Impact of Novel Foods on the Human Gut Microbiome: Current Status
Abstract
1. Introduction
2. Methodology
3. Microbiome
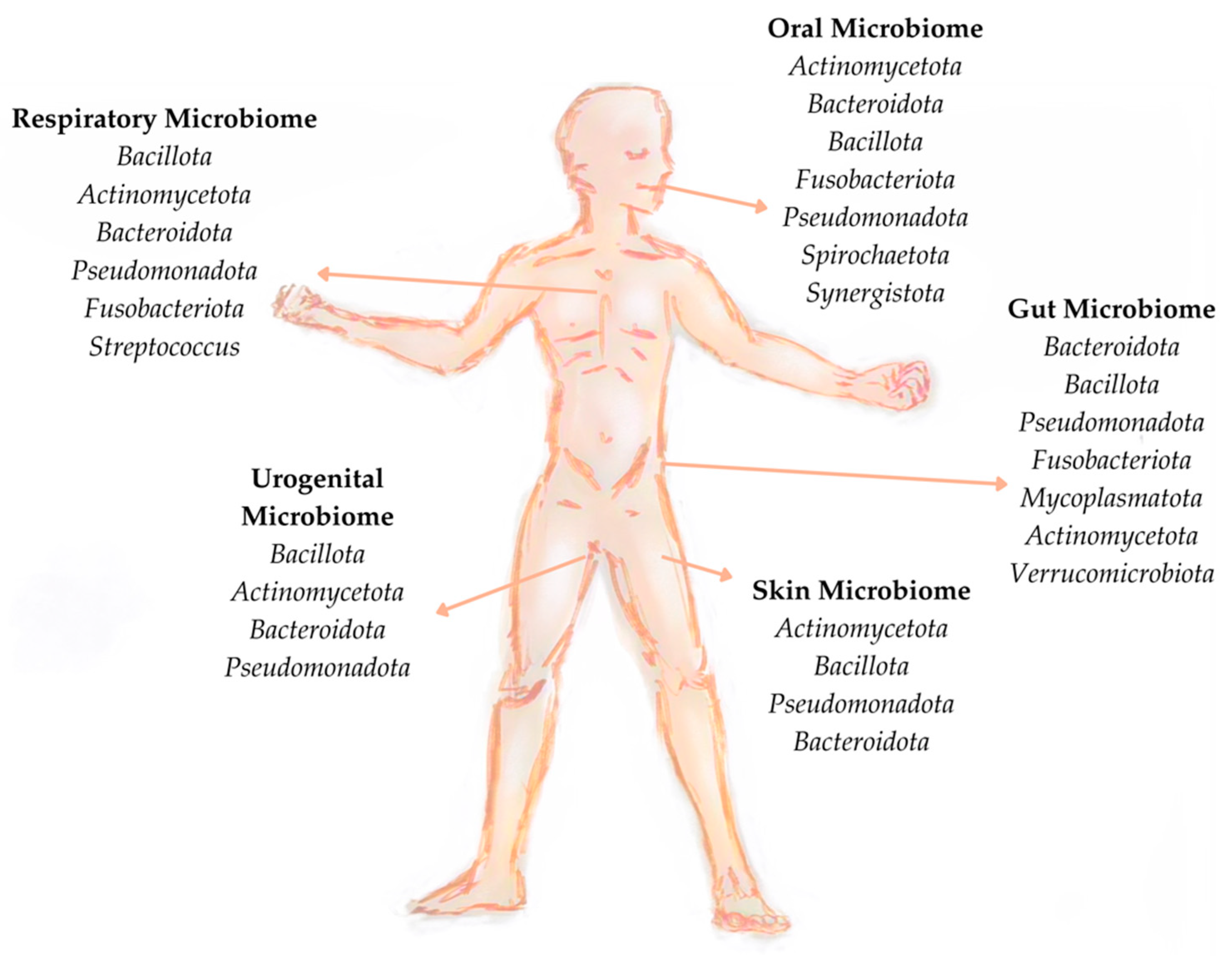
3.1. Microbiome Definition
3.2. Microbiome Characterisation
3.3. Factors Affecting Microbiome Composition
4. Foods and Microbiome
4.1. Nutrients
4.1.1. Carbohydrates
4.1.2. Proteins
4.1.3. Fats
5. Nutritional Supplements
5.1. Vitamins
5.2. Minerals
5.3. Prebiotics, Probiotics, Synbiotics, and Postbiotics
5.4. Essential Fatty Acids
5.5. Plant-Derived Bioactive Components
6. Functional Foods
7. Alternative Foods
8. Diet
9. New Eating Habits
10. Effects of Novel Foods on the Human Gut Microbiome
11. Critical Analysis and Future Directions
12. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lederberg, J.; Mccray, A.T. Ome Sweet Omics—A Genealogical Treasury of Words. Scientist 2001, 15, 8. [Google Scholar]
- Berg, G.; Rybakova, D.; Fischer, D.; Cernava, T.; Vergès, M.-C.C.; Charles, T.; Chen, X.; Cocolin, L.; Eversole, K.; Corral, G.H.; et al. Microbiome definition re-visited: Old concepts and new challenges. Microbiome 2020, 8, 103. [Google Scholar] [CrossRef]
- Zhang, P. Influence of Foods and Nutrition on the Gut Microbiome and Implications for Intestinal Health. Int. J. Mol. Sci. 2022, 23, 9588. [Google Scholar] [CrossRef]
- Cox, T.O.; Lundgren, P.; Nath, K.; Thaiss, C.A. Metabolic control by the microbiome. Genome Med. 2022, 14, 80. [Google Scholar] [CrossRef] [PubMed]
- Olofsson, L.E.; Bäckhed, F. The Metabolic Role and Therapeutic Potential of the Microbiome. Endocr. Rev. 2022, 43, 907–926. [Google Scholar] [CrossRef]
- Zheng, D.; Liwinski, T.; Elinav, E. Interaction between microbiota and immunity in health and disease. Cell Res. 2020, 30, 492–506. [Google Scholar] [CrossRef]
- Rooks, M.G.; Garrett, W.S. Gut microbiota, metabolites and host immunity. Nat. Rev. Immunol. 2016, 16, 341–352. [Google Scholar] [CrossRef]
- Durack, J.; Lynch, S.V. The gut microbiome: Relationships with disease and opportunities for therapy. J. Exp. Med. 2019, 216, 20–40. [Google Scholar] [CrossRef]
- Tang, W.W.; Bäckhed, F.; Landmesser, U.; Hazen, S.L. Intestinal Microbiota in Cardiovascular Health and Disease: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2019, 73, 2089–2105. [Google Scholar] [CrossRef]
- Ogunrinola, G.A.; Oyewale, J.O.; Oshamika, O.O.; Olasehinde, G.I. The Human Microbiome and Its Impacts on Health. Int. J. Microbiol. 2020, 2020, 8045646. [Google Scholar] [CrossRef]
- Aggarwal, N.; Kitano, S.; Puah, G.R.Y.; Kittelmann, S.; Hwang, I.Y.; Chang, M.W. Microbiome and Human Health: Current Understanding, Engineering, and Enabling Technologies. Chem. Rev. 2023, 123, 31–72. [Google Scholar] [CrossRef] [PubMed]
- Charles, T.C.; Dastogeer, K.M.G. Editorial: Plant microbiome: Diversity, functions, and applications. Front. Microbiol. 2022, 13, 1039212. [Google Scholar] [CrossRef]
- European Commission; Directorate-General for Research and Innovation; Bizzo, G.; Fabbri, K.; Gajdzinska, M. Food 2030—Pathways for Action 2.0—R&I Policy as a Driver for Sustainable, Healthy, Climate Resilient and Inclusive Food Systems; Publications Office of the European Union: Luxembourg, 2023; Available online: https://data.europa.eu/doi/10.2777/365011 (accessed on 15 April 2024).
- Turroni, S.; Benítez-Páez, A. Editorial: Remodeling Composition and Function of Microbiome by Dietary Strategies—Functional Foods Perspective. Front. Nutr. 2021, 8, 811102. [Google Scholar] [CrossRef] [PubMed]
- FAO; WHO. New Food Sources and Production Systems: Need for Codex Attention and Guidance? Joint FAO/WHO Food Standards Programme. Codex Alimentarius Commission, Forty-Fourth Session, 8–17 November 2021. Available online: https://www.fao.org/fao-who-codexalimentarius/sh-proxy/jp/?lnk=1&url=https%253A%252F%252Fworkspace.fao.org%252Fsites%252Fcodex%252FMeetings%252FCX-701-44%252FWorking%2BDocuments%252Fcac44_15.Add.1e.pdf (accessed on 6 August 2024).
- Siegrist, M.; Hartmann, C. Consumer acceptance of novel food technologies. Nat. Food 2020, 1, 343–350. [Google Scholar] [CrossRef]
- Singla, M.; Sit, N. Application of ultrasound in combination with other technologies in food processing: A review. Ultrason. Sonochemistry 2021, 73, 105506. [Google Scholar] [CrossRef] [PubMed]
- Tuorila, H.; Hartmann, C. Consumer responses to novel and unfamiliar foods. Curr. Opin. Food Sci. 2019, 33, 1–8. [Google Scholar] [CrossRef]
- Regulation (EU) 2015/2283 of the European Parliament and of the Council of 25 November 2015 on Novel Foods, Amending Regulation (EU) No 1169/2011 of the European Parliament and of the Council and Repealing Regulation (EC) No 258/97 of the European Parliament and of the Council and Commission Regulation (EC) No 1852/2001. 2015. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32015R2283 (accessed on 20 April 2024).
- Food Standard Agency (UK FSA). Introduction: 3D Printing Technologies in the Food System for Food Production and Packaging. 2023. Available online: https://www.food.gov.uk/research/introduction-3d-printing-technologies-in-the-food-system-for-food-production-and-packaging (accessed on 6 August 2024).
- Food Safety and Standards Authority of India. Food Safety and Standards (Approval of Non-Specified Food and Food Ingredients) Regulations. 2020. Available online: https://www.fssai.gov.in/upload/uploadfiles/files/FAQs_FSS_Approval_NonSpecified_Ingredients_06_05_2020.pdf (accessed on 6 August 2024).
- National Food Service Israel. New Food in Israel. National Food Service Israel. 2022. Available online: https://www.gov.il/en/pages/novel-food (accessed on 6 August 2024).
- GSO 2696: 2022; General Requirements for Novel Foods. GCC Standardization Organization, 2022. Available online: https://www.gso.org.sa/store/standards/GSO:803291/GSO%202696:2022?lang=en (accessed on 6 August 2024).
- Sun, J. The Regulation of Novel Food in China: The Tendency of Deregulation. Eur. Food Feed. Law Rev. 2015, 10, 442–448. [Google Scholar]
- Food Safety Commission of Japan. Basic Approach of the Safety Assessment of Food for Specified Health Uses (FOSHU). 2004. Available online: https://www.fsc.go.jp/english/what_we_do.data/basic_approach_of_foshu.pdf (accessed on 6 August 2024).
- Singapore Food Agency. Requirements for the Safety Assessment of Novel Foods and Novel Food Ingredients. SFA. 2023. Available online: https://www.sfa.gov.sg/docs/default-source/food-information/requirements-for-the-safety-assessment-of-novel-foods-and-novel-food-ingredients.pdf (accessed on 6 August 2024).
- FDA Thailand. Notification of the Ministry of Public Health (No. 376) B.E 2559, Re: Novel Food. 2016. Available online: https://en.fda.moph.go.th/media.php?id=517782064121126912&name=No.376_Re_Novel_food.pdf (accessed on 6 August 2024).
- Ministry of Food and Drug Safety. Novel Food. MFDS. 2018. Available online: https://www.nifds.go.kr/en/wpge/m_15/cont_02/cont_02_01_03.do (accessed on 6 August 2024).
- Food Standards Australia New Zealand. Regulation of Novel Foods, FSANZ. 2022. Available online: https://www.foodstandards.gov.au/business/novel#:~:text=Novel%20foods%20are%20non%2Dtraditional,Standards%20Code%20(the%20Code) (accessed on 6 August 2024).
- United States Food and Drug Administration. Novel Food Regulation: EFSA vs. FDA. 2022. Available online: https://safefood360.com/blog/novel-food-regulation-efsa-versus-fda/#:~:text=In%20the%20United%20States%2C%20no,Recognized%20as%20Safe%20(GRAS) (accessed on 6 August 2024).
- Health Canada. Guidelines for the Safety Assessment of Novel Foods. 2022. Available online: https://www.canada.ca/en/health-canada/services/food-nutrition/legislation-guidelines/guidance-documents/guidelines-safety-assessment-novel-foods-2006.html (accessed on 6 August 2024).
