Circulating Bacterial DNA as Plasma Biomarkers for Lung Cancer Early Detection
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cases and Specimens
2.2. Bacteria and Culture Conditions
2.3. Genomic DNA Isolation
2.4. Droplet Digital PCR (ddPCR) Analysis of Bacterial DNA Abundances
2.5. Statistical Analysis
3. Results
3.1. Bacteria Exhibited Different Abundances in Lung Tumor vs. Corresponing Normal Lung Tissues
3.2. Bacteria Displayed Increased Abundances in Plasma Samples of Patients Diagnosed with NSSCL
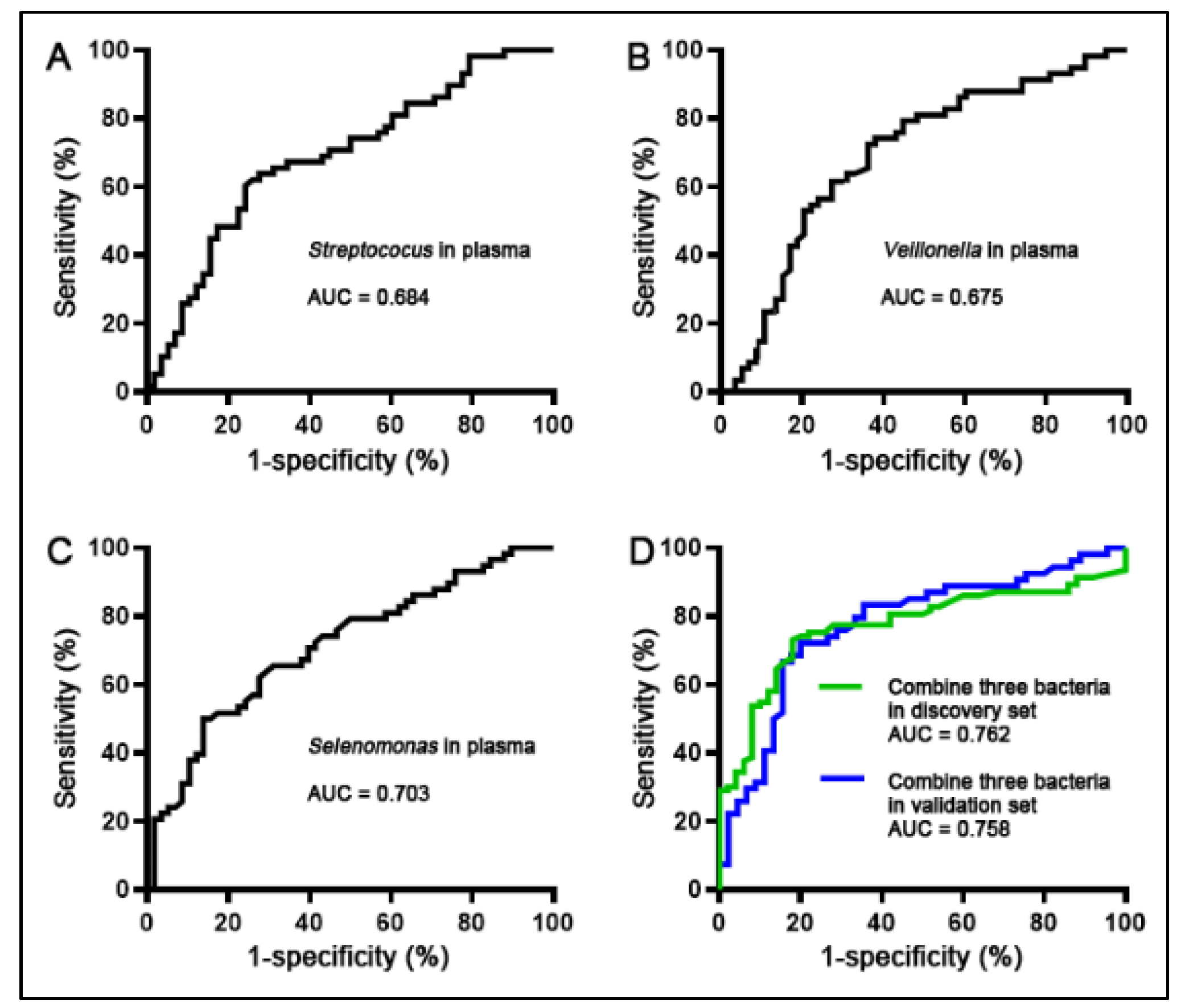
3.3. A Panel of Three Bacterial Genera Was Developed as Plasma-Based Biomarkers for NSCLC
3.4. Confirming the Biomarkers for the Diagnostic Signifcance in Different Cases and Controls
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aberle, D.R.; Adams, A.M.; Berg, C.D. Reduced lung-cancer mortality with low-dose computed tomographic screening. N. Engl. J. Med. 2011, 365, 395–409. [Google Scholar] [PubMed] [Green Version]
- Garrett, W.S. Cancer and the microbiota. Science 2015, 348, 80–86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greathouse, K.L.; White, J.R.; Vargas, A.J.; Bliskovsky, V.V.; Beck, J.A.; von Muhlinen, N.; Polley, E.C.; Bowman, E.D.; Khan, M.A.; Robles, A.I.; et al. Interaction between the microbiome and TP53 in human lung cancer. Genome Biol. 2018, 19, 123. [Google Scholar] [CrossRef] [PubMed]
