Synthetic Microbial Community Promotes Bacterial Communities Leading to Soil Multifunctionality in Desertified Land
Abstract
:1. Introduction
2. Materials and Methods
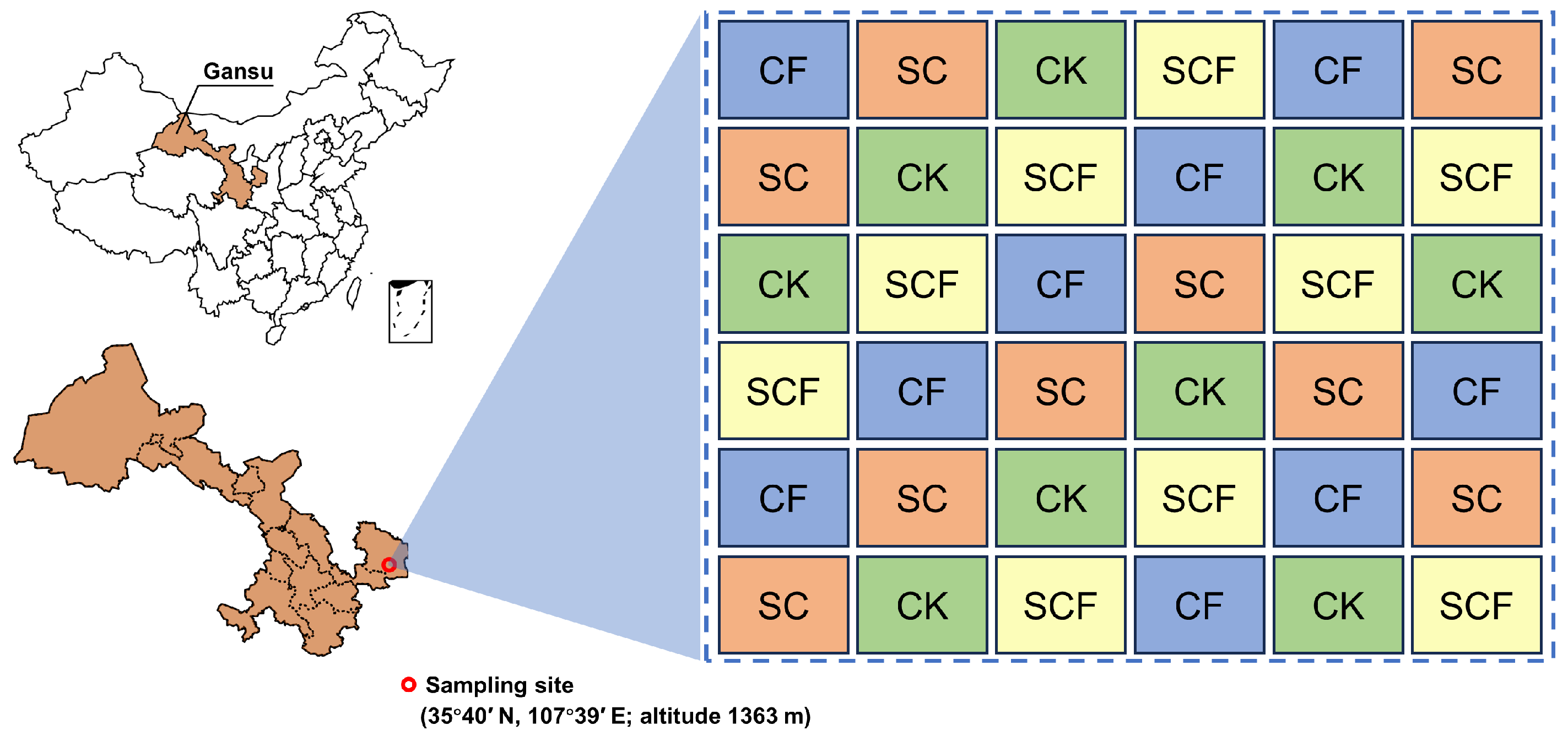
2.1. Field Experiments and Soil Sample Collection
2.2. Soil Physicochemical Properties and Enzyme Activities
2.3. Soil DNA Extraction and Amplicon Sequence Analysis
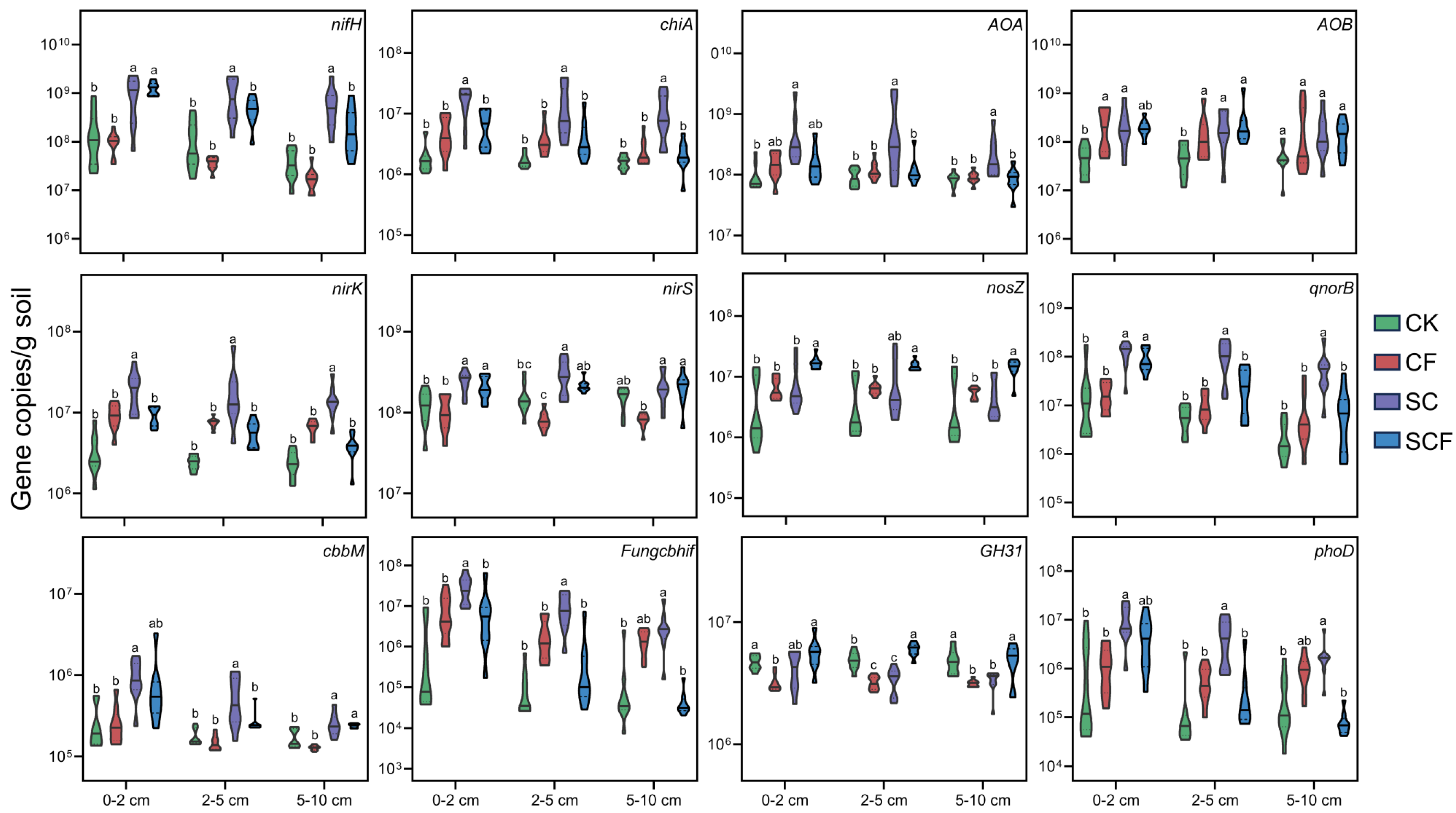
2.4. Quantitative Real-Time PCR (qRT-PCR)
2.5. Assessing Soil Multifunctionality
2.6. Data Analysis
3. Results
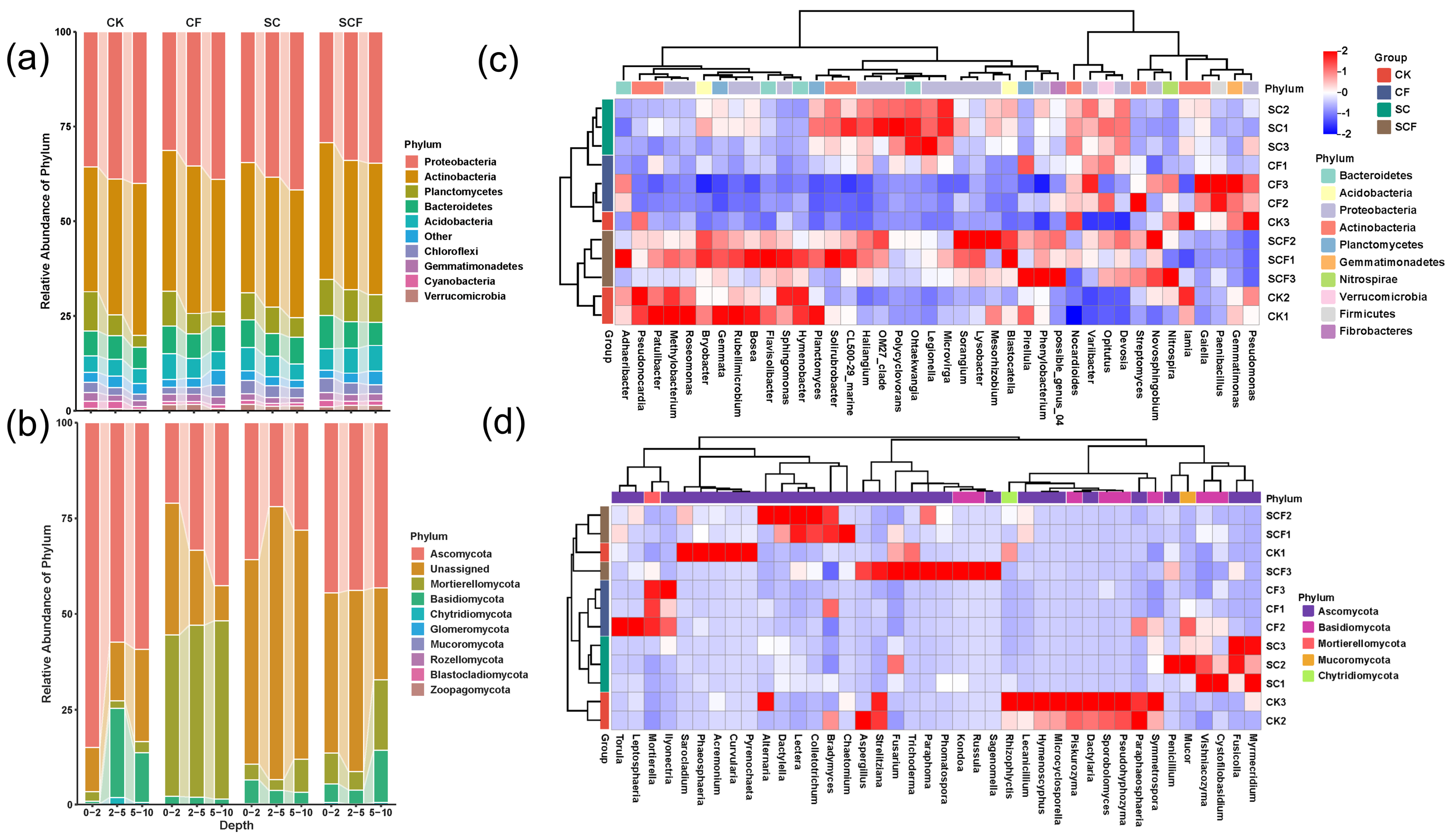
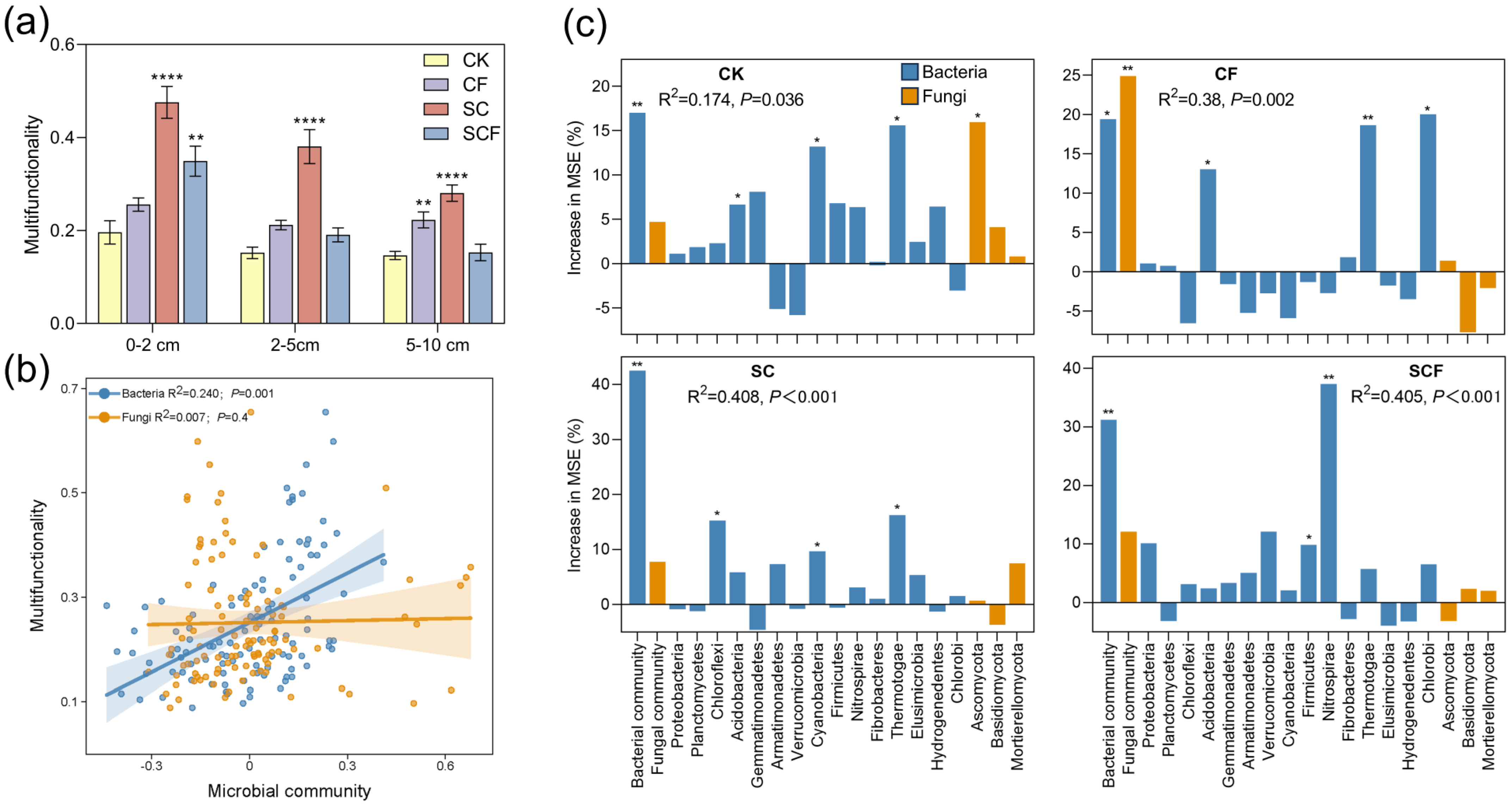
3.1. Variation in Bacterial and Fungal Community Diversity under Different Treatments
3.2. Differences in Microbial Composition under Different Treatments
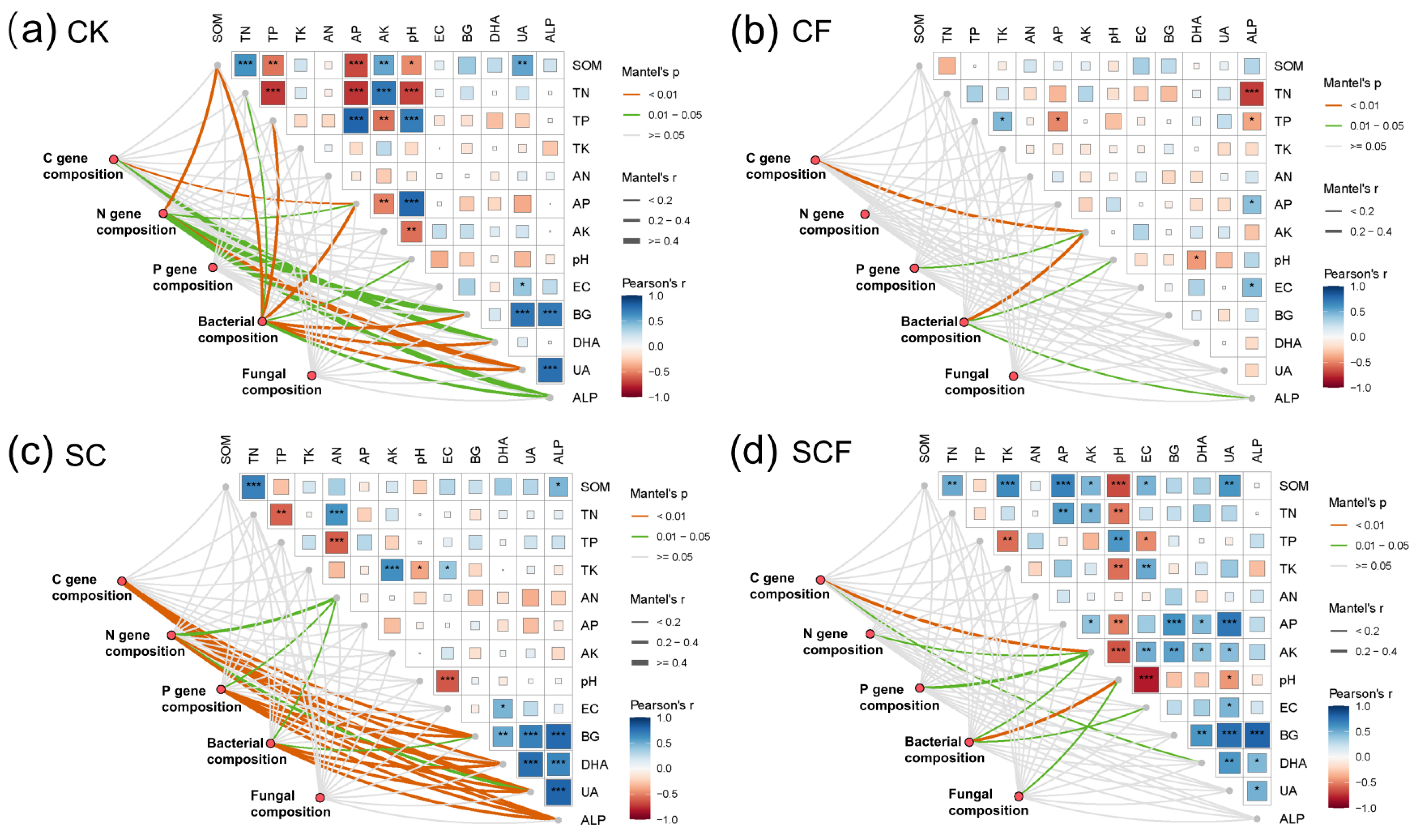
3.3. Correlation of Microbial Community with Functional Genes, Physicochemical Properties, and Enzyme Activities
3.4. Potential Microorganisms Driving Ecological Multifunctionality
4. Discussion
4.1. Fertilization Measures Have Altered Microbial Diversity and Composition
4.2. SynCom Enhanced the Link between Microorganisms and Soil Biological Properties
4.3. SynCom Increased the Contribution of Bacterial Communities to Soil Multifunctionality
4.4. Potentially Functional Microbial Taxa Play an Important Role in Ecological Multifunctionality
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bryan, B.A.; Gao, L.; Ye, Y.; Sun, X.; Connor, J.D.; Crossman, N.D.; Stafford-Smith, M.; Wu, J.; He, C.; Yu, D.; et al. China’s response to a national land-system sustainability emergency. Nature 2018, 559, 193–204. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Deng, D.; Zhang, L.; Zhu, X.; Chen, D.; Zhou, J. Soil and vegetation conditions changes following the different sand dune restoration measures on the Zoige Plateau. PLoS ONE 2019, 14, e0216975. