The Ambiguous Correlation of Blautia with Obesity: A Systematic Review
Abstract
:1. Introduction

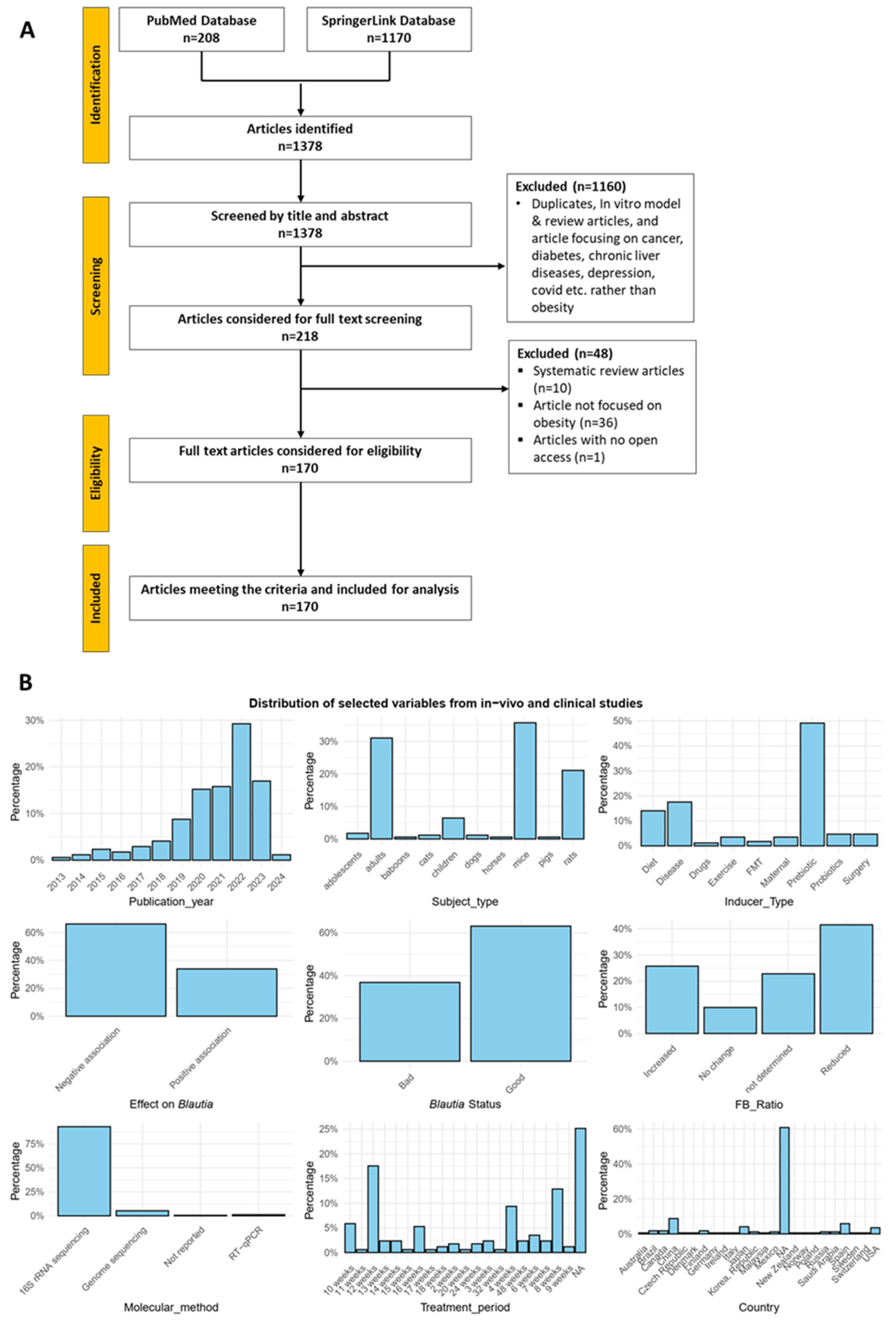
2. Methods
2.1. Literature Search Strategy
2.2. Study Selection
- Population: Animal models and human subjects
- Intervention: Any treatment or lifestyle interventions affecting gut microbiota
- Comparison: Factors inducing gut microbiota changes, including Firmicutes/Bacteroidetes (FB) ratio, molecular methods used, treatment period, and subjects’ country
- Outcome: Changes in Blautia abundance and obesity status
- Study design: Original research articles
2.3. Data Extraction
2.4. Bias Evaluation
2.5. Data Synthesis
3. Results
3.1. Studies Overview
3.2. Status and Dynamics of Blautia Population during Medical Treatment and Lifestyle Managements in Obese Individuals
3.2.1. Surgery
3.2.2. Diet
3.2.3. Exercise
3.2.4. Probiotics
3.2.5. Prebiotics
3.2.6. Fecal Microbial Transplant (FMT)
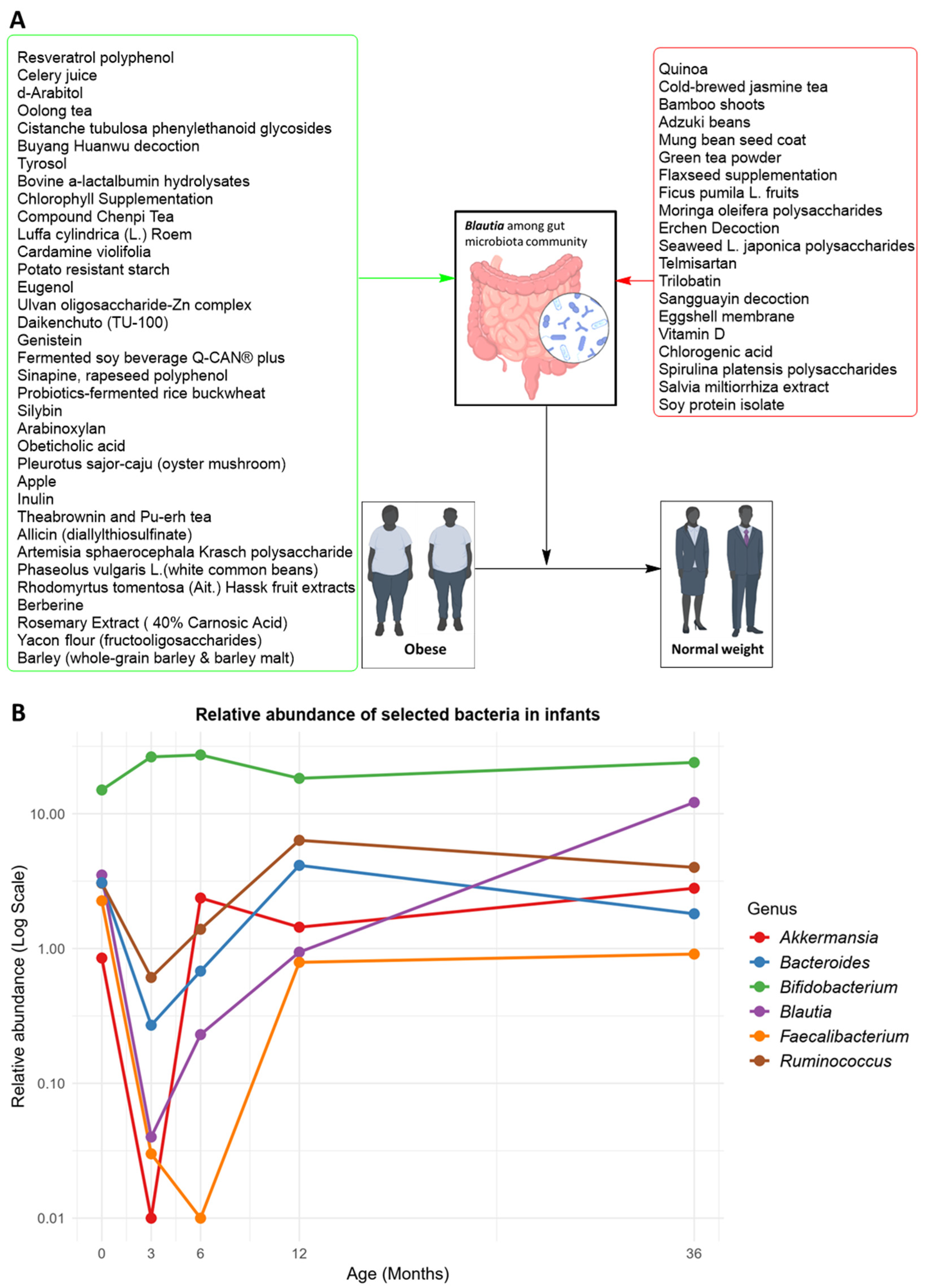
3.3. Association between Blautia and Obesity
3.3.1. Association of Blautia and Obesity in Children and Adolescents
3.3.2. Blautia and Obesity among Adult Subjects
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Romero-Corral, A.; Caples, S.M.; Lopez-Jimenez, F.; Somers, V.K. Interactions between obesity and obstructive sleep apnea: Implications for treatment. Chest 2010, 137, 711–719. [Google Scholar] [CrossRef]
- Tuomilehto, H.; Seppä, J.; Uusitupa, M. Obesity and obstructive sleep apnea—Clinical significance of weight loss. Sleep. Med. Rev. 2013, 17, 321–329. [Google Scholar] [CrossRef] [PubMed]
- Wilson, P.W.; D’Agostino, R.B.; Sullivan, L.; Parise, H.; Kannel, W.B. Overweight and obesity as determinants of cardiovascular risk: The Framingham experience. Arch. Intern. Med. 2002, 162, 1867–1872. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Huang, Y.; Xian, Y.; Zhu, S.; Jia, Z.; Liu, R.; Li, F.; Wei, J.W.; Wang, J.-G.; Liu, M.; et al. Association of body mass index with mortality and functional outcome after acute ischemic stroke. Sci. Rep. 2017, 7, 2507. [Google Scholar] [CrossRef] [PubMed]
- Schwenger, K.J.P.; Bolzon, C.M.; Li, C.; Allard, J.P. Non-alcoholic fatty liver disease and obesity: The role of the gut bacteria. Eur. J. Nutr. 2019, 58, 1771–1784. [Google Scholar] [CrossRef]
- Kulkarni, K.; Karssiens, T.; Kumar, V.; Pandit, H. Obesity and osteoarthritis. Maturitas 2016, 89, 22–28. [Google Scholar] [CrossRef]
- Bianco, A.; Nigro, E.; Monaco, M.L.; Matera, M.G.; Scudiero, O.; Mazzarella, G.; Daniele, A. The burden of obesity in asthma and COPD: Role of adiponectin. Pulm. Pharmacol. Ther. 2017, 43, 20–25. [Google Scholar] [CrossRef]
- Calhoun, K.C. Obesity and Infertility. In Obesity During Pregnancy in Clinical Practice; Nicholson, W., Baptiste-Roberts, K., Eds.; Springer: London, UK, 2014; pp. 11–31. [Google Scholar]
- Mutsaerts Meike, A.Q.; van Oers Anne, M.; Groen, H.; Burggraaff Jan, M.; Kuchenbecker Walter, K.H.; Perquin Denise, A.M.; Koks Carolien, A.M.; van Golde, R.; Kaaijk Eugenie, M.; Schierbeek Jaap, M.; et al. Randomized Trial of a Lifestyle Program in Obese Infertile Women. N. Engl. J. Med. 2016, 374, 1942–1953. [Google Scholar] [CrossRef] [PubMed]
- Schneider, K.L.; Baldwin, A.S.; Mann, D.M.; Schmitz, N. Depression, obesity, eating behavior, and physical activity. J. Obes. 2012, 2012, 517358. [Google Scholar] [CrossRef]
