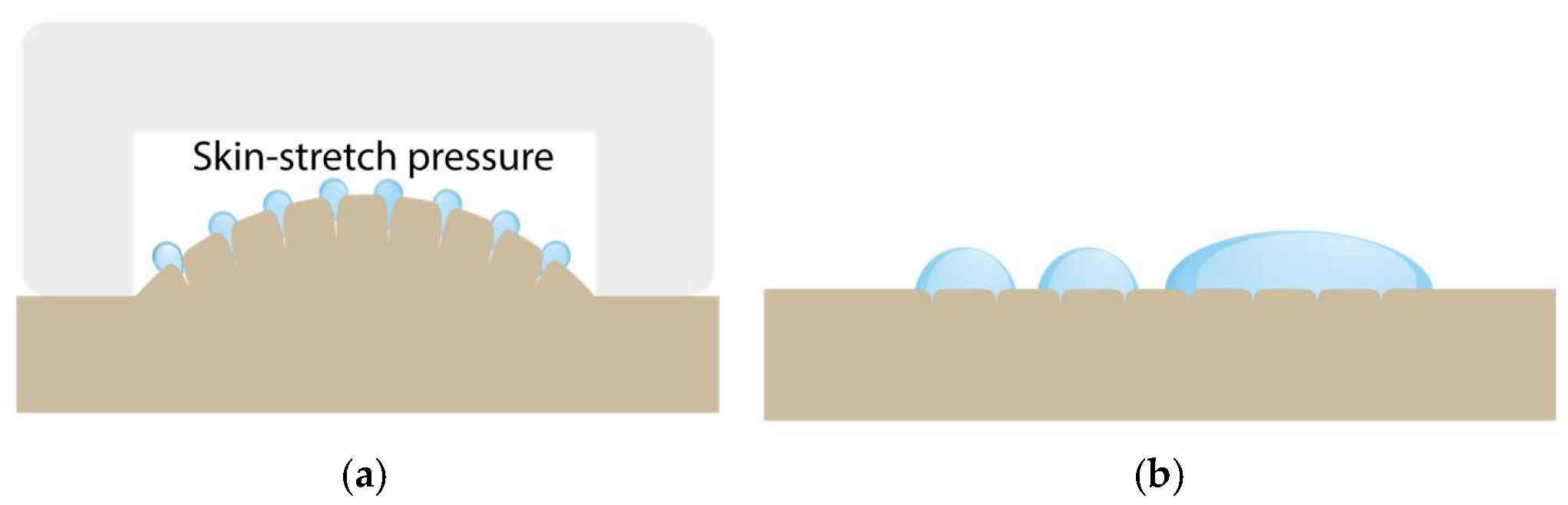
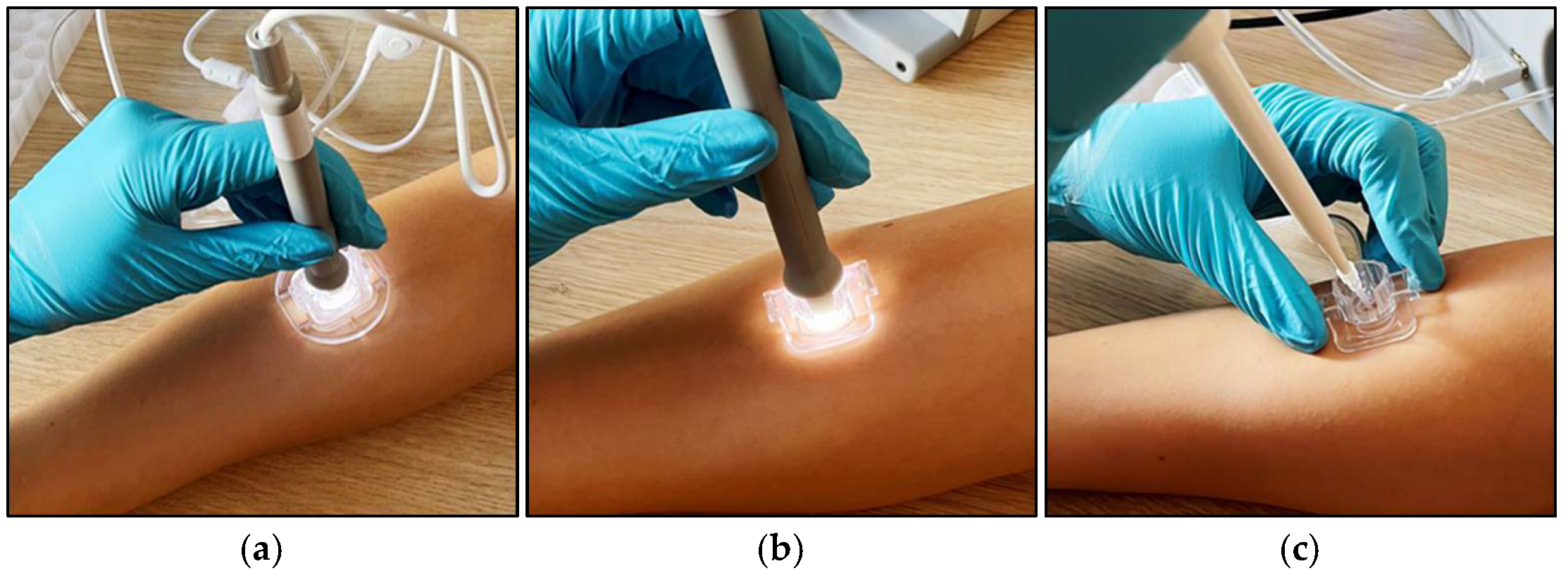
2.1.3. Sub-Pressure-Assisted Skin Penetration
2.1.4. Sub-Pressure-Assisted dISF Extraction
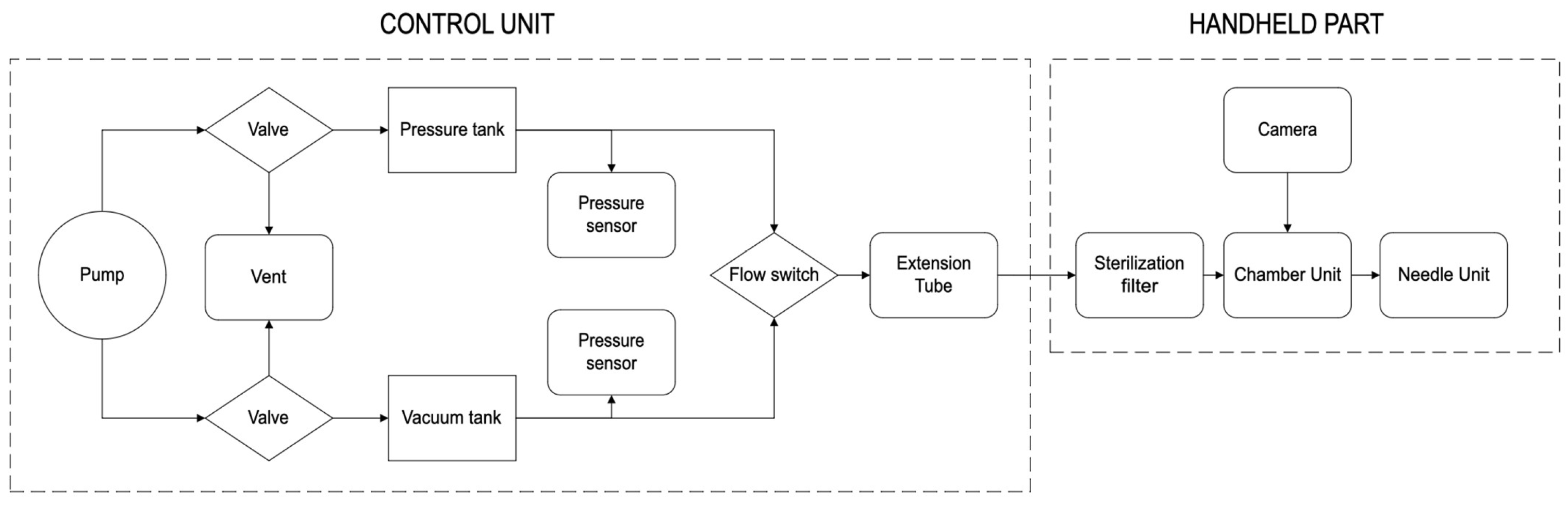
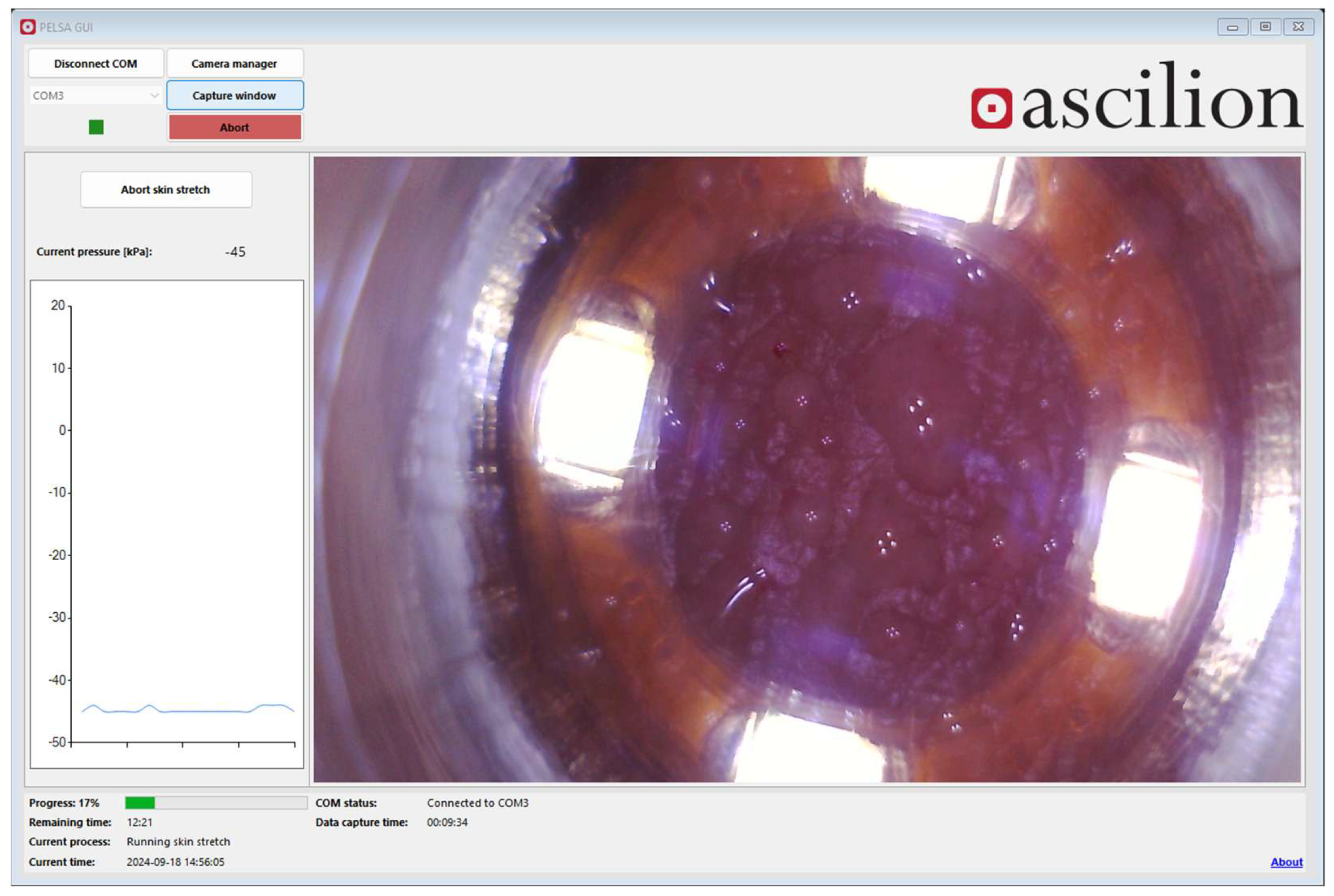
2.1.5. Design of the dISF Extraction Device (PELSA System)
2.1.6. System Configuration and Phases During Sampling of dISF
2.2. Examination of dISF-Derived Biomarkers in Heart Failure Patients and Comparison with Biomarker Levels in Blood
2.2.1. Study Design
2.2.2. Statistics
Statistical Analysis of the Primary Endpoint
Statistical Analysis of the Secondary Endpoint
- (a)
- For all biomarkers, a Pearson correlation coefficient including a two-sided 95% confidence interval will be calculated. In the case of non-normally distributed values, the Spearman correlation coefficient will be used. If sufficient (i.e., ≥10), patients with dermal interstitial fluid levels above the limit of quantification will be available, and a linear regression with blood levels as the dependent and dermal interstitial fluid levels as the independent variable will be performed.