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Dang, X.; Zhou, Y.; Hou, Z.; Li, X.; Jiang, H.; Zhou, D.; Wang, J.; Hai, C.; Zhou, R. Using sediment grain size characteristics to assess effectiveness of mechanical sand barriers in reducing erosion. Sci. Rep. 2020, 10, 14009. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ge, Q.; Geng, X.; Wang, Z.; Gao, L.; Bryan, B.A.; Chen, S.; Su, Y.; Cai, D.; Ye, J.; et al. Unintended consequences of combating desertification in China. Nat. Commun. 2023, 14, 1139. [Google Scholar] [CrossRef] [PubMed]
- Tong, Z.; Quan, G.; Wan, L.; He, F.; Li, X. The effect of fertilizers on biomass and biodiversity on a semi-arid grassland of Northern China. Sustainability 2019, 11, 2854. [Google Scholar] [CrossRef]
- Millett, J.; Foot, G.W.; Svensson, B.M. Nitrogen deposition and prey nitrogen uptake control the nutrition of the carnivorous plant Drosera rotundifolia. Sci. Total Environ. 2015, 512–513, 631–636. [Google Scholar] [CrossRef] [PubMed]
- de Souza, R.S.C.; Armanhi, J.S.L.; Arruda, P. From Microbiome to Traits: Designing Synthetic Microbial Communities for Improved Crop Resiliency. Front. Plant Sci. 2020, 11, 1179. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Bai, X.; Jiao, S.; Li, Y.; Li, P.; Yang, Y.; Zhang, H.; Wei, G. A simplified synthetic community rescues Astragalus mongholicus from root rot disease by activating plant-induced systemic resistance. Microbiome 2021, 9, 217. [Google Scholar] [CrossRef]
- Li, Y.B.; Li, Q.; Yang, J.J.; Lü, X.T.; Liang, W.J.; Han, X.G.; Bezemer, M. Home-field advantages of litter decomposition increase with increasing N deposition rates: A litter and soil perspective. Funct. Ecol. 2017, 31, 1792–1801. [Google Scholar] [CrossRef]
- Carlstrom, C.I.; Field, C.M.; Bortfeld-Miller, M.; Muller, B.; Sunagawa, S.; Vorholt, J.A. Synthetic microbiota reveal priority effects and keystone strains in the Arabidopsis phyllosphere. Nat. Ecol. Evol. 2019, 3, 1445–1454. [Google Scholar] [CrossRef]
