Probiotics in Periodontal and Peri-Implant Health Management: Biofilm Control, Dysbiosis Reversal, and Host Modulation
Abstract
1. Introduction
2. Periodontal Microbiota
2.1. Periodontal Microbiota in Healthy Conditions
2.1.1. Periodontal Microbiota around Natural Teeth in Healthy Conditions
2.1.2. Periodontal Microbiota around Dental Implants in Healthy Conditions
2.2. Periodontal Microbiota in Pathological Conditions
2.2.1. Periodontal Microbiota in Gingivitis and Periodontitis
2.2.2. Periodontal Microbiota in Peri-Implantitis
3. Probiotics
3.1. Probiotics Functions
3.2. Probiotics Mechanisms of Action
4. Probiotics’ Effect in Periodontal and Peri-Implant Diseases
4.1. Probiotics’ Effects on Gingivitis: Current Evidence
4.2. Probiotics’ Effects on Periodontitis: Current Evidence
4.3. Probiotics’ Effect in Peri-Implantitis: Current Evidence
5. Probiotics in Periodontitis Treatment
5.1. Probiotics as Adjuncts to Periodontal Treatment: Measures of Biological and Clinical Outcomes
5.2. Probiotics as Adjuncts to Periodontal Treatment: Reported Biological and Clinical Outcomes
| Authors, Year | Type of the Article | Periodontitis Treatment | Periodontal Outcomes Measured | Main Results (Statistically Significant) |
|---|---|---|---|---|
| Gruner et al., 2016 [102] | Systematic review and meta-analysis |
-Probiotics -Placebo | PPD BoP PI GI |
Current evidence is sufficient for recommending probiotics in gingivitis and periodontitis management Probiotics significantly reduced bleeding-on-probing and gingival index, but not plaque index |
| Vives-Soler et al., 2020 [103] | Systematic review | -Probiotics + nonsurgical treatments -Nonsurgical treatments + placebo | BoP PI PPD | Probiotics may provide supplementary clinical improvements to manual debridement in chronic periodontitis |
| Jayaramanet et al., 2016 [104] | Systematic review | -Probiotics + mechanical debridement -placebo + mechanical debridement | BoP PI PPD | Probiotics produce only short-term microbiologic and clinical benefits in periodontitis treatment |
| Matsubara et al., 2016 [88] | Systematic review | -Probiotics + mechanical debridement -mechanical debridement alone -mechanical debridement + placebo | PPD CAL BoP | Oral probiotics administration may be considered effective and safe adjunct to scaling and root planing |
| Canut-Del Gado et al., 2021 [105] | Systematic review | Probiotics + mechanical debridement -mechanical debridement + placebo | PPD BoP | Probiotics administration as adjuvants to periodontal treatment may aid in improving the clinical outcomes |
| Nga Ho et al., 2020 [106] | Systematic review | -Nonsurgical periodontal therapy + Probiotics -nonsurgical periodontal therapy alone | PPD CAL | Heterogenous evidence supports the long-term clinical benefit of probiotics as adjuncts to nonsurgical periodontal treatment |
| Ikram et al., 2018 [107] | Systematic review and meta-analysis |
-Scaling and root planning in the treatment of chronic periodontitis alone -Scaling and root planning in the treatment of chronic periodontitis + probiotics | PPD CAL | Adjunctive probiotics could result in additional benefits in CAL gain |
| Ng Ethan et al., 2022 [108] | Systematic review and meta-analysis | -Probiotics + mechanical debridement -mechanical debridement + placebo | PPD | Adjunctive probiotics should be considered safe and could offer beneficial effects compared to a placebo |
| Yanine et al., 2013 [109] | Systematic review | -Placebo vs. probiotics | Insufficient evidence currently supports the benefits of systemically-delivered probiotics in subjects suffering from periodontitis | |
| Gheisary, 2022 [110] | Systematic review and meta-analysis | -Prevention and treatment of periodontitis + probiotics -Prevention and treatment of periodontitis alone | PI GI PPD BoP | Probiotic administration improves clinical periodontal parameters |
6. Probiotics in Peri-Implantitis Treatment
| Authors, Year | Type of the Article | Peri-Implantitis Treatment | Periodontal Outcomes Measured | Main Results (Statistically Significant) |
|---|---|---|---|---|
| Zhao et al., 2021 [112] | Systematic review and meta-analysis | -Probiotic therapy + mechanical debridement -Mechanical debridement alone -Mechanical debridement + placebo | PPD BoP PI | No differences between the groups |
| Sayardoust et al., 2022 [74] | Systematic review and meta-analysis | -Probiotics -Probiotics + nonsurgical treatments -Nonsurgical treatments | BoP GI PPD | No differences between the groups |
| Arbildo-Vega et al., 2021 [113] | Systematic review and meta-analysis | -Lactobacillus reuteri + mechanical debridement -Mechanical debridement alone | BoP PI PPD | PPD improvement has been observed in the group using Lactobacillus reuteri |
| Gao et al., 2020 [114] | Systematic review and meta-analysis | -Lactobacillus + mechanical debridement (MD) -Mechanical debridement alone -Mechanical debridement + placebo | PPD PI BoP | Lactobacillus provided limited benefits in peri-implant mucositis for PD. No significant differences were found for PI and BOP |
