Association of Virulence, Biofilm, and Antimicrobial Resistance Genes with Specific Clonal Complex Types of Listeria monocytogenes
Abstract
1. Introduction
2. Materials and Methods
2.1. Strains
2.2. Whole-Genome Sequences
2.3. Pan-Genome Analysis
2.4. Genotypic Characterisation
2.5. Statistical Analysis
3. Results
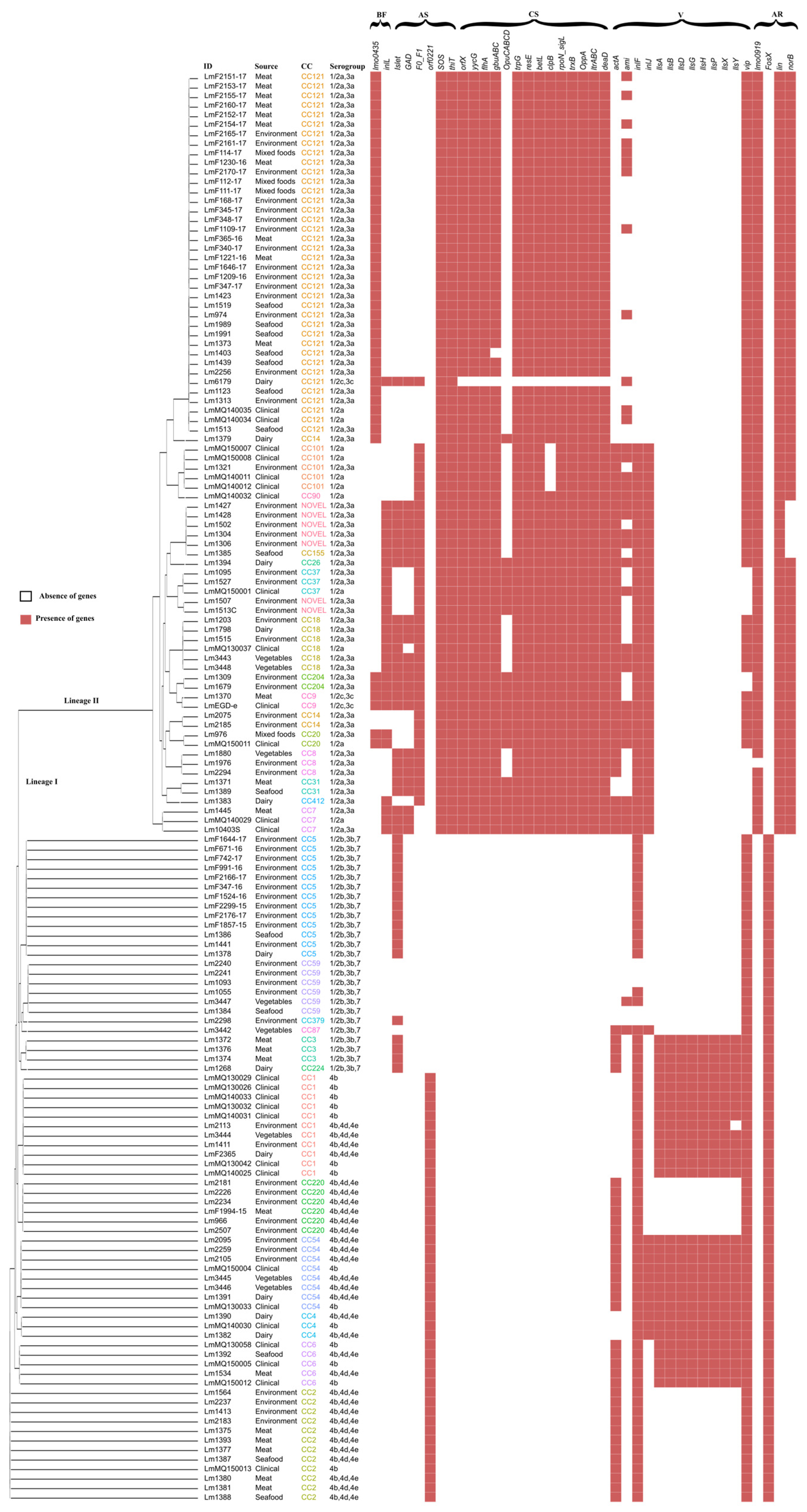
3.1. Clonal Distribution and Phylogenetic Clustering
3.2. Pan-Genome Analysis
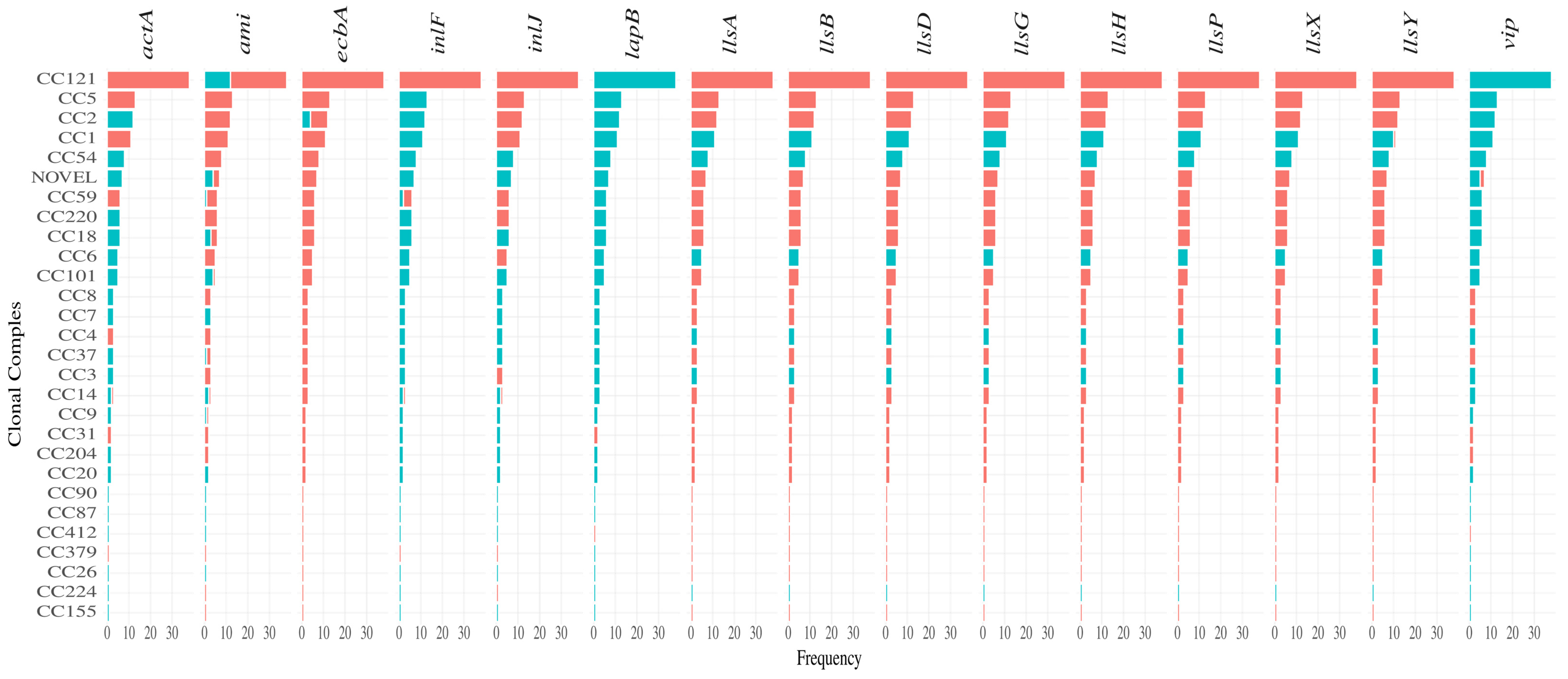
3.3. Presence of Virulence Genes and Their Genotype Association
3.4. Antimicrobial Resistance, Antibiotic Resistance, and Biofilm Formation Genes
3.5. Stress Tolerance Genes
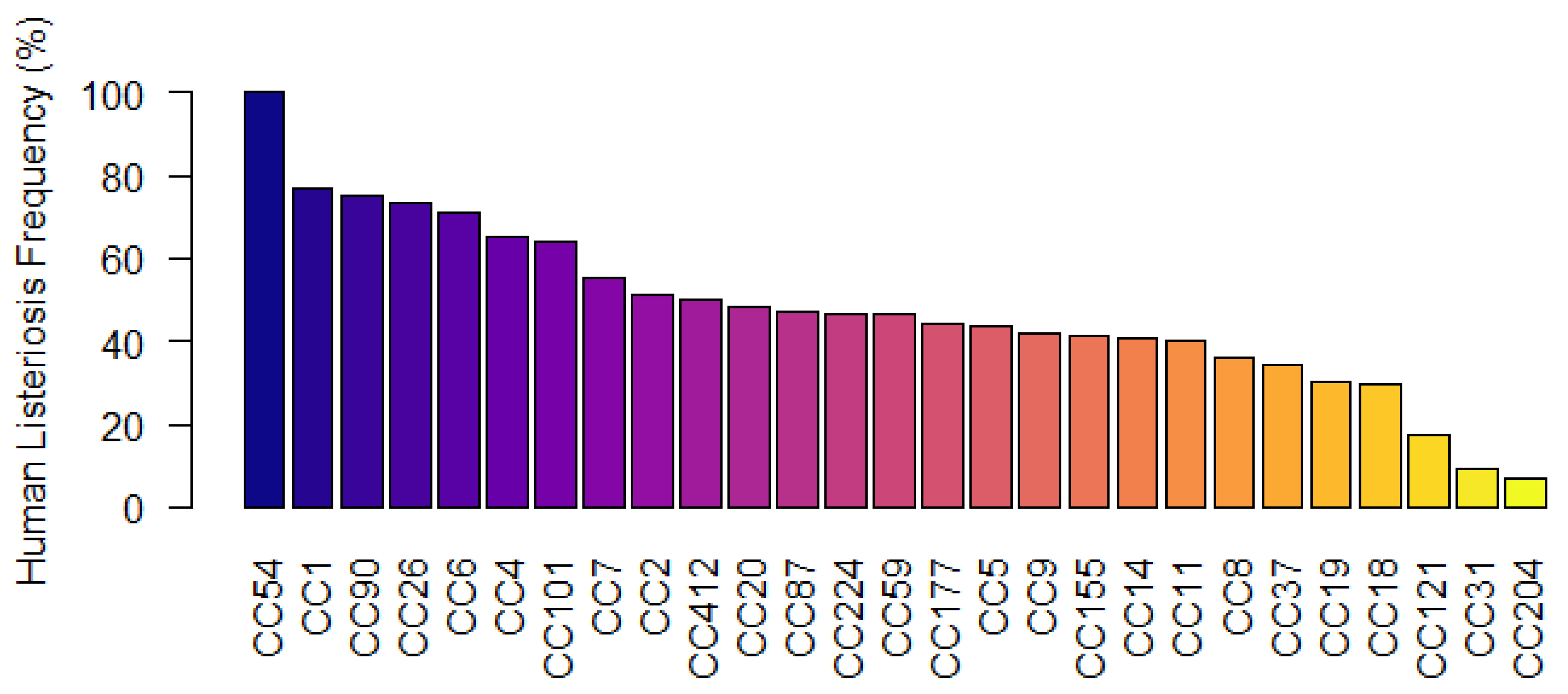
3.6. CC-Types Associated with Human Listeriosis
4. Discussion
4.1. Heterogeneity in Virulence Genes
4.2. Prevalence of Antimicrobial Resistance Genes
4.3. Stress Response and Biofilm Formation Genes
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- European Food Safety Authority (EFSA). EU One Health Zoonoses Report 2020. EFSA J. 2021, 19, e06971. [Google Scholar] [CrossRef]
- Arguedas-Villa, C.; Stephan, R.; Tasara, T. Evaluation of Cold Growth and Related Gene Transcription Responses Associated with Listeria monocytogenes Strains of Different Origins. Food Microbiol. 2010, 27, 653–660. [Google Scholar] [CrossRef]
- Myintzaw, P.; Pennone, V.; McAuliffe, O.; Begley, M.; Callanan, M. Variability in Cold Tolerance of Food and Clinical Listeria monocytogenes Isolates. Microorganisms 2023, 11, 65. [Google Scholar] [CrossRef]
- Cotter, P.D.; Ryan, S.; Gahan, C.G.M.; Hill, C. Presence of GadD1 Glutamate Decarboxylase in Selected Listeria monocytogenes Strains Is Associated with an Ability to Grow at Low PH. Appl. Environ. Microbiol. 2005, 71, 2832–2839. [Google Scholar] [CrossRef] [PubMed]
- Myintzaw, P.; Pennone, V.; McAuliffe, O.; Begley, M.; Callanan, M. Correlation of Organic Acid Tolerance and Genotypic Characteristics of Listeria monocytogenes Food and Clinical Isolates. Food Microbiol. 2022, 104, 104004. [Google Scholar] [CrossRef]
- Centorotola, G.; Guidi, F.; D’aurizio, G.; Salini, R.; Di Domenico, M.; Ottaviani, D.; Petruzzelli, A.; Fisichella, S.; Duranti, A.; Tonucci, F.; et al. Intensive Environmental Surveillance Plan for Listeria monocytogenes in Food Producing Plants and Retail Stores of Central Italy: Prevalence and Genetic Diversity. Foods 2021, 10, 1944. [Google Scholar] [CrossRef] [PubMed]
- Lourenço, A.; de Las Heras, A.; Scortti, M.; Vazquez-Boland, J.; Frank, J.F.; Brito, L. Comparison of Listeria monocytogenes Exoproteomes from Biofilm and Planktonic State: Lmo2504, a Protein Associated with Biofilms. Appl. Environ. Microbiol. 2013, 79, 6075–6082. [Google Scholar] [CrossRef]
- Moran-Gilad, J.; Sintchenko, V.; Pedersen, S.K.; Wolfgang, W.J.; Pettengill, J.; Strain, E.; Hendriksen, R.S. Proficiency Testing for Bacterial Whole Genome Sequencing: An End-User Survey of Current Capabilities, Requirements and Priorities. BMC Infect. Dis. 2015, 15, 174. [Google Scholar] [CrossRef] [PubMed]