- Jiang, M.; Delgado-Baquerizo, M.; Yuan, M.M.; Ding, J.; Yergeau, E.; Zhou, J.; Crowther, T.W.; Liang, Y. Home-based microbial solution to boost crop growth in low-fertility soil. New Phytol. 2023, 239, 752–765. [Google Scholar] [CrossRef] [PubMed]
- Fierer, N.; Schimel, J.P. Effects of drying–rewetting frequency on soil carbon and nitrogen transformations. Soil Biol. Biochem. 2002, 34, 777–787. [Google Scholar] [CrossRef]
- Meisner, A.; Leizeaga, A.; Rousk, J.; Bååth, E. Partial drying accelerates bacterial growth recovery to rewetting. Soil Biol. Biochem. 2017, 112, 269–276. [Google Scholar] [CrossRef]
- Pan, H.; Chen, M.; Feng, H.; Wei, M.; Song, F.; Lou, Y.; Cui, X.; Wang, H.; Zhuge, Y. Organic and inorganic fertilizers respectively drive bacterial and fungal community compositions in a fluvo-aquic soil in northern China. Soil Tillage Res. 2020, 198, 104540. [Google Scholar] [CrossRef]
- Grosskopf, T.; Soyer, O.S. Synthetic microbial communities. Curr. Opin. Microbiol. 2014, 18, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Niu, B.; Paulson, J.N.; Zheng, X.; Kolter, R. Simplified and representative bacterial community of maize roots. Proc. Natl. Acad. Sci. USA 2017, 114, E2450–E2459. [Google Scholar] [CrossRef] [PubMed]
- Jing, X.; Sanders, N.J.; Shi, Y.; Chu, H.; Classen, A.T.; Zhao, K.; Chen, L.; Shi, Y.; Jiang, Y.; He, J.S. The links between ecosystem multifunctionality and above- and belowground biodiversity are mediated by climate. Nat. Commun. 2015, 6, 8159. [Google Scholar] [CrossRef]
- van der Plas, F.; Hennecke, J.; Chase, J.M.; van Ruijven, J.; Barry, K.E. Universal beta-diversity–functioning relationships are neither observed nor expected. Trends Ecol. Evol. 2023, 38, 532–544. [Google Scholar] [CrossRef]