- Anvisa. Food-Market Authorizations. 2020. Available online: https://www.gov.br/anvisa/pt-br/english/regulation-of-products/food (accessed on 6 August 2024).
- Thakur, R.; Pristijono, P.; Scarlett, C.J.; Bowyer, M.; Singh, S.P.; Vuong, Q.V. Starch-based films: Major factors affecting their properties. Int. J. Biol. Macromol. 2019, 132, 1079–1089. [Google Scholar] [CrossRef]
- Pavli, F.; Argyri, A.A.; Skandamis, P.; Nychas, G.-J.; Tassou, C.; Chorianopoulos, N. Antimicrobial Activity of Oregano Essential Oil Incorporated in Sodium Alginate Edible Films: Control of Listeria monocytogenes and Spoilage in Ham Slices Treated with High Pressure Processing. Materials 2019, 12, 3726. [Google Scholar] [CrossRef]
- Siracusa, V.; Karpova, S.; Olkhov, A.; Zhulkina, A.; Kosenko, R.; Iordanskii, A. Gas Transport Phenomena and Polymer Dynamics in PHB/PLA Blend Films as Potential Packaging Materials. Polymers 2020, 12, 647. [Google Scholar] [CrossRef] [PubMed]
- Suhag, R.; Kumar, N.; Petkoska, A.T.; Upadhyay, A. Film formation and deposition methods of edible coating on food products: A review. Food Res. Int. 2020, 136, 109582. [Google Scholar] [CrossRef] [PubMed]
- Stewart, C.J. Diet-microbe-host interaction in early life. Science 2023, 381, 38–40. [Google Scholar] [CrossRef]
- Johnson, A.J.; Zheng, J.J.; Kang, J.W.; Saboe, A.; Knights, D.; Zivkovic, A.M. A Guide to Diet-Microbiome Study Design. Front. Nutr. 2020, 7, 79. [Google Scholar] [CrossRef]
- Olvera-Rosales, L.-B.; Cruz-Guerrero, A.-E.; Ramírez-Moreno, E.; Quintero-Lira, A.; Contreras-López, E.; Jaimez-Ordaz, J.; Castañeda-Ovando, A.; Añorve-Morga, J.; Calderón-Ramos, Z.-G.; Arias-Rico, J.; et al. Impact of the Gut Microbiota Balance on the Health–Disease Relationship: The Importance of Consuming Probiotics and Prebiotics. Foods 2021, 10, 1261. [Google Scholar] [CrossRef]
- Martin, S.E.; Kraft, C.S.; Ziegler, T.R.; Millson, E.C.; Rishishwar, L.; Martin, G.S. The Role of Diet on the Gut Microbiome, Mood, and Happiness. medRxiv 2023. [Google Scholar] [CrossRef]
- Yang, Q.; Liang, Q.; Balakrishnan, B.; Belobrajdic, D.P.; Feng, Q.-J.; Zhang, W. Role of Dietary Nutrients in the Modulation of Gut Microbiota: A Narrative Review. Nutrients 2020, 12, 381. [Google Scholar] [CrossRef] [PubMed]
- Leong, K.S.W.; Derraik, J.G.B.; Hofman, P.L.; Cutfield, W.S. Antibiotics, gut microbiome and obesity. Clin. Endocrinol. 2018, 88, 185–200. [Google Scholar] [CrossRef] [PubMed]
- Laursen, M.F. Gut Microbiota Development: Influence of Diet from Infancy to Toddlerhood. Ann. Nutr. Metab. 2021, 77, 21–34. [Google Scholar] [CrossRef] [PubMed]
- Dreher, M.L. Whole Fruits and Fruit Fiber Emerging Health Effects. Nutrients 2018, 10, 1833. [Google Scholar] [CrossRef]
- Peng, M.; Tabashsum, Z.; Anderson, M.; Truong, A.; Houser, A.K.; Padilla, J.; Akmel, A.; Bhatti, J.; Rahaman, S.O.; Biswas, D. Effectiveness of probiotics, prebiotics, and prebiotic-like components in common functional foods. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1908–1933. [Google Scholar] [CrossRef]
- Leeuwendaal, N.K.; Stanton, C.; O’toole, P.W.; Beresford, T.P. Fermented Foods, Health and the Gut Microbiome. Nutrients 2022, 14, 1527. [Google Scholar] [CrossRef]
- Taylor, B.C.; Lejzerowicz, F.; Poirel, M.; Shaffer, J.P.; Jiang, L.; Aksenov, A.; Litwin, N.; Humphrey, G.; Martino, C.; Miller-Montgomery, S.; et al. Consumption of Fermented Foods Is Associated with Systematic Differences in the Gut Microbiome and Metabolome. mSystems 2020, 5, e00901-19. [Google Scholar] [CrossRef] [PubMed]
- Bapteste, E.; Gérard, P.; Larose, C.; Blouin, M.; Not, F.; Campos, L.; Aïdan, G.; Selosse, M.A.; Adénis, M.S.; Bouchard, F.; et al. The Epistemic Revolution Induced by Microbiome Studies: An Interdisciplinary View. Biology 2021, 10, 651. [Google Scholar] [CrossRef] [PubMed]
- Yang, I.; Corwin, E.J.; Brennan, P.A.; Jordan, S.; Murphy, J.R.; Dunlop, A. The Infant Microbiome. Nurs. Res. 2016, 65, 76–88. [Google Scholar] [CrossRef]
- Ma, L.-C.; Zhao, H.-Q.; Wu, L.B.; Cheng, Z.-L.; Liu, C. Impact of the microbiome on human, animal, and environmental health from a One Health perspective. Sci. One Health 2023, 2, 100037. [Google Scholar] [CrossRef]
- Wilkinson, J.E.; Franzosa, E.A.; Everett, C.; Li, C.; Hu, F.B.; Wirth, D.F.; Song, M.; Chan, A.T.; Rimm, E.; Garrett, W.S.; et al. A framework for microbiome science in public health. Nat. Med. 2021, 27, 766–774. [Google Scholar] [CrossRef]
- Murphy, K.M.; Le, S.M.; Wilson, A.E.; Warner, D.A. The Microbiome as a Maternal Effect: A Systematic Review on Vertical Transmission of Microbiota. Integr. Comp. Biol. 2023, 63, 597–609. [Google Scholar] [CrossRef] [PubMed]
- Duncan, K.; Carey-Ewend, K.; Vaishnava, S. Spatial analysis of gut microbiome reveals a distinct ecological niche associated with the mucus layer. Gut Microbes 2021, 13, 1874815. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Ley, R.E.; Hamady, M.; Fraser-Liggett, C.M.; Knight, R.; Gordon, J.I. The Human Microbiome Project. Nature 2007, 449, 804–810. [Google Scholar] [CrossRef]
- Ursell, L.K.; Metcalf, J.L.; Parfrey, L.W.; Knight, R. Defining the human microbiome. Nutr. Rev. 2012, 70, S38–S44. [Google Scholar] [CrossRef]
- Marchesi, J.R.; Ravel, J. The vocabulary of microbiome research: A proposal. Microbiome 2015, 3, 31. [Google Scholar] [CrossRef] [PubMed]
- Sender, R.; Fuchs, S.; Milo, R. Are We Really Vastly Outnumbered? Revisiting the Ratio of Bacterial to Host Cells in Humans. Cell 2016, 164, 337–340. Available online: https://pubmed.ncbi.nlm.nih.gov/26824647/ (accessed on 30 April 2024). [CrossRef] [PubMed]
- Mancini, N.; Peri, F.; Rescigno, M.; Zanoni, I. Microbiome studies in the medical sciences and the need for closer multidisciplinary interplay. Sci. Signal. 2020, 13, eaba9911. [Google Scholar] [CrossRef]
- Henry, L.P.; Bruijning, M.; Forsberg, S.K.G.; Ayroles, J.F. Can the Microbiome Influence Host Evolutionary Trajectories? bioRxiv 2019. [Google Scholar] [CrossRef]
- Rosenberg, E. Current Knowledge, and Unanswered Questions. In Microbiomes, 1st ed.; Herolds, S., Ed.; Springer: Cham, Switzerland, 2021; pp. 1–431. [Google Scholar] [CrossRef]
- Muth, T.R.; Caplan, A.J. Microbiomes for All. Front. Microbiol. 2020, 11, 593472. [Google Scholar] [CrossRef]
- Mitreva, M. The Microbiome in Infectious Diseases. In Infectious Diseases, 2-Volume Set; Elsevier: Amsterdam, The Netherlands, 2020; pp. 68–74.e2. [Google Scholar] [CrossRef]
- Grace-Farfaglia, P.; Frazier, H.; Iversen, M.D. Essential Factors for a Healthy Microbiome: A Scoping Review. Int. J. Environ. Res. Public Health 2022, 19, 8361. [Google Scholar] [CrossRef]
- Gacesa, R.; Kurilshikov, A.; Vila, A.V.; Sinha, T.; Klaassen, M.A.Y.; Bolte, L.A.; Andreu-Sánchez, S.; Chen, L.; Collij, V.; Hu, S.; et al. Environmental factors shaping the gut microbiome in a Dutch population. Nature 2022, 604, 732–739. [Google Scholar] [CrossRef] [PubMed]
- Blum, H.E. The human microbiome. Adv. Med Sci. 2017, 62, 414–420. [Google Scholar] [CrossRef]
- Sender, R.; Fuchs, S.; Milo, R. Revised Estimates for the Number of Human and Bacteria Cells in the Body. PLoS Biol. 2016, 14, e1002533. [Google Scholar] [CrossRef]
- Ruan, W.; Engevik, M.A.; Spinler, J.K.; Versalovic, J. Healthy Human Gastrointestinal Microbiome: Composition and Function After a Decade of Exploration. Dig. Dis. Sci. 2020, 65, 695–705. [Google Scholar] [CrossRef]
- Mercer, E.M.; Ramay, H.R.; Moossavi, S.; Laforest-Lapointe, I.; Reyna, M.E.; Becker, A.B.; Simons, E.; Mandhane, P.J.; Turvey, S.E.; Moraes, T.J.; et al. Divergent maturational patterns of the infant bacterial and fungal gut microbiome in the first year of life are associated with inter-kingdom community dynamics and infant nutrition. Microbiome 2024, 12, 22. [Google Scholar] [CrossRef] [PubMed]
- Rautava, S. Early microbial contact, the breast milk microbiome and child health. J. Dev. Orig. Health Dis. 2016, 7, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Milani, C.; Duranti, S.; Bottacini, F.; Casey, E.; Turroni, F.; Mahony, J.; Belzer, C.; Delgado Palacio, S.; Arboleya Montes, S.; Mancabelli, L.; et al. The First Microbial Colonizers of the Human Gut: Composition, Activities, and Health Implications of the Infant Gut Microbiota. Microbiol. Mol. Biol. Rev. 2017, 81, e00036-17. [Google Scholar] [CrossRef]
- Park, J.Y.; Park, J.Y.; Yun, H.; Yun, H.; Lee, S.-B.; Lee, S.-B.; Kim, H.J.; Kim, H.J.; Jung, Y.H.; Jung, Y.H.; et al. Comprehensive characterization of maternal, fetal, and neonatal microbiomes supports prenatal colonization of the gastrointestinal tract. Sci. Rep. 2023, 13, 18905. [Google Scholar] [CrossRef]
- Cahana, I.; Iraqi, F.A. Impact of host genetics on gut microbiome: Take-home lessons from human and mouse studies. Anim. Model. Exp. Med. 2020, 3, 229–236. [Google Scholar] [CrossRef]
- Mills, S.; Lane, J.A.; Smith, G.J.; Grimaldi, K.A.; Ross, R.P.; Stanton, C. Precision Nutrition and the Microbiome Part II: Potential Opportunities and Pathways to Commercialisation. Nutrients 2019, 11, 1468. [Google Scholar] [CrossRef]
- Peroni, D.G.; Nuzzi, G.; Trambusti, I.; Di Cicco, M.E.; Comberiati, P. Microbiome Composition and Its Impact on the Development of Allergic Diseases. Front. Immunol. 2020, 11, 700. [Google Scholar] [CrossRef]
- Tiamani, K.; Luo, S.; Schulz, S.; Xue, J.; Costa, R.; Mirzaei, M.K.; Deng, L. The role of virome in the gastrointestinal tract and beyond. FEMS Microbiol. Rev. 2022, 46, fuac027. [Google Scholar] [CrossRef]
- Chibani, C.M.; Mahnert, A.; Borrel, G.; Almeida, A.; Werner, A.; Brugère, J.F.; Gribaldo, S.; Finn, R.D.; Schmitz, R.A.; Moissl-Eichinger, C. A Catalogue of 1,167 Genomes from the Human Gut Archaeome. Nat. Microbiol. 2022, 7, 48–61, Erratum in Nat. Microbiol. 2022, 7, 339. [Google Scholar] [CrossRef]