- Park, J.; Morley, T.S.; Kim, M.; Clegg, D.J.; Scherer, P.E. Obesity and cancer—Mechanisms underlying tumour progression and recurrence. Nat. Rev. Endocrinol. 2014, 10, 455–465. [Google Scholar] [CrossRef]
- Spinelli, A.; Buoncristiano, M.; Nardone, P.; Starc, G.; Hejgaard, T.; Júlíusson, P.B.; Fismen, A.-S.; Weghuber, D.; Musić Milanović, S.; García-Solano, M.; et al. Thinness, overweight, and obesity in 6- to 9-year-old children from 36 countries: The World Health Organization European Childhood Obesity Surveillance Initiative—COSI 2015–2017. Obes. Rev. 2021, 22, e13214. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.B.; Smith, M.S. Obesity Statistics. Prim. Care: Clin. Off. Pract. 2016, 43, 121–135. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Hou, D.; Fu, Y.; Xue, Y.; Guan, X.; Shen, Q. Adzuki Bean Alleviates Obesity and Insulin Resistance Induced by a High-Fat Diet and Modulates Gut Microbiota in Mice. Nutrients 2021, 13, 3240. [Google Scholar] [CrossRef]
- Pan, X.-F.; Wang, L.; Pan, A. Epidemiology and determinants of obesity in China. Lancet Diabetes Endocrinol. 2021, 9, 373–392. [Google Scholar] [CrossRef]
- Van Hul, M.; Cani, P.D. The gut microbiota in obesity and weight management: Microbes as friends or foe? Nat. Rev. Endocrinol. 2023, 19, 258–271. [Google Scholar] [CrossRef] [PubMed]
- Jandhyala, S.M.; Talukdar, R.; Subramanyam, C.; Vuyyuru, H.; Sasikala, M.; Nageshwar Reddy, D. Role of the normal gut microbiota. World J. Gastroenterol. 2015, 21, 8787–8803. [Google Scholar] [CrossRef]
- Stefura, T.; Zapała, B.; Gosiewski, T.; Skomarovska, O.; Dudek, A.; Pędziwiatr, M.; Major, P. Differences in Compositions of Oral and Fecal Microbiota between Patients with Obesity and Controls. Medicina 2021, 57, 678. [Google Scholar] [CrossRef]
- Sonnenburg, J.L.; Bäckhed, F. Diet–microbiota interactions as moderators of human metabolism. Nature 2016, 535, 56–64. [Google Scholar] [CrossRef]
- Chanda, D.; De, D. Meta-analysis reveals obesity associated gut microbial alteration patterns and reproducible contributors of functional shift. Gut Microbes 2024, 16, 2304900. [Google Scholar] [CrossRef]
- Hoseini Tavassol, Z.; Ejtahed, H.S.; Atlasi, R.; Saghafian, F.; Khalagi, K.; Hasani-Ranjbar, S.; Siadat, S.D.; Nabipour, I.; Ostovar, A.; Larijani, B. Alteration in Gut Microbiota Composition of Older Adults Is Associated with Obesity and Its Indices: A Systematic Review. J. Nutr. Health Aging 2023, 27, 817–823. [Google Scholar] [CrossRef]
- Lippert, K.; Kedenko, L.; Antonielli, L.; Kedenko, I.; Gemeier, C.; Leitner, M.; Kautzky-Willer, A.; Paulweber, B.; Hackl, E. Gut microbiota dysbiosis associated with glucose metabolism disorders and the metabolic syndrome in older adults. Benef. Microbes 2017, 8, 545–556. [Google Scholar] [CrossRef]
- Nishijima, S.; Suda, W.; Oshima, K.; Kim, S.-W.; Hirose, Y.; Morita, H.; Hattori, M. The gut microbiome of healthy Japanese and its microbial and functional uniqueness. DNA Res. 2016, 23, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Bamberger, C.; Rossmeier, A.; Lechner, K.; Wu, L.; Waldmann, E.; Fischer, S.; Stark, R.G.; Altenhofer, J.; Henze, K.; Parhofer, K.G. A Walnut-Enriched Diet Affects Gut Microbiome in Healthy Caucasian Subjects: A Randomized, Controlled Trial. Nutrients 2018, 10, 244. [Google Scholar] [CrossRef] [PubMed]
