The Prevalence, Coexistence, and Correlations between Seven Pathogens Detected by a PCR Method from South-Western Poland Dairy Cattle Suffering from Bovine Respiratory Disease
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection
- n—required sample size;
- Pexp—expected prevalence (0.74);
- d—desired absolute precision (0.1).
- n—number of samples submitted from the herd;
- p1—probability to detect at least one infected calf (0.95);
- N—number of animals in the herd;
- d—minimum number of affected animals expected in the herd (N × expected prevalence).
2.2. PCR Test
2.3. Statistical Analysis
2.4. Ethics Statement
3. Results
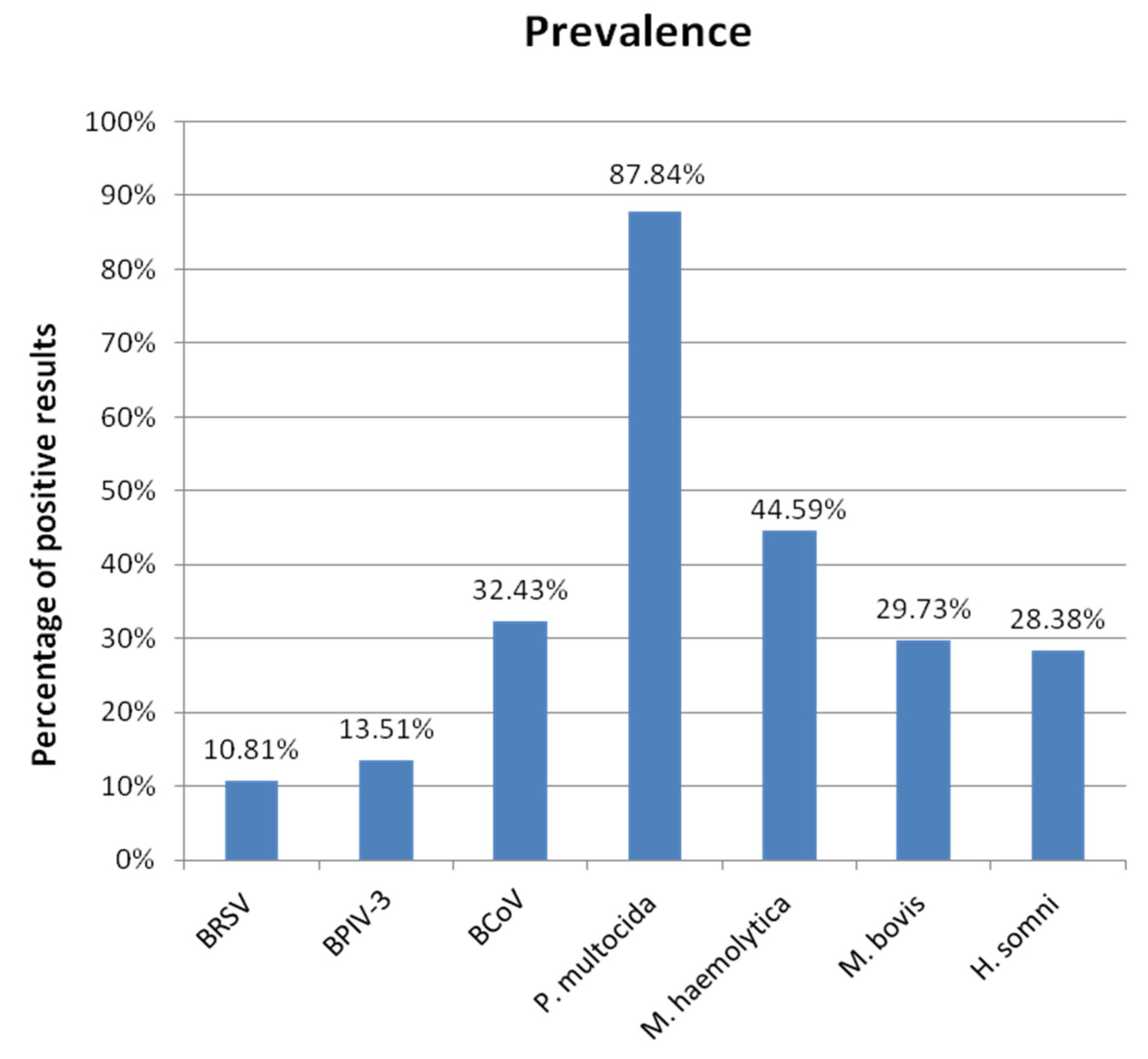
3.1. Prevalence
3.1.1. Overall Prevalence
3.1.2. Prevalence Depending on the Type of Samples
3.2. Occurrences
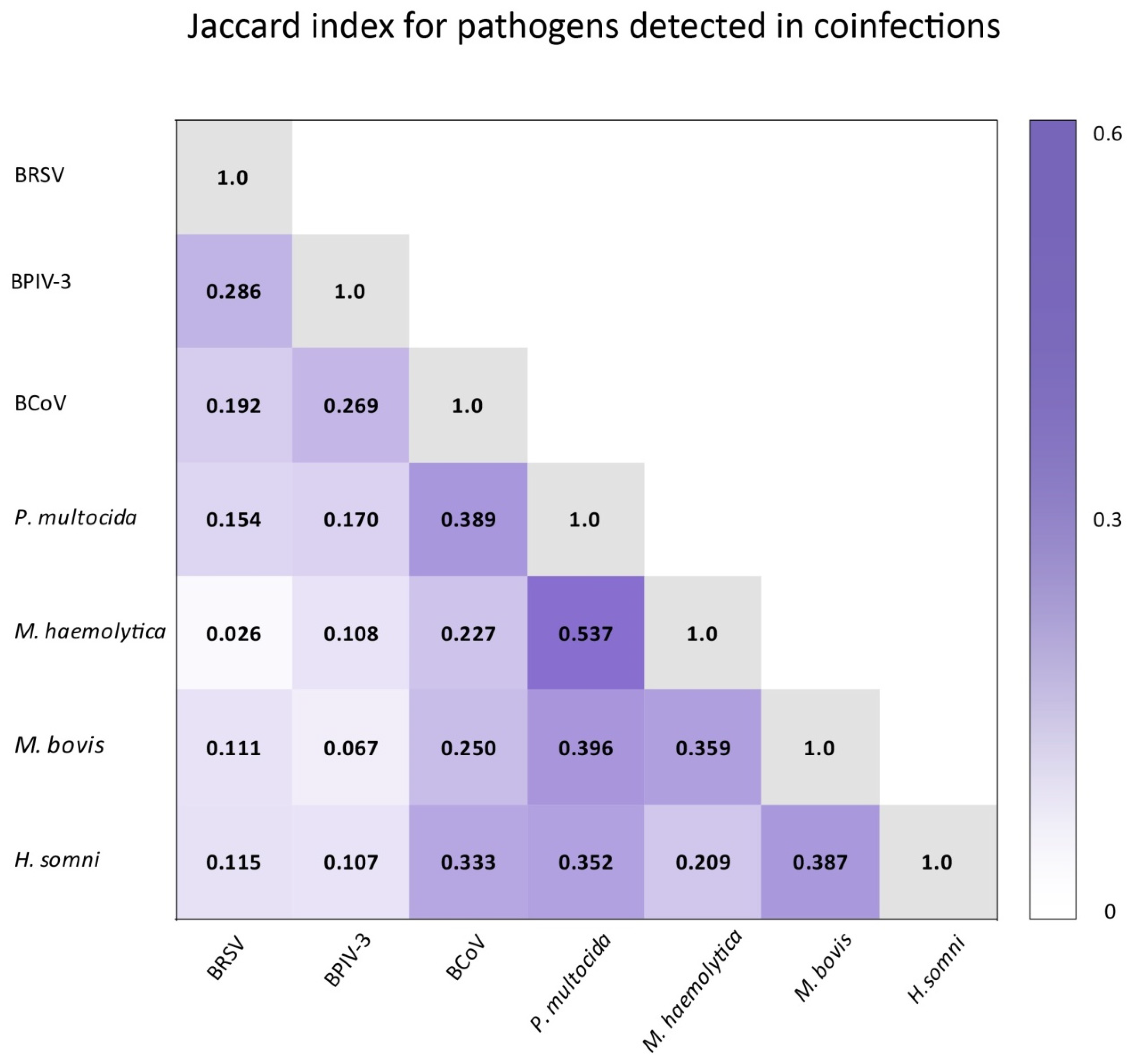
3.3. Correlations
3.4. Configurations
3.4.1. Occurred Configurations
3.4.2. Configurations in Bacterial-Only Infections and Coinfections
3.4.3. Coexistence
- M. haemolytica with P. multocida
- M. haemolytica with BCoV
- P. multocida with BCoV
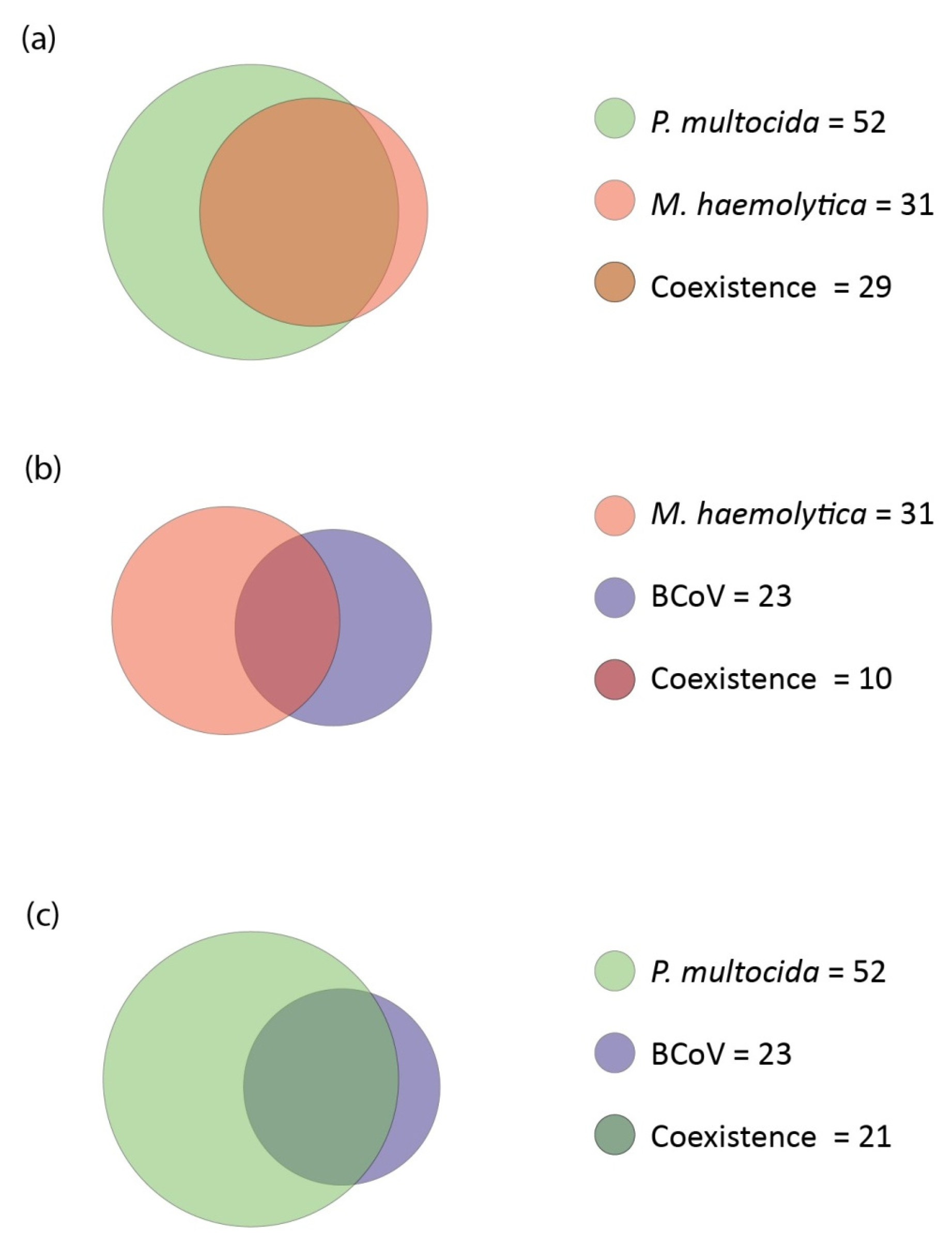
- M. bovis with H. somni
- BCoV with BPIV-3
- BRSV with BPIV-3

4. Discussion
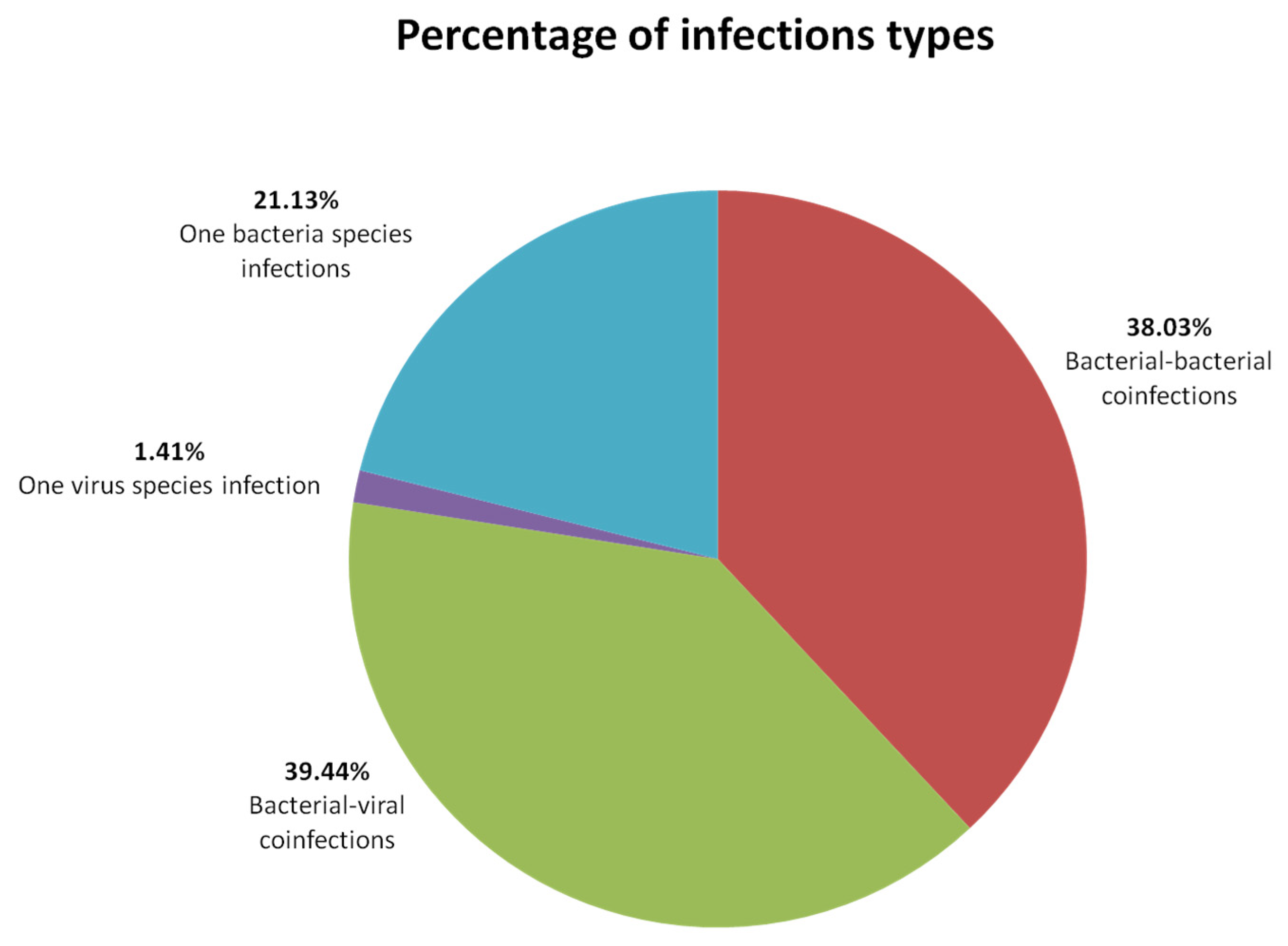
4.1. Infection Types
4.2. Prevalence
4.3. Coinfections and Configurations
4.4. Correlations
4.5. Coexistence
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Witkowska, D.; Ponieważ, A. The Effect of Housing System on Disease Prevalence and Productive Lifespan of Dairy Herds—A Case Study. Animals 2022, 12, 1610. [Google Scholar] [CrossRef] [PubMed]
- Andrés-Lasheras, S.; Ha, R.; Zaheer, R.; Lee, C.; Booker, C.W.; Dorin, C.; van Donkersgoed, J.; Deardon, R.; Gow, S.; Hannon, S.J.; et al. Prevalence and Risk Factors Associated with Antimicrobial Resistance in Bacteria Related to Bovine Respiratory Disease-A Broad Cross-Sectional Study of Beef Cattle at Entry into Canadian Feedlots. Front. Vet. Sci. 2021, 8, 692646. [Google Scholar] [CrossRef] [PubMed]
- Van der Poel, W.H.M.; Brand, A.; Kramps, J.A.; van Oirschot, J.T. Respiratory syncytial virus infections in human beings and in cattle. J. Infect. 1994, 29, 215–228. [Google Scholar] [CrossRef]
- Dubrovsky, S.A.; van Eenennaam, A.L.; Karle, B.M.; Rossitto, P.V.; Lehenbauer, T.W.; Aly, S.S. Bovine respiratory disease (BRD) cause-specific and overall mortality in preweaned calves on California dairies: The BRD 10K study. J. Dairy Sci. 2019, 102, 7320–7328. [Google Scholar] [CrossRef] [PubMed]