- FAO; UNESCO. Revised legend of the FAO-UNESCO soil map of the world. ISRIC Rep. 1988, 1, 1–109. [Google Scholar]
- Bao, S.D. Soil and Agricultural Chemistry Analysis; China Agriculture Press: Beijing, China, 2000. [Google Scholar]
- Elsas, J.D.V. Methods of Soil Analysis; John Wiley & Sons: New York, NY, USA, 1995; pp. 131–133. [Google Scholar]
- Ye, J.; Song, Z.; Wang, L.; Zhu, J. Metagenomic analysis of microbiota structure evolution in phytoremediation of a swine lagoon wastewater. Bioresour. Technol. 2016, 219, 439–444. [Google Scholar] [CrossRef]
- Zuo, T.; Wong, S.H.; Cheung, C.P.; Lam, K.; Lui, R.; Cheung, K.; Zhang, F.; Tang, W.; Ching, J.Y.L.; Wu, J.C.Y.; et al. Gut fungal dysbiosis correlates with reduced efficacy of fecal microbiota transplantation in Clostridium difficile infection. Nat. Commun. 2018, 9, 3663. [Google Scholar] [CrossRef]
- Liu, Y.-X.; Chen, L.; Ma, T.; Li, X.; Zheng, M.; Zhou, X.; Chen, L.; Qian, X.; Xi, J.; Lu, H.; et al. EasyAmplicon: An easy-to-use, open-source, reproducible, and community-based pipeline for amplicon data analysis in microbiome research. iMeta 2023, 2, e83. [Google Scholar] [CrossRef]
- Chen, S.; Wang, Y.; Gao, J.; Chen, X.; Qi, J.; Peng, Z.; Chen, B.; Pan, H.; Liang, C.; Liu, J.; et al. Agricultural tillage practice and rhizosphere selection interactively drive the improvement of soybean plant biomass. Plant Cell Environ. 2023, 46, 3542–3557. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.; Chen, B.; Gao, J.; Peng, Z.; Jiao, S.; Wei, G. Responses of soil bacterial community structure and function to dry–wet cycles more stable in paddy than in dryland agricultural ecosystems. Glob. Ecol. Biogeogr. 2022, 31, 362–377. [Google Scholar] [CrossRef]
- Delgado-Baquerizo, M.; Reich, P.B.; Trivedi, C.; Eldridge, D.J.; Abades, S.; Alfaro, F.D.; Bastida, F.; Berhe, A.A.; Cutler, N.A.; Gallardo, A.; et al. Multiple elements of soil biodiversity drive ecosystem functions across biomes. Nat. Ecol. Evol. 2020, 4, 210–220. [Google Scholar] [CrossRef]