- Sintchenko, V.; Holmes, E.C. The Role of Pathogen Genomics in Assessing Disease Transmission. BMJ 2015, 350, h1314. [Google Scholar] [CrossRef]
- Schürch, A.C.; van Schaik, W. Challenges and Opportunities for Whole-Genome Sequencing–Based Surveillance of Antibiotic Resistance. Ann. N. Y. Acad. Sci. 2017, 1388, 108–120. [Google Scholar] [CrossRef] [PubMed]
- Belén, A.; Pavón, I.; Maiden, M.C.J. Multilocus Sequence Typing. Methods Mol. Biol. 2009, 551, 129–140. [Google Scholar] [CrossRef]
- Caruso, M.; Fraccalvieri, R.; Pasquali, F.; Santagada, G.; Latorre, L.M.; Difato, L.M.; Miccolupo, A.; Normanno, G.; Parisi, A. Antimicrobial Susceptibility and Multilocus Sequence Typing of Listeria monocytogenes Isolated over 11 Years from Food, Humans, and the Environment in Italy. Foodborne Pathog. Dis. 2020, 17, 284–294. [Google Scholar] [CrossRef]
- Hilliard, A.; Leong, D.; O’Callaghan, A.; Culligan, E.P.; Morgan, C.A.; Delappe, N.; Hill, C.; Jordan, K.; Cormican, M.; Gahan, C.G.M. Genomic Characterization of Listeria monocytogenes Isolates Associated with Clinical Listeriosis and the Food Production Environment in Ireland. Genes 2018, 9, 171. [Google Scholar] [CrossRef]
- Félix, B.; Capitaine, K.; Te, S.; Felten, A.; Gillot, G.; Feurer, C.; Bosch, T. Van Den. Identification by High-Throughput Real-Time PCR of 30 Major Circulating Listeria monocytogenes Clonal Complexes in Europe. Am. Soc. Microbiol. 2023, 11, e03954-22. [Google Scholar] [CrossRef]
- Seemann, T. Prokka: Rapid Prokaryotic Genome Annotation. Bioinformatics 2014, 30, 2068–2069. [Google Scholar] [CrossRef]
- Tatusova, T.; Dicuccio, M.; Badretdin, A.; Chetvernin, V.; Nawrocki, E.P.; Zaslavsky, L.; Lomsadze, A.; Pruitt, K.D.; Borodovsky, M.; Ostell, J. NCBI Prokaryotic Genome Annotation Pipeline. Nucleic Acids Res. 2016, 44, 6614–6624. [Google Scholar] [CrossRef] [PubMed]
- Kuenne, C.; Billion, A.; Mraheil, M.A.; Strittmatter, A.; Daniel, R.; Goesmann, A.; Barbuddhe, S.; Hain, T.; Chakraborty, T. Reassessment of the Listeria monocytogenes Pan-Genome Reveals Dynamic Integration Hotspots and Mobile Genetic Elements as Major Components of the Accessory Genome. BMC Genom. 2013, 14, 47. [Google Scholar] [CrossRef] [PubMed]
- Maury, M.M.; Tsai, Y.-H.; Charlier, C.; Touchon, M.; Chenal-Francisque, V.; Leclercq, A.; Criscuolo, A.; Gaultier, C.; Roussel, S.; Brisabois, A.; et al. Uncovering Listeria monocytogenes Hypervirulence by Harnessing Its Biodiversity. Nat. Genet. 2016, 48, 308–313. [Google Scholar] [CrossRef]
- Sibanda, T.; Buys, E.M. Listeria monocytogenes Pathogenesis: The Role of Stress Adaptation. Microorganisms 2022, 10, 1522. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Nicaogáin, K.; McAuliffe, O.; Jordan, K.; O’Byrne, C. Phylogenetic and Phenotypic Analyses of a Collection of Food and Clinical Listeria monocytogenes Isolates Reveal Loss of Function of Sigma B from Several Clonal Complexes. Appl. Environ. Microbiol. 2022, 88, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Lauer, P.; Chow, M.Y.N.; Loessner, M.J.; Portnoy, D.A.; Calendar, R. Construction, Characterization, and Use of Two Listeria monocytogenes Site-Specific Phage Integration Vectors. J. Bacteriol. 2002, 184, 4177. [Google Scholar] [CrossRef]