| Silva et al., 2020 [115] | Systematic review | Effect of probiotics on peri-implant diseases | PPD BoP PI GI | No clinical effects of probiotics were observed |
| Pietri et al., 2020 [116] | Systematic review | Probiotic therapy among patients undergoing fixed orthodontic therapy | PI GI Halitosis | Many studies reported that probiotic therapy had a beneficial effect on PI, GI, and halitosis among patients undergoing fixed orthodontic therapy |
7. Probiotics in Orthodontic Treatment
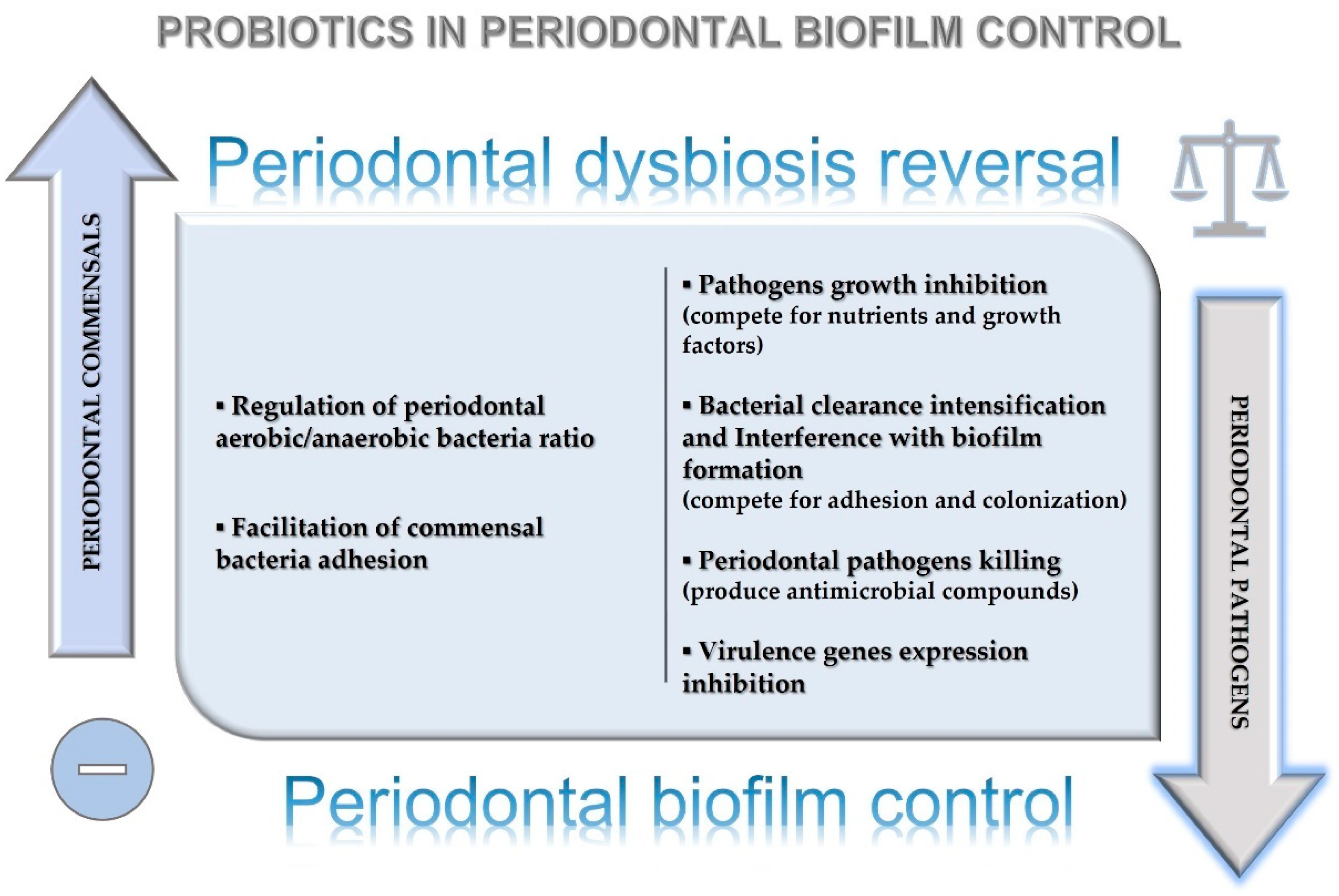
8. Probiotics’ Effects on Periodontal Dysbiosis Reversal and Biofilm Control around Natural Teeth and Dental Implants
9. Probiotics’ Effects on Host Modulation in Periodontal and Peri-Implant Tissues
10. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Radaic, A.; Kapila, Y.L. The oralome and its dysbiosis: New insights into oral microbiome-host interactions. Comput. Struct. Biotechnol. J. 2021, 19, 1335–1360. [Google Scholar] [CrossRef] [PubMed]
- Tonetti, M.S.; Greenwell, H.; Kornman, K.S. Staging and grading of periodontitis: Framework and proposal of a new classification and case definition. J. Periodontol. 2018, 89, S159–S172. [Google Scholar] [CrossRef] [PubMed]
- Sanz, M.; Teughels, W. Group A of European Workshop on Periodontology. Innovations in non-surgical periodontal therapy: Consensus Report of the Sixth European Workshop on Periodontology. J. Clin. Periodontol. 2008, 35 (Suppl. S8), 3–7. [Google Scholar] [CrossRef] [PubMed]
- Gasparro, R.; Qorri, E.; Valletta, A.; Masucci, M.; Sammartino, P.; Amato, A.; Marenzi, G. Non-Transfusional Hemocomponents: From Biology to the Clinic-A Literature Review. Bioengineering 2018, 5, 27. [Google Scholar] [CrossRef]
- Cortese, A.; Caggiano, M.; Carlino, F.; Pantaleo, G. Zygomatic fractures: Technical modifications for better aesthetic and functional results in older patients. Int. J. Surg. 2016, 33, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Di Spirito, F. Oral-Systemic Health and Disorders: Latest Prospects on Oral Antisepsis. Appl. Sci. 2022, 12, 8185. [Google Scholar] [CrossRef]
- Del Giudice, C.; Vaia, E.; Liccardo, D.; Marzano, F.; Valletta, A.; Spagnuolo, G.; Ferrara, N.; Rengo, C.; Cannavo, A.; Rengo, G. Infective Endocarditis: A Focus on Oral Microbiota. Microorganisms 2021, 9, 1218. [Google Scholar] [CrossRef]
- Hardan, L.; Bourgi, R.; Cuevas-Suárez, C.E.; Flores-Rodríguez, M.; Omaña-Covarrubias, A.; Nicastro, M.; Lazarescu, F.; Zarow, M.; Monteiro, P.; Jakubowicz, N.; et al. The Use of Probiotics as Adjuvant Therapy of Periodontal Treatment: A Systematic Review and Meta-Analysis of Clinical Trials. Pharmaceutics 2022, 14, 1017. [Google Scholar] [CrossRef]
- Nędzi-Góra, M.; Wroblewska, M.; Gorska, R. The Effect of Lactobacillus Salivarius SGL03 on Clinical and Microbiological Parameters in Periodontal Patients. Pol. J. Microbiol. 2020, 69, 441. [Google Scholar] [CrossRef]
- Di Spirito, F.; Toti, P.; Brevi, B.; Martuscelli, R.; Sbordone, L.; Sbordone, C. Computed tomography evaluation of jaw atrophies before and after surgical bone augmentation. Int. J. Clin. Dent. 2019, 12, 259–270. [Google Scholar]
- Liccardo, D.; Marzano, F.; Carraturo, F.; Guida, M.; Femminella, G.D.; Bencivenga, L.; Agrimi, J.; Addonizio, A.; Melino, I.; Valletta, A.; et al. Potential Bidirectional Relationship Between Periodontitis and Alzheimer’s Disease. Front. Physiol. 2020, 11, 683. [Google Scholar] [CrossRef] [PubMed]
- Di Spirito, F.; La Rocca, M.; De Bernardo, M.; Rosa, N.; Sbordone, C.; Sbordone, L. Possible Association of Periodontal Disease and Macular Degeneration: A Case-Control Study. Dent. J. 2020, 9, 1. [Google Scholar] [CrossRef] [PubMed]
- Di Spirito, F.; Argentino, S.; Martuscelli, R.; Sbordone, L. MRONJ incidence after multiple teeth extractions in patients taking oral bisphosphonates without “drug holiday”: A retrospective chart review. Oral Implantol. 2019, 12, 105–110. [Google Scholar]
- Di Spirito, F.; Iacono, V.J.; Iandolo, A.; Amato, A.; Sbordone, L. Evidence-based Recommendations on Periodontal Practice and the Management of Periodontal Patients during and after the COVID-19 Era: Challenging Infectious Diseases Spread by Air-borne Transmission. Open Dent. J. 2021, 15, 325–336. [Google Scholar] [CrossRef]
- Di Spirito, F.; Schiavo, L.; Pilone, V.; Lanza, A.; Sbordone, L.; D’Ambrosio, F. Periodontal and Periimplant Diseases and Systemically Administered Statins: A Systematic Review. Dent. J. 2021, 9, 100. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, F.; Giannobile, W.V.; Jung, R.E. Groups of the 2nd Osteology Foundation Consensus Meeting. Evidence-based knowledge on the aesthetics and maintenance of peri-implant soft tissues: Osteology Foundation Consensus Report Part 2-Effects of hard tissue augmentation procedures on the maintenance of peri-implant tissues. Clin. Oral Implants Res. 2018, 29 (Suppl. 15), 11–13. [Google Scholar] [CrossRef]