- Tsay, J.C.J.; Wu, B.G.; Badri, M.H.; Clemente, J.C.; Shen, N.; Meyn, P.; Li, Y.; Yie, T.-A.; Lhakhang, T.; Olsen, E.; et al. Airway Microbiota Is Associated with Upregulation of the PI3K Pathway in Lung Cancer. Am. J. Respir. Crit. Care Med. 2018, 198, 1188–1198. [Google Scholar] [CrossRef]
- Li, N.; Zhou, H.; Holden, V.K.; Deepak, J.; Dhilipkannah, P.; Todd, N.W.; Stass, S.A.; Jiang, F. Streptococcus pneumoniae promotes lung cancer development and progression. iScience 2023, 26, 105923. [Google Scholar] [CrossRef]
- Leng, Q.; Holden, V.; Deepak, J.; Todd, N.; Jiang, F. Microbiota Biomarkers for Lung Cancer. Diagnostics 2021, 11, 407. [Google Scholar] [CrossRef]
- Glyn, T.; Purcell, R. Circulating Bacterial DNA, a New Paradigm for Cancer Diagnostics. Front. Med. Lausanne 2022, 9, 831096. [Google Scholar] [CrossRef]
- Xiao, Q.; Lu, W.; Kong, X.; Shao, Y.W.; Hu, Y.; Wang, A.; Bao, H.; Cao, R.; Liu, K.; Wang, X.; et al. Alterations of circulating bacterial DNA in colorectal cancer and adenoma, A proof-of-concept study. Cancer Lett. 2021, 499, 201–208. [Google Scholar] [CrossRef]
- Messaritakis, I.; Vogiatzoglou, K.; Tsantaki, K.; Ntretaki, A.; Sfakianaki, M.; Koulouridi, A.; Tsiaoussis, J.; Mavroudis, D.; Souglakos, J. The Prognostic Value of the Detection of Microbial Translocation in the Blood of Colorectal Cancer Patients. Cancers 2020, 12, 1058. [Google Scholar] [CrossRef] [Green Version]