- Schmitz-Esser, S.; Müller, A.; Stessl, B.; Wagner, M. Genomes of Sequence Type 121 Listeria monocytogenes Strains Harbor Highly Conserved Plasmids and Prophages. Front. Microbiol. 2015, 6, 380. [Google Scholar] [CrossRef]
- Nelson, K.E.; Fouts, D.E.; Mongodin, E.F.; Ravel, J.; DeBoy, R.T.; Kolonay, J.F.; Rasko, D.A.; Angiuoli, S.V.; Gill, S.R.; Paulsen, I.T.; et al. Whole Genome Comparisons of Serotype 4b and 1/2a Strains of the Food-Borne Pathogen Listeria monocytogenes Reveal New Insights into the Core Genome Components of This Species. Nucleic Acids Res. 2004, 32, 2386–2395. [Google Scholar] [CrossRef]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A Flexible Trimmer for Illumina Sequence Data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Afgan, E.; Baker, D.; Bér´, B.; Batut, B.; Van Den Beek, M.; Bouvier, D.; Cech, M.; Chilton, J.; Clements, D.; Coraor, N.; et al. The Galaxy Platform for Accessible, Reproducible and Collaborative Biomedical Analyses: 2018 Update. Nucleic Acids Res. 2018, 46, 537–544. [Google Scholar] [CrossRef] [PubMed]
- Bankevich, A.; Nurk, S.; Antipov, D.; Gurevich, A.A.; Dvorkin, M.; Kulikov, A.S.; Lesin, V.M.; Nikolenko, S.I.; Pham, S.; Prjibelski, A.D.; et al. SPAdes: A New Genome Assembly Algorithm and Its Applications to Single-Cell Sequencing. J. Comput. Biol. 2012, 19, 455. [Google Scholar] [CrossRef] [PubMed]
- Page, A.J.; Cummins, C.A.; Hunt, M.; Wong, V.K.; Reuter, S.; Holden, M.T.G.; Fookes, M.; Falush, D.; Keane, J.A.; Parkhill, J. Roary: Rapid Large-Scale Prokaryote Pan Genome Analysis. Bioinformatics 2015, 31, 3691–3693. [Google Scholar] [CrossRef] [PubMed]
- Brynildsrud, O.; Bohlin, J.; Scheffer, L.; Eldholm, V. Rapid Scoring of Genes in Microbial Pan-Genome-Wide Association Studies with Scoary. Genome Biol. 2016, 17, 238. [Google Scholar] [CrossRef] [PubMed]
- Quainoo, S.; Coolen, J.P.M.; van Hijum, S.A.F.T.; Huynen, M.A.; Melchers, W.J.G.; van Schaik, W.; Wertheim, H.F.L. Whole-Genome Sequencing of Bacterial Pathogens: The Future of Nosocomial Outbreak Analysis. Clin. Microbiol. Rev. 2017, 30, 1015–1063. [Google Scholar] [CrossRef] [PubMed]
- Mafuna, T.; Matle, I.; Magwedere, K.; Pierneef, R.E.; Reva, O.N. Whole Genome-Based Characterization of Listeria monocytogenes Isolates Recovered from the Food Chain in South Africa. Front. Microbiol. 2021, 12, 669287. [Google Scholar] [CrossRef]
- Doumith, M.; Buchrieser, C.; Glaser, P.; Jacquet, C.; Martin, P. Differentiation of the Major Listeria monocytogenes Serovars by Multiplex PCR. J. Clin. Microbiol. 2004, 42, 3819–3822. [Google Scholar] [CrossRef] [PubMed]