- Zhang, Z.; Lu, Y.; Wei, G.; Jiao, S. Rare Species-Driven Diversity-Ecosystem Multifunctionality Relationships are Promoted by Stochastic Community Assembly. mBio 2022, 13, e0044922. [Google Scholar] [CrossRef] [PubMed]
- Fan, K.; Delgado-Baquerizo, M.; Guo, X.; Wang, D.; Wu, Y.; Zhu, M.; Yu, W.; Yao, H.; Zhu, Y.G.; Chu, H. Suppressed N fixation and diazotrophs after four decades of fertilization. Microbiome 2019, 7, 143. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Li, S.; Avera, B.N.; Strahm, B.D.; Badgley, B.D. Soil Bacterial and Fungal Communities Show Distinct Recovery Patterns during Forest Ecosystem Restoration. Appl. Environ. Microbiol. 2017, 83, e00966-17. [Google Scholar] [CrossRef]
- Yao, F.; Yang, S.; Wang, Z.; Wang, X.; Ye, J.; Wang, X.; DeBruyn, J.M.; Feng, X.; Jiang, Y.; Li, H. Microbial taxa distribution is associated with ecological trophic cascades along an elevation gradient. Front. Microbiol. 2017, 8, 2071. [Google Scholar] [CrossRef]
- Wu, X.; Zhang, T.; Zhao, J.; Wang, L.; Yang, D.; Li, G.; Xiu, W. Variation of Soil Bacterial and Fungal Communities from Fluvo-Aquic Soil Under Chemical Fertilizer Reduction Combined with Organic Materials in North China Plain. J. Soil Sci. Plant Nutr. 2021, 21, 349–363. [Google Scholar] [CrossRef]
- Xue, C.; Ryan Penton, C.; Zhu, C.; Chen, H.; Duan, Y.; Peng, C.; Guo, S.; Ling, N.; Shen, Q. Applied Soil Ecology Biology and Fertility of Soils Alterations in soil fungal community composition and network assemblage structure by different long-term fertilization regimes are correlated to the soil ionome. Biol. Fertil. Soils 2018, 54, 95–106. [Google Scholar] [CrossRef]
- Wolinska, A.; Podlewski, J.; Slomczewski, A.; Grzadziel, J.; Galazka, A.; Kuzniar, A. Fungal Indicators of Sensitivity and Resistance to Long-Term Maize Monoculture: A Culture-Independent Approach. Front. Microbiol. 2021, 12, 799378. [Google Scholar] [CrossRef] [PubMed]