- Forbes, J.D.; Bernstein, C.N.; Tremlett, H.; Van Domselaar, G.; Knox, N.C. A Fungal World: Could the Gut Mycobiome Be Involved in Neurological Disease? Front. Microbiol. 2019, 9, 3249. [Google Scholar] [CrossRef]
- Belvoncikova, P.; Splichalova, P.; Videnska, P.; Gardlik, R. The Human Mycobiome: Colonization, Composition and the Role in Health and Disease. J. Fungi 2022, 8, 1046. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Sugimura, N.; Burgermeister, E.; Ebert, M.P.; Zuo, T.; Lan, P. The gut virome: A new microbiome component in health and disease. EBioMedicine 2022, 81, 104113. [Google Scholar] [CrossRef]
- Spencer, L.; Olawuni, B.; Singh, P. Gut Virome: Role and Distribution in Health and Gastrointestinal Diseases. Front. Cell. Infect. Microbiol. 2022, 12, 836706. [Google Scholar] [CrossRef]
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. What Is the Healthy Gut Microbiota Composition? A Changing Ecosystem across Age, Environment, Diet, and Diseases. Microorganisms 2019, 7, 14. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Shi, X.; Xu, J.; Yuan, S.; Zheng, B.; Zhang, E.; Huang, G.; Li, G.; Jiang, G.; Gao, S.; et al. Characterization of the Genitourinary Microbiome of 1,165 Middle-Aged and Elderly Healthy Individuals. Front. Microbiol. 2021, 12, 673969. [Google Scholar] [CrossRef]
- Skowron, K.; Bauza-Kaszewska, J.; Kraszewska, Z.; Wiktorczyk-Kapischke, N.; Grudlewska-Buda, K.; Kwiecińska-Piróg, J.; Wałecka-Zacharska, E.; Radtke, L.; Gospodarek-Komkowska, E. Human Skin Microbiome: Impact of Intrinsic and Extrinsic Factors on Skin Microbiota. Microorganisms 2021, 9, 543. [Google Scholar] [CrossRef]
- Grice, E.A.; Kong, H.H.; Conlan, S.; Deming, C.B.; Davis, J.; Young, A.C.; NISC Comparative Sequencing Program; Bouffard, G.G.; Blakesley, R.W.; Murray, P.R.; et al. Topographical and Temporal Diversity of the Human Skin Microbiome. Science 2009, 324, 1190–1192. [Google Scholar] [CrossRef]
- Escapa, I.F.; Chen, T.; Huang, Y.; Gajare, P.; Dewhirst, F.E.; Lemon, K.P. New Insights into Human Nostril Microbiome from the Expanded Human Oral Microbiome Database (eHOMD): A Resource for the Microbiome of the Human Aerodigestive Tract. mSystems 2018, 3, e00187-18. [Google Scholar] [CrossRef]
- Manos, J. The human microbiome in disease and pathology. APMIS 2022, 130, 690–705. [Google Scholar] [CrossRef]
- Chattopadhyay, I.; Verma, M.; Panda, M. Role of Oral Microbiome Signatures in Diagnosis and Prognosis of Oral Cancer. Technol. Cancer Res. Treat. 2019, 18, 1533033819867354. [Google Scholar] [CrossRef]
- Sedghi, L.; DiMassa, V.; Harrington, A.; Lynch, S.V.; Kapila, Y.L. The oral microbiome: Role of key organisms and complex networks in oral health and disease. Periodontol. 2000 2021, 1, 107–131. [Google Scholar] [CrossRef]
- García, A.P.G.; Vidal, Y.L.; García, M.M.A. Microbioma oral: Variabilidad entre regiones y poblaciones. Rev. Fac. Med. 2022, 65, 8–19. [Google Scholar] [CrossRef]
- Bosch, A.A.; Levin, E.; van Houten, M.A.; Hasrat, R.; Kalkman, G.; Biesbroek, G.; Piters, W.A.d.S.; de Groot, P.-K.C.; Pernet, P.; Keijser, B.J.; et al. Development of Upper Respiratory Tract Microbiota in Infancy is Affected by Mode of Delivery. EBioMedicine 2016, 9, 336–345. [Google Scholar] [CrossRef]
- Stearns, J.C.; Davidson, C.J.; McKeon, S.; Whelan, F.J.; Fontes, M.E.; Schryvers, A.B.; Bowdish, D.M.; Kellner, J.D.; Surette, M.G. Culture and Molecular-Based Profiles Show Shifts in Bacterial Communities of the Upper Respiratory Tract That Occur with Age. ISME J. 2015, 5, 1246–1259, Erratum in ISME J. 2015, 5, 1268. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Lee, J.; Eor, J.Y.; Kwak, M.-J.; Huh, C.S.; Kim, Y. Effect of Consumption of Animal Products on the Gut Microbiome Composition and Gut Health. Food Sci. Anim. Resour. 2023, 43, 723–750. [Google Scholar] [CrossRef]
- Andrade, B.G.N.; Cuadrat, R.R.C.; Tonetti, F.R.; Kitazawa, H.; Villena, J. The role of respiratory microbiota in the protection against viral diseases: Respiratory commensal bacteria as next-generation probiotics for COVID-19. Biosci. Microbiota, Food Health 2022, 41, 94–102. [Google Scholar] [CrossRef]
- Kurilshikov, A.; Medina-Gomez, C.; Bacigalupe, R.; Radjabzadeh, D.; Wang, J.; Demirkan, A.; Le Roy, C.I.; Garay, J.A.R.; Finnicum, C.T.; Liu, X.; et al. Large-scale association analyses identify host factors influencing human gut microbiome composition. Nat. Genet. 2021, 53, 156–165. [Google Scholar] [CrossRef]
- Patangia, D.V.; Ryan, C.A.; Dempsey, E.; Ross, R.P.; Stanton, C. Impact of antibiotics on the human microbiome and consequences for host health. Microbiologyopen 2022, 1, e1260. [Google Scholar] [CrossRef]
- Bäckhed, F.; Roswall, J.; Peng, Y.; Feng, Q.; Jia, H.; Kovatcheva-Datchary, P.; Li, Y.; Xia, Y.; Xie, H.; Zhong, H.; et al. Dynamics and Stabilization of the Human Gut Microbiome during the First Year of Life. Cell Host Microbe 2015, 17, 690–703. [Google Scholar] [CrossRef]
- Mehta, V.; Nagu, P.; Inbaraj, B.S.; Sharma, M.; Parashar, A.; Sridhar, K. Epigenetics and Gut Microbiota Crosstalk: A potential Factor in Pathogenesis of Cardiovascular Disorders. Bioengineering 2022, 9, 798. [Google Scholar] [CrossRef]
- Fenga, C. Gut microbiota modulation: A tailored approach for the prevention of chronic diseases. Biomed. Rep. 2022, 16, 23. [Google Scholar] [CrossRef]
- Dutton, C.L.; Maisha, F.M.; Quinn, E.B.; Morales, K.L.; Moore, J.M.; Mulligan, C.J. Maternal Psychosocial Stress Is Associated with Reduced Diversity in the Early Infant Gut Microbiome. Microorganisms 2023, 11, 975. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, J.; Wu, J.; Zhu, Q.; Chen, C.; Li, Y. Implications of gut microbiota dysbiosis and fecal metabolite changes in psychologically stressed mice. Front. Microbiol. 2023, 14, 1124454. [Google Scholar] [CrossRef]
- Su, Q.; Liu, Q. Factors Affecting Gut Microbiome in Daily Diet. Front. Nutr. 2021, 8, 644138. [Google Scholar] [CrossRef]
- Daïen, C.I.; Pinget, G.V.; Tan, J.K.; Macia, L. Detrimental Impact of Microbiota-Accessible Carbohydrate-Deprived Diet on Gut and Immune Homeostasis: An Overview. Front. Immunol. 2017, 8, 548. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Png, E.; Gowans, M.; Ong, D.E.H.; de Sessions, P.F.; Song, J.; Nagarajan, N. Ectopic gut colonization: A metagenomic study of the oral and gut microbiome in Crohn’s disease. Gut Pathog. 2021, 13, 13. [Google Scholar] [CrossRef]
- Park, S.-Y.; Hwang, B.-O.; Lim, M.; Ok, S.-H.; Lee, S.-K.; Chun, K.-S.; Park, K.-K.; Hu, Y.; Chung, W.-Y.; Song, N.-Y. Oral–Gut Microbiome Axis in Gastrointestinal Disease and Cancer. Cancers 2021, 13, 2124. [Google Scholar] [CrossRef] [PubMed]
- Piccioni, A.; Covino, M.; Candelli, M.; Ojetti, V.; Capacci, A.; Gasbarrini, A.; Franceschi, F.; Merra, G. How Do Diet Patterns, Single Foods, Prebiotics and Probiotics Impact Gut Microbiota? Microbiol. Res. 2023, 14, 390–408. [Google Scholar] [CrossRef]
- Wilson, A.S.; Koller, K.R.; Ramaboli, M.C.; Nesengani, L.T.; Ocvirk, S.; Chen, C.; Flanagan, C.A.; Sapp, F.R.; Merritt, Z.T.; Bhatti, F.; et al. Diet and the Human Gut Microbiome: An International Review. Dig. Dis. Sci. 2020, 65, 723–740. [Google Scholar] [CrossRef]
- Jacky, D.; Bibi, C.; Meng, L.M.C.; Jason, F.; Gwendoline, T.; Jeremy, L.; Wie, C.C. Effects of OsomeFood Clean Label plant-based meals on the gut microbiome. BMC Microbiol. 2023, 23, 88. [Google Scholar] [CrossRef]
- Lerma-Aguilera, A.M.; Pérez-Burillo, S.; Navajas-Porras, B.; León, E.D.; Ruíz-Pérez, S.; Pastoriza, S.; Jiménez-Hernández, N.; Cämmerer, B.-M.; Rufián-Henares, J.; Gosalbes, M.J.; et al. Effects of different foods and cooking methods on the gut microbiota: An in vitro approach. Front. Microbiol. 2024, 14, 1334623. [Google Scholar] [CrossRef] [PubMed]
- Um, C.Y.; Peters, B.A.; Choi, H.S.; Oberstein, P.; Beggs, D.B.; Usyk, M.; Wu, F.; Hayes, R.B.; Gapstur, S.M.; McCullough, M.L.; et al. Grain, Gluten, and Dietary Fiber Intake Influence Gut Microbial Diversity: Data from the Food and Microbiome Longitudinal Investigation. Cancer Res. Commun. 2023, 3, 43–53. [Google Scholar] [CrossRef]
- Ecklu-Mensah, G.; Gilbert, J.; Devkota, S. Dietary Selection Pressures and Their Impact on the Gut Microbiome. Cell Mol. Gastroenterol. Hepatol. 2022, 13, 7–18. [Google Scholar] [CrossRef]
- Rinninella, E.; Cintoni, M.; Raoul, P.; Lopetuso, L.R.; Scaldaferri, F.; Pulcini, G.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. Food Components and Dietary Habits: Keys for a Healthy Gut Microbiota Composition. Nutrients 2019, 11, 2393. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Tester, R.F. Fructose, galactose and glucose—In health and disease. Clin. Nutr. ESPEN 2019, 33, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Mudgil, D.; Barak, S. Composition, properties and health benefits of indigestible carbohydrate polymers as dietary fiber: A review. Int. J. Biol. Macromol. 2013, 61, 1–6. [Google Scholar] [CrossRef]
- Suharoschi, R.; Pop, O.L.; Vlaic, R.A.; Muresan, C.I.; Muresan, C.C.; Cozma, A.; Sitar-Taut, A.V.; Vulturar, R.; Heghes, S.C.; Fodor, A.; et al. Dietary Fiber and Metabolism. In Dietary Fiber: Properties, Recovery, and Applications; Academic Press: Amsterdam, The Netherlands, 2019; pp. 59–77. [Google Scholar] [CrossRef]
- Lamothe, L.M.; Cantu-Jungles, T.M.; Chen, T.; Green, S.; Naqib, A.; Srichuwong, S.; Hamaker, B.R. Boosting the value of insoluble dietary fiber to increase gut fermentability through food processing. Food Funct. 2021, 12, 10658–10666. [Google Scholar] [CrossRef] [PubMed]