- Koulouridi, A.; Messaritakis, I.; Theodorakis, E.; Chondrozoumaki, M.; Sfakianaki, M.; Gouvas, N.; Tsiaoussis, J.; Mavroudis, D.; Tzardi, M.; Souglakos, J. Detection of Circulating Tumor Cells and Microbial DNA Fragments in Stage III Colorectal Cancer Patients under Three versus Six Months of Adjuvant Treatment. Cancers 2021, 13, 3552. [Google Scholar] [CrossRef]
- Poore, G.D.; Kopylova, E.; Zhu, Q.; Carpenter, C.; Fraraccio, S.; Wandro, S.; Kosciolek, T.; Janssen, S.; Metcalf, J.; Song, S.J.; et al. Microbiome analyses of blood and tissues suggest cancer diagnostic approach. Nature 2020, 579, 567–574. [Google Scholar] [CrossRef]
- Gou, X.; Zhang, Q.; More, S.; Bamunuarachchi, G.; Liang, Y.; Khan, F.H.; Maranville, R.; Zuniga, E.; Wang, C.; Liu, L. Repeated Exposure to Streptococcus pneumoniae Exacerbates Chronic Obstructive Pulmonary Disease. Am. J. Pathol. 2019, 189, 1711–1720. [Google Scholar] [CrossRef]
- Hosgood, H.D., 3rd; Sapkota, A.R.; Rothman, N.; Rohan, T.; Hu, W.; Xu, J.; Vermeulen, R.; He, X.; White, J.R.; Wu, G.; et al. The potential role of lung microbiota in lung cancer attributed to household coal burning exposures. Env. Mol. Mutagen 2014, 55, 643–651. [Google Scholar] [CrossRef] [Green Version]