- Nicolò, M.; Pirozzi, M.; Catalano, C.; Amato, M. Parodontiti associate a malattie sistemiche con deficit qualitativo della funzione fagocitaria. Nota I. Diabetemellito [Periodontitis associated with systemic diseases with qualitative deficiency of phagocyte function. I. Diabetes mellitus]. Minerva Stomatol. 1989, 38, 899–903. [Google Scholar]
- D’Ambrosio, F.; Di Spirito, F.; De Caro, F.; Lanza, A.; Passarella, D.; Sbordone, L. Adherence to Antibiotic Prescription of Dental Patients: The Other Side of the Antimicrobial Resistance. Healthcare 2022, 10, 1636. [Google Scholar] [CrossRef]
- D’Ambrosio, F.; Di Spirito, F.; Amato, A.; Caggiano, M.; Lo Giudice, R.; Martina, S. Attitudes towards Antibiotic Prescription and Antimicrobial Resistance Awareness among Italian Dentists: What Are the Milestones? Healthcare 2022, 10, 1585. [Google Scholar] [CrossRef]
- Barone, A.; Chatelain, S.; Derchi, G.; Di Spirito, F.; Martuscelli, R.; Porzio, M.; Sbordone, L. Effectiveness of antibiotics in preventing alveolitis after erupted tooth extraction: A retrospective study. Oral Dis. 2020, 26, 967–973. [Google Scholar] [CrossRef]
- Ganguly, N.; Bhattacharya, S.; Sesikeran, B.; Nair, G.; Ramakrishna, B.; Sachdev, H.; Batish, V.; Kanagasabapathy, A.; Muthuswamy, V.; Kathuria, S. ICMR-DBT Guidelines for Evaluation of Probiotics in Food. Indian J. Med. Res. 2011, 134, 22–25. [Google Scholar]
- Seminario-Amez, M.; López-López, J.; Estrugo-Devesa, A.; Ayuso-Montero, R.; Jané-Salas, E. Probiotics and oral health: A systematic review. Med. Oral Patol. Oral y Cir. Buccal 2018, 22, e282–e288. [Google Scholar] [CrossRef] [PubMed]
- Bizzini, B.; Pizzo, G.; Scapagnini, G.; Nuzzo, D.; Vasto, S. Probiotics and oral health. Curr. Pharm. Des. 2012, 18, 5522–5531. [Google Scholar] [CrossRef]
- Costacurta, M.; Sicuro, L.; Margiotta, S.; Ingrasciotta, I.; Docimo, R. Clinical Effects of Lactobacillus reuteri Probiotic in Treatment of Chronic Periodontitis. A Randomized, Controlled Trial. Oral Implantol. 2018, 11, 191–198. [Google Scholar]
- Ikram, S.; Hassan, N.; Baig, S.; Borges, K.J.J.; Raffat, M.A.; Akram, Z. Effect of Local Probiotic (Lactobacillus reuteri) vs Systemic Antibiotic Therapy as an Adjunct to Non-surgical Periodontal Treatment in Chronic Periodontitis. J. Investig. Clin. Dent. 2019, 10, 12393. [Google Scholar] [CrossRef] [PubMed]
- Danser, M.M.; Gómez, S.M.; Van der Weijden, G.A. Tongue coating and tongue brushing: A literature review. Int. J. Dent. Hyg. 2003, 1, 151–158. [Google Scholar] [CrossRef]
- Aas, J.A.; Paster, B.J.; Stokes, L.N.; Olsen, I.; Dewhirst, F.E. Defining the normal bacterial flora of the oral cavity. J. Clin. Microbiol. 2005, 43, 5721–5732. [Google Scholar] [CrossRef]
- Kolenbrander, P.E.; Andersen, R.N.; Blehert, D.S.; Egland, P.G.; Foster, J.S.; Palmer, R.J., Jr. Communication among oral bacteria. Microbiol. Mol. Biol. Rev. 2002, 66, 486–505. [Google Scholar] [CrossRef]
- Curtis, M.A.; Diaz, P.A.; Van Dyke, T.E. The role of the microbiota in periodontal disease. Periodontology 2000 2020, 83, 14–25. [Google Scholar] [CrossRef]
- Joseph, S.; Curtis, M.A. Microbial transitions from health to disease. Periodontology 2000 2021, 86, 201–209. [Google Scholar] [CrossRef]
- Moore, W.E.; Moore, L.V. The bacteria of periodontal diseases. Periodontol 2000 1994, 5, 66–77. [Google Scholar] [CrossRef] [PubMed]
- Heitz-Mayfield, L.J.; Salvi, G.E.; Botticelli, D.; Mombelli, A.; Faddy, M.; Lang, N.P. Implant Complication Research Group. Anti-infective treatment of peri-implant mucositis: A randomised controlled clinical trial. Clin. Oral Implants Res. 2011, 22, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Kotsakis, G.A.; Olmedo, D.G. Peri-implantitis is not periodontitis: Scientific discoveries shed light on microbiome-biomaterial interactions that may determine disease phenotype. Periodontology 2000 2021, 86, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Li, R.; Zeng, X. Predictive modeling of gingivitis severity and susceptibility via oral microbiota. ISME J. 2014, 8, 1768–1780. [Google Scholar] [CrossRef] [PubMed]
- Stephen, A.S.; Dhadwal, N.; Nagala, V.; Gonzales-Marin, C.; Gillam, D.G.; Bradshaw, D.J.; Burnett, G.R.; Allaker, R.P. Interdental and subgingival microbiota may affect the tongue microbial ecology and oral malodour in health, gingivitis and periodontitis. J. Periodontal Res. 2021, 56, 1174–1184. [Google Scholar] [CrossRef] [PubMed]
- Schincaglia, G.P.; Hong, B.Y.; Rosania, A.; Barasz, J.; Thompson, A.; Sobue, T.; Panagakos, F.; Burleson, J.A.; Dongari-Bagtzoglou, A.; Diaz, P.I. Clinical, immune, and microbiome traits of gingivitis and peri-implant mucositis. J. Dent. Res. 2017, 96, 47–55. [Google Scholar] [CrossRef]