- Hingston, P.; Chen, J.; Dhillon, B.K.; Laing, C.; Bertelli, C.; Gannon, V.; Tasara, T.; Allen, K.; Brinkman, F.S.L.; Truelstrup Hansen, L.; et al. Genotypes Associated with Listeria monocytogenes Isolates Displaying Impaired or Enhanced Tolerances to Cold, Salt, Acid, or Desiccation Stress. Front. Microbiol. 2017, 8, 369. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Zheng, D.; Jin, Q.; Chen, L.; Yang, J. VFDB 2019: A Comparative Pathogenomic Platform with an Interactive Web Interface. Nucleic Acids Res. 2019, 47, D687–D692. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zheng, D.; Liu, B.; Yang, J.; Jin, Q. VFDB 2016: Hierarchical and Refined Dataset for Big Data Analysis—10 Years On. Nucleic Acids Res. 2016, 44, D694–D697. [Google Scholar] [CrossRef]
- Reis, O.; Sousa, S.; Camejo, A.; Villiers, V.; Gouin, E.; Cossart, P.; Cabanes, D. LapB, a Novel Listeria monocytogenes LPXTG Surface Adhesin, Required for Entry into Eukaryotic Cells and Virulence. J. Infect. Dis. 2010, 202, 551–562. [Google Scholar] [CrossRef]
- Wagner, E.; Fagerlund, A.; Thalguter, S.; Jensen, M.R.; Heir, E.; Møretrø, T.; Moen, B.; Langsrud, S.; Rychli, K. Deciphering the Virulence Potential of Listeria monocytogenes in the Norwegian Meat and Salmon Processing Industry by Combining Whole Genome Sequencing and in Vitro Data. Int. J. Food Microbiol. 2022, 383, 109962. [Google Scholar] [CrossRef]
- Caliandro, R.; de Diego, I.; Gomis-Rüth, F.X. Crystal Structure Report of the ImmR Transcriptional Regulator DNA-Binding Domain of the Bacillus subtilis ICEBs1 Transposon. Sci. Rep. 2022, 12, 5258. [Google Scholar] [CrossRef]
- Løvdal, T.; Brandal, L.T.; Sundaram, A.Y.M.; Naseer, U.; Roth, B.; Lunestad, B.T. Small-Scale Comparative Genomic Analysis of Listeria monocytogenes Isolated from Environments of Salmon Processing Plants and Human Cases in Norway. Hygiene 2021, 1, 43–55. [Google Scholar] [CrossRef]
- Mota, M.I.; Vázquez, S.; Cornejo, C.; D’Alessandro, B.; Braga, V.; Caetano, A.; Betancor, L.; Varela, G. Does Shiga Toxin-Producing Escherichia coli and Listeria monocytogenes Contribute Significantly to the Burden of Antimicrobial Resistance in Uruguay? Front. Vet. Sci. 2020, 7, 583930. [Google Scholar] [CrossRef]
- Lüth, S.; Halbedel, S.; Rosner, B.; Wilking, H.; Holzer, A.; Roedel, A.; Dieckmann, R.; Vincze, S.; Prager, R.; Flieger, A.; et al. Backtracking and Forward Checking of Human Listeriosis Clusters Identified a Multiclonal Outbreak Linked to Listeria monocytogenes in Meat Products of a Single Producer. Emerg. Microbes Infect. 2020, 9, 1600–1608. [Google Scholar] [CrossRef]
- Thedieck, K.; Hain, T.; Mohamed, W.; Tindall, B.J.; Nimtz, M.; Chakraborty, T.; Wehland, J.; Jänsch, L. The MprF Protein Is Required for Lysinylation of Phospholipids in Listerial Membranes and Confers Resistance to Cationic Antimicrobial Peptides (CAMPs) on Listeria monocytogenes. Mol. Microbiol. 2006, 62, 1325–1339. [Google Scholar] [CrossRef]
