Learning from the Invaders: What Viruses Teach Us about RNA-Based Regulation in Microbes
Abstract
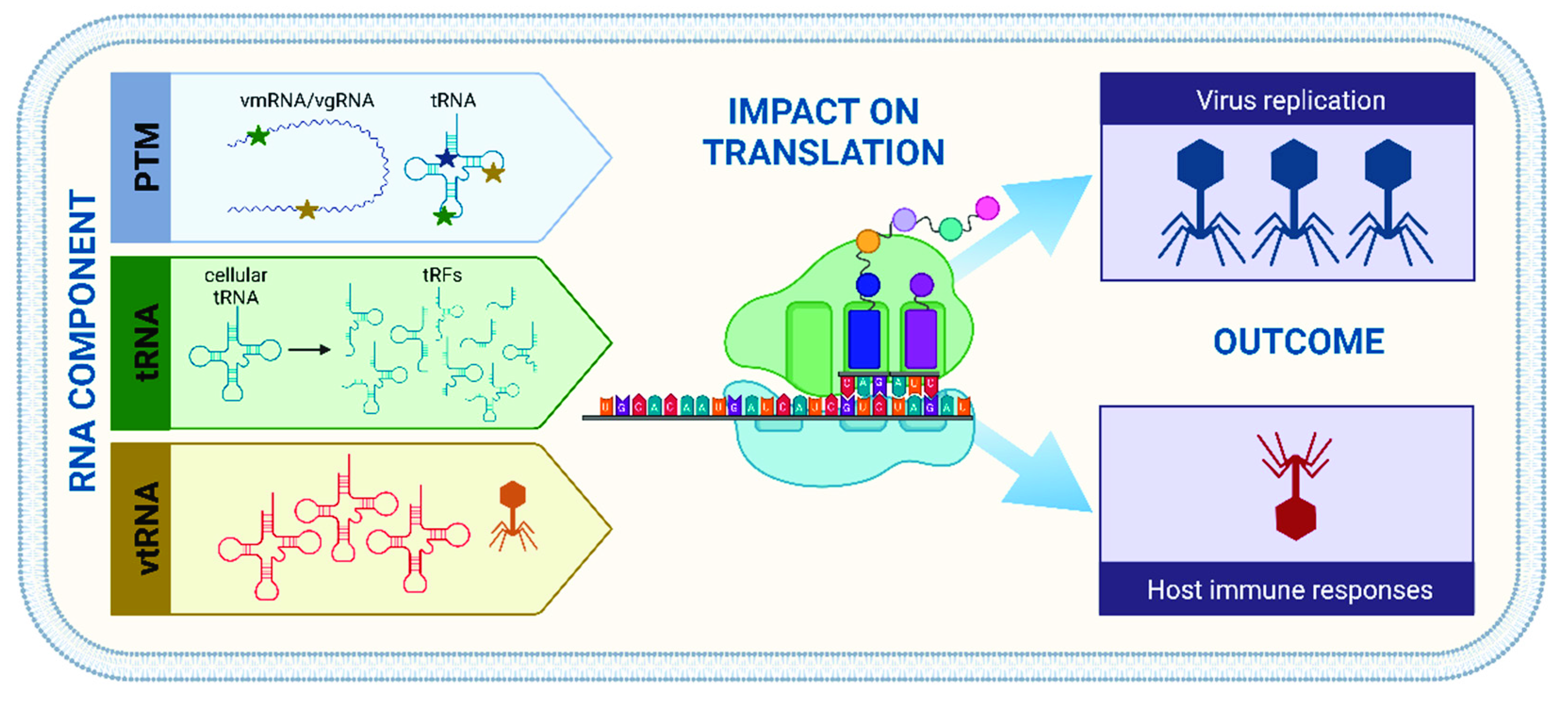
:1. Introduction
2. Tools of the Trade—RNA in the Viroscope
2.1. How Modifications Modulate Cellular Functions
2.2. Translation Potluck—Bring Your Own tRNAs
3. Outlook
Funding
Acknowledgments
Conflicts of Interest
References
- Rowlands, D.J. A Brief History of Virology. In Encyclopedia of Virology, 4th ed.; Bamford, D.H., Zuckerman, M., Eds.; Academic Press: Cambridge, MA, USA, 2021; Volume 1, pp. 3–13. [Google Scholar]
- Li, N.; Rana, T.M. Regulation of antiviral innate immunity by chemical modification of viral RNA. Wiley Interdiscipl. Rev. RNA 2022, e1720. [Google Scholar] [CrossRef]
- Jin, D.; Musier-Forsyth, K. Role of host tRNAs and aminoacyl-tRNA synthetases in retroviral replication. J. Biol. Chem. 2019, 294, 5352–5364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, X.; Xie, Y.; Zhang, S.; Song, X.; Xiao, B.; Yan, Z. tRNA-derived fragments: Mechanisms underlying their regulation of gene expression and potential applications as therapeutic targets in cancers and virus infections. Theranostics 2021, 11, 461–469. [Google Scholar] [CrossRef] [PubMed]
- Dreher, T.W. Viral tRNAs and tRNA-like structures. Wiley Interdiscip. Rev. RNA 2010, 1, 402–414. [Google Scholar] [CrossRef]
- Nunes, A.; Ribeiro, D.R.; Marques, M.; Santos, M.A.S.; Ribeiro, D.; Soares, A.R. Emerging Roles of tRNAs in RNA Virus Infections. Trends. Biochem. Sci. 2020, 45, 794–805. [Google Scholar] [CrossRef]
- Wu, S.; Li, X.; Wang, G. tRNA-like structures and their functions. FEBS J. 2022, 289, 5089–5099. [Google Scholar] [CrossRef]
- Boccaletto, P.; Stefaniak, F.; Ray, A.; Cappannini, A.; Mukherjee, S.; Purta, E.; Kurkowska, M.; Shirvanizadeh, N.; Destefanis, E.; Groza, P.; et al. MODOMICS: A database of RNA modification pathways. 2021 update. Nucleic Acids Res. 2022, 50, D231–D235. [Google Scholar] [CrossRef]
- Koh, C.S.; Sarin, L.P. Transfer RNA modification and infection—Implications for pathogenicity and host responses. Biochim. Biophys. Acta Gene Regul. Mech. 2018, 1861, 419–432. [Google Scholar] [CrossRef]
- Zinshteyn, B.; Gilbert, W.V. Loss of a conserved tRNA anticodon modification perturbs cellular signaling. PLoS Genet. 2013, 9, e1003675. [Google Scholar] [CrossRef] [Green Version]
- Alings, F.; Sarin, L.P.; Fufezan, C.; Drexler, H.C.; Leidel, S.A. An evolutionary approach uncovers a diverse response of tRNA 2-thiolation to elevated temperatures in yeast. RNA 2015, 21, 202–212. [Google Scholar] [CrossRef]