- Goto, T. Airway Microbiota as a Modulator of Lung Cancer. Int. J. Mol. Sci. 2020, 21, 3044. [Google Scholar] [CrossRef] [PubMed]
- Kovaleva, O.V.; Romashin, D.; Zborovskaya, I.B.; Davydov, M.M.; Shogenov, M.S.; Gratchev, A. Human Lung Microbiome on the Way to Cancer. J. Immunol. Res. 2019, 2019, 1394191. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Sung, J.Y.; Yong, D.; Chun, J.; Kim, S.Y.; Song, J.H.; Chung, K.S.; Kim, E.Y.; Jung, J.Y.; Kang, Y.A.; et al. Characterization of microbiome in bronchoalveolar lavage fluid of patients with lung cancer comparing with benign mass like lesions. Lung Cancer 2016, 102, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Bingula, R.; Filaire, M.; Radosevic-Robin, N.; Berthon, J.Y.; Bernalier-Donadille, A.; Vasson, M.P.; Thivat, E.; Kwiatkowski, F.; Filaire, E. Characterisation of gut, lung, and upper airways microbiota in patients with non-small cell lung carcinoma, Study protocol for case-control observational trial. Medicine Baltim. 2018, 97, e13676. [Google Scholar] [CrossRef]
- Kim, D.; Hofstaedter, C.E.; Zhao, C.; Mattei, L.; Tanes, C.; Clarke, E.; Lauder, A.; Sherrill-Mix, S.; Chehoud, C.; Kelsen, J.; et al. Optimizing methods and dodging pitfalls in microbiome research. Microbiome 2017, 5, 52. [Google Scholar] [CrossRef] [Green Version]