- Duval, M.; Dar, D.; Carvalho, F.; Rocha, E.P.C.; Sorek, R.; Cossart, P. HflXr, a Homolog of a Ribosome-Splitting Factor, Mediates Antibiotic Resistance. Proc. Natl. Acad. Sci. USA 2018, 115, 13359–13364. [Google Scholar] [CrossRef]
- Dreux, N.; Albagnac, C.; Sleator, R.D.; Hill, C.; Carlin, F.; Morris, C.E.; Nguyen-The, C. Glycine Betaine Improves Listeria monocytogenes Tolerance to Desiccation on Parsley Leaves Independent of the Osmolyte Transporters BetL, Gbu and OpuC. J. Appl. Microbiol. 2008, 104, 1221–1227. [Google Scholar] [CrossRef]
- Roberts, A.J.; Wiedmann, M. Pathogen, Host and Environmental Factors Contributing to the Pathogenesis of Listeriosis. Cell. Mol. Life Sci. 2003, 60, 904–918. [Google Scholar] [CrossRef] [PubMed]
- Bergis, H.; Bonanno, L.; Asséré, A.; Lombard, B. EURL Lm Technical Guidance Document on Challenge Tests and Durability Studies for Assessing Shelf-Life of Ready-to-Eat Foods Related to Listeria monocytogenes; EURL Lm: Maisons-Alfort, France, 2021; Volume 4, p. 60. [Google Scholar]
- Li, H.; Wang, P.; Lan, R.; Luo, L.; Cao, X.; Wang, Y.; Wang, Y.; Li, H.; Zhang, L.; Ji, S.; et al. Risk Factors and Level of Listeria Monocytogenes Contamination of Raw Pork in Retail Markets in China. Front. Microbiol. 2018, 9, 1090. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Molina, A.; Cobo-Díaz, J.F.; López, M.; Prieto, M.; de Toro, M.; Alvarez-Ordóñez, A. Unraveling the Emergence and Population Diversity of Listeria monocytogenes in a Newly Built Meat Facility through Whole Genome Sequencing. Int. J. Food Microbiol. 2021, 340, 109043. [Google Scholar] [CrossRef] [PubMed]
- Fagerlund, A.; Langsrud, S.; Moen, B.; Heir, E. Complete Genome Sequences of Six Listeria monocytogenes Sequence Type 9 Isolates from Meat Processing Plants in Norway. Am. Soc. Microbiol. 2018, 6, 9–10. [Google Scholar] [CrossRef] [PubMed]
- Muchaamba, F.; Stephan, R.; Tasara, T. Β-Phenylethylamine as a Natural Food Additive Shows Antimicrobial Activity Against Listeria monocytogenes on Ready-to-Eat Foods. Foods 2020, 9, 1363. [Google Scholar] [CrossRef]
- Davis, M.L.; Ricke, S.C.; Donaldson, J.R. Establishment of Listeria monocytogenes in the Gastrointestinal Tract. Microorganisms 2019, 7, 75. [Google Scholar] [CrossRef]
- Guidi, F.; Chiaverini, A.; Repetto, A.; Lorenzetti, C.; Centorotola, G.; Bazzucchi, V.; Palombo, B.; Gattuso, A.; Pomilio, F.; Blasi, G. Hyper-Virulent Listeria monocytogenes Strains Associated with Respiratory Infections in Central Italy. Front. Cell. Infect. Microbiol. 2021, 11, 765540. [Google Scholar] [CrossRef]
- Pillich, H.; Puri, M.; Chakraborty, T. The Actin Cytoskeleton and Bacterial Infection. In Current Topics in Microbiology and Immunology; Springer: Berlin/Heidelberg, Germany, 2016; Volume 399, pp. 113–132. [Google Scholar]
- Smith, A.; Moorhouse, E.; Monaghan, J.; Taylor, C.; Singleton, I. Sources and Survival of Listeria monocytogenes on Fresh, Leafy Produce. J. Appl. Microbiol. 2018, 125, 930–942. [Google Scholar] [CrossRef]