- Murray, G.M.; More, S.J.; Sammin, D.; Casey, M.J.; McElroy, M.C.; O’Neill, R.G.; Byrne, W.J.; Earley, B.; Clegg, T.A.; Ball, H.; et al. Pathogens, patterns of pneumonia, and epidemiologic risk factors associated with respiratory disease in recently weaned cattle in Ireland. J. Vet. Diagn. Investig. 2017, 29, 20–34. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, R.; Mooney, J.; Connaghan, E.; Furphy, C.; Graham, D.A. Patterns of detection of respiratory viruses in nasal swabs from calves in Ireland: A retrospective study. Vet. Rec. 2014, 175, 351. [Google Scholar] [CrossRef]
- Studer, E.; Schönecker, L.; Meylan, M.; Stucki, D.; Dijkman, R.; Holwerda, M.; Glaus, A.; Becker, J. Prevalence of BRD-Related Viral Pathogens in the Upper Respiratory Tract of Swiss Veal Calves. Animals 2021, 11, 1940. [Google Scholar] [CrossRef] [PubMed]
- Pratelli, A.; Cirone, F.; Capozza, P.; Trotta, A.; Corrente, M.; Balestrieri, A.; Buonavoglia, C. Bovine respiratory disease in beef calves supported long transport stress: An epidemiological study and strategies for control and prevention. Res. Vet. Sci. 2021, 135, 450–455. [Google Scholar] [CrossRef]
- Pratelli, A.; Padalino, B. Editorial: Evolving Prospects of Bovine Respiratory Diseases and Management in Feedlot Cattle. Front. Vet. Sci. 2022, 9, 854844. [Google Scholar] [CrossRef]
- Taylor, J.D.; Fulton, R.W.; Lehenbauer, T.W.; Step, D.L.; Confer, A.W. The epidemiology of bovine respiratory disease: What is the evidence for predisposing factors? Can. Vet. J. Rev. Vet. Can. 2010, 51, 1095–1102. [Google Scholar]
- Nickell, J.S.; White, B.J. Metaphylactic antimicrobial therapy for bovine respiratory disease in stocker and feedlot cattle. Vet. Clin. N. Am. Food Anim. Pract. 2010, 26, 285–301. [Google Scholar] [CrossRef] [PubMed]
- Confer, A.W. Update on bacterial pathogenesis in BRD. Anim. Health Res. Rev. 2009, 10, 145–148. [Google Scholar] [CrossRef] [PubMed]
- Guterbock, W.M. The impact of BRD: The current dairy experience. Anim. Health Res. Rev. 2014, 15, 130–134. [Google Scholar] [CrossRef]
- Akalu, M.; Vemulapati, B.; Abayneh, T.; Degefa, T.; Deresse, G.; Gelaye, E. Serotyping, antibiogram, and detection of bacterial pathogens associated with bovine respiratory disease in selected areas of Ethiopia. Ir. Vet. J. 2022, 75, 3. [Google Scholar] [CrossRef]
- Fanelli, A.; Cirilli, M.; Lucente, M.S.; Zarea, A.A.K.; Buonavoglia, D.; Tempesta, M.; Greco, G. Fatal Calf Pneumonia Outbreaks in Italian Dairy Herds Involving Mycoplasma bovis and Other Agents of BRD Complex. Front. Vet. Sci. 2021, 8, 742785. [Google Scholar] [CrossRef] [PubMed]