- Damon, J.R.; Pincus, D.; Ploegh, H.L. tRNA thiolation links translation to stress responses in Saccharomyces cerevisiae. Mol. Biol. Cell 2015, 26, 270–282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shigi, N.; Sakaguchi, Y.; Suzuki, T.; Watanabe, K. Identification of two tRNA thiolation genes required for cell growth at extremely high temperatures. J. Biol. Chem. 2006, 281, 14296–14306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chavarria, N.E.; Hwang, S.; Cao, S.; Fu, X.; Holman, M.; Elbanna, D.; Rodriguez, S.; Arrington, D.; Englert, M.; Uthandi, S.; et al. Archaeal Tuc1/Ncs6 homolog required for wobble uridine tRNA thiolation is associated with ubiquitin-proteasome, translation, and RNA processing system homologs. PLoS ONE 2014, 9, e99104. [Google Scholar] [CrossRef] [Green Version]
- Shippy, D.C.; Eakley, N.M.; Bochsler, P.N.; Chopra, A.K.; Fadl, A.A. Biological and virulence characteristics of Salmonella enterica serovar Typhimurium following deletion of glucose-inhibited division (gidA) gene. Microb. Pathog. 2011, 50, 303–313. [Google Scholar] [CrossRef] [PubMed]
- Sinha, H.; David, L.; Pascon, R.C.; Clauder-Munster, S.; Krishnakumar, S.; Nguyen, M.; Shi, G.; Dean, J.; Davis, R.W.; Oefner, P.J.; et al. Sequential elimination of major-effect contributors identifies additional quantitative trait loci conditioning high-temperature growth in yeast. Genetics 2008, 180, 1661–1670. [Google Scholar] [CrossRef] [Green Version]
- Cho, K.H.; Caparon, M.G. tRNA modification by GidA/MnmE is necessary for Streptococcus pyogenes virulence: A new strategy to make live attenuated strains. Infect. Immun. 2008, 76, 3176–3186. [Google Scholar] [CrossRef] [Green Version]
- Chionh, Y.H.; McBee, M.; Babu, I.R.; Hia, F.; Lin, W.; Zhao, W.; Cao, J.; Dziergowska, A.; Malkiewicz, A.; Begley, T.J.; et al. tRNA-mediated codon-biased translation in mycobacterial hypoxic persistence. Nat. Commun. 2016, 7, 13302. [Google Scholar] [CrossRef] [Green Version]
- Dodds, P.N.; Rathjen, J.P. Plant immunity: Towards an integrated view of plant-pathogen interactions. Nat. Rev. Genet. 2010, 11, 539–548. [Google Scholar] [CrossRef]
- Jenner, R.G.; Young, R.A. Insights into host responses against pathogens from transcriptional profiling. Nat. Rev. Microbiol. 2005, 3, 281–294. [Google Scholar] [CrossRef]
- Lemaitre, B.; Girardin, S.E. Translation inhibition and metabolic stress pathways in the host response to bacterial pathogens. Nat. Rev. Microbiol. 2013, 11, 365–369. [Google Scholar] [CrossRef]
- Netzband, R.; Pager, C.T. Epitranscriptomic marks: Emerging modulators of RNA virus gene expression. Wiley Interdiscipl. Rev. RNA 2020, 11, e1576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gokhale, N.S.; Horner, S.M. RNA modifications go viral. PLoS Pathog. 2017, 13, e1006188. [Google Scholar] [CrossRef] [Green Version]
- Hao, H.; Liu, W.; Miao, Y.; Ma, L.; Yu, B.; Liu, L.; Yang, C.; Zhang, K.; Chen, Z.; Yang, J.; et al. N4-acetylcytidine regulates the replication and pathogenicity of enterovirus 71. Nucleic Acids Res. 2022, 50, 9339–9354. [Google Scholar] [CrossRef]
- Korniy, N.; Samatova, E.; Anokhina, M.M.; Peske, F.; Rodnina, M.V. Mechanisms and biomedical implications of -1 programmed ribosome frameshifting on viral and bacterial mRNAs. FEBS Lett. 2019, 593, 1468–1482. [Google Scholar] [CrossRef] [PubMed]