- Vitaglione, P.; Mennella, I.; Ferracane, R.; Goldsmith, F.; Guice, J.; Page, R.; Raggio, A.M.; Coulon, D.; Martin, R.; Keenan, M. Gut fermentation induced by a resistant starch rich whole grain diet explains serum concentration of dihydroferulic acid and hippuric acid in a model of ZDF rats. J. Funct. Foods 2019, 53, 286–291. [Google Scholar] [CrossRef]
- Soliman, G.A. Dietary Fiber, Atherosclerosis, and Cardiovascular Disease. Nutrients 2019, 11, 1155. [Google Scholar] [CrossRef]
- Swann, O.G.; Kilpatrick, M.; Breslin, M.; Oddy, W.H. Dietary fiber and its associations with depression and inflammation. Nutr. Rev. 2020, 78, 394–411. [Google Scholar] [CrossRef]
- Sonnenburg, E.D.; Smits, S.A.; Tikhonov, M.; Higginbottom, S.K.; Wingreen, N.S.; Sonnenburg, J.L. Diet-induced extinctions in the gut microbiota compound over generations. Nature 2016, 529, 212–215. [Google Scholar] [CrossRef] [PubMed]
- Steimle, A.; Neumann, M.; Grant, E.T.; Turner, J.D.; Desai, M.S. Concentrated Raw Fibers Enhance the Fiber-Degrading Capacity of a Synthetic Human Gut Microbiome. Int. J. Mol. Sci. 2021, 22, 6855. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Bhat, Z.F.; Gounder, R.S.; Ahmed, I.A.M.; Al-Juhaimi, F.Y.; Ding, Y.; Bekhit, A.E.D. Effect of Dietary Protein and Processing on Gut Microbiota—A Systematic Review. Nutrients 2022, 14, 453. [Google Scholar] [CrossRef] [PubMed]
- Di Rosa, C.; Altomare, A.; Terrigno, V.; Carbone, F.; Tack, J.; Cicala, M.; Guarino, M.P.L. Constipation-Predominant Irritable Bowel Syndrome (IBS-C): Effects of Different Nutritional Patterns on Intestinal Dysbiosis and Symptoms. Nutrients 2023, 15, 1647. [Google Scholar] [CrossRef] [PubMed]
- Kazura, W.; Michalczyk, K.; Stygar, D. The Relationship between the Source of Dietary Animal Fats and Proteins and the Gut Microbiota Condition and Obesity in Humans. Nutrients 2023, 15, 3082. [Google Scholar] [CrossRef]
- Ashkar, F.; Wu, J. Effects of Food Factors and Processing on Protein Digestibility and Gut Microbiota. J. Agric. Food Chem. 2023, 71, 8685–8698. [Google Scholar] [CrossRef]
- David, L.A.; Maurice, C.F.; Carmody, R.N.; Gootenberg, D.B.; Button, J.E.; Wolfe, B.E.; Ling, A.V.; Devlin, A.S.; Varma, Y.; Fischbach, M.A.; et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014, 505, 559–563. [Google Scholar] [CrossRef]
- Ghosh, T.S.; Rampelli, S.; Jeffery, I.B.; Santoro, A.; Neto, M.; Capri, M.; Giampieri, E.; Jennings, A.; Candela, M.; Turroni, S.; et al. Mediterranean diet intervention alters the gut microbiome in older people reducing frailty and improving health status: The NU-AGE 1-year dietary intervention across five European countries. Gut 2020, 69, 1218–1228. [Google Scholar] [CrossRef]
- Sidhu, S.R.K.; Kok, C.W.; Kunasegaran, T.; Ramadas, A. Effect of Plant-Based Diets on Gut Microbiota: A Systematic Review of Interventional Studies. Nutrients 2023, 15, 1510. [Google Scholar] [CrossRef]
- Ma, N.; Tian, Y.; Wu, Y.; Ma, X. Contributions of the Interaction Between Dietary Protein and Gut Microbiota to Intestinal Health. Curr. Protein Pept. Sci. 2017, 18, 795–808. [Google Scholar] [CrossRef]
- De Souza, A.Z.Z.; Zambom, A.Z.; Abboud, K.Y.; Reis, S.K.; Tannihão, F.; Guadagnini, D.; Saad, M.J.; Prada, P.O. Oral supplementation with l-glutamine alters gut microbiota of obese and overweight adults: A pilot study. Nutrition 2015, 31, 884–889. [Google Scholar] [CrossRef]
- Duan, Y.; Zeng, L.; Zheng, C.; Song, B.; Li, F.; Kong, X.; Xu, K. Inflammatory Links Between High Fat Diets and Diseases. Front. Immunol. 2018, 9, 2649. [Google Scholar] [CrossRef]
- Wolters, M.; Ahrens, J.; Romaní-Pérez, M.; Watkins, C.; Sanz, Y.; Benítez-Páez, A.; Stanton, C.; Günther, K. Dietary fat, the gut microbiota, and metabolic health—A systematic review conducted within the MyNewGut project. Clin. Nutr. 2019, 38, 2504–2520. [Google Scholar] [CrossRef] [PubMed]
- Rohr, M.W.; Narasimhulu, C.A.; Rudeski-Rohr, T.A.; Parthasarathy, S. Negative Effects of a High-Fat Diet on Intestinal Permeability: A Review. Adv. Nutr. 2020, 11, 77–91. [Google Scholar] [CrossRef]
- Bailén, M.; Bressa, C.; Martínez-López, S.; González-Soltero, R.; Lominchar, M.G.M.; Juan, C.S.; Larrosa, M. Microbiota Features Associated with a High-Fat/Low-Fiber Diet in Healthy Adults. Front. Nutr. 2020, 7, 583608. [Google Scholar] [CrossRef] [PubMed]
- López-Salazar, V.; Tapia, M.S.; Tobón-Cornejo, S.; Díaz, D.; Alemán-Escondrillas, G.; Granados-Portillo, O.; Noriega, L.; Tovar, A.R.; Torres, N. Consumption of soybean or olive oil at recommended concentrations increased the intestinal microbiota diversity and insulin sensitivity and prevented fatty liver compared to the effects of coconut oil. J. Nutr. Biochem. 2021, 94, 108751. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wu, G.; Zhao, L.; Wang, W. Nutritional Modulation of Gut Microbiota Alleviates Severe Gastrointestinal Symptoms in a Patient with Post-Acute COVID-19 Syndrome. mBio 2022, 13, e0380121. [Google Scholar] [CrossRef] [PubMed]
- Pham, V.T.; Dold, S.; Rehman, A.; Bird, J.K.; Steinert, R.E. Vitamins, the gut microbiome and gastrointestinal health in humans. Nutr. Res. 2021, 95, 35–53. [Google Scholar] [CrossRef]
- Huda, M.N.; Ahmad, S.M.; Kalanetra, K.M.; Taft, D.H.; Alam, M.J.; Khanam, A.; Raqib, R.; Underwood, M.A.; Mills, D.A.; Stephensen, C.B. Neonatal Vitamin A Supplementation and Vitamin A Status Are Associated with Gut Microbiome Composition in Bangladeshi Infants in Early Infancy and at 2 Years of Age. J. Nutr. 2019, 149, 1075–1088. [Google Scholar] [CrossRef]
- Tabatabaeizadeh, S.-A.; Fazeli, M.; Meshkat, Z.; Khodashenas, E.; Esmaeili, H.; Mazloum, S.; Ferns, G.A.; Abdizadeh, M.F.; Ghayour-Mobarhan, M. The effects of high doses of vitamin D on the composition of the gut microbiome of adolescent girls. Clin. Nutr. ESPEN 2020, 35, 103–108. [Google Scholar] [CrossRef]
- Carrothers, J.M.; York, M.A.; Brooker, S.L.; Lackey, K.A.; Williams, J.E.; Shafii, B.; Price, W.J.; Settles, M.L.; McGuire, M.A.; McGuire, M.K. Fecal Microbial Community Structure Is Stable over Time and Related to Variation in Macronutrient and Micronutrient Intakes in Lactating Women. J. Nutr. 2015, 145, 2379–2388. [Google Scholar] [CrossRef]
- Tang, M.; Frank, D.N.; Sherlock, L.; Ir, D.; Robertson, C.E.; Krebs, N.F. Effect of Vitamin E With Therapeutic Iron Supplementation on Iron Repletion and Gut Microbiome in US Iron Deficient Infants and Toddlers. J. Pediatr. Gastroenterol. Nutr. 2016, 63, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Krause, L.; Somerset, S. Associations between micronutrient intakes and gut microbiota in a group of adults with cystic fibrosis. Clin. Nutr. 2017, 36, 1097–1104. [Google Scholar] [CrossRef]
- Bielik, V.; Kolisek, M. Bioaccessibility and Bioavailability of Minerals in Relation to a Healthy Gut Microbiome. Int. J. Mol. Sci. 2021, 22, 6803. [Google Scholar] [CrossRef] [PubMed]
- Sauer, A.K.; Grabrucker, A.M. Zinc Deficiency During Pregnancy Leads to Altered Microbiome and Elevated Inflammatory Markers in Mice. Front. Neurosci. 2019, 13, 1295. [Google Scholar] [CrossRef] [PubMed]
- Reed, S.; Neuman, H.; Moscovich, S.; Glahn, R.P.; Koren, O.; Tako, E. Chronic Zinc Deficiency Alters Chick Gut Microbiota Composition and Function. Nutrients 2015, 7, 9768–9784. [Google Scholar] [CrossRef] [PubMed]
- González, A.; Gálvez, N.; Martín, J.; Reyes, F.; Pérez-Victoria, I.; Dominguez-Vera, J.M. Identification of the key excreted molecule by Lactobacillus fermentum related to host iron absorption. Food Chem. 2017, 228, 374–380. [Google Scholar] [CrossRef]
- Sjödin, K.S.; Domellöf, M.; Lagerqvist, C.; Hernell, O.; Lönnerdal, B.; Szymlek-Gay, E.A.; Sjödin, A.; West, C.E.; Lind, T. Administration of ferrous sulfate drops has significant effects on the gut microbiota of iron-sufficient infants: A randomised controlled study. Gut 2019, 68, 2095–2097. [Google Scholar] [CrossRef]
- Trautvetter, U.; Camarinha-Silva, A.; Jahreis, G.; Lorkowski, S.; Glei, M. High phosphorus intake and gut-related parameters—Results of a randomized placebo-controlled human intervention study. Nutr. J. 2018, 17, 23. [Google Scholar] [CrossRef]
- Piuri, G.; Zocchi, M.; Della Porta, M.; Ficara, V.; Manoni, M.; Zuccotti, G.V.; Pinotti, L.; Maier, J.A.; Cazzola, R. Magnesium in Obesity, Metabolic Syndrome, and Type 2 Diabetes. Nutrients 2021, 13, 320. [Google Scholar] [CrossRef]
- Valcheva, R.; Dieleman, L.A. Prebiotics: Definition and protective mechanisms. Best Pract. Res. Clin. Gastroenterol. 2016, 30, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Blaak, E.E.; Canfora, E.E.; Theis, S.; Frost, G.; Groen, A.K.; Mithieux, G.; Nauta, A.; Scott, K.; Stahl, B.; Van Harsselaar, J.; et al. Short chain fatty acids in human gut and metabolic health. Benef. Microbes 2020, 11, 411–455. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef] [PubMed]
- Precup, G.; Pocol, C.B.; Teleky, B.-E.; Vodnar, D.C. Awareness, Knowledge, and Interest about Prebiotics—A Study among Romanian Consumers. Int. J. Environ. Res. Public Health 2022, 19, 1208. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, M.; Azcarate-Peril, M.A.; Barnard, A.; Benoit, V.; Grimaldi, R.; Guyonnet, D.; Holscher, H.D.; Hunter, K.; Manurung, S.; Obis, D.; et al. Shaping the Future of Probiotics and Prebiotics. Trends Microbiol. 2021, 29, 667–685. [Google Scholar] [CrossRef]
- Chandrasekaran, P.; Weiskirchen, S.; Weiskirchen, R. Effects of Probiotics on Gut Microbiota: An Overview. Int. J. Mol. Sci. 2024, 11, 6022. [Google Scholar] [CrossRef]
- Pasolli, E.; De Filippis, F.; Mauriello, I.E.; Cumbo, F.; Walsh, A.M.; Leech, J.; Cotter, P.D.; Segata, N.; Ercolini, D. Large-scale genome-wide analysis links lactic acid bacteria from food with the gut microbiome. Nat. Commun. 2020, 11, 2610. [Google Scholar] [CrossRef]