- Ozato, N.; Saito, S.; Yamaguchi, T.; Katashima, M.; Tokuda, I.; Sawada, K.; Katsuragi, Y.; Kakuta, M.; Imoto, S.; Ihara, K.; et al. Blautia genus associated with visceral fat accumulation in adults 20–76 years of age. NPJ Biofilms Microbiomes 2019, 5, 28. [Google Scholar] [CrossRef]
- Liu, C.; Finegold, S.M.; Song, Y.; Lawson, P.A. Reclassification of Clostridium coccoides, Ruminococcus hansenii, Ruminococcus hydrogenotrophicus, Ruminococcus luti, Ruminococcus productus and Ruminococcus schinkii as Blautia coccoides gen. nov., comb. nov., Blautia hansenii comb. nov., Blautia hydrogenotrophica comb. nov., Blautia luti comb. nov., Blautia producta comb. nov., Blautia schinkii comb. nov. and description of Blautia wexlerae sp. nov., isolated from human faeces. Int. J. Syst. Evol. Microbiol. 2008, 58, 1896–1902. [Google Scholar] [CrossRef] [PubMed]
- Ozato, N.; Yamaguchi, T.; Mori, K.; Katashima, M.; Kumagai, M.; Murashita, K.; Katsuragi, Y.; Tamada, Y.; Kakuta, M.; Imoto, S.; et al. Two Blautia Species Associated with Visceral Fat Accumulation: A One-Year Longitudinal Study. Biology 2022, 11, 318. [Google Scholar] [CrossRef]
- Hosomi, K.; Saito, M.; Park, J.; Murakami, H.; Shibata, N.; Ando, M.; Nagatake, T.; Konishi, K.; Ohno, H.; Tanisawa, K.; et al. Oral administration of Blautia wexlerae ameliorates obesity and type 2 diabetes via metabolic remodeling of the gut microbiota. Nat. Commun. 2022, 13, 4477. [Google Scholar] [CrossRef] [PubMed]
- Benítez-Páez, A.; Gómez Del Pugar, E.M.; López-Almela, I.; Moya-Pérez, Á.; Codoñer-Franch, P.; Sanz, Y. Depletion of Blautia Species in the Microbiota of Obese Children Relates to Intestinal Inflammation and Metabolic Phenotype Worsening. mSystems 2020, 5. [Google Scholar] [CrossRef]
- Wu, X.R.; Chen, Z.Z.; Dong, X.L.; Zhao, Q.P.; Cai, J. A Novel Symbiotic Formulation Reduces Obesity and Concomitant Metabolic Syndrome in Rats by Raising the Relative Abundance of Blautia. Nutrients 2023, 15, 956. [Google Scholar] [CrossRef]
- Xing, H.; Zhang, Y.; Krämer, M.; Kissmann, A.K.; Henkel, M.; Weil, T.; Knippschild, U.; Rosenau, F. A Polyclonal Selex Aptamer Library Directly Allows Specific Labelling of the Human Gut Bacterium Blautia producta without Isolating Individual Aptamers. Molecules 2022, 27, 5693. [Google Scholar] [CrossRef]
- Vazquez-Moreno, M.; Perez-Herrera, A.; Locia-Morales, D.; Dizzel, S.; Meyre, D.; Stearns, J.C.; Cruz, M. Association of gut microbiome with fasting triglycerides, fasting insulin and obesity status in Mexican children. Pediatr. Obes. 2021, 16, e12748. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Liang, J.; Su, Y.; Wang, J.; Amakye, W.K.; Pan, J.; Chu, X.; Ma, B.; Song, Y.; Li, Y.; et al. The associations of the gut microbiome composition and short-chain fatty acid concentrations with body fat distribution in children. Clin. Nutr. 2021, 40, 3379–3390. [Google Scholar] [CrossRef] [PubMed]
- Dai, D.; Zhu, J.; Sun, C.; Li, M.; Liu, J.; Wu, S.; Ning, K.; He, L.-J.; Zhao, X.-M.; Chen, W.-H. GMrepo v2: A curated human gut microbiome database with special focus on disease markers and cross-dataset comparison. Nucleic Acids Res. 2021, 50, D777–D784. [Google Scholar] [CrossRef]
- Wu, S.; Sun, C.; Li, Y.; Wang, T.; Jia, L.; Lai, S.; Yang, Y.; Luo, P.; Dai, D.; Yang, Y.-Q.; et al. GMrepo: A database of curated and consistently annotated human gut metagenomes. Nucleic Acids Res. 2019, 48, D545–D553. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- O’Connor, A.M.; Sargeant, J.M. Critical Appraisal of Studies Using Laboratory Animal Models. ILAR J. 2014, 55, 405–417. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Altman, D.G. Assessing Risk of Bias in Included Studies. In Cochrane Handbook for Systematic Reviews of Interventions; Higgins, J.P., Green, S., Eds.; WILEY Online Library: Hoboken, NJ, USA, 2008; pp. 187–241. [Google Scholar] [CrossRef]