- Diaz, P.I.; Hoare, A.; Hong, B.Y. Subgingival microbiome shifts and community dynamics in periodontal diseases. J. Calif. Dent. Assoc. 2016, 44, 421–435. [Google Scholar]
- Di Spirito, F.; Lo Giudice, R.; Amato, M.; Di Palo, M.P.; D’Ambrosio, F.; Amato, A.; Martina, S. Inflammatory, Reactive, and Hypersensitivity Lesions Potentially Due to Metal Nanoparticles from Dental Implants and Supported Restorations: An Umbrella Review. Appl. Sci. 2022, 12, 11208. [Google Scholar] [CrossRef]
- Gupta, G. Probiotics and periodontal health. J. Med. Life 2009, 4, 387–394. [Google Scholar]
- Houle, M.A.; Grenier, D.; Plamondon, P.; Nakayama, K. The collagenase activity of Porphyromonas gingivalis is due to Arg-gingipain. FEMS Microbiol. Lett. 2003, 221, 181–185. [Google Scholar] [CrossRef]
- Dabdoub, S.M.; Tsigarida, A.A.; Kumar, P.S. Patient-specific analysis of periodontal and peri-implant microbiomes. J. Dent. Res. 2013, 92, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Persson, G.R.; Renvert, S. Cluster of bacteria associated with peri-implantitis. Clin. Implant Dent. Relat. Res. 2014, 16, 783–793. [Google Scholar] [CrossRef] [PubMed]
- Sahrmann, P.; Gilli, F.; Wiedemeier, D.B.; Attin, T.; Schmidlin, P.R.; Karygianni, L. The Microbiome of Peri-Implantitis: A Systematic Review and Meta-Analysis. Microorganisms 2020, 8, 661. [Google Scholar] [CrossRef]
- Eick, S.; Ramseier, C.A.; Rothenberger, K.; Brägger, U.; Buser, D.; Salvi, G.E. Microbiota at teeth and implants in partially edentulous patients. A 10-year retrospective study. Clin. Oral Implants Res. 2016, 27, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2004, 11, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Binda, S.; Hill, C.; Johansen, E.; Obis, D.; Pot, B.; Sanders, M.E.; Tremblay, A.; Ouwehand, A.C. Criteria to Qualify Microorganisms as "Probiotic" in Foods and Dietary Supplements. Front. Microbiol. 2020, 11, 1662. [Google Scholar] [CrossRef] [PubMed]
- Fijan, S. Microorganisms with claimed probiotic properties: An overview of recent literature. Int. J. Environ. Res. Public Health 2014, 11, 4745–4767. [Google Scholar] [CrossRef]
- Setbo, E.; Campbell, K.; O’Cuiv, P.; Hubbard, R. Utility of Probiotics for Maintenance or Improvement of Health Status in Older People—A Scoping Review. J. Nutr. Health Aging 2019, 23, 364–372. [Google Scholar] [CrossRef]
- Flint, H.J.; Duncan, S.H.; Louis, P. The impact of nutrition on intestinal bacterial communities. Curr. Opin. Microbiol. 2017, 38, 59–65. [Google Scholar] [CrossRef]
- Del Piano, M.; Carmagnola, S.; Anderloni, A.; Andorno, S.; Ballarè, M.; Balzarini, M.; Montino, F.; Orsello, M.; Pagliarulo, M.; Sartori, M.; et al. The use of probiotics in healthy volunteers with evacuation disorders and hard stools: A double- blind, randomized, placebo-controlled study. J. Clin. Gastroenterol. 2010, 44, 30–34. [Google Scholar] [CrossRef]
- Fang, H.R.; Zhang, G.Q.; Cheng, J.Y.; Li, Z.Y. Efficacy of Lactobacillus- supplemented triple therapy for Helicobacter pylori infection in children: A meta-analysis of randomized controlled trials. Eur. J. Pediatr. 2019, 178, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Chong, P.P.; Chin, V.K.; Looi, C.Y.; Wong, W.F.; Madhavan, P.; Yong, V.C. The microbiome and irritable bowel syndrome—A review on the pathophysiology, current research and future therapy. Front. Microbiol. 2019, 10, 1136. [Google Scholar] [CrossRef]
- Beserra, B.T.; Fernandes, R.; do Rosario, V.A.; Mocellin, M.C.; Kuntz, M.G.; Trindade, E.B. A systematic review and meta-analysis of the prebiotics and synbiotics effects on glycaemia, insulin concentrations and lipid parameters in adult patients with overweight or obesity. Clin. Nutr. 2015, 34, 845–858. [Google Scholar] [CrossRef]
- Torres-Maravilla, E.; Boucard, A.S.; Mohseni, A.H.; Taghinezhad, S.S.; Cortes-Perez, N.G.; Bermúdez-Humarán, L.G. Role of Gut Microbiota and Probiotics in Colorectal Cancer: Onset and Progression. Microorganisms 2021, 9, 1021. [Google Scholar] [CrossRef] [PubMed]
- Sanders, M.E.; Benson, A.; Lebeer, S.; Merenstein, D.J.; Klaenhammer, T.R. Shared mechanisms among probiotic taxa: Implications for general probiotic claims. Curr. Opin. Biotechnol. 2018, 49, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Sanders, M.E.; Merenstein, D.J.; Reid, G.; Gibson, G.R.; Rastall, R.A. Probiotics and prebiotics in intestinal health and disease: From biology to the clinic. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 605–616. [Google Scholar] [CrossRef] [PubMed]
- Costabile, A.; Walton, G.E.; Tzortzis, G.; Vulevic, J.; Charalampopoulos, D.; Gibson, G.R. Effects of orange juice formulation on prebiotic functionality using an in vitro colonic model system. PLoS ONE 2015, 10, 0121955. [Google Scholar] [CrossRef]
- Kotz, C.M.; Furne, J.K.; Savaiano, D.A.; Levitt, M.D. Factors affecting the ability of a high beta- galactosidase yogurt to enhance lactose absorption. J. Dairy Sci. 1994, 77, 3538–3544. [Google Scholar] [CrossRef]