- (b)
- The proportion of patients with increased (difference to first/previous measurement ≥ 0) and decreased (difference to first/previous measurement < 0) values (blood level and dermal interstitial fluid levels) will be analyzed descriptively.
- (c)
- The difference in the proportion of patients with increased or decreased values (blood level and dermal interstitial fluid levels), either compared to the first or previous measurement, will be analyzed using a Boschloo test. The absolute numerical differences will also be analyzed using linear regression if the values for all patients are available. The difference in blood levels will be the dependent variable and the difference in dermal interstitial fluid levels as well as the blood levels from the first or previous measurement will be the two independent variables. Further, Pearson or Spearman correlation coefficients including 95% confidence intervals will be calculated for the differences in dermal interstitial fluid and blood levels.
- (d)
- A dISF sampling volume of at least 3 μL in at least 90% of the sampling procedures is defined as a fulfillment of the performance of the PELSA system according to its intended use. No dedicated statistical analysis is needed for an analysis of this endpoint.
- (e)
- The safety of the PELSA system in relation to its intended use is defined as the absence of the documentation of any device-related adverse event or serious adverse event. No dedicated statistical analysis is needed for the evaluation of this endpoint.
2.2.3. Biomaterial Processing and Laboratory Analysis
dISF Processing
Blood Processing
dISF and Blood Analysis with Standard Laboratory Methods
Olink®-Based dISF and Blood Analysis
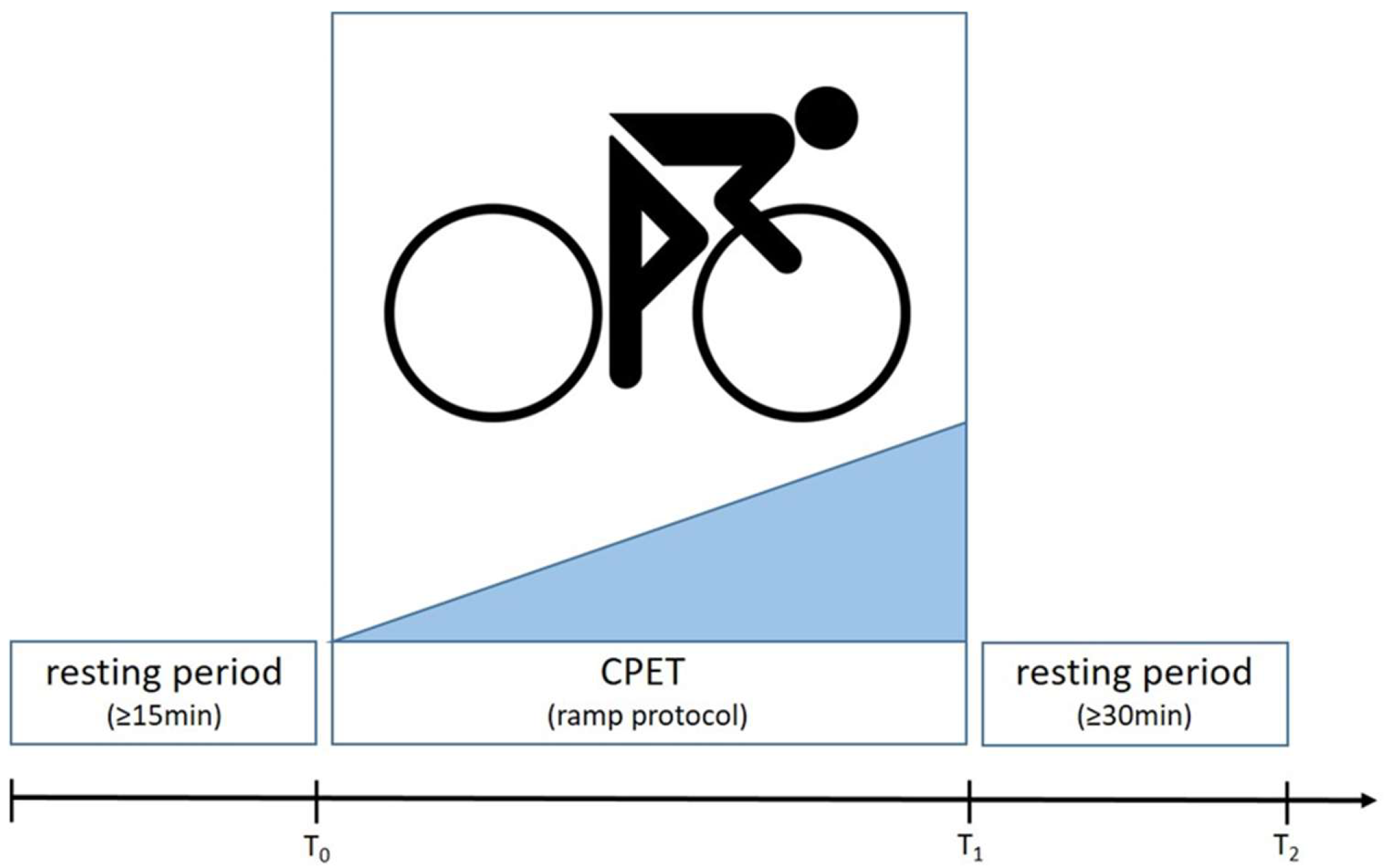
2.2.4. Cardiopulmonary Exercise Testing
2.2.5. Measures for Quality Assurance
3. Results
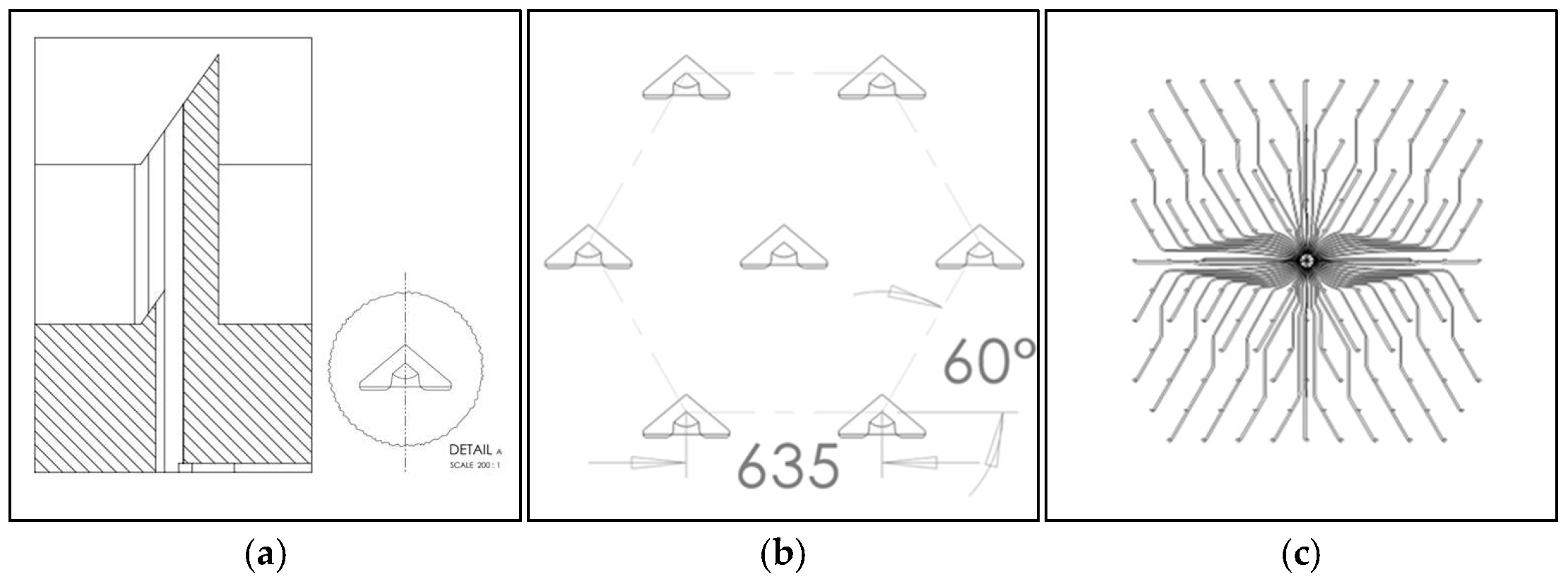
3.1. Silicon Microneedle Technology as the Base of an Innovative dISF Extraction Device
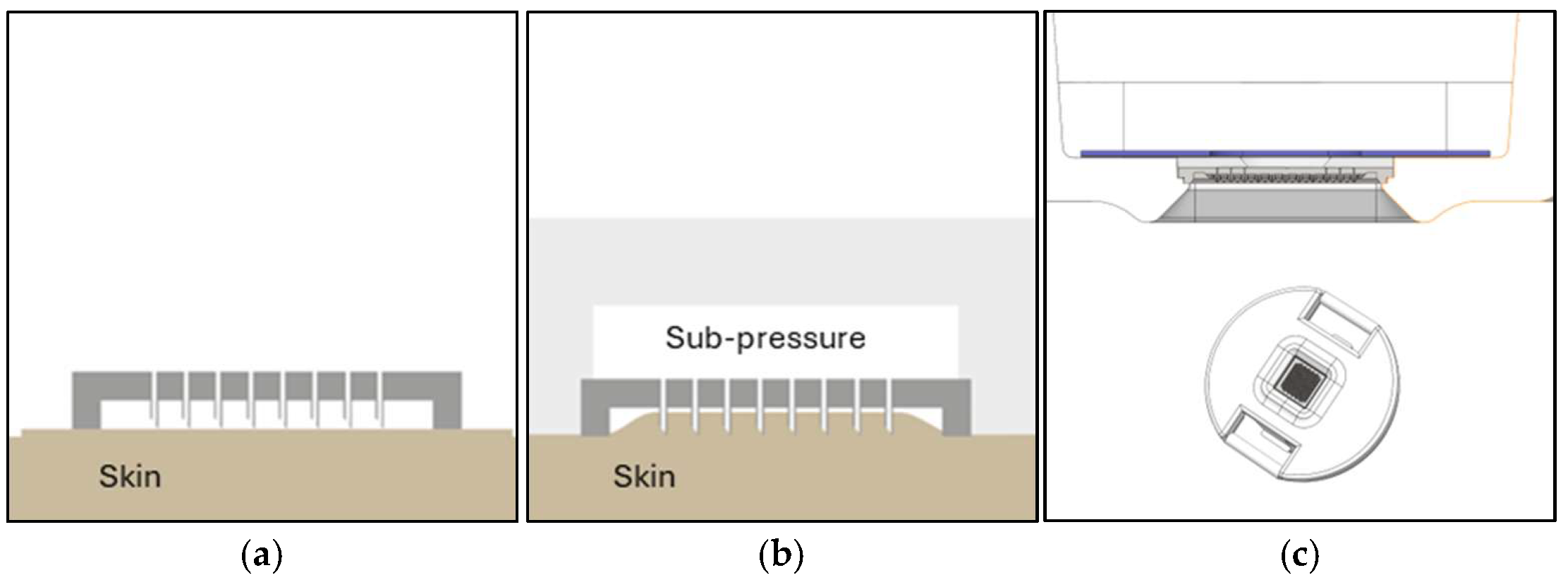
3.2. System/Device for dISF Extraction
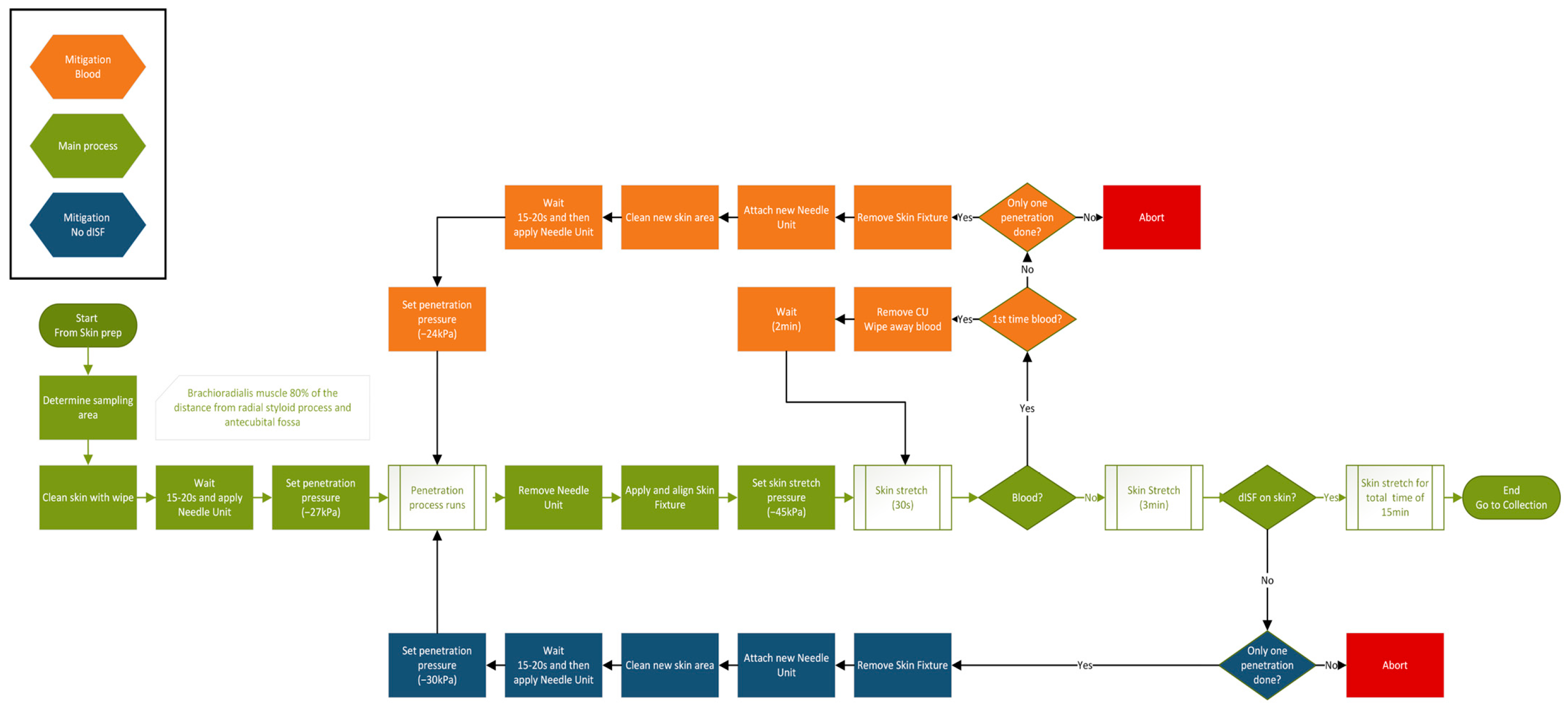
3.3. Protocol for dISF Extraction
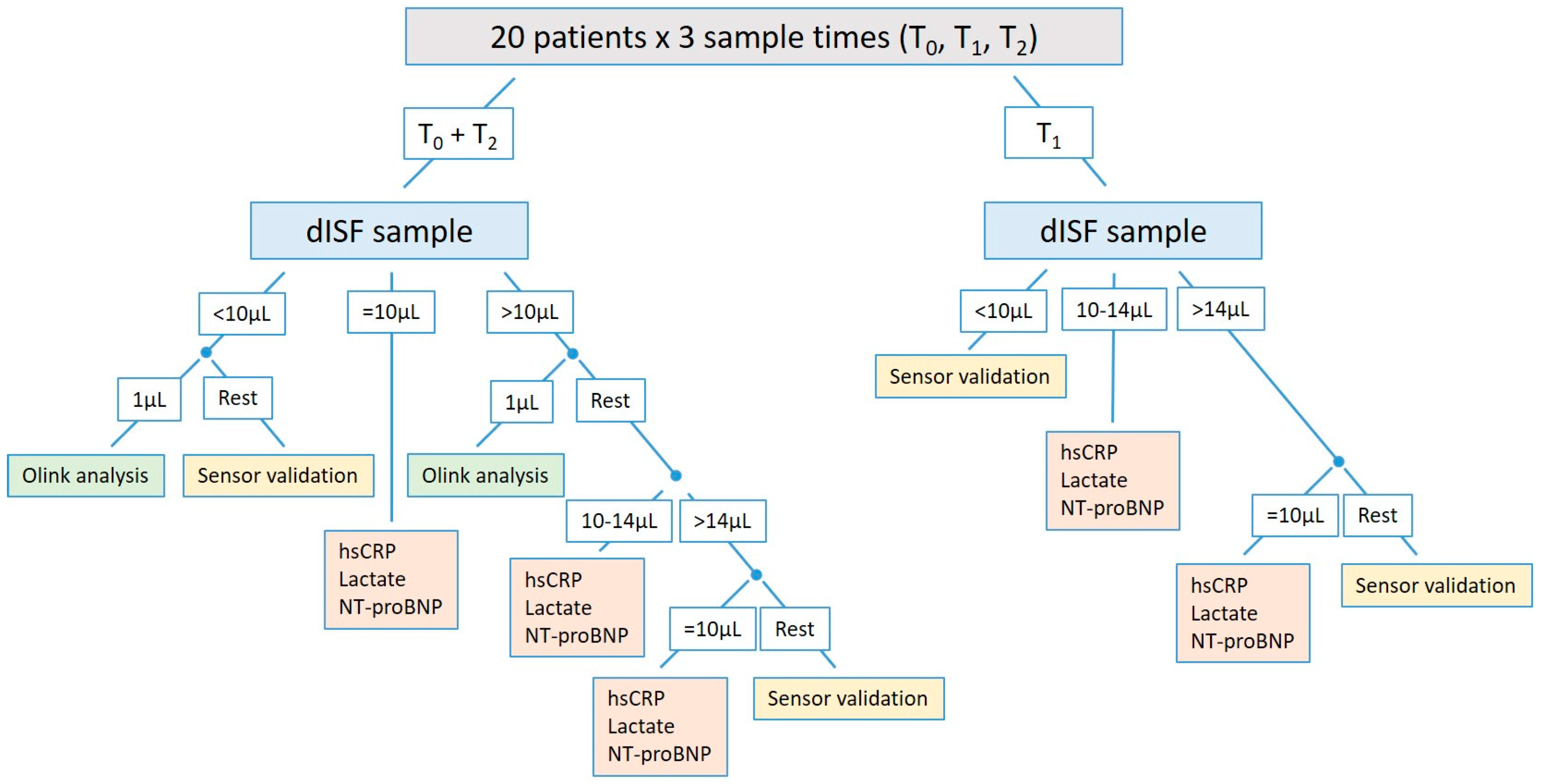
3.4. A Comprehensive Study Design to Investigate Biomarkers in dISF
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CPET | cardiopulmonary exercise test |
| dISF | dermal interstitial fluid |
| eCRF | electronic case report form |
| GUI | graphical user interface |
| hsCRP | high-sensitivity C-reactive protein |
| MEMS | micro-electro-mechanical systems |
| NT-proBNP | N-terminal pro-brain-type natriuretic peptide |
| SOP | standard operating procedure |
Appendix A
Appendix B
Appendix C
- List of biomarkers analyzed by the Olink Target 96 Cardiovascular II panel.
- 2,4-dienoyl-CoA reductase, mitochondrial (DECR1), A disintegrin and metalloproteinase with thrombospondin motifs 13 (ADAMTS13), Advanced glycosylation end product-specific receptor (AGER), Agouti-related protein (AGRP), Alpha-L-iduronidase (IDUA), Angiopoietin-1 (ANGPT1), Angiopoietin-1 