- Ďásková, N.; Modos, I.; Krbcová, M.; Kuzma, M.; Pelantová, H.; Hradecký, J.; Heczková, M.; Bratová, M.; Videňská, P.; Šplíchalová, P.; et al. Multi-omics signatures in new-onset diabetes predict metabolic response to dietary inulin: Findings from an observational study followed by an interventional trial. Nutr. Diabetes 2023, 13, 7. [Google Scholar] [CrossRef] [PubMed]
- Gu, I.; Lam, W.S.; Marasini, D.; Brownmiller, C.; Savary, B.J.; Lee, J.A.; Carbonero, F.; Lee, S.O. In Vitro Fecal Fermentation Patterns of Arabinoxylan from Rice Bran on Fecal Microbiota from Normal-Weight and Overweight/Obese Subjects. Nutrients 2021, 13, 2052. [Google Scholar] [CrossRef]
- Mei, X.; Li, Y.; Zhang, X.; Zhai, X.; Yang, Y.; Li, Z.; Li, L. Maternal Phlorizin Intake Protects Offspring from Maternal Obesity-Induced Metabolic Disorders in Mice via Targeting Gut Microbiota to Activate the SCFA-GPR43 Pathway. J. Agric. Food Chem. 2024, 72, 4703–4725. [Google Scholar] [CrossRef]
- Gómez-Pérez, A.M.; Ruiz-Limón, P.; Salas-Salvadó, J.; Vioque, J.; Corella, D.; Fitó, M.; Vidal, J.; Atzeni, A.; Torres-Collado, L.; Álvarez-Sala, A.; et al. Gut microbiota in nonalcoholic fatty liver disease: A PREDIMED-Plus trial sub analysis. Gut Microbes 2023, 15, 2223339. [Google Scholar] [CrossRef]
- Sánchez-Alcoholado, L.; Gutiérrez-Repiso, C.; Gómez-Pérez, A.M.; García-Fuentes, E.; Tinahones, F.J.; Moreno-Indias, I. Gut microbiota adaptation after weight loss by Roux-en-Y gastric bypass or sleeve gastrectomy bariatric surgeries. Surg. Obes. Relat. Dis. 2019, 15, 1888–1895. [Google Scholar] [CrossRef]
- O’Neill, L.; Pandya, V.; Grigoryan, Z.; Patel, R.; DeSipio, J.; Judge, T.; Phadtare, S. Effects of Milkfat on the Gut Microbiome of Patients After Bariatric Surgery, a Pilot Study. Obes. Surg. 2022, 32, 480–488. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Rubio, Á.; Soluyanova, P.; Moro, E.; Quintás, G.; Rienda, I.; Periañez, M.D.; Painel, A.; Vizuete, J.; Pérez-Rojas, J.; Castell, J.V.; et al. Gut Microbiota and Plasma Bile Acids Associated with Non-Alcoholic Fatty Liver Disease Resolution in Bariatric Surgery Patients. Nutrients 2023, 15, 3187. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Lei, O.K.; Nie, J.; Shi, Q.; Xu, Y.; Kong, Z. Effects of Low-Carbohydrate Diet and Exercise Training on Gut Microbiota. Front. Nutr. 2022, 9, 884550. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.; Jeon, K.J.; Kim, H.K.; Han, S.N. Effect of a 12-week weight management program on the clinical characteristics and dietary intake of the young obese and the contributing factors to the successful weight loss. Nutr. Res. Pract. 2014, 8, 571–579. [Google Scholar] [CrossRef] [PubMed]
- Jastreboff, A.M.; Aronne, L.J.; Ahmad, N.N.; Wharton, S.; Connery, L.; Alves, B.; Kiyosue, A.; Zhang, S.; Liu, B.; Bunck, M.C.; et al. Tirzepatide Once Weekly for the Treatment of Obesity. N. Engl. J. Med. 2022, 387, 205–216. [Google Scholar] [CrossRef] [PubMed]