- Suez, J.; Zmora, N.; Segal, E.; Elinav, E. The pros, cons, and many unknowns of probiotics. Nat. Med. 2019, 25, 716–729. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Alonso, M.; Camorlinga, A.A.; Messiah, S.E.; Marroquin, E. Effect of adding probiotics to an antibiotic intervention on the human gut microbial diversity and composition: A systematic review. J. Med Microbiol. 2022, 71, 001625. [Google Scholar] [CrossRef]
- Kumari, M.; Singh, P.; Nataraj, B.H.; Kokkiligadda, A.; Naithani, H.; Ali, S.A.; Behare, P.V.; Nagpal, R. Fostering next-generation probiotics in human gut by targeted dietary modulation: An emerging perspective. Food Res. Int. 2021, 150, 110716. [Google Scholar] [CrossRef]
- Swanson, K.S.; Gibson, G.R.; Hutkins, R.; Reimer, R.A.; Reid, G.; Verbeke, K.; Scott, K.P.; Holscher, H.D.; Azad, M.B.; Delzenne, N.M.; et al. The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of synbiotics. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 687–701. [Google Scholar] [CrossRef]
- Hibberd, A.; Yde, C.; Ziegler, M.; Honoré, A.; Saarinen, M.; Lahtinen, S.; Stahl, B.; Jensen, H.; Stenman, L. Probiotic or synbiotic alters the gut microbiota and metabolism in a randomised controlled trial of weight management in overweight adults. Benef. Microbes 2019, 10, 121–136. [Google Scholar] [CrossRef]
- Sergeev, I.N.; Aljutaily, T.; Walton, G.; Huarte, E. Effects of Synbiotic Supplement on Human Gut Microbiota, Body Composition and Weight Loss in Obesity. Nutrients 2020, 12, 222. [Google Scholar] [CrossRef] [PubMed]
- Thorakkattu, P.; Khanashyam, A.C.; Shah, K.; Babu, K.S.; Mundanat, A.S.; Deliephan, A.; Deokar, G.S.; Santivarangkna, C.; Nirmal, N.P. Postbiotics: Current Trends in Food and Pharmaceutical Industry. Foods 2022, 11, 3094. [Google Scholar] [CrossRef] [PubMed]
- Moradi, M.; Kousheh, S.A.; Almasi, H.; Alizadeh, A.; Guimarães, J.T.; Yılmaz, N.; Lotfi, A. Postbiotics produced by lactic acid bacteria: The next frontier in food safety. Compr. Rev. Food Sci. Food Saf. 2020, 19, 3390–3415. [Google Scholar] [CrossRef]
- De Marco, S.; Sichetti, M.; Muradyan, D.; Piccioni, M.; Traina, G.; Pagiotti, R.; Pietrella, D. Probiotic Cell-Free Supernatants Exhibited Anti-Inflammatory and Antioxidant Activity on Human Gut Epithelial Cells and Macrophages Stimulated with LPS. Evid. Based Complement. Altern. Med. 2018, 2018, 1756308. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, A.; Aghebati-Maleki, L.; Homayouni-Rad, A. The promising biological role of postbiotics derived from probiotic Lactobacillus species in reproductive health. Crit. Rev. Food Sci. Nutr. 2022, 32, 8829–8841. [Google Scholar] [CrossRef]
- Fang, F.; Li, Y.; Lu, X.; Wu, K.; Zhou, L.; Sun, Y.; Wu, J.; Gao, J. Effect of potential postbiotics derived from food-isolated Lactobacillus parabuchneri on different enterotypes of human gut microbiome. LWT 2023, 182, 114782. [Google Scholar] [CrossRef]
- Costantini, L.; Molinari, R.; Farinon, B.; Merendino, N. Impact of Omega-3 Fatty Acids on the Gut Microbiota. Int. J. Mol. Sci. 2017, 18, 2645. [Google Scholar] [CrossRef]
- Yang, P.; Jiang, Y.; Fischer, S.M. Prostaglandin E3 metabolism and cancer. Cancer Lett. 2014, 348, 1–11. [Google Scholar] [CrossRef]
- Ochoa-Repáraz, J.; Kasper, L.H. The Second Brain: Is the Gut Microbiota a Link Between Obesity and Central Nervous System Disorders? Curr. Obes. Rep. 2016, 5, 51–64. [Google Scholar] [CrossRef]
- Watson, H.; Mitra, S.; Croden, F.C.; Taylor, M.; Wood, H.M.; Perry, S.L.; Spencer, J.A.; Quirke, P.; Toogood, G.J.; Lawton, C.L.; et al. A randomised trial of the effect of omega-3 polyunsaturated fatty acid supplements on the human intestinal microbiota. Gut 2018, 67, 1974–1983. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Pan, S.; Li, F.; Xu, X.; Xing, H. Plant-Derived Bioactive Compounds and Potential Health Benefits: Involvement of the Gut Microbiota and Its Metabolic Activity. Biomolecules 2022, 12, 1871. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.-Z.; Li, X.-Y.; Wang, S.; Shen, L.; Ji, H.-F. Bidirectional interactions between curcumin and gut microbiota in transgenic mice with Alzheimer’s disease. Appl. Microbiol. Biotechnol. 2020, 104, 3507–3515. [Google Scholar] [CrossRef] [PubMed]
- Islam, T.; Koboziev, I.; Albracht-Schulte, K.; Mistretta, B.; Scoggin, S.; Yosofvand, M.; Moussa, H.; Zabet-Moghaddam, M.; Ramalingam, L.; Gunaratne, P.H.; et al. Curcumin Reduces Adipose Tissue Inflammation and Alters Gut Microbiota in Diet-Induced Obese Male Mice. Mol. Nutr. Food Res. 2021, 65, e2100274. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; You, J.; Wang, Z.; Liu, Y.; Wang, B.; Du, M.; Zou, T. Curcumin alleviates high-fat diet-induced hepatic steatosis and obesity in association with modulation of gut microbiota in mice. Food Res. Int. 2021, 143, 110270. [Google Scholar] [CrossRef]
- Peterson, C.T.; Vaughn, A.R.; Sharma, V.; Chopra, D.; Mills, P.J.; Peterson, S.N.; Sivamani, R.K. Effects of Turmeric and Curcumin Dietary Supplementation on Human Gut Microbiota: A Double-Blind, Randomized, Placebo-Controlled Pilot Study. J. Evid. Based Integr. Med. 2018, 23, 2515690X18790725. [Google Scholar] [CrossRef]
- Tang, P.C.-T.; Ng, Y.-F.; Ho, S.; Gyda, M.; Chan, S.-W. Resveratrol and cardiovascular health—Promising therapeutic or hopeless illusion? Pharmacol. Res. 2014, 90, 88–115. [Google Scholar] [CrossRef]
- Zhao, W.; Huang, X.; Han, X.; Hu, D.; Hu, X.; Li, Y.; Huang, P.; Yao, W. Resveratrol Suppresses Gut-Derived NLRP3 Inflammasome Partly through Stabilizing Mast Cells in a Rat Model. Mediat. Inflamm. 2018, 2018, 6158671. [Google Scholar] [CrossRef]
- Chen, M.-L.; Yi, L.; Zhang, Y.; Zhou, X.; Ran, L.; Yang, J.; Zhu, J.-D.; Zhang, Q.-Y.; Mi, M.-T. Resveratrol Attenuates Trimethylamine- N -Oxide (TMAO)-Induced Atherosclerosis by Regulating TMAO Synthesis and Bile Acid Metabolism via Remodeling of the Gut Microbiota. mBio 2016, 7, e02210-15. [Google Scholar] [CrossRef]
- Alrafas, H.R.; Busbee, P.B.; Nagarkatti, M.; Nagarkatti, P.S. Resveratrol modulates the gut microbiota to prevent murine colitis development through induction of Tregs and suppression of Th17 cells. J. Leukoc. Biol. 2019, 106, 467–480. [Google Scholar] [CrossRef]
- Lavefve, L.; Howard, L.R.; Carbonero, F. Berry polyphenols metabolism and impact on human gut microbiota and health. Food Funct. 2020, 11, 45–65. [Google Scholar] [CrossRef] [PubMed]
- Song, M.-Y.; Wang, J.-H.; Eom, T.; Kim, H. Schisandra chinensis fruit modulates the gut microbiota composition in association with metabolic markers in obese women: A randomized, double-blind placebo-controlled study. Nutr. Res. 2015, 35, 655–663. [Google Scholar] [CrossRef] [PubMed]
- Temple, N.J. A rational definition for functional foods: A perspective. Front. Nutr. 2022, 9, 957516. [Google Scholar] [CrossRef] [PubMed]
- Essa, M.M.; Bishir, M.; Bhat, A.; Chidambaram, S.B.; Al-Balushi, B.; Hamdan, H.; Govindarajan, N.; Freidland, R.P.; Qoronfleh, M.W. Functional foods and their impact on health. J. Food Sci. Technol. 2023, 60, 820–834. [Google Scholar] [CrossRef] [PubMed]
- Xia, H.; Liu, C.; Li, C.-C.; Fu, M.; Takahashi, S.; Hu, K.-Q.; Aizawa, K.; Hiroyuki, S.; Wu, G.; Zhao, L.; et al. Dietary Tomato Powder Inhibits High-Fat Diet–Promoted Hepatocellular Carcinoma with Alteration of Gut Microbiota in Mice Lacking Carotenoid Cleavage Enzymes. Cancer Prev. Res. 2018, 11, 797–810. [Google Scholar] [CrossRef]
- Farag, M.A.; Abdelwareth, A.; Sallam, I.E.; El Shorbagi, M.; Jehmlich, N.; Fritz-Wallace, K.; Schäpe, S.S.; Rolle-Kampczyk, U.; Ehrlich, A.; Wessjohann, L.A.; et al. Metabolomics reveals impact of seven functional foods on metabolic pathways in a gut microbiota model. J. Adv. Res. 2020, 23, 47–59. [Google Scholar] [CrossRef]
- Kaczmarek, J.L.; Liu, X.; Charron, C.S.; Novotny, J.A.; Jeffery, E.H.; Seifried, H.E.; Ross, S.A.; Miller, M.J.; Swanson, K.S.; Holscher, H.D. Broccoli consumption affects the human gastrointestinal microbiota. J. Nutr. Biochem. 2019, 63, 27–34. [Google Scholar] [CrossRef]
- Huang, L.; Zheng, T.; Hui, H.; Xie, G. Soybean isoflavones modulate gut microbiota to benefit the health weight and metabolism. Front. Cell. Infect. Microbiol. 2022, 12, 8695. [Google Scholar] [CrossRef]
- Cladis, D.P.; Simpson, A.M.R.; Cooper, K.J.; Nakatsu, C.H.; Ferruzzi, M.G.; Weaver, C.M. Blueberry polyphenols alter gut microbiota & phenolic metabolism in rats. Food Funct. 2021, 12, 2442–2456. [Google Scholar] [CrossRef]
- Sivamani, R.K.; Chakkalakal, M.; Pan, A.; Nadora, D.; Min, M.; Dumont, A.; Burney, W.A.; Chambers, C.J. Prospective Randomized, Double-Blind, Placebo-Controlled Study of a Standardized Oral Pomegranate Extract on the Gut Microbiome and Short-Chain Fatty Acids. Foods 2023, 13, 15. [Google Scholar] [CrossRef] [PubMed]
- García-Mantrana, I.; Calatayud, M.; Romo-Vaquero, M.; Espín, J.C.; Selma, M.V.; Collado, M.C. Urolithin Metabotypes Can Determine the Modulation of Gut Microbiota in Healthy Individuals by Tracking Walnuts Consumption over Three Days. Nutrients 2019, 11, 2483. [Google Scholar] [CrossRef]
- Dhillon, J.; Li, Z.; Ortiz, R.M. Almond Snacking for 8 wk Increases Alpha-Diversity of the Gastrointestinal Microbiome and Decreases Bacteroides fragilis Abundance Compared with an Isocaloric Snack in College Freshmen. Curr. Dev. Nutr. 2019, 3, nzz079. [Google Scholar] [CrossRef]
- Toribio-Mateas, M.A.; Bester, A.; Klimenko, N. Impact of Plant-Based Meat Alternatives on the Gut Microbiota of Consumers: A Real-World Study. Foods 2021, 10, 2040. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Yang, C.; Liu, B.; Lin, L.; Sarker, S.D.; Nahar, L.; Yu, H.; Cao, H.; Xiao, J. Bioactive compounds from marine macroalgae and their hypoglycemic benefits. Trends Food Sci. Technol. 2018, 72, 1–12. [Google Scholar] [CrossRef]