receptor (TEK), Angiotensin-converting enzyme 2 (ACE2), Bone morphogenetic protein 6 (BMP6), Brother of CDO (BOC), C-C motif chemokine 17 (CCL17), C-C motif chemokine 3 (CCL3), CD40 ligand (CD40LG), Carbonic anhydrase 5A, mitochondrial (CA5A), Carcinoembryonic antigen-related cell adhesion molecule 8 (CEACAM8), Cathepsin L1 (CTSL), Chymotrypsin-C (CTRC), Cobalamin binding intrinsic factor (CBLIF), Decorin (DCN), Dickkopf-related protein 1 (DKK1), Fatty acid-binding protein, intestinal (FABP2), Fibroblast growth factor 21 (FGF21), Fibroblast growth factor 23 (FGF23), Follistatin (FST), Galectin-9 (LGALS9), Gastrotropin (FABP6), Growth-regulated alpha protein (CXCL1), Growth/differentiation factor 2 (GDF2), Heat shock protein beta-1 (HSPB1), Heme oxygenase 1 (HMOX1), Hepatitis A virus cellular receptor 1 (HAVCR1), Hydroxyacid oxidase 1 (HAO1), Integrin beta-1-binding protein 2 (ITGB1BP2), Interleukin-1 receptor antagonist protein (IL1RN), Interleukin-1 receptor-like 2 (IL1RL2), Interleukin-17D (IL17D), Interleukin-18 (IL18), Interleukin-27 (EBI3_IL27), Interleukin-4 receptor subunit alpha (IL4R), Interleukin-6 (IL6), Kit ligand (KITLG), Lactoylglutathione lyase (GLO1), Leptin (LEP), Lipoprotein lipase (LPL), Low affinity immunoglobulin gamma Fc region receptor II-b (FCGR2B), Lymphotactin (XCL1), Macrophage metalloelastase (MMP12), Macrophage receptor MARCO (MARCO), Matrilysin (MMP7), NF-kappa-B essential modulator (IKBKG), Natriuretic peptides B (NPPB), Osteoclast-associated immunoglobulin-like