- Yadav, J.; Liang, T.; Qin, T.; Nathan, N.; Schwenger, K.J.P.; Pickel, L.; Xie, L.; Lei, H.; Winer, D.A.; Maughan, H.; et al. Gut microbiome modified by bariatric surgery improves insulin sensitivity and correlates with increased brown fat activity and energy expenditure. Cell Rep. Med. 2023, 4, 101051. [Google Scholar] [CrossRef]
- Gasmi, A.; Bjørklund, G.; Mujawdiya, P.K.; Semenova, Y.; Dosa, A.; Piscopo, S.; Pen, J.J.; Gasmi Benahmed, A.; Costea, D.O. Gut microbiota in bariatric surgery. Crit. Rev. Food Sci. Nutr. 2023, 63, 9299–9314. [Google Scholar] [CrossRef]
- Ocaña-Wilhelmi, L.; Martín-Núñez, G.M.; Ruiz-Limón, P.; Alcaide, J.; García-Fuentes, E.; Gutiérrez-Repiso, C.; Tinahones, F.J.; Moreno-Indias, I. Gut Microbiota Metabolism of Bile Acids Could Contribute to the Bariatric Surgery Improvements in Extreme Obesity. Metabolites 2021, 11, 733. [Google Scholar] [CrossRef]
- Lin, W.; Wen, L.; Wen, J.; Xiang, G. Effects of Sleeve Gastrectomy on Fecal Gut Microbiota and Short-Chain Fatty Acid Content in a Rat Model of Polycystic Ovary Syndrome. Front. Endocrinol. 2021, 12, 747888. [Google Scholar] [CrossRef]
- Haal, S.; Guman, M.S.S.; de Brauw, L.M.; van Veen, R.N.; Schouten, R.; Fockens, P.; Gerdes, V.E.A.; Dijkgraaf, M.G.W.; Voermans, R.P. Ursodeoxycholic acid for the prevention of symptomatic gallstone disease after bariatric surgery: Statistical analysis plan for a randomised controlled trial (UPGRADE trial). Trials 2020, 21, 676. [Google Scholar] [CrossRef] [PubMed]
- Jie, Z.; Yu, X.; Liu, Y.; Sun, L.; Chen, P.; Ding, Q.; Gao, Y.; Zhang, X.; Yu, M.; Liu, Y.; et al. The Baseline Gut Microbiota Directs Dieting-Induced Weight Loss Trajectories. Gastroenterology 2021, 160, 2029–2042.e16. [Google Scholar] [CrossRef]
- Cuevas-Sierra, A.; Milagro, F.I.; Guruceaga, E.; Cuervo, M.; Goni, L.; García-Granero, M.; Martinez, J.A.; Riezu-Boj, J.I. A weight-loss model based on baseline microbiota and genetic scores for selection of dietary treatments in overweight and obese population. Clin. Nutr. 2022, 41, 1712–1723. [Google Scholar] [CrossRef]
- Wang, H.; Lv, X.; Zhao, S.; Yuan, W.; Zhou, Q.; Sadiq, F.A.; Zhao, J.; Lu, W.; Wu, W. Weight Loss Promotion in Individuals with Obesity through Gut Microbiota Alterations with a Multiphase Modified Ketogenic Diet. Nutrients 2023, 15, 4163. [Google Scholar] [CrossRef] [PubMed]
- Pataky, Z.; Genton, L.; Spahr, L.; Lazarevic, V.; Terraz, S.; Gaïa, N.; Rubbia-Brandt, L.; Golay, A.; Schrenzel, J.; Pichard, C. Impact of Hypocaloric Hyperproteic Diet on Gut Microbiota in Overweight or Obese Patients with Nonalcoholic Fatty Liver Disease: A Pilot Study. Dig. Dis. Sci. 2016, 61, 2721–2731. [Google Scholar] [CrossRef] [PubMed]
- Yuan, W.; Lu, W.; Wang, H.; Wu, W.; Zhou, Q.; Chen, Y.; Lee, Y.K.; Zhao, J.; Zhang, H.; Chen, W. A multiphase dietetic protocol incorporating an improved ketogenic diet enhances weight loss and alters the gut microbiome of obese people. Int. J. Food Sci. Nutr. 2022, 73, 238–250. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Song, W.; Yuan, W.; Zhou, Q.; Sadiq, F.A.; Zhao, J.; Wu, W.; Lu, W. Modulating the Human Gut Microbiota through Hypocaloric Balanced Diets: An Effective Approach for Managing Obesity. Nutrients 2023, 15, 3101. [Google Scholar] [CrossRef]
- Medawar, E.; Haange, S.B.; Rolle-Kampczyk, U.; Engelmann, B.; Dietrich, A.; Thieleking, R.; Wiegank, C.; Fries, C.; Horstmann, A.; Villringer, A.; et al. Gut microbiota link dietary fiber intake and short-chain fatty acid metabolism with eating behavior. Transl. Psychiatry 2021, 11, 500. [Google Scholar] [CrossRef]