- Salter, S.J.; Cox, M.J.; Turek, E.M.; Calus, S.T.; Cookson, W.O.; Moffatt, M.F.; Turner, P.; Parkhill, J.; Loman, N.J.; Walker, A.W. Reagent and laboratory contamination can critically impact sequence-based microbiome analyses. BMC Biol. 2014, 12, 87. [Google Scholar] [CrossRef] [Green Version]
- Gomes, S.; Cavadas, B.; Ferreira, J.C.; Marques, P.I.; Monteiro, C.; Sucena, M.; Sousa, C.; Rodrigues, L.V.; Teixeira, G.; Pinto, P.; et al. Profiling of lung microbiota discloses differences in adenocarcinoma and squamous cell carcinoma. Sci. Rep. 2019, 9, 12838. [Google Scholar] [CrossRef] [Green Version]
- Yan, X.; Yang, M.; Liu, J.; Gao, R.; Hu, J.; Li, J.; Zhang, L.; Shi, Y.; Guo, H.; Cheng, J.; et al. Discovery and validation of potential bacterial biomarkers for lung cancer. Am. J. Cancer Res. 2015, 5, 3111–3122. [Google Scholar]
- Rodriguez, R.M.; Hernandez, B.Y.; Menor, M.; Deng, Y.; Khadka, V.S. The landscape of bacterial presence in tumor and adjacent normal tissue across 9 major cancer types using TCGA exome sequencing. Comput. Struct. Biotechnol. J. 2020, 18, 631–641. [Google Scholar] [CrossRef]
- Pasquereau-Kotula, E.; Martins, M.; Aymeric, L.; Dramsi, S. Significance of Streptococcus gallolyticus subsp. gallolyticus Association With Colorectal Cancer. Front. Microbiol. 2018, 9, 614. [Google Scholar] [CrossRef] [PubMed]