- He, K.; Xu, Y.; He, G.; Zhao, X.H.; Wang, C.P.; Li, S.J.; Zhou, G.K.; Hu, R.B. Combined application of acidic biochar and fertilizer synergistically enhances Miscanthus productivity in coastal saline-alkaline soil. Sci. Total Environ. 2023, 893, 164811. [Google Scholar] [CrossRef] [PubMed]
- Aydogan, E.L.; Moser, G.; Muller, C.; Kampfer, P.; Glaeser, S.P. Long-Term Warming Shifts the Composition of Bacterial Communities in the Phyllosphere of Galium album in a Permanent Grassland Field-Experiment. Front. Microbiol. 2018, 9, 144. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.; Dou, Y.; Yang, X.; An, S. Soil microbial community and their functional genes during grassland restoration. J. Environ. Manag. 2023, 325, 116488. [Google Scholar] [CrossRef] [PubMed]
- Allegrini, M.; Morales, M.E.; Villamil, M.B.; Zabaloy, M.C. Ammonia Oxidizing Prokaryotes Respond Differently to Fertilization and Termination Methods in Common Oat’s Rhizosphere. Front. Microbiol. 2021, 12, 746524. [Google Scholar] [CrossRef] [PubMed]
- Odum, E.P. The strategy of ecosystem development. Science 1969, 164, 262–270. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Hu, Y.; Peng, P.; Zhu, Z.; Atere, C.T.; O’donnell, A.G.; Wu, J.; Ge, T. Effect of P stoichiometry on the abundance of nitrogen-cycle genes in phosphorus-limited paddy soil. Biol. Fertil. Soils 2017, 53, 767–776. [Google Scholar] [CrossRef]
- Huang, X.; Jiang, H.; Li, Y.; Ma, Y.; Tang, H.; Ran, W.; Shen, Q. The role of poorly crystalline iron oxides in the stability of soil aggregate-associated organic carbon in a rice–wheat cropping system. Geoderma 2016, 279, 1–10. [Google Scholar] [CrossRef]