- Rettedal, E.A.; Cree, J.M.E.; Adams, S.E.; MacRae, C.; Skidmore, P.M.L.; Cameron-Smith, D.; Gant, N.; Blenkiron, C.; Merry, T.L. Short-term high-intensity interval training exercise does not affect gut bacterial community diversity or composition of lean and overweight men. Exp. Physiol. 2020, 105, 1268–1279. [Google Scholar] [CrossRef]
- Quiroga, R.; Nistal, E.; Estébanez, B.; Porras, D.; Juárez-Fernández, M.; Martínez-Flórez, S.; García-Mediavilla, M.V.; de Paz, J.A.; González-Gallego, J.; Sánchez-Campos, S.; et al. Exercise training modulates the gut microbiota profile and impairs inflammatory signaling pathways in obese children. Exp. Mol. Med. 2020, 52, 1048–1061. [Google Scholar] [CrossRef]
- Matei, D.; Trofin, D.; Iordan, D.A.; Onu, I.; Condurache, I.; Ionite, C.; Buculei, I. The Endocannabinoid System and Physical Exercise. Int. J. Mol. Sci. 2023, 24, 1989. [Google Scholar] [CrossRef]
- Meyer, J.D.; Crombie, K.M.; Cook, D.B.; Hillard, C.J.; Koltyn, K.F. Serum Endocannabinoid and Mood Changes after Exercise in Major Depressive Disorder. Med. Sci. Sports Exerc. 2019, 51, 1909–1917. [Google Scholar] [CrossRef]
- Di Marzo, V.; Després, J.-P. CB1 antagonists for obesity—What lessons have we learned from rimonabant? Nat. Rev. Endocrinol. 2009, 5, 633–638. [Google Scholar] [CrossRef] [PubMed]
- Alhouayek, M.; Muccioli, G.G. Harnessing the anti-inflammatory potential of palmitoylethanolamide. Drug Discov. Today 2014, 19, 1632–1639. [Google Scholar] [CrossRef]
- Couch, D.G.; Cook, H.; Ortori, C.; Barrett, D.; Lund, J.N.; O’Sullivan, S.E. Palmitoylethanolamide and Cannabidiol Prevent Inflammation-induced Hyperpermeability of the Human Gut In Vitro and In Vivo-A Randomized, Placebo-controlled, Double-blind Controlled Trial. Inflamm. Bowel Dis. 2019, 25, 1006–1018. [Google Scholar] [CrossRef]
- Minichino, A.; Jackson, M.A.; Francesconi, M.; Steves, C.J.; Menni, C.; Burnet, P.W.J.; Lennox, B.R. Endocannabinoid system mediates the association between gut-microbial diversity and anhedonia/amotivation in a general population cohort. Mol. Psychiatry 2021, 26, 6269–6276. [Google Scholar] [CrossRef] [PubMed]
- Holmberg, S.M.; Feeney, R.H.; Prasoodanan, P.K.V.; Puértolas-Balint, F.; Singh, D.K.; Wongkuna, S.; Zandbergen, L.; Hauner, H.; Brandl, B.; Nieminen, A.I.; et al. The gut commensal Blautia maintains colonic mucus function under low-fiber consumption through secretion of short-chain fatty acids. Nat. Commun. 2024, 15, 3502. [Google Scholar] [CrossRef] [PubMed]
- Rashidi, A.; Peled, J.U.; Ebadi, M.; Rehman, T.U.; Elhusseini, H.; Marcello, L.T.; Halaweish, H.; Kaiser, T.; Holtan, S.G.; Khoruts, A.; et al. Protective Effect of Intestinal Blautia Against Neutropenic Fever in Allogeneic Transplant Recipients. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2022, 75, 1912–1920. [Google Scholar] [CrossRef]
- Zhao, K.; Qiu, L.; He, Y.; Tao, X.; Zhang, Z.; Wei, H. Alleviation Syndrome of High-Cholesterol-Diet-Induced Hypercholesterolemia in Mice by Intervention with Lactiplantibacillus plantarum WLPL21 via Regulation of Cholesterol Metabolism and Transportation as Well as Gut Microbiota. Nutrients 2023, 15, 2600. [Google Scholar] [CrossRef]
- Yan, J.; Li, J.; Xue, Q.; Xie, S.; Jiang, J.; Li, P.; Du, B. Bacillus sp. DU-106 ameliorates type 2 diabetes by modulating gut microbiota in high-fat-fed and streptozotocin-induced mice. J. Appl. Microbiol. 2022, 133, 3126–3138. [Google Scholar] [CrossRef]