- Hashem, Y.M.; Mousa, W.S.; Abdeen, E.E.; Abdelkhalek, H.M.; Nooruzzaman, M.; El-Askary, A.; Ismail, K.A.; Megahed, A.M.; Abdeen, A.; Soliman, E.A.; et al. Prevalence and Molecular Characterization of Mycoplasma Species, Pasteurella multocida, and Staphylococcus aureus Isolated from Calves with Respiratory Manifestations. Animals 2022, 12, 312. [Google Scholar] [CrossRef]
- Sarchet, J.J.; Pollreisz, J.P.; Bechtol, D.T.; Blanding, M.R.; Saltman, R.L.; Taube, P.C. Limitations of bacterial culture, viral PCR, and tulathromycin susceptibility from upper respiratory tract samples in predicting clinical outcome of tulathromycin control or treatment of bovine respiratory disease in high-risk feeder heifers. PLoS ONE 2022, 17, e0247213. [Google Scholar] [CrossRef]
- Kishimoto, M.; Tsuchiaka, S.; Rahpaya, S.S.; Hasebe, A.; Otsu, K.; Sugimura, S.; Kobayashi, S.; Komatsu, N.; Nagai, M.; Omatsu, T.; et al. Development of a one-run real-time PCR detection system for pathogens associated with bovine respiratory disease complex. J. Vet. Med. Sci. 2017, 79, 517–523. [Google Scholar] [CrossRef] [Green Version]
- Pardon, B.; de Bleecker, K.; Dewulf, J.; Callens, J.; Boyen, F.; Catry, B.; Deprez, P. Prevalence of respiratory pathogens in diseased, non-vaccinated, routinely medicated veal calves. Vet. Rec. 2011, 169, 278. [Google Scholar] [CrossRef]
- Saegerman, C.; Gaudino, M.; Savard, C.; Broes, A.; Ariel, O.; Meyer, G.; Ducatez, M.F. Influenza D virus in respiratory disease in Canadian, province of Québec, cattle: Relative importance and evidence of new reassortment between different clades. Transbound. Emerg. Dis. 2022, 69, 1227–1245. [Google Scholar] [CrossRef]
- Mekata, H.; Yamamoto, M.; Hamabe, S.; Tanaka, H.; Omatsu, T.; Mizutani, T.; Hause, B.M.; Okabayashi, T. Molecular epidemiological survey and phylogenetic analysis of bovine influenza D virus in Japan. Transbound. Emerg. Dis. 2018, 65, e355–e360. [Google Scholar] [CrossRef] [PubMed]
- Van der Fels-Klerx, H.J.; Saatkamp, H.W.; Verhoeff, J.; Dijkhuizen, A.A. Effects of bovine respiratory disease on the productivity of dairy heifers quantified by experts. Livest. Prod. Sci. 2002, 75, 157–166. [Google Scholar] [CrossRef]
- Lowie, T.; van Leenen, K.; Jourquin, S.; Pas, M.L.; Bokma, J.; Pardon, B. Differences in the association of cough and other clinical signs with ultrasonographic lung consolidation in dairy, veal, and beef calves. J. Dairy Sci. 2022, 105, 6111–6124. [Google Scholar] [CrossRef] [PubMed]