receptor (OSCAR), Oxidized low-density lipoprotein receptor 1 (OLR1), P-selectin glycoprotein ligand 1 (SELPLG), Pappalysin-1 (PAPPA), Pentraxin-related protein PTX3 (PTX3), Placenta growth factor (PGF), Platelet-derived growth factor subunit B (PDGFB), Poly [ADP-ribose] polymerase 1 (PARP1), Polymeric immunoglobulin receptor (PIGR), Pro-adrenomedullin (ADM), Pro-interleukin-16 (IL16), Programmed cell death 1 ligand 2 (PDCD1LG2), Proheparin-binding EGF-like growth factor (HBEGF), Prolargin (PRELP), Prostasin (PRSS8), Protein AMBP (AMBP), Protein-glutamine gamma-glutamyltransferase 2 (TGM2), Proteinase-activated receptor 1 (F2R), Proto-oncogene tyrosine-protein kinase Src (SRC), Renin (REN), SLAM family member 5 (CD84), SLAM family member 7 (SLAMF7), Serine protease 27 (PRSS27), Serine/threonine-protein kinase 4 (STK4), Serpin A12 (SERPINA12), Somatotropin (GH1), Sortilin (SORT1), Spondin-2 (SPON2), Superoxide dismutase [Mn], mitochondrial (SOD2), T-cell surface glycoprotein CD4 (CD4), Thrombomodulin (THBD), Thrombopoietin (THPO), Thrombospondin-2 (THBS2), Tissue factor (F3), Tumor necrosis factor receptor superfamily member 10A (TNFRSF10A), Tumor necrosis factor receptor, superfamily member 10B (TNFRSF10B), Tumor necrosis factor receptor superfamily member 11A (TNFRSF11A), Tumor necrosis factor receptor superfamily member 13B (TNFRSF13B), Tyrosine-protein kinase Mer (MERTK), V-set and immunoglobulin domain-containing protein 2 (VSIG2), and Vascular endothelial growth factor D (VEGFD).