- Larsen, N.; Vogensen, F.K.; Gøbel, R.J.; Michaelsen, K.F.; Forssten, S.D.; Lahtinen, S.J.; Jakobsen, M. Effect of Lactobacillus salivarius Ls-33 on fecal microbiota in obese adolescents. Clin. Nutr. 2013, 32, 935–940. [Google Scholar] [CrossRef]
- Shibata, M.; Ozato, N.; Tsuda, H.; Mori, K.; Kinoshita, K.; Katashima, M.; Katsuragi, Y.; Nakaji, S.; Maeda, H. Mouse Model of Anti-Obesity Effects of Blautia hansenii on Diet-Induced Obesity. Curr. Issues Mol. Biol. 2023, 45, 7147–7160. [Google Scholar] [CrossRef] [PubMed]
- Bäumler, A.J.; Sperandio, V. Interactions between the microbiota and pathogenic bacteria in the gut. Nature 2016, 535, 85–93. [Google Scholar] [CrossRef]
- Li, Y.; Liu, W.; Xu, Z.Z.; Xiao, J.X.; Zong, A.Z.; Qiu, B.; Jia, M.; Liu, L.N.; Xu, T.C. Research on the mechanism of microwave-toughened starch on glucolipid metabolism in mice. Food Funct. 2020, 11, 9789–9800. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Liu, Q.; Hao, M.; Zhai, X.; Chen, J. Effects of neutral polysaccharide from Platycodon grandiflorum on high-fat diet-induced obesity via the regulation of gut microbiota and metabolites. Front. Endocrinol. 2023, 14, 1078593. [Google Scholar] [CrossRef]
- Kim, Y.; Han, H.; Oh, Y.; Shin, H.; Park, G.; Park, S.; Manthey, J.A.; Kim, Y.; Kim, Y. A combination of rebaudioside A and neohesperidin dihydrochalcone suppressed weight gain by regulating visceral fat and hepatic lipid metabolism in ob/ob mice. Food Sci. Biotechnol. 2024, 33, 913–923. [Google Scholar] [CrossRef]
- Guzzardi, M.A.; Ederveen, T.H.A.; Rizzo, F.; Weisz, A.; Collado, M.C.; Muratori, F.; Gross, G.; Alkema, W.; Iozzo, P. Maternal pre-pregnancy overweight and neonatal gut bacterial colonization are associated with cognitive development and gut microbiota composition in pre-school-age offspring. Brain Behav. Immun. 2022, 100, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Lahtinen, P.; Juuti, A.; Luostarinen, M.; Niskanen, L.; Liukkonen, T.; Tillonen, J.; Kössi, J.; Ilvesmäki, V.; Viljakka, M.; Satokari, R.; et al. Effectiveness of Fecal Microbiota Transplantation for Weight Loss in Patients With Obesity Undergoing Bariatric Surgery: A Randomized Clinical Trial. JAMA Netw. Open 2022, 5, e2247226. [Google Scholar] [CrossRef] [PubMed]
- Ghorbani, Y.; Schwenger, K.J.P.; Sharma, D.; Jung, H.; Yadav, J.; Xu, W.; Lou, W.; Poutanen, S.; Hota, S.S.; Comelli, E.M.; et al. Effect of faecal microbial transplant via colonoscopy in patients with severe obesity and insulin resistance: A randomized double-blind, placebo-controlled Phase 2 trial. Diabetes Obes. Metab. 2023, 25, 479–490. [Google Scholar] [CrossRef]
- Zhang, Z.; Mocanu, V.; Cai, C.; Dang, J.; Slater, L.; Deehan, E.C.; Walter, J.; Madsen, K.L. Impact of Fecal Microbiota Transplantation on Obesity and Metabolic Syndrome—A Systematic Review. Nutrients 2019, 11, 2291. [Google Scholar] [CrossRef]
- Becetti, I.; Lauze, M.; Lee, H.; Bredella, M.A.; Misra, M.; Singhal, V. Changes in Branched-Chain Amino Acids One Year after Sleeve Gastrectomy in Youth with Obesity and Their Association with Changes in Insulin Resistance. Nutrients 2023, 15, 3801. [Google Scholar] [CrossRef]
- Kitsy, A.; Carney, S.; Vivar, J.C.; Knight, M.S.; Pointer, M.A.; Gwathmey, J.K.; Ghosh, S. Effects of Leucine Supplementation and Serum Withdrawal on Branched-Chain Amino Acid Pathway Gene and Protein Expression in Mouse Adipocytes. PLoS ONE 2014, 9, e102615. [Google Scholar] [CrossRef] [PubMed]