- Zhang, R.; Zhao, X.; Zuo, X.; Degen, A.A.; Li, Y.; Liu, X.; Luo, Y.; Qu, H.; Lian, J.; Wang, R. Drought-induced shift from a carbon sink to a carbon source in the grasslands of Inner Mongolia, China. Catena 2020, 195, 104845. [Google Scholar] [CrossRef]
- Oliverio, A.M.; Geisen, S.; Delgado-Baquerizo, M.; Maestre, F.T.; Turner, B.L.; Fierer, N. The global-scale distributions of soil protists and their contributions to belowground systems. Sci. Adv. 2020, 6, eaax8787. [Google Scholar] [CrossRef]
- Chen, H.; Yang, T.; Xia, Q.; Bowman, D.; Williams, D.; Walker, J.T.; Shi, W. The extent and pathways of nitrogen loss in turfgrass systems: Age impacts. Sci. Total Environ. 2018, 637–638, 746–757. [Google Scholar] [CrossRef]
- Deutschmann, I.M.; Lima-Mendez, G.; Krabberod, A.K.; Raes, J.; Vallina, S.M.; Faust, K.; Logares, R. Disentangling environmental effects in microbial association networks. Microbiome 2021, 9, 232. [Google Scholar]
- Meyer, S.T.; Ptacnik, R.; Hillebrand, H.; Bessler, H.; Buchmann, N.; Ebeling, A.; Eisenhauer, N.; Engels, C.; Fischer, M.; Halle, S.; et al. Biodiversity-multifunctionality relationships depend on identity and number of measured functions. Nat. Ecol. Evol. 2018, 2, 44–49. [Google Scholar] [CrossRef]
- Karimi, B.; Terrat, S.; Dequiedt, S.; Saby, N.P.A.; Horriguel, W.; Lelièvre, M.; Nowak, V.; Jolivet, C.; Arrouays, D.; Wincker, P.; et al. Biogeography of soil bacteria and archaea across France. Sci. Adv. 2018, 4, eaat1808. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Baquerizo, M.; Maestre, F.T.; Reich, P.B.; Jeffries, T.C.; Gaitan, J.J.; Encinar, D.; Berdugo, M.; Campbell, C.D.; Singh, B.K. Microbial diversity drives multifunctionality in terrestrial ecosystems. Nat. Commun. 2016, 7, 10541. [Google Scholar] [CrossRef] [PubMed]
- Jiao, S.; Lu, Y.H.; Wei, G.H. Soil multitrophic network complexity enhances the link between biodiversity and multifunctionality in agricultural systems. Glob. Chang. Biol. 2022, 28, 140–153. [Google Scholar] [CrossRef]