- Yang, C.-F.; Lai, S.-S.; Chen, Y.-H.; Liu, D.; Liu, B.; Ai, C.; Wan, X.-Z.; Gao, L.-Y.; Chen, X.-H.; Zhao, C. Anti-diabetic effect of oligosaccharides from seaweed Sargassum confusum via JNK-IRS1/PI3K signalling pathways and regulation of gut microbiota. Food Chem. Toxicol. 2019, 131, 110562. [Google Scholar] [CrossRef]
- Li, S.; Wang, L.; Liu, B.; He, N. Unsaturated alginate oligosaccharides attenuated obesity-related metabolic abnormalities by modulating gut microbiota in high-fat-diet mice. Food Funct. 2020, 11, 4773–4784. [Google Scholar] [CrossRef]
- Guerrero-Wyss, M.; Yans, C.; Boscán-González, A.; Duran, P.; Parra-Soto, S.; Angarita, L. Durvillaea antarctica: A Seaweed for Enhancing Immune and Cardiometabolic Health and Gut Microbiota Composition Modulation. Int. J. Mol. Sci. 2023, 24, 10779. [Google Scholar] [CrossRef]
- Young, W.; Arojju, S.K.; McNeill, M.R.; Rettedal, E.; Gathercole, J.; Bell, N.; Payne, P. Feeding Bugs to Bugs: Edible Insects Modify the Human Gut Microbiome in an in vitro Fermentation Model. Front. Microbiol. 2020, 11, 1763. [Google Scholar] [CrossRef]
- Vamanu, E.; Dinu, L.D.; Pelinescu, D.R.; Gatea, F. Therapeutic Properties of Edible Mushrooms and Herbal Teas in Gut Microbiota Modulation. Microorganisms 2021, 9, 1262. [Google Scholar] [CrossRef]
- Tung, Y.-T.; Pan, C.-H.; Chien, Y.-W.; Huang, H.-Y. Edible Mushrooms: Novel Medicinal Agents to Combat Metabolic Syndrome and Associated Diseases. Curr. Pharm. Des. 2020, 26, 4970–4981. [Google Scholar] [CrossRef] [PubMed]
- Xue, Z.; Ma, Q.; Chen, Y.; Lu, Y.; Wang, Y.; Jia, Y.; Zhang, M.; Chen, H. Structure characterization of soluble dietary fiber fractions from mushroom Lentinula edodes (Berk.) Pegler and the effects on fermentation and human gut microbiota in vitro. Food Res. Int. 2020, 129, 108870. [Google Scholar] [CrossRef] [PubMed]
- Mitsou, E.K.; Saxami, G.; Stamoulou, E.; Kerezoudi, E.; Terzi, E.; Koutrotsios, G.; Bekiaris, G.; Zervakis, G.I.; Mountzouris, K.C.; Pletsa, V.; et al. Effects of Rich in Β-Glucans Edible Mushrooms on Aging Gut Microbiota Characteristics: An In Vitro Study. Molecules 2020, 25, 2806. [Google Scholar] [CrossRef]
- Valentino, V.; Magliulo, R.; Farsi, D.; Cotter, P.D.; O’Sullivan, O.; Ercolini, D.; De Filippis, F. Fermented foods, their microbiome and its potential in boosting human health. Microb. Biotechnol. 2024, 17, e14428. [Google Scholar] [CrossRef]
- Stephanie, S.; Kartawidjajaputra, F.; Silo, W.; Yogiara, Y.; Suwanto, A. Tempeh consumption enhanced beneficial bacteria in the human gut. Food Res. 2019, 3, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Thriene, K.; Hansen, S.S.; Binder, N.; Michels, K.B. Effects of Fermented Vegetable Consumption on Human Gut Microbiome Diversity—A Pilot Study. Fermentation 2022, 8, 118. [Google Scholar] [CrossRef]
- Barber, T.M.; Kabisch, S.; Pfeiffer, A.F.H.; Weickert, M.O. The Effects of the Mediterranean Diet on Health and Gut Microbiota. Nutrients 2023, 15, 2150. [Google Scholar] [CrossRef]
- De Filippis, F.; Pellegrini, N.; Vannini, L.; Jeffery, I.B.; La Storia, A.; Laghi, L.; Serrazanetti, D.I.; Di Cagno, R.; Ferrocino, I.; Lazzi, C.; et al. High-level adherence to a Mediterranean diet beneficially impacts the gut microbiota and associated metabolome. Gut 2016, 65, 1812–1821. [Google Scholar] [CrossRef]
- Khavandegar, A.; Heidarzadeh, A.; Angoorani, P.; Hasani-Ranjbar, S.; Ejtahed, H.-S.; Larijani, B.; Qorbani, M. Adherence to the Mediterranean diet can beneficially affect the gut microbiota composition: A systematic review. BMC Med Genom. 2024, 17, 1791. [Google Scholar] [CrossRef]
- Garcia-Mantrana, I.; Selma-Royo, M.; Alcantara, C.; Collado, M.C. Shifts on Gut Microbiota Associated to Mediterranean Diet Adherence and Specific Dietary Intakes on General Adult Population. Front. Microbiol. 2018, 9, 890. [Google Scholar] [CrossRef]
- Gallè, F.; Valeriani, F.; Cattaruzza, M.S.; Gianfranceschi, G.; Liguori, R.; Antinozzi, M.; Mederer, B.; Liguori, G.; Spica, V.R. Mediterranean Diet, Physical Activity and Gut Microbiome Composition: A Cross-Sectional Study among Healthy Young Italian Adults. Nutrients 2020, 12, 2164. [Google Scholar] [CrossRef]
- Veronese, N.; Reginster, J.-Y. The effects of calorie restriction, intermittent fasting and vegetarian diets on bone health. Aging Clin. Exp. Res. 2019, 31, 753–758. [Google Scholar] [CrossRef]
- Wang, H.; Wei, C.-X.; Min, L.; Zhu, L.-Y. Good or bad: Gut bacteria in human health and diseases. Biotechnol. Biotechnol. Equip. 2018, 32, 1075–1080. [Google Scholar] [CrossRef]
- Hjorth, M.F.; Blædel, T.; Bendtsen, L.Q.; Lorenzen, J.K.; Holm, J.B.; Kiilerich, P.; Roager, H.M.; Kristiansen, K.; Larsen, L.H.; Astrup, A. Prevotella-to-Bacteroides ratio predicts body weight and fat loss success on 24-week diets varying in macronutrient composition and dietary fiber: Results from a post-hoc analysis. Int. J. Obes. 2019, 43, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Tomova, A.; Bukovsky, I.; Rembert, E.; Yonas, W.; Alwarith, J.; Barnard, N.D.; Kahleova, H. The Effects of Vegetarian and Vegan Diets on Gut Microbiota. Front. Nutr. 2019, 6, 47. [Google Scholar] [CrossRef] [PubMed]
- Losasso, C.; Eckert, E.M.; Mastrorilli, E.; Villiger, J.; Mancin, M.; Patuzzi, I.; Di Cesare, A.; Cibin, V.; Barrucci, F.; Pernthaler, J.; et al. Assessing the Influence of Vegan, Vegetarian and Omnivore Oriented Westernized Dietary Styles on Human Gut Microbiota: A Cross Sectional Study. Front. Microbiol. 2018, 9, 317. [Google Scholar] [CrossRef] [PubMed]
- Ang, Q.Y.; Alexander, M.; Newman, J.C.; Tian, Y.; Cai, J.; Upadhyay, V.; Turnbaugh, J.A.; Verdin, E.; Hall, K.D.; Leibel, R.L.; et al. Ketogenic Diets Alter the Gut Microbiome Resulting in Decreased Intestinal Th17 Cells. Cell 2020, 181, 1263–1275.e16. [Google Scholar] [CrossRef]
- Attaye, I.; van Oppenraaij, S.; Warmbrunn, M.V.; Nieuwdorp, M. The Role of the Gut Microbiota on the Beneficial Effects of Ketogenic Diets. Nutrients 2022, 14, 191. [Google Scholar] [CrossRef]
- Basciani, S.; Camajani, E.; Contini, S.; Persichetti, A.; Risi, R.; Bertoldi, L.; Strigari, L.; Prossomariti, G.; Watanabe, M.; Mariani, S.; et al. Very-Low-Calorie Ketogenic Diets with Whey, Vegetable, or Animal Protein in Patients With Obesity: A Randomized Pilot Study. J. Clin. Endocrinol. Metab. 2020, 105, dgaa336. [Google Scholar] [CrossRef]
- Zouhal, H.; Saeidi, A.; Salhi, A.; Li, H.; Essop, M.F.; Laher, I.; Rhibi, F.; Amani-Shalamzari, S.; Ben Abderrahman, A. Exercise Training and Fasting: Current Insights. Open Access J. Sports Med. 2020, 11, 1–28. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, R. The Effect of Fasting on Human Metabolism and Psychological Health. Dis. Markers 2022, 2022, 5653739. [Google Scholar] [CrossRef] [PubMed]
- Zeb, F.; Wu, X.; Chen, L.; Fatima, S.; Haq, I.-U.; Chen, A.; Majeed, F.; Feng, Q.; Li, M. Effect of time-restricted feeding on metabolic risk and circadian rhythm associated with gut microbiome in healthy males. Br. J. Nutr. 2020, 123, 1216–1226. [Google Scholar] [CrossRef]
- Zeevi, D.; Korem, T.; Zmora, N.; Israeli, D.; Rothschild, D.; Weinberger, A.; Ben-Yacov, O.; Lador, D.; Avnit-Sagi, T.; Lotan-Pompan, M.; et al. Personalized Nutrition by Prediction of Glycemic Responses. Cell 2015, 163, 1079–1094. [Google Scholar] [CrossRef] [PubMed]
- Mendes-Soares, H.; Raveh-Sadka, T.; Azulay, S.; Edens, K.; Ben-Shlomo, Y.; Cohen, Y.; Ofek, T.; Bachrach, D.; Stevens, J.; Colibaseanu, D.; et al. Assessment of a Personalized Approach to Predicting Postprandial Glycemic Responses to Food Among Individuals Without Diabetes. JAMA Netw. Open 2019, 2, e188102. [Google Scholar] [CrossRef]
- Berry, S.; Valdes, A.; Davies, R.; Al Khatib, H.; Delahanty, L.; Drew, D.; Tan Chan, A.; Segata, N.; Franks, P.; Spector, T. Large Inter-individual Variation in Postprandial Lipemia Follow-ing a Mixed Meal in over 1000 Twins and Singletons from the UK and US: The PREDICT I Study (OR19-06-19). Curr. Dev. Nutr. 2019, 3, nzz046-OR19. [Google Scholar] [CrossRef]
- Kolodziejczyk, A.A.; Zheng, D.; Elinav, E. Diet–microbiota interactions and personalized nutrition. Nat. Rev. Microbiol. 2019, 17, 742–753. [Google Scholar] [CrossRef]
- Post, M.J.; Levenberg, S.; Kaplan, D.L.; Genovese, N.; Fu, J.; Bryant, C.J.; Negowetti, N.; Verzijden, K.; Moutsatsou, P. Scientific, sustainability and regulatory challenges of cultured meat. Nat. Food 2020, 1, 403–415. [Google Scholar] [CrossRef]
- Singh, T.; Shukla, S.; Kumar, P.; Wahla, V.; Bajpai, V.K.; Rather, I.A. Application of Nanotechnology in Food Science: Perception and Overview. Front. Microbiol. 2017, 8, 1501. [Google Scholar] [CrossRef]
- Yemmireddy, V.; Adhikari, A.; Moreira, J. Effect of ultraviolet light treatment on microbiological safety and quality of fresh produce: An overview. Front. Nutr. 2022, 9, 871243. [Google Scholar] [CrossRef]
- Augustin, M.A.; Hartley, C.J.; Maloney, G.; Tyndall, S. Innovation in precision fermentation for food ingredients. Crit. Rev. Food Sci. Nutr. 2024, 64, 6218–6238. [Google Scholar] [CrossRef]
- Avgoustaki, D.D.; Xydis, G. How Energy Innovation in Indoor Vertical Farming Can Improve Food Security, Sustainability, and Food Safety? In Advances in Food Security and Sustainability; Cohen, M.J., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; Volume 5, pp. 1–51. [Google Scholar] [CrossRef]
- Norum, B. World’s First 3D Printing Restaurant Set for London. The Standard. 2016. Available online: https://www.standard.co.uk/going-out/restaurants/world-s-first-3d-printing-restaurant-set-for-london-a3294871.html (accessed on 6 August 2024).