- Sacco, R.E.; McGill, J.L.; Pillatzki, A.E.; Palmer, M.V.; Ackermann, M.R. Respiratory syncytial virus infection in cattle. Vet. Pathol. 2014, 51, 427–436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Closs, G.; Dechow, C. The effect of calf-hood pneumonia on heifer survival and subsequent performance. Livest. Sci. 2017, 205, 5–9. [Google Scholar] [CrossRef]
- Wang, M.; Schneider, L.G.; Hubbard, K.J.; Smith, D.R. Cost of bovine respiratory disease in preweaned calves on US beef cow-calf operations (2011–2015). J. Am. Vet. Med. Assoc. 2018, 253, 624–631. [Google Scholar] [CrossRef]
- Chai, J.; Capik, S.F.; Kegley, B.; Richeson, J.T.; Powell, J.G.; Zhao, J. Bovine respiratory microbiota of feedlot cattle and its association with disease. Vet. Res. 2022, 53, 4. [Google Scholar] [CrossRef]
- Centeno-Martinez, R.E.; Glidden, N.; Mohan, S.; Davidson, J.L.; Fernández-Juricic, E.; Boerman, J.P.; Schoonmaker, J.; Pillai, D.; Koziol, J.; Ault, A.; et al. Identification of bovine respiratory disease through the nasal microbiome. Anim. Microbiome 2022, 4, 15. [Google Scholar] [CrossRef]
- Timsit, E.; Workentine, M.; van der Meer, F.; Alexander, T. Distinct bacterial metacommunities inhabit the upper and lower respiratory tracts of healthy feedlot cattle and those diagnosed with bronchopneumonia. Vet. Microbiol. 2018, 221, 105–113. [Google Scholar] [CrossRef]
- Winnicki, S.; Jugowar, L.J. Housing systems for dairy cattle in the Wielkopolska region–Current status and prospects. Przegląd Hod. 2013, 6, 15–17. [Google Scholar]
- Thrusfield, M.; Brown, H.; Christley, R. Veterinary Epidemiology, 4th ed; Wiley Blackwell: Oxford, UK, 2018. [Google Scholar]
- Paller, T.; Hostnik, P.; Pogačnik, M.; Toplak, I. The prevalence of ten pathogens detected by a real-time pcr method in nasal swab samples collected from live cattle with respiratory disease. Slov. Vet. Res. 2017, 54, 101–107. [Google Scholar]
- Oliveira, T.E.S.; Scuisato, G.S.; Pelaquim, I.F.; Cunha, C.W.; Cunha, L.S.; Flores, E.F.; Pretto-Giordano, L.G.; Lisbôa, J.A.N.; Alfieri, A.A.; Saut, J.P.E.; et al. The Participation of a Malignant Catarrhal Fever Virus and Mycoplasma bovis in the Development of Single and Mixed Infections in Beef and Dairy Cattle with Bovine Respiratory Disease. Front. Vet. Sci. 2021, 8, 691448. [Google Scholar] [CrossRef]