References
- Heikenfeld, J.; Jajack, A.; Feldman, B.; Granger, S.W.; Gaitonde, S.; Begtrup, G.; Katchman, B.A. Accessing analytes in biofluids for peripheral biochemical monitoring. Nat. Biotechnol. 2019, 37, 407–419. [Google Scholar] [CrossRef] [PubMed]
- Samant, P.P.; Niedzwiecki, M.M.; Raviele, N.; Tran, V.; Mena-Lapaix, J.; Walker, D.I.; Felner, E.I.; Jones, D.P.; Miller, G.W.; Prausnitz, M.R. Sampling interstitial fluid from human skin using a microneedle patch. Sci. Transl. Med. 2020, 12, 571. [Google Scholar] [CrossRef]
- Tran, B.Q.; Miller, P.R.; Taylor, R.M.; Boyd, G.; Mach, P.M.; Rosenzweig, C.N.; Baca, J.T.; Polsky, R.; Glaros, T. Proteomic Characterization of Dermal Interstitial Fluid Extracted Using a Novel Microneedle-Assisted Technique. J. Proteome Res. 2018, 17, 479–485. [Google Scholar] [CrossRef]
- Baumann, K.Y.; Church, M.K.; Clough, G.F.; Quist, S.R.; Schmelz, M.; Skov, P.S.; Anderson, C.D.; Tannert, L.K.; Giménez-Arnau, A.M.; Frischbutter, S.; et al. Skin microdialysis: Methods, applications and future opportunities—An EAACI position paper. Clin. Transl. Allergy 2019, 9, 1–13. [Google Scholar] [CrossRef]
- Kiistala, U. Suction blister device for separation of viable epidermis from dermis. J. Investig. Dermatol. 1968, 50, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Wang, C.; Lu, F.; Du, L.; Fang, Z.; Guo, X.; Liu, J.-T.; Chen, C.-J.; Zhao, Z. A Flexible Interdigital Electrode Used in Skin Penetration Promotion and Evaluation with Electroporation and Reverse Iontophoresis Synergistically. Sensors 2018, 18, 1431. [Google Scholar] [CrossRef] [PubMed]
- Wiig, H.; Heir, S.; Aukland, K. Colloid osmotic pressure of interstitial fluid in rat subcutis and skeletal muscle: Comparison of various wick sampling techniques. Acta Physiol. Scand. 1988, 133, 167–175. [Google Scholar] [CrossRef]
- Wiig, H.; Aukland, K.; Tenstad, O. Isolation of interstitial fluid from rat mammary tumors by a centrifugation method. Am. J. Physiol. Heart Circ. Physiol. 2003, 284, 416–424. [Google Scholar] [CrossRef]
- Barber, C.; Boiko, S. Tape stripping: Investigational, diagnostic, and therapeutic uses in dermatology. Clin. Dermatol. 2022, 40, 355–362. [Google Scholar] [CrossRef]
- Henry, S.; McAllister, D.V.; Allen, M.G.; Prausnitz, M.R. Microfabricated microneedles: A novel approach to transdermal drug delivery. J. Pharm. Sci. 1998, 87, 922–925. [Google Scholar] [CrossRef]
- Saifullah, K.M.; Faraji Rad, Z. Sampling Dermal Interstitial Fluid Using Microneedles: A Review of Recent Developments in Sampling Methods and Microneedle-Based Biosensors. Adv. Mater. Interfaces 2023, 10, 2201763. [Google Scholar] [CrossRef]
- Gill, H.S.; Denson, D.D.; Burris, B.A.; Prausnitz, M.R. Effect of microneedle design on pain in human volunteers. Clin. J. Pain. 2008, 24, 585–594. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, R.; Jung, H.; Caffarel-Salvador, E.; Gill, H. Introduction to microneedles. J. Mater. Chem. B 2023, 11, 9323. [Google Scholar] [CrossRef]
- Tucak, A.; Sirbubalo, M.; Hindija, L.; Rahić, O.; Hadžiabdić, J.; Muhamedagić, K.; Čekić, A.; Vranić, E. Microneedles: Characteristics, Materials, Production Methods and Commercial Development. Micromachines 2020, 11, 961. [Google Scholar] [CrossRef]
- Samant, P.P.; Prausnitz, M.R. Mechanisms of sampling interstitial fluid from skin using a microneedle patch. Proc. Natl. Acad. Sci. USA 2018, 115, 4583–4588. [Google Scholar] [CrossRef]
- Tehrani, F.; Teymourian, H.; Wuerstle, B.; Kavner, J.; Patel, R.; Furmidge, A.; Aghavali, R.; Hosseini-Toudeshki, H.; Brown, C.; Zhang, F.; et al. An integrated wearable microneedle array for the continuous monitoring of multiple biomarkers in interstitial fluid. Nat. Biomed. Eng. 2022, 6, 1214–1224. [Google Scholar] [CrossRef]
- Ribet, F.; Bendes, A.; Fredolini, C.; Dobielewski, M.; Böttcher, M.; Beck, O.; Schwenk, J.M.; Stemme, G.; Roxhed, N. Microneedle Patch for Painless Intradermal Collection of Interstitial Fluid Enabling Multianalyte Measurement of Small Molecules, SARS-CoV-2 Antibodies, and Protein Profiling. Adv. Healthc. Mater. 2023, 12, 2202564. [Google Scholar] [CrossRef] [PubMed]
- Petersen, K. Silicon as a Mechanical Material. Proc. IEEE 1982, 70, 420–457. [Google Scholar] [CrossRef]
- Liljeholm, J.; Renlund, M.; Ebefors, T.; Rangsten, P.; Niklaus, F. High-yield and High-volume Fabrication of Silicon Microneedles Intended for Interstitial Fluid Extraction. In Proceedings of the 4th International Conference on Microneedles, London, UK, 23–25 May 2016. [Google Scholar]