- Kumar, K.; Gambhir, G.; Dass, A.; Tripathi, A.K.; Singh, A.; Jha, A.K.; Yadava, P.; Choudhary, M.; Rakshit, S. Genetically modified crops: Current status and future prospects. Planta 2020, 251, 91. [Google Scholar] [CrossRef]
- Eilam, Y.; Khattib, H.; Pintel, N.; Avni, D. Microalgae—Sustainable Source for Alternative Proteins and Functional Ingredients Promoting Gut and Liver Health. Glob. Challenges 2023, 7, 2200177. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, A.P.F.; Bragotto, A.P.A. Microalgae-based products: Food and public health. Futur. Foods 2022, 6, 100157. [Google Scholar] [CrossRef]
- Sá, A.G.A.; Moreno, Y.M.F.; Carciofi, B.A.M. Food processing for the improvement of plant proteins digestibility. Crit. Rev. Food Sci. Nutr. 2020, 60, 3367–3386. [Google Scholar] [CrossRef] [PubMed]
- Ashwell, M.; Gibson, S.; Bellisle, F.; Buttriss, J.; Drewnowski, A.; Fantino, M.; Gallagher, A.M.; de Graaf, K.; Goscinny, S.; Hardman, C.A.; et al. Expert consensus on low-calorie sweeteners: Facts, research gaps and suggested actions. Nutr. Res. Rev. 2020, 33, 145–154. [Google Scholar] [CrossRef]
- Hashempour-Baltork, F.; Khosravi-Darani, K.; Hosseini, H.; Farshi, P.; Reihani, S.F.S. Mycoproteins as safe meat substitutes. J. Clean. Prod. 2020, 253, 119958. [Google Scholar] [CrossRef]
- Magara, H.J.O.; Niassy, S.; Ayieko, M.A.; Mukundamago, M.; Egonyu, J.P.; Tanga, C.M.; Kimathi, E.K.; Ongere, J.O.; Fiaboe, K.K.M.; Hugel, S.; et al. Edible Crickets (Orthoptera) Around the World: Distribution, Nutritional Value, and Other Benefits—A Review. Front. Nutr. 2021, 7, 537915. [Google Scholar] [CrossRef] [PubMed]
- De Domenico, S.; De Rinaldis, G.; Paulmery, M.; Piraino, S.; Leone, A. Barrel Jellyfish (Rhizostoma pulmo) as Source of Antioxidant Peptides. Mar. Drugs 2019, 17, 134. [Google Scholar] [CrossRef]
- Wang, X.; Qi, Y.; Zheng, H. Dietary Polyphenol, Gut Microbiota, and Health Benefits. Antioxidants 2022, 11, 1212. [Google Scholar] [CrossRef]
- Abouelela, M.E.; Helmy, Y.A. Next-Generation Probiotics as Novel Therapeutics for Improving Human Health: Current Trends and Future Perspectives. Microorganisms 2024, 12, 430. [Google Scholar] [CrossRef]
- van Huis, A. Insects as food and feed, a new emerging agricultural sector: A review. J. Insects Food Feed. 2020, 6, 27–44. [Google Scholar] [CrossRef]
- Blumfield, M.; Abbott, K.; Duve, E.; Cassettari, T.; Marshall, S.; Fayet-Moore, F. Examining the health effects and bioactive components in Agaricus bisporus mushrooms: A scoping review. J. Nutr. Biochem. 2020, 84, 108453. [Google Scholar] [CrossRef] [PubMed]
- Thøgersen, R.; Castro-Mejía, J.L.; Sundekilde, U.K.; Hansen, L.H.; Gray, N.; Kuhnle, G.; Jørgensen, N.R.; Hansen, A.K.; Nielsen, D.S.; Bertram, H.C. Inulin and milk mineral fortification of a pork sausage exhibits distinct effects on the microbiome and biochemical activity in the gut of healthy rats. Food Chem. 2020, 331, 127291. [Google Scholar] [CrossRef]
- Deng, J.; Yun, J.; Gu, Y.; Yan, B.; Yin, B.; Huang, C. Evaluating the In Vitro and In Vivo Prebiotic Effects of Different Xylo-Oligosaccharides Obtained from Bamboo Shoots by Hydrothermal Pretreatment Combined with Endo-Xylanase Hydrolysis. Int. J. Mol. Sci. 2023, 24, 13422. [Google Scholar] [CrossRef] [PubMed]
- Chambers, E.S.; Byrne, C.S.; Morrison, D.J.; Murphy, K.G.; Preston, T.; Tedford, C.; Garcia-Perez, I.; Fountana, S.; Serrano-Contreras, J.I.; Holmes, E.; et al. Dietary supplementation with inulin-propionate ester or inulin improves insulin sensitivity in adults with overweight and obesity with distinct effects on the gut microbiota, plasma metabolome and systemic inflammatory responses: A randomised cross-over trial. Gut 2019, 68, 1430–1438. [Google Scholar] [CrossRef]
- Refael, G.; Riess, H.T.; Levi, C.S.; Magzal, F.; Tamir, S.; Koren, O.; Lesmes, U. Responses of the human gut microbiota to physiologically digested insect powders or isolated chitin thereof. Futur. Foods 2022, 6, 100197. [Google Scholar] [CrossRef]
- Lu, X.; Xu, H.; Fang, F.; Liu, J.; Wu, K.; Zhang, Y.; Wu, J.; Gao, J. In vitro effects of two polysaccharide fractions from Laminaria japonica on gut microbiota and metabolome. Food Funct. 2023, 14, 3379–3390. [Google Scholar] [CrossRef]
- Charoensiddhi, S.; Conlon, M.; Methacanon, P.; Thayanukul, P.; Hongsprabhas, P.; Zhang, W. Gut microbiome modulation and gastrointestinal digestibility in vitro of polysaccharide-enriched extracts and seaweeds from Ulva rigida and Gracilaria fisheri. J. Funct. Foods 2022, 96, 105204. [Google Scholar] [CrossRef]
- Jia, L.; Peng, X.; Deng, Z.; Zhang, B.; Li, H. The structural characterization of polysaccharides from three cultivars of Moringa oleifera Lam. root and their effects on human intestinal microflora. Food Biosci. 2023, 52, 102482. [Google Scholar] [CrossRef]
- Stull, V.J.; Finer, E.; Bergmans, R.S.; Febvre, H.P.; Longhurst, C.; Manter, D.K.; Patz, J.A.; Weir, T.L. Impact of Edible Cricket Consumption on Gut Microbiota in Healthy Adults, a Double-blind, Randomized Crossover Trial. Sci. Rep. 2018, 8, 10762. [Google Scholar] [CrossRef]
- Kleigrewe, K.; Haack, M.; Baudin, M.; Ménabréaz, T.; Crovadore, J.; Masri, M.; Beyrer, M.; Andlauer, W.; Lefort, F.; Dawid, C.; et al. Dietary Modulation of the Human Gut Microbiota and Metabolome with Flaxseed Preparations. Int. J. Mol. Sci. 2022, 23, 10473. [Google Scholar] [CrossRef] [PubMed]
- Nikoloudaki, O.; Celano, G.; Polo, A.; Cappello, C.; Granehäll, L.; Costantini, A.; Vacca, M.; Speckmann, B.; Di Cagno, R.; Francavilla, R.; et al. Novel probiotic preparation with in vivo gluten-degrading activity and potential modulatory effects on the gut microbiota. Microbiol. Spectr. 2024, 12, e0352423. [Google Scholar] [CrossRef] [PubMed]
- Jang, Y.J.; Min, B.; Lim, J.H.; Kim, B.-Y. In Vitro Evaluation of Probiotic Properties of Two Novel Probiotic Mixtures, Consti-Biome and Sensi-Biome. J. Microbiol. Biotechnol. 2023, 33, 1149–1161. [Google Scholar] [CrossRef] [PubMed]
- Guse, K.; Sharma, A.; Weyenberg, E.; Davison, S.; Ma, Y.; Choi, Y.; Johnson, A.J.; Chen, C.; Gomez, A. Regular consumption of lacto-fermented vegetables has greater effects on the gut metabolome compared with the microbiome. Gut Microbiome 2023, 4, e11. [Google Scholar] [CrossRef]
- Tamargo, A.; Cueva, C.; Laguna, L.; Moreno-Arribas, M.; Muñoz, L.A. Understanding the impact of chia seed mucilage on human gut microbiota by using the dynamic gastrointestinal model simgi®. J. Funct. Foods 2018, 50, 104–111. [Google Scholar] [CrossRef]
- Tindall, A.M.; McLimans, C.J.; Petersen, K.S.; Kris-Etherton, P.M.; Lamendella, R. Walnuts and Vegetable Oils Containing Oleic Acid Differentially Affect the Gut Microbiota and Associations with Cardiovascular Risk Factors: Follow-up of a Randomized, Controlled, Feeding Trial in Adults at Risk for Cardiovascular Disease. J. Nutr. 2020, 150, 806–817. [Google Scholar] [CrossRef]
- Jin, M.Y.; Wu, X.Y.; Li, M.Y.; Li, X.T.; Huang, R.M.; Sun, Y.M.; Xu, Z.L. Noni (Morinda citrifolia L.) Fruit Polysaccharides Regulated IBD Mice Via Targeting Gut Microbiota: Association of JNK/ERK/NF-κB Signaling Pathways. J. Agric. Food Chem. 2021, 69, 10151–10162. [Google Scholar] [CrossRef]
- Siddiqui, N.Z.; Rehman, A.U.; Yousuf, W.; Khan, A.I.; Farooqui, N.A.; Zang, S.; Xin, Y.; Wang, L. Effect of crude polysaccharide from seaweed, Dictyopteris divaricata (CDDP) on gut microbiota restoration and anti-diabetic activity in streptozotocin (STZ)-induced T1DM mice. Gut Pathog. 2022, 14, 39. [Google Scholar] [CrossRef]
- Hu, J.; Li, Y.; Pakpour, S.; Wang, S.; Pan, Z.; Liu, J.; Wei, Q.; She, J.; Cang, H.; Zhang, R.X. Dose Effects of Orally Administered Spirulina Suspension on Colonic Microbiota in Healthy Mice. Front. Cell. Infect. Microbiol. 2019, 9, 243. [Google Scholar] [CrossRef]
- Zhao, R.; Hu, Q.; Ma, G.; Su, A.; Xie, M.; Li, X.; Chen, G.; Zhao, L. Effects of Flammulina velutipes polysaccharide on immune response and intestinal microbiota in mice. J. Funct. Foods 2019, 56, 255–264. [Google Scholar] [CrossRef]
- Khan, I.; Huang, G.; Li, X.; Leong, W.; Xia, W.; Hsiao, W.W. Mushroom polysaccharides from Ganoderma lucidum and Poria cocos reveal prebiotic functions. J. Funct. Foods 2018, 41, 191–201. [Google Scholar] [CrossRef]
- Jiang, T.; Li, Y.; Li, L.; Liang, T.; Du, M.; Yang, L.; Yang, J.; Yang, R.; Zhao, H.; Chen, M.; et al. Bifidobacterium longum 070103 Fermented Milk Improve Glucose and Lipid Metabolism Disorders by Regulating Gut Microbiota in Mice. Nutrients 2022, 14, 4050. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.Q.; Ong, H.C.; Yong, A.M.H.; Fattori, V.; Mukherjee, K. Addressing the safety of new food sources and production systems. Compr. Rev. Food Sci. Food Saf. 2024, 23, e13341. [Google Scholar] [CrossRef] [PubMed]

| Region/Country | Definition | Reference |
|---|---|---|
| European Union | Food produced using recent technologies and production processes or that is or has been traditionally consumed outside the EU and has not been consumed in the EU to a significant degree. | [19] |
| United Kingdom of Great Britain and Northern Ireland | A food that has not been significantly consumed by humans in the UK before 15 May 1997 is classified as a “novel food” under UK legislation. | [20] |
| India | A food may lack a history of human consumption, or its ingredients and sources may not have been previously consumed. Alternatively, it could be a food or ingredient produced using recent technology or innovative engineering processes. These methods may alter the size, composition, or structure of the food or its ingredients, potentially affecting its nutritional value, metabolism, or the presence of undesirable substances. | [21] |
| Israel | A newly developed, innovative food created through modern technologies and production methods, or a food traditionally consumed outside of Israel. | [22] |
| Gulf Cooperation Council | Novel food is composed of, isolated from, or produced through cell culture or tissue culture derived from animals, plants, microorganisms, fungi, or algae. | [23] |
| China | Any food not traditionally consumed in China, including organisms, extracts from organisms, food ingredients with modified structures, or newly developed food components. | [24] |
| Japan | Foods with no history of safe use or those produced using new processes must undergo a novel food safety assessment. | [25] |
| Singapore | Foods that have not been widely consumed as food for at least 20 years, whether within Singapore or internationally. | [26] |
| Thailand | Any substance used as food or a food ingredient that has been widely consumed by humans for less than 15 years based on scientific or reliable evidence, or that has undergone a production process not currently in use, where this process significantly alters the composition or structure of the food, impacting its nutritional value, metabolism, or the presence of undesirable substances. | [27] |
| Republic of Korea | Food ingredients that have no history of consumption in Korea. | [28] |
| Australia and Nueva Zelanda | Novel foods, which are not traditionally consumed, must be evaluated by Food Standards Australia New Zealand to ensure their safety before they can be introduced into the food supply. | [29] |
| United States of America | There is no explicit definition for novel foods; however, any new food substance must receive premarket approval from the US FDA unless it is classified as Generally Recognized as Safe (GRAS). | [30] |
| Canada | Novel foods fall into three major categories: Substances with no history of safe use as food. Foods manufactured, prepared, preserved, or packaged using new processes. Foods derived from plants, animals, or microorganisms that have been genetically modified. | [31] |
| Brazil | Novel foods are substances with no history of consumption in Brazil, or foods containing substances that are already consumed but at levels significantly higher than those currently observed in regular diets. These foods require premarket approval, which may be renewed every five years. | [32] |
| Definition | Key Issues | Author | Reference |
|---|---|---|---|