- Pardon, B.; Buczinski, S. Bovine Respiratory Disease Diagnosis: What Progress Has Been Made in Infectious Diagnosis? Vet. Clin. N. Am. Food Anim. Pract. 2020, 36, 425–444. [Google Scholar] [CrossRef]
- Klima, C.L.; Zaheer, R.; Cook, S.R.; Booker, C.W.; Hendrick, S.; Alexander, T.W.; McAllister, T.A. Pathogens of bovine respiratory disease in North American feedlots conferring multidrug resistance via integrative conjugative elements. J. Clin. Microbiol. 2014, 52, 438–448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jamali, H.; Rezagholipour, M.; Fallah, S.; Dadrasnia, A.; Chelliah, S.; Velappan, R.D.; Wei, K.S.C.; Ismail, S. Prevalence, characterization and antibiotic resistance of Pasteurella multocida isolated from bovine respiratory infection. Vet. J. 2014, 202, 381–383. [Google Scholar] [CrossRef] [PubMed]
- Schönecker, L.; Schnyder, P.; Schüpbach-Regula, G.; Meylan, M.; Overesch, G. Prevalence and antimicrobial resistance of opportunistic pathogens associated with bovine respiratory disease isolated from nasopharyngeal swabs of veal calves in Switzerland. Prev. Vet. Med. 2020, 185, 105182. [Google Scholar] [CrossRef] [PubMed]
- Timsit, E.; Hallewell, J.; Booker, C.; Tison, N.; Amat, S.; Alexander, T.W. Prevalence and antimicrobial susceptibility of Mannheimia haemolytica, Pasteurella multocida, and Histophilus somni isolated from the lower respiratory tract of healthy feedlot cattle and those diagnosed with bovine respiratory disease. Vet. Microbiol. 2017, 208, 118–125. [Google Scholar] [CrossRef]
- Headley, S.A.; Okano, W.; Balbo, L.C.; Marcasso, R.A.; Oliveira, T.E.; Alfieri, A.F.; Negri Filho, L.C.; Michelazzo, M.Z.; Rodrigues, S.C.; Baptista, A.L.; et al. Molecular survey of infectious agents associated with bovine respiratory disease in a beef cattle feedlot in southern Brazil. J. Vet. Diagn. Investig. 2018, 30, 249–251. [Google Scholar] [CrossRef] [Green Version]
- Hodnik, J.J.; Ježek, J.; Starič, J. Coronaviruses in cattle. Trop. Anim. Health Prod. 2020, 52, 2809–2816. [Google Scholar] [CrossRef]
- Van Leenen, K.; van Driessche, L.; de Cremer, L.; Masmeijer, C.; Boyen, F.; Deprez, P.; Pardon, B. Comparison of bronchoalveolar lavage fluid bacteriology and cytology in calves classified based on combined clinical scoring and lung ultrasonography. Prev. Vet. Med. 2020, 176, 104901. [Google Scholar] [CrossRef]