- Jungfleisch, J.; Bottcher, R.; Tallo-Parra, M.; Perez-Vilaro, G.; Merits, A.; Novoa, E.M.; Diez, J. CHIKV infection reprograms codon optimality to favor viral RNA translation by altering the tRNA epitranscriptome. Nat. Commun. 2022, 13, 4725. [Google Scholar] [CrossRef] [PubMed]
- Maynard, N.D.; Macklin, D.N.; Kirkegaard, K.; Covert, M.W. Competing pathways control host resistance to virus via tRNA modification and programmed ribosomal frameshifting. Mol. Syst. Biol. 2012, 8, 567. [Google Scholar] [CrossRef] [PubMed]
- Maynard, N.D.; Birch, E.W.; Sanghvi, J.C.; Chen, L.; Gutschow, M.V.; Covert, M.W. A forward-genetic screen and dynamic analysis of lambda phage host-dependencies reveals an extensive interaction network and a new anti-viral strategy. PLoS Genet. 2010, 6, e1001017. [Google Scholar] [CrossRef] [Green Version]
- Goldfarb, A.; Daniel, V. Transcriptional control of two gene subclusters in the tRNA operon of bacteriophage T4. Nature 1980, 286, 418–420. [Google Scholar] [CrossRef]
- Daniel, V.; Sarid, S.; Littauer, U.Z. Amino acid acceptor activity of bacteriophage T4 transfer RNA. FEBS Lett. 1968, 2, 39–41. [Google Scholar] [CrossRef] [Green Version]
- McClain, W.H.; Guthrie, C.; Barrell, B.G. Eight transfer RNAs induced by infection of Escherichia coli with bacteriophage T4. Proc. Natl. Acad. Sci. USA 1972, 69, 3703–3707. [Google Scholar] [CrossRef]
- Morgado, S.; Vicente, A.C. Global In-Silico Scenario of tRNA Genes and Their Organization in Virus Genomes. Viruses 2019, 11, 180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, J.Y.; Fang, W.; Miranda-Sanchez, F.; Brown, J.M.; Kauffman, K.M.; Acevero, C.M.; Bartel, D.P.; Polz, M.F.; Kelly, L. Degradation of host translational machinery drives tRNA acquisition in viruses. Cell Syst. 2021, 12, 771–779.e775. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, G.; Askora, A.; Blanc-Mathieu, R.; Kawasaki, T.; Li, Y.; Nakano, M.; Ogata, H.; Yamada, T. Xanthomonas citri jumbo phage XacN1 exhibits a wide host range and high complement of tRNA genes. Sci. Rep. 2018, 8, 4486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fermin, G. Host Range, Host–Virus Interactions, and Virus Transmission. In Viruses; Tennant, P., Fermin, G., Foster, J.E., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 101–134. [Google Scholar]
- Chen, Y.; Bao, X. Respiratory Syncytial Virus Induces a Functional tRNA-derived Fragment to Promote Infection by Targeting SYNE2. J. Immunol. 2020, 93, 1. [Google Scholar]