- Bergholz, T.M.; Shah, M.K.; Burall, L.S.; Rakic-Martinez, M.; Datta, A.R. Genomic and Phenotypic Diversity of Listeria monocytogenes Clonal Complexes Associated with Human Listeriosis. Appl. Microbiol. Biotechnol. 2018, 102, 3475–3485. [Google Scholar] [CrossRef] [PubMed]
- Lake, F.B.; van Overbeek, L.S.; Baars, J.J.P.; Koomen, J.; Abee, T.; den Besten, H.M.W. Genomic Characteristics of Listeria monocytogenes Isolated during Mushroom (Agaricus bisporus) Production and Processing. Int. J. Food Microbiol. 2021, 360, 109438. [Google Scholar] [CrossRef] [PubMed]
- Ireton, K.; Mortuza, R.; Gyanwali, G.C.; Gianfelice, A.; Hussain, M. Role of Internalin Proteins in the Pathogenesis of Listeria monocytogenes. Mol. Microbiol. 2021, 116, 1407–1419. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, P.; Halvorsen, E.M.; Ammendolia, D.A.; Mor-vaknin, N.; Riordan, M.X.D.O.; Brumell, J.H.; Markovitz, D.M.; Higgins, D.E. Invasion of the Brain by Listeria monocytogenes Is Mediated by InlF and Host Cell Vimentin. MBio 2018, 9, e00160-18. [Google Scholar] [CrossRef]
- Vilchis-Rangel, R.E.; Espinoza-Mellado, M.D.R.; Salinas-Jaramillo, I.J.; Martinez-Peña, M.D.; Rodas-Suárez, O.R. Association of Listeria monocytogenes LIPI-1 and LIPI-3 Marker LlsX with Invasiveness. Curr. Microbiol. 2019, 76, 637–643. [Google Scholar] [CrossRef]
- Muchaamba, F.; Eshwar, A.K.; von Ah, U.; Stevens, M.J.A.; Tasara, T. Evolution of Listeria monocytogenes During a Persistent Human Prosthetic Hip Joint Infection. Front. Microbiol. 2020, 11, 1726. [Google Scholar] [CrossRef]
- Wilson, A.; Gray, J.; Scott Chandry, P.; Fox, E.M. Phenotypic and Genotypic Analysis of Antimicrobial Resistance among Listeria monocytogenes Isolated from Australian Food Production Chains. Genes 2018, 9, 80. [Google Scholar] [CrossRef]
- Baquero, F.; Lanza, V.F.; Duval, M.; Coque, T.M. Ecogenetics of Antibiotic Resistance in Listeria monocytogenes. Mol. Microbiol. 2020, 113, 570–579. [Google Scholar] [CrossRef]
- Hughes, D.; Andersson, D.I. Environmental and Genetic Modulation of the Phenotypic Expression of Antibiotic Resistance. FEMS Microbiol. Rev. 2017, 41, 374–391. [Google Scholar] [CrossRef]
- Yu, W.; Huang, Y.; Ying, C.; Zhou, Y.; Zhang, L.; Zhang, J.; Chen, Y.; Qiu, Y. Analysis of Genetic Diversity and Antibiotic Options for Clinical Listeria monocytogenes Infections in China. Open Forum Infect. Dis. 2021, 8, ofab177. [Google Scholar] [CrossRef]
- Popowska, M.; Krawczyk-balska, A.; Ostrowski, R. InlL from Listeria monocytogenes Is Involved in Biofilm Formation and Adhesion to Mucin. Front. Microbiol. 2017, 8, 660. [Google Scholar] [CrossRef]
- Fagerlund, A.; Wagner, E.; Møretrø, T.; Heir, E.; Moen, B.; Rychli, K.; Langsrud, S. Pervasive Listeria monocytogenes Is Common in the Norwegian. Appl. Environ. Microbiol. 2022, 88, e00861-22. [Google Scholar] [PubMed]
- European Centre for Disease Prevention and Control (ECDC). Surveillance Atlas of Infectious Diseases. Available online: https://atlas.ecdc.europa.eu/public/index.aspx (accessed on 15 August 2022).