- Klaenhammer, T.R.; Kleerebezem, M.; Kopp, M.V.; Rescigno, M. The impact of probiotics and prebiotics on the immune system. Nat. Rev. Immunol. 2012, 12, 728–734. [Google Scholar] [CrossRef]
- Przemska-Kosicka, A.; Childs, C.E.; Enani, S.; Maidens, C.; Dong, H.; Dayel, I.B.; Tuohy, K.; Todd, S.; Gosney, M.A.; Yaqoob, P. Effect of a synbiotic on the response to seasonal influenza vaccination is strongly influenced by degree of immunosenescence. Immun. Ageing 2016, 13, 6. [Google Scholar] [CrossRef]
- Vitetta, L.; Saltzman, E.T.; Thomsen, M.; Nikov, T.; Hall, S. Adjuvant probiotics and the intestinal microbiome: Enhancing vaccines and immuno therapy outcomes. Vaccines 2017, 5, 50. [Google Scholar] [CrossRef] [PubMed]
- Aoudia, N.; Rieu, A.; Briandet, R.; Deschamps, J.; Chluba, J.; Jego, G.; Garrido, C.; Guzzo, J. Biofilms of Lactobacillus plantarum and Lactobacillus fermentum: Effect on stress responses, antagonistic effects on pathogen growth and immunomodulatory properties. Food Microbiol. 2016, 53, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Rios-Covian, D.; Ruas-Madiedo, P.; Margolles, A.; Gueimonde, M.; De Los Reyes-gavilán, C.G.; Salazar, N. Intestinal short chain fatty acids and their link with diet and human health. Front. Microbiol. 2016, 7, 185. [Google Scholar] [CrossRef] [PubMed]
- Van Baarlen, P.; Wells, J.M.; Kleerebezem, M. Regulation of intestinal homeostasis and immunity with probiotic lactobacilli. Trends Immunol. 2013, 34, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.; Yun, M.; Oh, Y.J.; Choi, H.J. Mind- altering with the gut: Modulation of the gut-brain axis with probiotics. J. Microbiol. 2018, 56, 172–182. [Google Scholar] [CrossRef]
- Reid, G. Disentangling what we know about microbes and mental health. Front. Endocrinol. 2019, 10, 81. [Google Scholar] [CrossRef]
- Liang, S.; Wang, T.; Hu, X.; Luo, L.; Li, W.; Wu, X.; Duan, Y.; Jin, F. Administration of Lactobacillus helveticus NS8 improves behavioral, cognitive, and biochemical aberrations caused by chronic restraint stress. Neuroscience 2015, 310, 561–577. [Google Scholar] [CrossRef]
- Guo, Q.; Goldenberg, J.Z.; Humphrey, C.; El Dib, R.; Johnston, B.C. Probiotics for the prevention of pediatric antibiotic-associated diarrhea. Cochrane Database Syst. Rev. 2019, 4, CD004827. [Google Scholar] [CrossRef]
- Albouy, J.P.; Abrahamsson, I.; Berglundh, T. Spontaneous progression of experimental peri-implantitis at implants with different surface characteristics: An experimental study in dogs. J. Clin. Periodontol. 2012, 39, 182–187. [Google Scholar] [CrossRef]
- Fickl, S.; Nannmark, U.; Schlagenhauf, U.; Hürzeler, M.B.; Kebschull, M. Porcine dermal matrix in the treatment of dehiscence-type defects--an experimental split-mouth animal trial. Clin. Oral Implants Res. 2015, 26, 799–805. [Google Scholar] [CrossRef]
- Pontoriero, R.; Tonelli, M.P.; Carnevale, G.; Mombelli, A.; Nyman, S.R.; Lang, N.P. Experimentally induced peri-implant mucositis. A clinical study in humans. Clin. Oral Implants Res. 1994, 5, 254–259. [Google Scholar] [CrossRef]
- Flichy-Fernández, A.J.; Ata-Ali, J.; Alegre-Domingo, T.; Candel-Martí, E.; Ata-Ali, F.; Palacio, J.R.; Peñarrocha-Diago, M. The effect of orally administered probiotic Lactobacillus reuteri-containing tablets in peri-implant mucositis: A double-blind randomized controlled trial. J. Periodontal Res. 2015, 50, 775–785. [Google Scholar] [CrossRef]
- Sayardoust, S.; Johansson, A.; Jönsson, D. Do Probiotics Cause a Shift in the Microbiota of Dental Implants-A Systematic Review and Meta-Analysis. Front. Cell. Infect. Microbiol. 2022, 12, 823985. [Google Scholar] [CrossRef]
- Alkaya, B.; Laleman, I.; Keceli, S.; Ozcelik, O.; Cenk Haytac, M.; Teughels, W. Clinical effects of probiotics containing Bacillus species on gingivitis: A pilot randomized controlled trial. J. Periodontal Res. 2017, 52, 497–504. [Google Scholar] [CrossRef]
- Toiviainen, A.; Jalasvuori, H.; Lahti, E.; Gursoy, U.; Salminen, S.; Fontana, M.; Flannagan, S.; Eckert, G.; Kokaras, A.; Paster, B.; et al. Impact of orally administered lozenges with Lactobacillus rhamnosus GG and Bifidobacterium animalis subsp. lactis BB-12 on the number of salivary mutans streptococci, amount of plaque, gingival inflammation and the oral microbiome in healthy adults. Clin. Oral Investig. 2015, 19, 77–83. [Google Scholar] [CrossRef]
- Sabatini, S.; Lauritano, D.; Candotto, V.; Silvestre, F.; Nardi, G. Oral probiotics in the management of gingivitis in diabetic patients: A double blinded randomized controlled study. J. Biol. Regul. Homeost. Agents 2017, 31, 197–202. [Google Scholar]
- Kuru, B.E.; Laleman, I.; Yalnızoglu, T.; Kuru, L.; Teughels, W. The influence of a Bifidobacterium animalis probiotic on gingival health: A randomized controlled clinical trial. J. Periodontol. 2017, 88, 1115–1123. [Google Scholar] [CrossRef]