- Deng, J.; Ptashkin, R.N.; Chen, Y.; Cheng, Z.; Liu, G.; Phan, T.; Deng, X.; Zhou, J.; Lee, I.; Lee, Y.S.; et al. Respiratory Syncytial Virus Utilizes a tRNA Fragment to Suppress Antiviral Responses Through a Novel Targeting Mechanism. Mol. Ther. 2015, 23, 1622–1629. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Selitsky, S.R.; Baran-Gale, J.; Honda, M.; Yamane, D.; Masaki, T.; Fannin, E.E.; Guerra, B.; Shirasaki, T.; Shimakami, T.; Kaneko, S.; et al. Small tRNA-derived RNAs are increased and more abundant than microRNAs in chronic hepatitis B and C. Sci. Rep. 2015, 5, 7675. [Google Scholar] [CrossRef] [Green Version]
- Avcilar-Kucukgoze, I.; Kashina, A. Hijacking tRNAs From Translation: Regulatory Functions of tRNAs in Mammalian Cell Physiology. Front. Mol. Biosci. 2020, 7, 610617. [Google Scholar] [CrossRef]
- Fricker, R.; Brogli, R.; Luidalepp, H.; Wyss, L.; Fasnacht, M.; Joss, O.; Zywicki, M.; Helm, M.; Schneider, A.; Cristodero, M.; et al. A tRNA half modulates translation as stress response in Trypanosoma brucei. Nat. Commun. 2019, 10, 118. [Google Scholar] [CrossRef] [Green Version]
- Miller, E.S.; Kutter, E.; Mosig, G.; Arisaka, F.; Kunisawa, T.; Ruger, W. Bacteriophage T4 genome. Microbiol. Mol. Biol. Rev. MMBR 2003, 67, 86–156, table of contents. [Google Scholar] [CrossRef] [Green Version]
- Hofer, K.; Jaschke, A. Epitranscriptomics: RNA Modifications in Bacteria and Archaea. Microbiol. Spectr. 2018, 6. [Google Scholar] [CrossRef]
- Pilotto, S.; Werner, F. How to Shut Down Transcription in Archaea during Virus Infection. Microorganisms 2022, 10, 1824. [Google Scholar] [CrossRef] [PubMed]
- Gregorova, P.; Sipari, N.H.; Sarin, L.P. Broad-range RNA modification analysis of complex biological samples using rapid C18-UPLC-MS. RNA Biol. 2021, 18, 1382–1389. [Google Scholar] [CrossRef] [PubMed]
- Hagelskamp, F.; Borland, K.; Ramos, J.; Hendrick, A.G.; Fu, D.; Kellner, S. Broadly applicable oligonucleotide mass spectrometry for the analysis of RNA writers and erasers in vitro. Nucleic Acids Res. 2020, 48, e41. [Google Scholar] [CrossRef] [PubMed]
- Ingolia, N.T.; Hussmann, J.A.; Weissman, J.S. Ribosome Profiling: Global Views of Translation. Cold Spring Harb. Perspect. Biol. 2019, 11, a032698. [Google Scholar] [CrossRef] [PubMed]
- Gelsinger, D.R.; Dallon, E.; Reddy, R.; Mohammad, F.; Buskirk, A.R.; DiRuggiero, J. Ribosome profiling in archaea reveals leaderless translation, novel translational initiation sites, and ribosome pausing at single codon resolution. Nucleic Acids Res. 2020, 48, 5201–5216. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarin, L.P. Learning from the Invaders: What Viruses Teach Us about RNA-Based Regulation in Microbes. Microorganisms 2022, 10, 2106. https://doi.org/10.3390/microorganisms10112106
Sarin LP. Learning from the Invaders: What Viruses Teach Us about RNA-Based Regulation in Microbes. Microorganisms. 2022; 10(11):2106. https://doi.org/10.3390/microorganisms10112106
Chicago/Turabian StyleSarin, L. Peter. 2022. "Learning from the Invaders: What Viruses Teach Us about RNA-Based Regulation in Microbes" Microorganisms 10, no. 11: 2106. https://doi.org/10.3390/microorganisms10112106