- Centres for Disease Control and Prevention (CDC). Pathogen Surveillance—Listeria. Available online: https://www.cdc.gov/listeria/pdf/listeriainitiativeoverview_508.pdf (accessed on 27 April 2023).
- Cabanes, D.; Dussurget, O.; Dehoux, P.; Cossart, P. Auto, a Surface Associated Autolysin of Listeria monocytogenes Required for Entry into Eukaryotic Cells and Virulence. Mol. Microbiol. 2004, 51, 1601–1614. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Xu, J.; Chen, Y.; Zhu, Z.; He, F. An Insight into the Genome of a Listeria monocytogenes Strain Isolated from a Bloodstream Infection and Phylogenetic Analysis. J. Clin. Lab. Anal. 2021, 35, e23824. [Google Scholar] [CrossRef]
- Hein, I.; Klinger, S.; Dooms, M.; Flekna, G.; Stessl, B.; Leclercq, A.; Hill, C.; Allerberger, F.; Wagner, M. Stress Survival Islet 1 (SSI-1) Survey in Listeria monocytogenes Reveals an Insert Common to Listeria innocua in Sequence Type 121 L. Monocytogenes Strains. Appl. Environ. Microbiol. 2011, 77, 2169–2173. [Google Scholar] [CrossRef]
- Cotter, P.D.; Gahan, C.G.M.; Hill, C. Analysis of the Role of the Listeria monocytogenes F0F1-ATPase Operon in the Acid Tolerance Response. Int. J. Food Microbiol. 2000, 60, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Cotter, P.D.; Emerson, N.; Gahan, C.G.M.; Hill, C. Identification and Disruption of LisRK, a Genetic Locus Encoding a Two-Component Signal Transduction System Involved in Stress Tolerance and Virulence in Listeria monocytogenes. J. Bacteriol. 1999, 181, 6840. [Google Scholar] [CrossRef]
- Pöntinen, A.; Lindström, M.; Skurnik, M.; Korkeala, H. Screening of the Two-Component-System Histidine Kinases of Listeria monocytogenes EGD-e. LiaS Is Needed for Growth under Heat, Acid, Alkali, Osmotic, Ethanol and Oxidative Stresses. Food Microbiol. 2017, 65, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Wiedmann, M.; Arvik, T.J.; Hurley, R.J.; Boor, K.J. General Stress Transcription Factor ΣB and Its Role in Acid Tolerance and Virulence of Listeria monocytogenes. J. Bacteriol. 1998, 180, 3650. [Google Scholar] [CrossRef]
- Chen, J.; Cheng, C.; Xia, Y.; Zhao, H.; Fang, C.; Shan, Y.; Wu, B.; Fang, W. Lmo0036, an Ornithine and Putrescine Carbamoyltransferase in Listeria monocytogenes, Participates in Arginine Deiminase and Agmatine Deiminase Pathways and Mediates Acid Tolerance. Microbiology 2011, 157, 3150–3161. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Jiang, L.; Chen, Q.; Zhao, H.; Luo, X.; Chen, X.; Fang, W. Lmo0038 Is Involved in Acid and Heat Stress Responses and Specific for Listeria monocytogenes Lineages i and II, and Listeria Ivanovii. Foodborne Pathog. Dis. 2009, 6, 365–376. [Google Scholar] [CrossRef] [PubMed]
- Ryan, S.; Begley, M.; Gahan, C.G.M.; Hill, C. Molecular Characterization of the Arginine Deiminase System in Listeria monocytogenes: Regulation and Role in Acid Tolerance. Environ. Microbiol. 2009, 11, 432–445. [Google Scholar] [CrossRef] [PubMed]
- van der Veen, S.; van Schalkwijk, S.; Molenaar, D.; de Vos, W.M.; Abee, T.; Wells-Bennik, M.H.J. The SOS Response of Listeria monocytogenes Is Involved in Stress Resistance and Mutagenesis. Microbiology 2010, 156, 374–384. [Google Scholar] [CrossRef]
- Van Der Veen, S.; Moezelaar, R.; Abee, T.; Wells-Bennik, M.H.J. The Growth Limits of a Large Number of Listeria monocytogenes Strains at Combinations of Stresses Show Serotype- and Niche-Specific Traits. J. Appl. Microbiol. 2008, 105, 1246–1258. [Google Scholar] [CrossRef]
- Madeo, M.; O’Riordan, N.; Fuchs, T.M.; Utratna, M.; Karatzas, K.A.G.; O’Byrne, C.P. Thiamine Plays a Critical Role in the Acid Tolerance of Listeria monocytogenes. FEMS Microbiol. Lett. 2012, 326, 137–143. [Google Scholar] [CrossRef]
- Borezee, E.; Pellegrini, E.; Berche, P. OppA of Listeria monocytogenes, an Oligopeptide-Binding Protein Required for Bacterial Growth at Low Temperature and Involved in Intracellular Survival. Infect. Immun. 2000, 68, 7069–7077. [Google Scholar] [CrossRef]
- Angelidis, A.S.; Smith, G.M. Role of the Glycine Betaine and Carnitine Transporters in Adaptation of Listeria monocytogenes to Chill Stress in Defined Medium. Appl. Environ. Microbiol. 2003, 69, 7492–7498. [Google Scholar] [CrossRef]
- Liu, S.; Graham, J.E.; Bigelow, L.; Morse, P.D.; Wilkinson, B.J. Identification of Listeria monocytogenes Genes Expressed in Response to Growth at Low Temperature. Appl. Environ. Microbiol. 2002, 68, 1697–1705. [Google Scholar] [CrossRef]