- Akram, Z.; Shafqat, S.S.; Aati, S.; Kujan, O.; Fawzy, A. Clinical efficacy of probiotics in the treatment of gingivitis: A systematic review and meta-analysis. Aust. Dent. J. 2020, 65, 12–20. [Google Scholar] [CrossRef]
- Slots, J. Herpesviruses in periodontal diseases. Periodontol 2000 2005, 38, 33–62. [Google Scholar] [CrossRef]
- Borrell, L.N.; Papapanou, P.N. Analytical epidemiology of periodontitis. J. Clin. Periodontol. 2005, 32 (Suppl. S6), 132–158. [Google Scholar] [CrossRef]
- Teughels, W.; Newman, M.G.; Coucke, W.; Haffajee, A.D.; Van Der Mei, H.C.; Haake, S.K.; Schepers, E.; Cassiman, J.J.; Van Eldere, J.; van Steenberghe, D.; et al. Guided periodontal pocket recolonization: A proof of concept. J. Dent. Res. 2007, 86, 1078–1082. [Google Scholar] [CrossRef] [PubMed]
- Nackaerts, O.; Jacobs, R.; Quirynen, M.; Rober, M.; Sun, Y.; Teughels, W. Replacement therapy for periodontitis: Pilot radiographic evaluation in a dog model. J. Clin. Periodontol. 2008, 35, 1048–1052. [Google Scholar] [CrossRef] [PubMed]
- Grudianov, A.I.; Dmitrieva, N.A.; Fomenko, E.V. Use of probiotics Bifidumbacterin and Acilact in tablets in therapy of periodontal inflammations. Stomatologiia 2002, 81, 39–43. [Google Scholar] [PubMed]
- Riccia, D.N.; Bizzini, F.; Perilli, M.G.; Polimeni, A.; Trinchieri, V.; Amicosante, G.; Cifone, M.G. Anti-inflammatory effects of Lactobacillus brevis (CD2) on periodontal disease. Oral Dis. 2007, 13, 376–385. [Google Scholar] [CrossRef] [PubMed]
- Staab, B.; Eick, S.; Knöfler, G.; Jentsch, H. The influence of a probiotic milk drink on the development of gingivitis: A pilot study. J. Clin. Periodontol. 2009, 36, 850–856. [Google Scholar] [CrossRef] [PubMed]
- Mulla, M.; Hegde, S.; Koshy, A.; Mulla, M. Effect of Probiotic Lactobacillus salivarius on Peri-Implantitis Pathogenic Bacteria: An In Vitro Study. Cureus 2021, 13, e20808. [Google Scholar] [CrossRef]
- Butera, A.; Gallo, S.; Maiorani, C.; Molino, D.; Chiesa, A.; Preda, C.; Esposito, F.; Scribante, A. Probiotic Alternative to Chlorhexidine in Periodontal Therapy: Evaluation of Clinical and Microbiological Parameters. Microorganisms 2020, 9, 69. [Google Scholar] [CrossRef]
- Matsubara, V.H.; Bandara, H.M.; Ishikawa, K.H.; Mayer, M.P.; Samaranayake, L.P. The role of probiotic bacteria in managing periodontal disease: A systematic review. Expert Rev. Anti-Infect. Ther. 2016, 14, 643–655. [Google Scholar] [CrossRef]
- Löe, H.; Silness, J. Periodontal disease in pregnancy I. Prevalence and severity. Acta Odontol. Scand. 1963, 21, 533–551. [Google Scholar] [CrossRef]
- Silness, J.; Löe, H. Periodontal disease in pregnancy II. Correlation between oral hygiene and periodontal condition. Acta Odontol. Scand. 1964, 22, 121–135. [Google Scholar] [CrossRef]
- Socransky, S.S.; Haffajee, A.D. Periodontal microbial ecology. Periodontol 2000 2005, 38, 135–187. [Google Scholar] [CrossRef] [PubMed]
- Berezow, A.B.; Darveau, R.P. Microbial shift and periodontitis. Periodontology 2000 2011, 55, 36–47. [Google Scholar] [CrossRef] [PubMed]
- Socransky, S.S.; Haffajee, A.D.; Smith, C.; Martin, L.; Haffajee, J.A.; Uzel, N.G.; Goodson, J.M. Use of checkerboard DNA–DNA hybridization to study complex microbial ecosystems. Oral Microbiol. Immunol. 2004, 19, 352–362. [Google Scholar] [CrossRef] [PubMed]
- Beikler, T.; Peters, U.; Prior, K.; Eisenacher, M.; Flemmig, T.F. Gene expression in periodontal tissues following treatment. BMC Med. Genom. 2008, 1, 30. [Google Scholar] [CrossRef] [PubMed]
- Mayanagi, G.; Kimura, M.; Nakaya, S.; Hirata, H.; Sakamoto, M.; Benno, Y.; Shimauchi, H. Probiotic effects of orally administered Lactobacillus salivarius WB21-containing tablets on periodontopathic bacteria: A double-blinded, placebocontrolled, randomized clinical trial. J. Clin. Periodontol. 2009, 36, 506–513. [Google Scholar] [CrossRef]
- İnce, G.; Gürsoy, H.; İpçi, Ş.D.; Cakar, G.; Emekli-Alturfan, E.; Yılmaz, S. Clinical and Biochemical Evaluation of Lozenges Containing Lactobacillus reuteri as an Adjunct to Non-Surgical Periodontal Therapy in Chronic Periodontitis. J. Periodontol. 2015, 86, 746–754. [Google Scholar] [CrossRef]
- Iniesta, M.; Herrera, D.; Montero, E.; Zurbriggen, M.; Matos, A.R.; Marín, M.J.; Sánchez-Beltrán, M.C.; Llama-Palacio, A.; Sanz, M. Probiotic effects of orally administered Lactobacillus reuteri-containing tablets on the subgingival and salivary microbiota in patients with gingivitis. A randomized clinical trial. J. Clin. Periodontol. 2012, 39, 736–744. [Google Scholar] [CrossRef]
- Haukioja, A. Probiotics and oral health. Eur. J. Dent. 2010, 4, 348–355. [Google Scholar] [CrossRef]
- Laleman, I.; Teughels, W. Probiotics in the dental practice: A review. Quintessence Int. 2015, 46, 255–264. [Google Scholar] [CrossRef]