- Bello, S.; Vengoechea, J.J.; Ponce-Alonso, M.; Figueredo, A.L.; Mincholé, E.; Rezusta, A.; Gambó, P.; Pastor, J.M.; Galeano, J.; del Camp, R. Core Microbiota in Central Lung Cancer With Streptococcal Enrichment as a Possible Diagnostic Marker. Arch. Bronconeumol. Engl. Ed. 2020, 57, 681–689. [Google Scholar] [CrossRef] [PubMed]
- Zozaya-Valdés, E.; Wong, S.Q.; Raleigh, J.; Hatzimihalis, A.; Ftouni, S.; Papenfuss, A.T.; Sandhu, S.; Dawson, M.A.; Dawson, S.-J. Detection of cell-free microbial DNA using a contaminant-controlled analysis framework. Genome Biol. 2021, 22, 187. [Google Scholar] [CrossRef] [PubMed]
- Goto, T. Microbiota and lung cancer. Semin. Cancer Biol. 2022, 86, 1–10. [Google Scholar] [CrossRef]
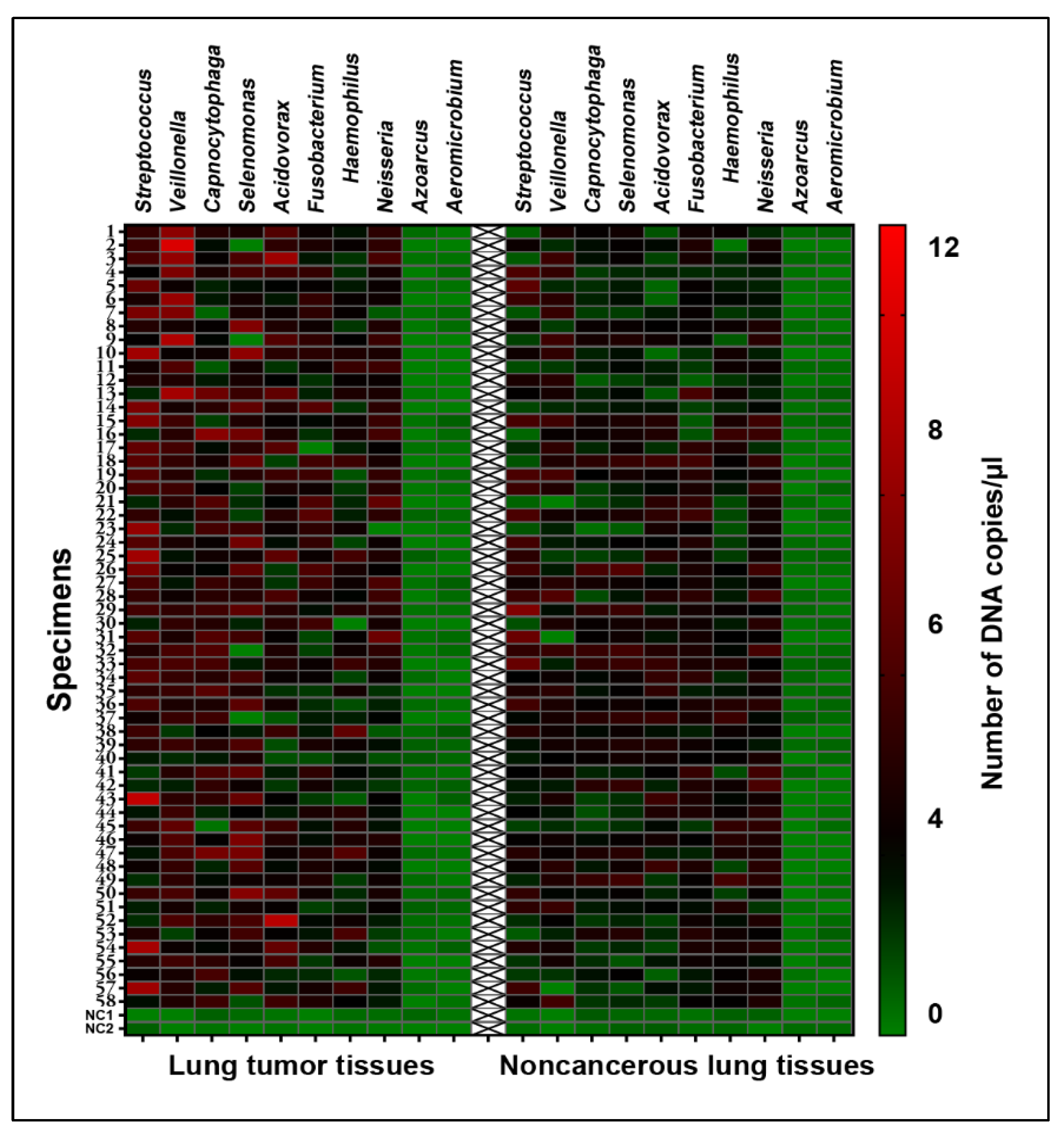
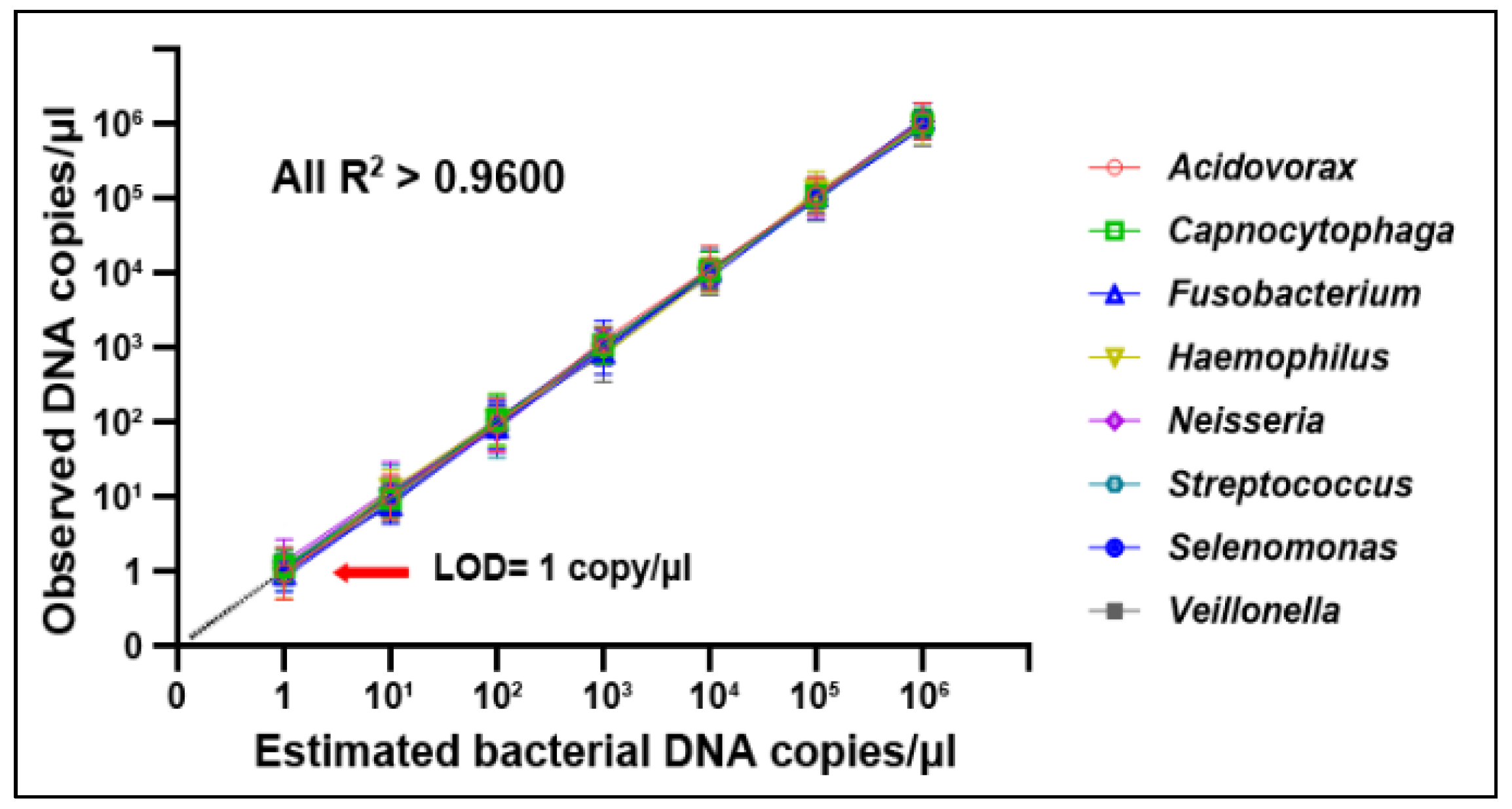
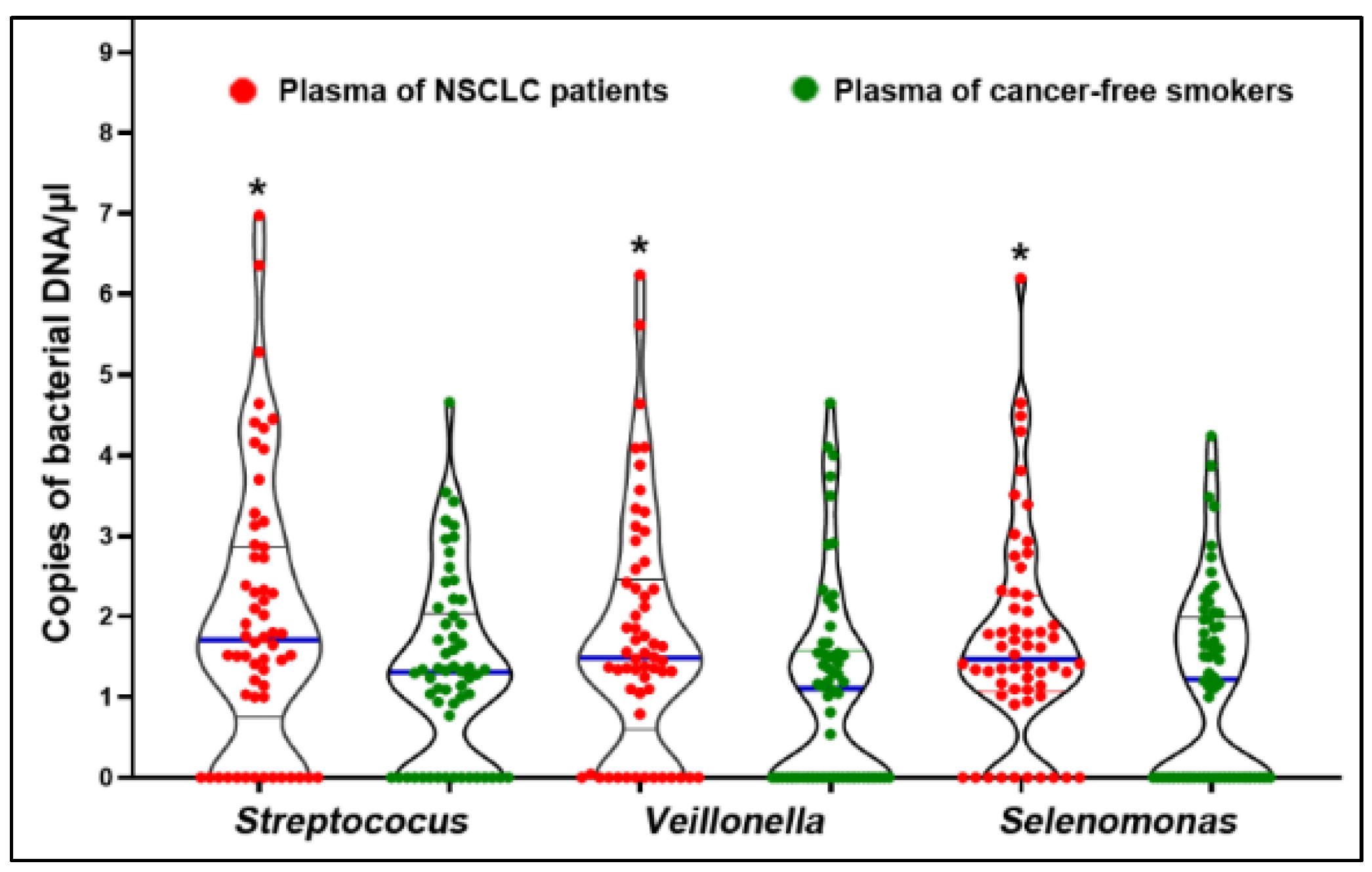
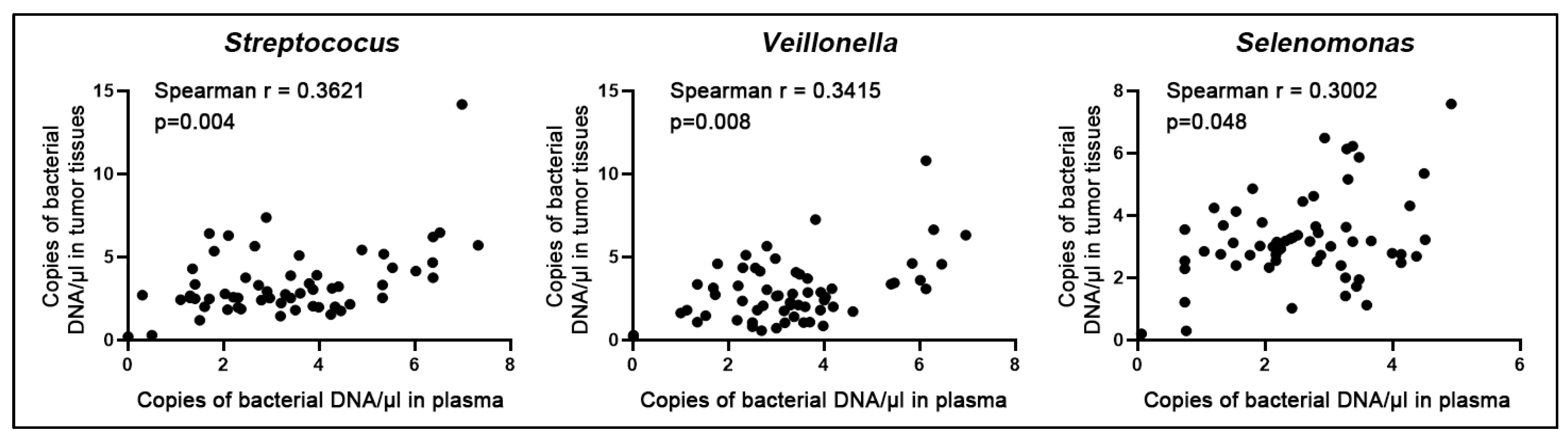
| Bacterial Genera | Mean (Standard Deviation) in Lung Tumor Tissues | Mean (Standard Deviation) in Noncancerous Lung Tissues | p Values |
|---|---|---|---|
| Acidovorax | 2.325 (1.376) | 1.865 (0.899) | 0.003780 |
| Capnocytophaga | 3.467 (1.187) | 1.759 (0.832) | 0.000382 |
| Fusobacterium | 3.256 (1.066) | 2.355 (0.776) | 0.959197 |
| Haemophilus | 2.075 (0.988) | 1.982 (0.818) | 0.309746 |
| Neisseria | 2.347 (1.001) | 2.41 (0.8120) | 0.969772 |
| Streptococcus | 3.245 (1.695) | 2.151 (1.353) | 0.000483 |
| Selenomonas | 2.885 (1.606) | 2.115 (0.857) | 0.000256 |
| Veillonella | 3.179 (1.544) | 2.453 (1.053) | 0.000042 |