- Frucchi, A.P.S.; Dall Agnol, A.M.; Bronkhorst, D.E.; Beuttemmuller, E.A.; Alfieri, A.A.; Alfieri, A.F. Bovine Coronavirus Co-infection and Molecular Characterization in Dairy Calves with or Without Clinical Respiratory Disease. Front. Vet. Sci. 2022, 9, 895492. [Google Scholar] [CrossRef] [PubMed]
- Urban-Chmiel, R.; Wernicki, A.; Puchalski, A.; Dec, M.; Stęgierska, D.; Grooms, D.L.; Barbu, N.I. Detection of bovine respiratory syncytial virus infections in young dairy and beef cattle in Poland. Vet. Q. 2015, 35, 33–36. [Google Scholar] [CrossRef] [PubMed]
- Szacawa, E.; Niemczuk, K.; Dudek, K.; Bednarek, D.; Rosales, R.; Ayling, R. Mycoplasma bovis infections and co-infections with other Mycoplasma spp. with different clinical manifestations in affected cattle herds in eastern region of Poland. Bull. Vet. Inst. Pulawy 2015, 59, 331–338. [Google Scholar] [CrossRef] [Green Version]
- Doyle, D.; Credille, B.; Lehenbauer, T.W.; Berghaus, R.; Aly, S.S.; Champagne, J.; Blanchard, P.; Crossley, B.; Berghaus, L.; Cochran, S.; et al. Agreement among 4 Sampling Methods to Identify Respiratory Pathogens in Dairy Calves with Acute Bovine Respiratory Disease. J. Vet. Intern. Med. 2017, 31, 954–959. [Google Scholar] [CrossRef]
- Zhang, M.; Hill, J.E.; Fernando, C.; Alexander, T.W.; Timsit, E.; van der Meer, F.; Huang, Y. Respiratory viruses identified in western Canadian beef cattle by metagenomic sequencing and their association with bovine respiratory disease. Transbound. Emerg. Dis. 2019, 66, 1379–1386. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Hill, J.E.; Alexander, T.W.; Huang, Y. The nasal viromes of cattle on arrival at western Canadian feedlots and their relationship to development of bovine respiratory disease. Transbound. Emerg. Dis. 2021, 68, 2209–2218. [Google Scholar] [CrossRef] [PubMed]
- McMullen, C.; Alexander, T.W.; Orsel, K.; Timsit, E. Progression of nasopharyngeal and tracheal bacterial microbiotas of feedlot cattle during development of bovine respiratory disease. Vet. Microbiol. 2020, 248, 108826. [Google Scholar] [CrossRef]
- Thomas, A.; Dizier, I.; Trolin, A.; Mainil, J.; Linden, A. Comparison of sampling procedures for isolating pulmonary mycoplasmas in cattle. Vet. Res. Commun. 2002, 26, 333–339. [Google Scholar] [CrossRef]
- Van Driessche, L.; Valgaeren, B.R.; Gille, L.; Boyen, F.; Ducatelle, R.; Haesebrouck, F.; Deprez, P.; Pardon, B. A Deep Nasopharyngeal Swab Versus Nonendoscopic Bronchoalveolar Lavage for Isolation of Bacterial Pathogens from Preweaned Calves with Respiratory Disease. J. Vet. Intern. Med. 2017, 31, 946–953. [Google Scholar] [CrossRef] [Green Version]