- Brøndsted, L.; Kallipolitis, B.H.; Ingmer, H.; Knöchel, S. KdpE and a Putative RsbQ Homologue Contribute to Growth of Listeria monocytogenes at High Osmolarity and Low Temperature. FEMS Microbiol. Lett. 2003, 219, 233–239. [Google Scholar] [CrossRef]
- Becker, L.A.; Evans, S.N.; Hutkins, R.W.; Benson, A.K. Role of ΣB in Adaptation of Listeria monocytogenes to Growth at Low Temperature. J. Bacteriol. 2000, 182, 7083–7087. [Google Scholar] [CrossRef] [PubMed]
- Christiansen, J.K.; Larsen, M.H.; Ingmer, H.; Søgaard-Andersen, L.; Kallipolitis, B.H. The RNA-Binding Protein Hfq of Listeria monocytogenes: Role in Stress Tolerance and Virulence. J. Bacteriol. 2004, 186, 3355–3362. [Google Scholar] [CrossRef] [PubMed]
- Knudsen, G.M.; Olsen, J.E.; Dons, L. Characterization of DegU, a Response Regulator in Listeria monocytogenes, Involved in Regulation of Motility and Contributes to Virulence. FEMS Microbiol. Lett. 2004, 240, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Schmid, B.; Klumpp, J.; Raimann, E.; Loessner, M.J.; Stephan, R.; Tasara, T. Role of Cold Shock Proteins in Growth of Listeria monocytogenes under Cold and Osmotic Stress Conditions. Appl. Environ. Microbiol. 2009, 75, 1621–1627. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Kathariou, S. Differentiation of Epidemic-Associated Strains of Listeria monocytogenes by Restriction Fragment Length Polymorphism in a Gene Region Essential for Growth at Low Temperatures (4 °C). Appl. Environ. Microbiol. 1995, 61, 4310–4314. [Google Scholar] [CrossRef] [PubMed]
- Markkula, A.; Mattila, M.; Lindström, M.; Korkeala, H. Genes Encoding Putative DEAD-Box RNA Helicases in Listeria monocytogenes EGD-e Are Needed for Growth and Motility at 3 °C. Environ. Microbiol. 2012, 14, 2223–2232. [Google Scholar] [CrossRef] [PubMed]
- Pöntinen, A.; Markkula, A.; Lindström, M.; Korkeala, H. Two-Component-System Histidine Kinases Involved in Growth of Listeria monocytogenes EGD-e at Low Temperatures. Appl. Environ. Microbiol. 2015, 81, 3994–4004. [Google Scholar] [CrossRef]
- Mattila, M.; Lindström, M.; Somervuo, P.; Markkula, A.; Korkeala, H. Role of FlhA and MotA in Growth of Listeria monocytogenes at Low Temperatures. Int. J. Food Microbiol. 2011, 148, 177–183. [Google Scholar] [CrossRef]


| ID | Query (bp) | Accession | Identity (%) | Predicted Function |
|---|---|---|---|---|
| group_3 | 609 | WP_242211654 | 100 | LPXTG * cell-wall-anchored domain-containing protein |
| group_877 | 3276 | WP_003726175.1 | 100 | carbohydrate-binding protein |
| group_71 | 306 | EEO3657903.1 | 100 | transposase [Listeria monocytogenes] protein |
| group_1254 | 1515 | WP_012951969.1 | 100 | putative DNA-binding-domain-containing protein |
| group_10 | 2754 | WP_014601151.1 | 99.89 | autolysin Ami * |
| immR_1 | 423 | WP_003724014.1 | 100 | helix-turn-helix transcriptional regulator protein |
| group_902 | 210 | WP_003733688.1 | 100 | helix-turn-helix domain-containing protein |
| group_12 | 516 | EAC8754143.1 | 100 | GW-domain-containing glycosaminoglycan-binding protein |
| immA | 426 | EDD2318653.1 | 100 | ImmA */IrrE family metallo-endopeptidase protein |
| group_3548 | 204 | WP_222949761.1 | 100 | hypothetical protein |
| ispDF | 2592 | EEW21888.1 | 100 | LOW-QUALITY PROTEIN: peptidoglycan-bound protein |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Myintzaw, P.; Pennone, V.; McAuliffe, O.; Begley, M.; Callanan, M. Association of Virulence, Biofilm, and Antimicrobial Resistance Genes with Specific Clonal Complex Types of Listeria monocytogenes. Microorganisms 2023, 11, 1603. https://doi.org/10.3390/microorganisms11061603
Myintzaw P, Pennone V, McAuliffe O, Begley M, Callanan M. Association of Virulence, Biofilm, and Antimicrobial Resistance Genes with Specific Clonal Complex Types of Listeria monocytogenes. Microorganisms. 2023; 11(6):1603. https://doi.org/10.3390/microorganisms11061603
Chicago/Turabian StyleMyintzaw, Peter, Vincenzo Pennone, Olivia McAuliffe, Máire Begley, and Michael Callanan. 2023. "Association of Virulence, Biofilm, and Antimicrobial Resistance Genes with Specific Clonal Complex Types of Listeria monocytogenes" Microorganisms 11, no. 6: 1603. https://doi.org/10.3390/microorganisms11061603
APA StyleMyintzaw, P., Pennone, V., McAuliffe, O., Begley, M., & Callanan, M. (2023). Association of Virulence, Biofilm, and Antimicrobial Resistance Genes with Specific Clonal Complex Types of Listeria monocytogenes. Microorganisms, 11(6), 1603. https://doi.org/10.3390/microorganisms11061603