| Sensitivity (95% CI) | Specificity (95% CI) | |
|---|---|---|
| Selenomonas | 70.69% (57.27% to 81.91%) | 60.34% (46.64% to 72.95%) |
| Streptococus | 67.24% (53.66% to 78.99%) | 65.52% (51.88% to 77.51%) |
| Veillonella | 62.07% (48.37% to 74.49%) | 70.69% (57.27% to 81.91%) |
| Combined analysis of the three bacterial genera in discovery set | 75.27% (65.24% to 83.63%) * | 78.00% (64.04% to 88.47%) * |
| Combined analysis of the three bacterial genera in validation set | 74.07% (60.35% to 85.04%) * | 76.33% (60.46% to 87.12%) * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, H.; Liao, J.; Leng, Q.; Chinthalapally, M.; Dhilipkannah, P.; Jiang, F. Circulating Bacterial DNA as Plasma Biomarkers for Lung Cancer Early Detection. Microorganisms 2023, 11, 582. https://doi.org/10.3390/microorganisms11030582
Zhou H, Liao J, Leng Q, Chinthalapally M, Dhilipkannah P, Jiang F. Circulating Bacterial DNA as Plasma Biomarkers for Lung Cancer Early Detection. Microorganisms. 2023; 11(3):582. https://doi.org/10.3390/microorganisms11030582
Chicago/Turabian StyleZhou, Huifen, Jipei Liao, Qixin Leng, Molangur Chinthalapally, Pushpa Dhilipkannah, and Feng Jiang. 2023. "Circulating Bacterial DNA as Plasma Biomarkers for Lung Cancer Early Detection" Microorganisms 11, no. 3: 582. https://doi.org/10.3390/microorganisms11030582