- Bassel, L.L.; Kaufman, E.I.; Alsop, S.-N.A.; Buchan, J.; Hewson, J.; McCandless, E.E.; Tiwari, R.; Sharif, S.; Vulikh, K.; Caswell, J.L. Effect of aerosolized bacterial lysate on development of naturally occurring respiratory disease in beef calves. J. Vet. Intern. Med. 2021, 35, 655–665. [Google Scholar] [CrossRef]
- Kudirkiene, E.; Aagaard, A.K.; Schmidt, L.M.B.; Pansri, P.; Krogh, K.M.; Olsen, J.E. Occurrence of major and minor pathogens in calves diagnosed with bovine respiratory disease. Vet. Microbiol. 2021, 259, 109135. [Google Scholar] [CrossRef] [PubMed]
- Klima, C.L.; Holman, D.B.; Ralston, B.J.; Stanford, K.; Zaheer, R.; Alexander, T.W.; McAllister, T.A. Lower Respiratory Tract Microbiome and Resistome of Bovine Respiratory Disease Mortalities. Microb. Ecol. 2019, 78, 446–456. [Google Scholar] [CrossRef] [PubMed]
- Zeineldin, M.; A Elolimy, A.; Barakat, R. Meta-analysis of bovine respiratory microbiota: Link between respiratory microbiota and bovine respiratory health. FEMS Microbiol. Ecol. 2020, 96, 127. [Google Scholar] [CrossRef] [PubMed]



| Correlations | |||||||
|---|---|---|---|---|---|---|---|
| BRSV | BCoV | Mannheimia haemolytica | Pasteurella multocida | Histophilus somni | Mycoplasma bovis | ||
| BPIV-3 | p | 0.010 | 0.011 | 1.000 | 1.000 | 1.000 | 0.713 |
| ϕ | 0.33 | 0.32 | 0.04 | 0.03 | 0.01 | 0.08 | |
| BRSV | p | 0.103 | 0.068 * | 0.584 | 0.680 | 0.688 | |
| ϕ | 0.22 | 0.23 * | 0.130 | 0.070 | 0.06 | ||
| BCoV | p | 0.726 | 1.000 | 0.021 | 0.311 | ||
| ϕ | 0.04 | 0.01 | 0.27 | 0.12 | |||
| Mannheimia haemolytica | p | 1.000 | 1.000 | 0.032 | |||
| ϕ | <0.01 | 0.02 | 0.25 | ||||
| Pasteurella multocida | p | 1.000 | 0.265 | ||||
| ϕ | 0.05 | 0.15 | |||||
| Histophilus somni | p | 0.001 | |||||
| ϕ | 0.38 | ||||||
| Configurations | |||||
|---|---|---|---|---|---|
| No. of Pathogen in Configura-tions | Pathogens Name | No. of Positive Results | % of All Positive Results | ||
| All (71) | NS (45) | TW (26) | |||
| 1 | BCoV | 1 | 1 | 0 | 1.41% |
| M. haemolytica | 2 | 2 | 0 | 2.82% | |
| P. multocida | 13 | 9 | 4 | 18.31% | |
| 2 | M. haemolytica, BPIV-3 | 1 | 1 | 0 | 1.41% |
| P. multocida, BPIV-3 | 1 | 1 | 0 | 1.41% | |
| P. multocida, BRSV | 2 | 1 | 1 | 2.82% | |
| P. multocida, BCoV | 3 | 3 | 0 | 4.23% | |
| P. multocida, M. haemolytica | 10 | 7 | 3 | 14.08% | |
| P. multocida, H. somni | 3 | 2 | 1 | 4.23% | |
| P. multocida, M. bovis | 2 | 0 | 2 | 2.82% | |
| 3 | BPIV-3, BCoV, P. multocida | 1 | 1 | 0 | 1.41% |