- Livingstone, P.G.; Morphew, R.M.; Cookson, A.R.; Whitworth, D.E. Genome Analysis, Metabolic Potential, and Predatory Capabilities of Herpetosiphon llansteffanense sp. nov. Appl. Environ. Microbiol. 2018, 84, e01040-18. [Google Scholar] [CrossRef]
- Asiloglu, R.; Kenya, K.; Samuel, S.O.; Sevilir, B.; Murase, J.; Suzuki, K.; Harada, N. Top-down effects of protists are greater than bottom-up effects of fertilisers on the formation of bacterial communities in a paddy field soil. Soil Biol. Biochem. 2021, 156, 108186. [Google Scholar] [CrossRef]
- Zeng, Z.Q.; Zhuang, W.Y. Three New Species of Fusicolla (Hypocreales) from China. J. Fungi 2023, 9, 572. [Google Scholar] [CrossRef]
- Zhu, Q.; Wang, N.; Duan, B.; Wang, Q.; Wang, Y. Rhizosphere bacterial and fungal communities succession patterns related to growth of poplar fine roots. Sci. Total Environ. 2021, 756, 143839. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hao, X.; Gu, Y.; Zhang, H.; Wang, X.; Liu, X.; Chen, C.; Wang, C.; Zhang, X.; Liu, X.; Shen, X. Synthetic Microbial Community Promotes Bacterial Communities Leading to Soil Multifunctionality in Desertified Land. Microorganisms 2024, 12, 1117. https://doi.org/10.3390/microorganisms12061117
Hao X, Gu Y, Zhang H, Wang X, Liu X, Chen C, Wang C, Zhang X, Liu X, Shen X. Synthetic Microbial Community Promotes Bacterial Communities Leading to Soil Multifunctionality in Desertified Land. Microorganisms. 2024; 12(6):1117. https://doi.org/10.3390/microorganisms12061117
Chicago/Turabian StyleHao, Xinwei, Yazhou Gu, Hongzhi Zhang, Xiao Wang, Xiaozhen Liu, Chunlei Chen, Congcong Wang, Xiaoqing Zhang, Xingyu Liu, and Xihui Shen. 2024. "Synthetic Microbial Community Promotes Bacterial Communities Leading to Soil Multifunctionality in Desertified Land" Microorganisms 12, no. 6: 1117. https://doi.org/10.3390/microorganisms12061117