- Hu, D.; Zhong, T.; Dai, Q. Clinical efficacy of probiotics as an adjunctive therapy to scaling and root planning in the management of periodontitis: A systematic review and meta-analysis of randomized controlled trails. J. Evid.-Based Dent. Pract. 2021, 21, 101547. [Google Scholar] [CrossRef]
- Gruner, D.; Paris, S.; Schwendicke, F. Probiotics for managing caries and periodontitis: Systematic review and meta-analysis. J. Dent. 2016, 48, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Vives-Soler, A.; Chimenos-Küstner, E. Effect of probiotics as a complement to non-surgical periodontal therapy in chronic periodontitis: A systematic review. Med. Oral Patol. Oral Cir. Bucal 2020, 25, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Jayaram, P.; Chatterjee, A.; Raghunathan, V. Probiotics in the treatment of periodontal disease: A systematic review. J. Indian Soc. Periodontol. 2016, 20, 488–495. [Google Scholar] [CrossRef]
- Canut-Delgado, N.; Giovannoni, M.L.; Chimenos-Küstner, E. Are probiotics a possible treatment of periodontitis? Probiotics against periodontal disease: A systematic review. Br. Dent. J. 2021; advance online publication. [Google Scholar] [CrossRef]
- Tang, H.W.; Abbasiliasi, S.; Murugan, P.; Tam, Y.J.; Ng, H.S.; Tan, J.S. Influence of freeze-drying and spray-drying preservation methods on survivability rate of different types of protectants encapsulated Lactobacillus acidophilus FTDC 3081. Biosci. Biotechnol. Biochem. 2020, 84, 1913–1920. [Google Scholar] [CrossRef] [PubMed]
- Ikram, S.; Hassan, N.; Raffat, M.A.; Mirza, S.; Akram, Z. Systematic review and meta-analysis of double-blind, placebo-controlled, randomized clinical trials using probiotics in chronic periodontitis. J. Investig. Clin. Dent. 2018, 9, e12338. [Google Scholar] [CrossRef]
- Ng, E.; Tay, J.; Saffari, S.E.; Lim, L.P.; Chung, K.M.; Ong, M. Adjunctive probiotics after periodontal debridement versus placebo: A systematic review and meta-analysis. Acta Odontol. Scand. 2022, 80, 81–90. [Google Scholar] [CrossRef]
- Yanine, N.; Araya, I.; Brignardello-Petersen, R.; Carrasco-Labra, A.; González, A.; Preciado, A.; Villanueva, J.; Sanz, M.; Martin, C. Effects of probiotics in periodontal diseases: A systematic review. Clin. Oral Investig. 2013, 17, 1627–1634. [Google Scholar] [CrossRef]
- Gheisary, Z.; Mahmood, R.; Harri Shivanantham, A.; Liu, J.; Lieffers, J.; Papagerakis, P.; Papagerakis, S. The Clinical, Microbiological, and Immunological Effects of Probiotic Supplementation on Prevention and Treatment of Periodontal Diseases: A Systematic Review and Meta-Analysis. Nutrients 2022, 14, 1036. [Google Scholar] [CrossRef]
- Sargolzaei, N.; Arab, H.; Gerayeli, M.; Ivani, F. Evaluation of the Topical Effect of Probiotic Mouthwash in the Treatment of Patients with Peri-Implant Mucositis. J. Long-Term Eff. Med. Implants 2022, 32, 85–91. [Google Scholar] [CrossRef]
- Zhao, R.; Hu, H.; Wang, Y.; Lai, W.; Jian, F. Efficacy of Probiotics as Adjunctive Therapy to Nonsurgical Treatment of Peri-Implant Mucositis: A Systematic Review and Meta-Analysis. Front. Pharmacol. 2021, 11, 541752. [Google Scholar] [CrossRef]
- Arbildo-Vega, H.I.; Panda, S.; Bal, A.; Mohanty, R.; Rendón-Alvarado, A.; Das, A.C.; Cruzado-Oliva, F.H.; Infantes-Ruíz, E.D.; Manfredi, B.; Vásquez-Rodrigo, H.; et al. Clinical effectiveness of Lactobacillus reuteri in the treatment of peri-implant diseases: A systematic review and meta-analysis. J. Biol. Regul. Homeost. Agents 2021, 35 (Suppl. S1), 79–88. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Yu, S.; Zhu, X.; Yan, Y.; Zhang, Y.; Pei, D. Does Probiotic Lactobacillus Have an Adjunctive Effect in the Nonsurgical Treatment of Peri-Implant Diseases? A Systematic Review and Meta-analysis. J. Evid.-Based Dent. Pract. 2020, 20, 101398. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.P.; Cordeiro, T.O.; da Costa, R.A.; Martins, A.; Dantas, E.M.; Gurgel, B.; Lins, R. Effect of Adjunctive Probiotic Therapy on the Treatment of Peri-implant Diseases—A Systematic Review. J. Int. Acad. Periodontol. 2020, 22, 137–145. [Google Scholar]
- Pietri, F.K.; Rossouw, P.E.; Javed, F.; Michelogiannakis, D. Role of Probiotics in Oral Health Maintenance Among Patients Undergoing Fixed Orthodontic Therapy: A Systematic Review of Randomized Controlled Clinical Trials. Probiotics Antimicrob. Proteins 2020, 12, 1349–1359. [Google Scholar] [CrossRef] [PubMed]
- Goyal, N.; Shamanna, P.U.; Varughese, S.T.; Abraham, R.; Antony, B.; Emmatty, R.; Paul, P. Effects of amine fluoride and probiotic mouthwash on levels of Porphyromonas gingivalis in orthodontic patients: A randomized controlled trial. J. Indian Soc. Periodontol. 2019, 23, 339–344. [Google Scholar] [CrossRef]
- Amato, A. Oral-Systemic Health and Disorders: Latest Advances on Oral–Gut–Lung Microbiome Axis. Appl. Sci. 2022, 12, 8213. [Google Scholar] [CrossRef]
- Shah, S.S.; Nambiar, S.; Kamath, D.; Suman, E.; Unnikrishnan, B.; Desai, A.; Mahajan, S.; Dhawan, K.K. Comparative Evaluation of Plaque Inhibitory and Antimicrobial Efficacy of Probiotic and Chlorhexidine Oral Rinses in Orthodontic Patients: A Randomized Clinical Trial. Int. J. Dent. 2019, 1964158. [Google Scholar] [CrossRef]