| BPIV-3, BRSV, P. multocida | 1 | 1 | 0 | 1.41% | |
| BCoV, H. somni, M. bovis | 1 | 0 | 1 | 1.41% | |
| BCoV, P. multocida, H. somni | 3 | 3 | 0 | 4.23% | |
| BCoV, P. multocida, M. haemolytica | 2 | 1 | 1 | 2.82% | |
| BCoV, M. haemolytica, H. somni | 1 | 0 | 1 | 1.41% | |
| M. haemolytica, P. multocida, M. bovis | 5 | 3 | 2 | 7.04% | |
| M. haemolytica, P. multocida., H. somni | 1 | 1 | 0 | 1.41% | |
| P. multocida, H. somni, M. bovis | 2 | 0 | 2 | 2.82% | |
| 4 | BPIV-3, BRSV, BCoV, P. multocida | 1 | 1 | 0 | 1.41% |
| BPIV-3, BCoV, P. multocida, M. haemolytica | 2 | 1 | 1 | 2.82% | |
| BRSV, BCoV, P. multocida, M. bovis | 1 | 0 | 1 | 1.41% | |
| BCoV, P. multocida, M. haemolytica, M. bovis | 2 | 1 | 1 | 2.82% | |
| P. multocida, M. haemolytica, H. somni, M. bovis | 4 | 1 | 3 | 5.63% | |
| 5 | BPIV-3, BRSV, BCoV, P. multocida, H. somni | 1 | 1 | 0 | 1.41% |
| BPIV-3, BCoV, P. multocida, H. somni, M. bovis | 1 | 1 | 0 | 1.41% | |
| BRSV, BCoV, P. multocida, H. somni, M. bovis | 1 | 1 | 0 | 1.41% | |
| BCoV, P. multocida, H. somni, M. haemolytica, M. bovis | 2 | 1 | 1 | 2.82% | |
| 6 | not detected | - | - | - | - |
| 7 | BPIV-3, BRSV, BCoV, P. multocida, M. haemolytica, H. somni, M. bovis | 1 | 0 | 1 | 1.41% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lachowicz-Wolak, A.; Klimowicz-Bodys, M.D.; Płoneczka-Janeczko, K.; Bykowy, M.; Siedlecka, M.; Cinciała, J.; Rypuła, K. The Prevalence, Coexistence, and Correlations between Seven Pathogens Detected by a PCR Method from South-Western Poland Dairy Cattle Suffering from Bovine Respiratory Disease. Microorganisms 2022, 10, 1487. https://doi.org/10.3390/microorganisms10081487
Lachowicz-Wolak A, Klimowicz-Bodys MD, Płoneczka-Janeczko K, Bykowy M, Siedlecka M, Cinciała J, Rypuła K. The Prevalence, Coexistence, and Correlations between Seven Pathogens Detected by a PCR Method from South-Western Poland Dairy Cattle Suffering from Bovine Respiratory Disease. Microorganisms. 2022; 10(8):1487. https://doi.org/10.3390/microorganisms10081487
Chicago/Turabian StyleLachowicz-Wolak, Agnieszka, Małgorzata D. Klimowicz-Bodys, Katarzyna Płoneczka-Janeczko, Marek Bykowy, Magdalena Siedlecka, Jagoda Cinciała, and Krzysztof Rypuła. 2022. "The Prevalence, Coexistence, and Correlations between Seven Pathogens Detected by a PCR Method from South-Western Poland Dairy Cattle Suffering from Bovine Respiratory Disease" Microorganisms 10, no. 8: 1487. https://doi.org/10.3390/microorganisms10081487