- Renlund, M.; Hillmering, M.; Franzén, M.; Rangsten, P. Rapid Extraction of Interstitial Fluid using Sharp Hollow Silicon Microneedles. In Proceedings of the 5th International Conference on Microneedles, Vancouver, BC, Canada, 29 May–1 June 2018. [Google Scholar]
- Hillmering, M.; Rangsten, P.; Renlund, M. Device for Sampling of Dermal Interstitial Fluid for Biomedical Research. In Proceedings of the 6th International Conference on Microneedles—Online Conference, Seoul, Republic of Korea, 10–11 November 2020. [Google Scholar]
- Renlund, M.; Hillmering, M.; Engman, A.; Zymberi, G.; Gill, E.; Rangsten, P. PELSA—A Medical Device for Sampling of Dermal Interstitial Fluid. In Proceedings of the 7th International Conference on Microneedles, Washington, DC, USA, 15–17 May 2023. [Google Scholar]
- Rangsten, P.; Hillmering, M.; Engman, A.; Renlund, M. Microneedle in the European Project DIGIPREDICT. In Proceedings of the 7th International Conference on Microneedles, Washington, DC, USA, 15–17 May 2023. [Google Scholar]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkiene, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef]
- Alis, R.; Sanchis-Gomar, F.; Primo-Carrau, C.; Lozano-Calve, S.; Dipalo, M.; Aloe, R.; Blesa, J.R.; Romagnoli, M.; Lippi, G. Hemoconcentration induced by exercise: Revisiting the Dill and Costill equation. Scand. J. Med. Sci. Sports 2015, 25, e630–e637. [Google Scholar] [CrossRef]
- Yao, W.; Li, Y.; Ding, G. Interstitial Fluid Flow: The Mechanical Environment of Cells and Foundation of Meridians. Evid. Based Complement. Altern. Med. 2012. [Google Scholar] [CrossRef] [PubMed]
- Vitello, J.D.; Ripper, R.M.; Fettiplace, M.R.; Weinberg, G.L.; Vitello, J.M. Blood Density Is Nearly Equal to Water Density: A Validation Study of the Gravimetric Method of Measuring Intraoperative Blood Loss. J. Vet. Med. 2015. [Google Scholar] [CrossRef] [PubMed]
- Campbell, I. Physiology of fluid balance. Anaesth. Intensive Care Med. 2009, 10, 593–596. [Google Scholar] [CrossRef]
- Dörr, M.; Kaczmarek, S. DZHK-SOP-K-07—Spiroergometrie V1.2; Deutsches Zentrum für Herz-Kreislauf-Forschung e.V.: Berlin, Germany, 2022. [Google Scholar]
- Santini, L.; Adamo, F.; Mahfouz, K.; Colaiaco, C.; Finamora, I.; De Lucia, C.; Danisi, N.; Gentile, S.; Sorrentino, C.; Romano, M.G.; et al. Remote Management of Heart Failure in Patients with Implantable Devices. Diagnostics 2024, 14, 2554. [Google Scholar] [CrossRef]
- Koehler, F.; Koehler, K.; Deckwart, O.; Prescher, S.; Wegscheider, K.; Kirwan, B.A.; Winkler, S.; Vettorazzi, E.; Bruch, L.; Oeff, M.; et al. Efficacy of telemedical interventional management in patients with heart failure (TIM-HF2): A randomised, controlled, parallel-group, unmasked trial. Lancet 2018, 392, 1047–1057. [Google Scholar] [CrossRef]
- Benes, J.; Kotrc, M.; Conrad, M.J.; Kautzner, J.; Melenovsky, V.; Jarolim, P. Exercise dynamics of cardiac biomarkers and hemoconcentration in patients with chronic systolic heart failure. J. Card. Fail. 2020, 26, 1100–1105. [Google Scholar] [CrossRef]





| Inclusion Criteria | Exclusion Criteria |
|---|---|
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Renlund, M.; Kopp Fernandes, L.; Rangsten, P.; Hillmering, M.; Mosel, S.; Issa, Z.; Falk, V.; Meyer, A.; Schoenrath, F. Use of a Silicon Microneedle Chip-Based Device for the Extraction and Subsequent Analysis of Dermal Interstitial Fluid in Heart Failure Patients. Diagnostics 2025, 15, 989. https://doi.org/10.3390/diagnostics15080989
Renlund M, Kopp Fernandes L, Rangsten P, Hillmering M, Mosel S, Issa Z, Falk V, Meyer A, Schoenrath F. Use of a Silicon Microneedle Chip-Based Device for the Extraction and Subsequent Analysis of Dermal Interstitial Fluid in Heart Failure Patients. Diagnostics. 2025; 15(8):989. https://doi.org/10.3390/diagnostics15080989
Chicago/Turabian StyleRenlund, Markus, Laurenz Kopp Fernandes, Pelle Rangsten, Mikael Hillmering, Sara Mosel, Ziad Issa, Volkmar Falk, Alexander Meyer, and Felix Schoenrath. 2025. "Use of a Silicon Microneedle Chip-Based Device for the Extraction and Subsequent Analysis of Dermal Interstitial Fluid in Heart Failure Patients" Diagnostics 15, no. 8: 989. https://doi.org/10.3390/diagnostics15080989
APA StyleRenlund, M., Kopp Fernandes, L., Rangsten, P., Hillmering, M., Mosel, S., Issa, Z., Falk, V., Meyer, A., & Schoenrath, F. (2025). Use of a Silicon Microneedle Chip-Based Device for the Extraction and Subsequent Analysis of Dermal Interstitial Fluid in Heart Failure Patients. Diagnostics, 15(8), 989. https://doi.org/10.3390/diagnostics15080989