- Megha, S.; Shalini, G.; Varsha, S.A.; Abhishek, D.; Neetu, J. Effect of short-term placebo-controlled consumption of probiotic yoghurt and Indian curd on the Streptococcus mutans level in children undergoing fixed interceptive orthodontic therapy. Turk. J. Orthod. 2019, 32, 16–21. [Google Scholar] [CrossRef]
- Goyal, B.M.S.; Nota, A.; Albani, F.; Marchetti, E.; Gatto, R.; Marzo, G.; Quinzi, V.; Tecco, S. Salivary levels of Streptococcus mutans and Lactobacilli and other salivary indices in patients wearing clear aligners versus fixed orthodontic appliances: An observational study. PLoS ONE 2020, 15, 0228798. [Google Scholar] [CrossRef]
- Gizani, S.; Petsi, G.; Twetman, S.; Caroni, C.; Makou, M.; Papagianoulis, L. Effect of the probiotic bacterium Lactobacillus reuteri on white spot lesion development in orthodontic patients. Eur. J. Orthod. 2016, 38, 85–89. [Google Scholar] [CrossRef]
- Hadj-Hamou, R.; Senok, A.C.; Athanasiou, A.E.; Kaklamanos, E.G. Do probiotics promote oral health during orthodontic treatment with fixed appliances? A systematic review. BMC Oral Health 2020, 20, 126. [Google Scholar] [CrossRef] [PubMed]
- Schlagenhauf, U.; Jockel-Schneider, Y. Probiotics in the management of gingivitis and periodontitis. A review. Front. Dent. Med. 2021, 2, 1–11. [Google Scholar] [CrossRef]
- Donos, N.; Calciolari, E.; Brusselaers, N.; Goldoni, M.; Bostanci, N.; Belibasakis, G.N. The adjunctive use of host modulators in non-surgical periodontal therapy. A systematic review of randomized, placebo-controlled clinical studies. J. Clin. Periodontol. 2020, 47, 199–238. [Google Scholar] [CrossRef] [PubMed]
- Balta, M.G.; Papathanasiou, E.; Blix, I.J.; Van Dyke, T.E. Host Modulation and Treatment of Periodontal Disease. J. Dent. Res. 2021, 100, 798–809. [Google Scholar] [CrossRef] [PubMed]
- Hajishengallis, G.; Chavakis, T.; Lambris, J.D. Current understanding of periodontal disease pathogenesis and targets for host-modulation therapy. Periodontology 2000 2020, 84, 14–34. [Google Scholar] [CrossRef] [PubMed]
- Bunte, K.; Beikler, T. Th17 Cells and the IL-23/IL-17 Axis in the Pathogenesis of Periodontitis and Immune-Mediated Inflammatory Diseases. Int. J. Mol. Sci. 2019, 20, 3394. [Google Scholar] [CrossRef]
- Kaźmierczyk-Winciorek, M.; Nędzi-Góra, M.; Słotwińska, S.M. The immunomodulating role of probiotics in the prevention and treatment of oral diseases. Cent.-Eur. J. Immunol. 2021, 46, 99–104. [Google Scholar] [CrossRef]
- Abusleme, L.; Moutsopoulos, N.M. IL-17: Overview and role in oral immunity and microbiome. Oral Dis. 2017, 23, 854–865. [Google Scholar] [CrossRef]
- Valeri, M.; Raffatellu, M. Cytokines IL-17 and IL-22 in the host response to infection. Pathog. Dis. 2016, 74, ftw111. [Google Scholar] [CrossRef]
- Awang, R.A.; Lappin, D.F.; MacPherson, A.; Riggio, M.; Robertson, D.; Hodge, P.; Ramage, G.; Culshaw, S.; Preshaw, P.M.; Taylor, J.; et al. Clinical associations between IL-17 family cytokines and periodontitis and potential differential roles for IL-17A and IL-17E in periodontal immunity. Inflamm. Res. 2014, 63, 1001–1012. [Google Scholar] [CrossRef]
- Zenobia, C.; Hajishengallis, G. Basic biology and role of interleukin-17 in immunity and inflammation. Periodontology 2000 2015, 69, 142–159. [Google Scholar] [CrossRef] [PubMed]
- Bifulco, M.; Amato, M.; Gangemi, G.; Marasco, M.; Caggiano, M.; Amato, A.; Pisanti, S. Dental care and dentistry practice in the Medieval Medical School of Salerno. Brit. Dent. J. 2016, 221, 87–89. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amato, M.; Di Spirito, F.; D’Ambrosio, F.; Boccia, G.; Moccia, G.; De Caro, F. Probiotics in Periodontal and Peri-Implant Health Management: Biofilm Control, Dysbiosis Reversal, and Host Modulation. Microorganisms 2022, 10, 2289. https://doi.org/10.3390/microorganisms10112289
Amato M, Di Spirito F, D’Ambrosio F, Boccia G, Moccia G, De Caro F. Probiotics in Periodontal and Peri-Implant Health Management: Biofilm Control, Dysbiosis Reversal, and Host Modulation. Microorganisms. 2022; 10(11):2289. https://doi.org/10.3390/microorganisms10112289
Chicago/Turabian StyleAmato, Massimo, Federica Di Spirito, Francesco D’Ambrosio, Giovanni Boccia, Giuseppina Moccia, and Francesco De Caro. 2022. "Probiotics in Periodontal and Peri-Implant Health Management: Biofilm Control, Dysbiosis Reversal, and Host Modulation" Microorganisms 10, no. 11: 2289. https://doi.org/10.3390/microorganisms10112289
APA StyleAmato, M., Di Spirito, F., D’Ambrosio, F., Boccia, G., Moccia, G., & De Caro, F. (2022). Probiotics in Periodontal and Peri-Implant Health Management: Biofilm Control, Dysbiosis Reversal, and Host Modulation. Microorganisms, 10(11), 2289. https://doi.org/10.3390/microorganisms10112289