| Communities of symbiotic and pathogenic microorganisms that share the human body space. | Introduces the coexistence of symbionts and pathogenic microorganisms that cohabit in the human body. | Lederberg and McCray | [1] |
| Includes all microorganisms and their genetic material in a human organism as well as the environmental conditions that affect them. | The influence on host physiology and health focuses on a specific environment. | Duncan et al., | [53] |
| Collection of microorganisms that live in various habitats of the human body, each with distinct functions that affect health and disease. | Diversity of habitats within the human body and their distinct functions. | Turnbaugh et al. | [54] |
| Set of genes harbouring the microbial communities that coexist with humans in the gut. | Variability in the composition of the microbiome between individuals and over time within a single person. | Ursell et al. | [55] |
| A complete habitat includes microorganisms (bacteria, archaea, lower and higher eukaryotes, and viruses), their genomes (genes), and the surrounding environmental conditions. | They encompass both biotic and abiotic factors in specific environments. | Marchesi and Ravel | [56] |
| The human microbiome encompasses all microorganisms residing in the human body, estimated at around 39 trillion bacteria, which surpasses the number of human cells. | Specific number of bacteria compared to human cells. | Sender, Fuchs, and Milo | [57] |
| This concept applies not only to bacteria-mediated conditions in the gut but also to viral, fungal, and host–microbe interactions throughout the human body. This includes environmental microbial communities that interact with human health, such as those in the built environment, livestock, agriculture, and pets. | Potential in public health to discover new biomarkers, therapeutics, and molecular mechanisms in human populations. | Wilkinson et al. | [51] |
| The microbiome is a community of microorganisms, including bacteria, fungi, archaea, protists, and viruses, whose genetic material it found within or on a specific host organism. These microorganisms interact with each other and their environment to create distinct ecological niches. Microbiomes evolve over time and across different scales, and are intricately connected with macro-ecosystems that include eukaryotic hosts, where they play a vital role in overall functioning and health. | It covers the diversity of microorganisms, their functions, and their interactions within a habitat with different physicochemical properties. | Berg et al. | [2] |
| Interactive and fluctuating communities of microbes colonise and develop on surfaces, including those associated with host organisms. | Vertical transmission refers to the transfer of microorganisms from mother to child during pregnancy, childbirth, and breastfeeding. | Murphy et al. | [52] |
| A complete community of microorganisms inhabits a given environment, such as the human body, animals, or the environment. | The holistic concept in the field of One Health facilitates interactions between humans, animals, and the environment, along with co-evolution, co-development, comet metabolism, and co-regulation with humans and animal partners. | Ma et al. | [50] |
| Collection of dynamic microbial communities inhabiting diverse anatomical locations in the human body. These microbial communities co-evolve with the host and play key roles in promoting human health. | A diverse array of microorganisms, including bacteria, archaea, fungi, viruses, and mobile genetic elements, have been identified. These microorganisms influence host physiology and play a crucial role in metabolism and immune system development. | Aggarwal | [11] |
| Classification | Type | Description | Reference |
|---|---|---|---|
| Foods and food ingredients derived from new production process | Cell-based food production | In vitro cultivation of animal cells followed by processing into products that resemble conventionally sourced meat. | [225] |
| Nanotechnology in food | Use of nanotechnology to improve the texture, taste, preservation, and nutritional properties of foods. | [226] | |
| UV-treated food | Exposure of food to UV-C radiation, which has a wavelength of between 200 and 280 nanometres. | [227] | |
| Precision fermentation | Use of genetically engineered microorganisms to produce food substances in a bioreactor, usually using simple starting materials such as sugar and glycerol. | [228] | |
| Vertical farming | New modality in indoor farming that has gained popularity in recent years due to increasing urbanisation coupled with food security concerns. | [229] | |
| 3D printing of foods | The use of a computer-controlled robotic process to construct solid food layers and fusing these layers together using physical or chemical methods. | [230] | |
| Foods and food ingredients with a new or intentionally modified primary molecular structure | Genetically modified organisms (GMOs) | Foods that have been genetically altered to enhance their performance, resistance, or nutritional value. | [231] |
| Foods and food ingredients consisting of or isolated from microorganisms, fungi, or algae | Microbial protein | Proteins obtained from bacteria, fungi, and microalgae. | [232] |
| Seaweed and microalgae | Seaweed and microalgae used as a source of protein, natural omega-3 long-chain fatty acids, soluble dietary fibres, vitamins, and minerals. | [233] | |
| Foods and food ingredients consisting of or isolated from plants or their parts | Plant-derived proteins | Derived from plant materials through chemical and mechanical processing, which effectively removes carbohydrates, lipids, and other non-protein components, resulting in a mixture where protein is the primary component. | [234] |
| Sugar substitutes | Ingredients or products that replace traditional food components. | [235] | |
| Plant-based alternatives | Primarily consuming plant-based foods, including fruits, vegetables, nuts, seeds, legumes, and whole grains. | [236] | |
| New food ingredients isolated from animals | Insects | Edible insects used as a source of protein and other essential nutrients. | [237] |
| Jellyfish | Edible species typically have low carbohydrate and lipid content, high protein levels (primarily collagen), and different essential minerals. | [238] |
| Food | Effect in Gut Microbiome | Type of Study | Reference |
|---|---|---|---|
| Bamboo (insoluble dietary fibre) | Increases Bifidobacterium adolescentis and Lactobacillus acidophilus | In vitro | [244] |
| Inulin supplementation Inulin-propionate ester supplementation | Increase Actinomycetota and decrease Clostridia with inulin supplementation. Higher proportion of Anaerostipes hadrus, Bifidobacterium faecale, and Bacteroides caccae. Lower proportion of Blautia obeum, Blautia luti, Oscillibacter spp., Blautia faecis, and Ruminococcus faecis. Higher proportion of Bacteroides uniformis, Bacteroides xylanisolvens, and Fusicatenibacter saccharivorans. Lower proportion of Blautia obeum, Anaerostipes hadrus, Bifidobacterium faecale, Prevotella copri, and Eubacterium ruminantium | In vivo | [245] |
| Powder from crickets Acheta domestica, silkworm pupae Bombyx mori, isolated chitin | Fluctuations in the relative abundance of Pseudomonadota, Bacteroidota, and Actinomycetota and the families Bifidobacteriaceae, Prevotellaceae, and Lachnospiraceae. | In vitro | [246] |
| Chitin induces the growth of Ruminococcaceae, and Lachnospiraceae, Faecalibacterium, and Roseburia | |||
| Two Laminaria japonica polysaccharides | Fraction with lower molecular weight is better at promoting the proliferation of Akkermansiaceae. Fraction with higher molecular weight reduces the Bacillota/Bacteroidota ratio and increases the content of Bacteroidaceae and Tannerellaceae | In vitro | [247] |
| Red seaweed (Gracilaria fisheri) | Increases the diversity of Roseburia and Faecalibacterium | In vitro | [248] |
| Moringa oleifera | Increases the abundance of Bacteroidota and decreases the abundance of Pseudomonadota | In vitro | [249] |
| Cricket flour (prebiotic) | Bacteroidota and Bacillota make up ~90% of sequences at phylum level.Increases Actinomycetota (Bifidobacterium animalis), decreases Lactobacillus reuteri, Leuconostoc, and Acidaminococcus genus | In vivo | [250] |
| Flaxseed supplementation | Decreases the abundance of Bacillota, Pseudomonadota, Bacteroidota | In vitro | [251] |
| Novel probiotic preparation | Most differentially abundant genera (87%) were identified in volunteers who consumed probiotics, including Coprococcus, Streptococcus, and Lactococcus | In vivo | [252] |
| Two novel probiotic mixtures (Consti-Biome and Sensi-Biome) | Consti-Biome and Sensi-Biome compete with Staphylococcus aureus and Escherichia coli, and prevent them from adhering to HT-29 cells | In vitro | [253] |
| Lacto-fermented vegetables | Increase Leuconostoc mesenteroides, Parabacteroides distasonis, Lachnospira, Ruminococcaceae, Coproccocus, Blautia, Clostridiales, Coprobacillus, AlphaPseudomonadota, Rhodotorula mucilaginosa, Penicillium, Starmerella, Cryptococcus laurentii, Vishniacozyma carnescens | In vivo | [254] |
| Chia mucilage | Increases Enterococcus spp. and Lactobacillus spp. | In vitro | [255] |
| Walnut | Increases the relative abundance of Roseburia, Eubacterium eligens group, Lachnospiraceae, and Leuconostocaceae | In vivo | [256] |
| Noni (Morinda citrifolia L.) fruit | Increases Enterobacteria, BetaPseudomonadota, Verrucomicrobiota, and reduces Gram-positive bacteria | In vivo | [257] |
| Plant-based OsomeFood Clean Label meal (fungi, algae, nuts, and plant protein) | Increases Faecalibacterium prausnitzii, Prevotella CAG 5226, Roseburia hominis, Roseburia sp. CAG 182 | In vivo | [107] |
| Studies in murine models with projection in humans | |||
| Bamboo (insoluble dietary fibre) | Increases Streptomyces and Bacteroides. Decreases the abundance of Alcaligenes and Pasteurella | In vivo | [244] |
| Crude polysaccharide from Dictyopteris divaricata seaweed | Increases the population of Bacillota, Bacteroidota, and Lactobacillus | In vivo | [258] |
| Food supplement of Arthrospira platensis | Clostridium XIVa, Barnesiella, Desulfovribrio, and Eubacterium show significant differences in abundance across the treatment groups | In vivo | [259] |
| Edible mushroom (Flammulina velu polysaccharides) | Elevates the relative abundance of Bacteroidota and reduce the relative abundance of Bacillota | In vivo | [260] |
| Edible mushroom (Ganoderma lucidum and Poria cocos polysaccharides) | The abundance of Porphyromonadaceae decreases in the group treated with G. lucidum polysaccharides. Eubacteriaceae family increases in the groups treated with G. lucidum and P. cocos polysaccharides | In vivo | [261] |
| Bifidobacterium longum 070103 fermented milk | Increase in Bacillota, Bacteroides, and Actinomycetes. The main microbiota consists of norank_f_Muribaculaceae, Allobaculum, Lactobacillus, Dubosiella, Faecalibaculum, and Bifidobacterium | In vivo | [262] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez, A.; Velázquez, L.; Díaz, R.; Huaiquipán, R.; Pérez, I.; Muñoz, A.; Valdés, M.; Sepúlveda, N.; Paz, E.; Quiñones, J. Impact of Novel Foods on the Human Gut Microbiome: Current Status. Microorganisms 2024, 12, 1750. https://doi.org/10.3390/microorganisms12091750
Martínez A, Velázquez L, Díaz R, Huaiquipán R, Pérez I, Muñoz A, Valdés M, Sepúlveda N, Paz E, Quiñones J. Impact of Novel Foods on the Human Gut Microbiome: Current Status. Microorganisms. 2024; 12(9):1750. https://doi.org/10.3390/microorganisms12091750
Chicago/Turabian StyleMartínez, Ailín, Lidiana Velázquez, Rommy Díaz, Rodrigo Huaiquipán, Isabela Pérez, Alex Muñoz, Marcos Valdés, Néstor Sepúlveda, Erwin Paz, and John Quiñones. 2024. "Impact of Novel Foods on the Human Gut Microbiome: Current Status" Microorganisms 12, no. 9: 1750. https://doi.org/10.3390/microorganisms12091750
APA StyleMartínez, A., Velázquez, L., Díaz, R., Huaiquipán, R., Pérez, I., Muñoz, A., Valdés, M., Sepúlveda, N., Paz, E., & Quiñones, J. (2024). Impact of Novel Foods on the Human Gut Microbiome: Current Status. Microorganisms, 12(9), 1750. https://doi.org/10.3390/microorganisms12091750





_MARCO_VALDES.jpg)