- Melnik, B.C. Leucine signaling in the pathogenesis of type 2 diabetes and obesity. World J. Diabetes 2012, 3, 38–53. [Google Scholar] [CrossRef]
- Lee, Y.; Cho, J.Y.; Cho, K.Y. Serum, Urine, and Fecal Metabolome Alterations in the Gut Microbiota in Response to Lifestyle Interventions in Pediatric Obesity: A Non-Randomized Clinical Trial. Nutrients 2023, 15, 2184. [Google Scholar] [CrossRef]
- Liu, X.; Mao, B.; Gu, J.; Wu, J.; Cui, S.; Wang, G.; Zhao, J.; Zhang, H.; Chen, W. Blautia—A new functional genus with potential probiotic properties? Gut Microbes 2021, 13, 1875796. [Google Scholar] [CrossRef]
- Yuan, X.; Zhang, Y.; Lin, X.; Yang, X.; Chen, R. Association of gut microbiota and glucose metabolism in children with disparate degrees of adiposity. Pediatr. Obes. 2023, 18, e13009. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.Y. Association of gut microbiota with obesity in children and adolescents. Clin. Exp. Pediatr. 2023, 66, 148–154. [Google Scholar] [CrossRef]
- Galley, J.D.; Bailey, M.; Dush, C.K.; Schoppe-Sullivan, S.; Christian, L.M. Maternal obesity is associated with alterations in the gut microbiome in toddlers. PLoS ONE 2014, 9, e113026. [Google Scholar] [CrossRef]
- Squillario, M.; Bonaretti, C.; La Valle, A.; Di Marco, E.; Piccolo, G.; Minuto, N.; Patti, G.; Napoli, F.; Bassi, M.; Maghnie, M.; et al. Gut-microbiota in children and adolescents with obesity: Inferred functional analysis and machine-learning algorithms to classify microorganisms. Sci. Rep. 2023, 13, 11294. [Google Scholar] [CrossRef]
- Ruiz, A.; Cerdó, T.; Jáuregui, R.; Pieper, D.H.; Marcos, A.; Clemente, A.; García, F.; Margolles, A.; Ferrer, M.; Campoy, C.; et al. One-year calorie restriction impacts gut microbial composition but not its metabolic performance in obese adolescents. Environ. Microbiol. 2017, 19, 1536–1551. [Google Scholar] [CrossRef] [PubMed]
- Morgado, M.C.; Sousa, M.; Coelho, A.B.; Costa, J.A.; Seabra, A. Exploring Gut Microbiota and the Influence of Physical Activity Interventions on Overweight and Obese Children and Adolescents: A Systematic Review. Healthcare 2023, 11, 2459. [Google Scholar] [CrossRef] [PubMed]
- Kasai, C.; Sugimoto, K.; Moritani, I.; Tanaka, J.; Oya, Y.; Inoue, H.; Tameda, M.; Shiraki, K.; Ito, M.; Takei, Y.; et al. Comparison of the gut microbiota composition between obese and non-obese individuals in a Japanese population, as analyzed by terminal restriction fragment length polymorphism and next-generation sequencing. BMC Gastroenterol. 2015, 15, 100. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Li, J.; Zhang, Y.; Philip, A.; Shi, E.; Chi, X.; Meng, J. Influence of glucose fermentation on CO2 assimilation to acetate in homoacetogen Blautia coccoides GA-1. J. Ind. Microbiol. Biotechnol. 2015, 42, 1217–1224. [Google Scholar] [CrossRef]
- Ang, Z.; Ding, J.L. GPR41 and GPR43 in Obesity and Inflammation–Protective or Causative? Front. Immunol. 2016, 7, 28. [Google Scholar] [CrossRef]
- Kimura, I.; Ozawa, K.; Inoue, D.; Imamura, T.; Kimura, K.; Maeda, T.; Terasawa, K.; Kashihara, D.; Hirano, K.; Tani, T. The gut microbiota suppresses insulin-mediated fat accumulation via the short-chain fatty acid receptor GPR43. Nat. Commun. 2013, 4, 1829. [Google Scholar] [CrossRef]
- Mo, R.; Zhang, M.; Wang, H.; Liu, T.; Zhang, G.; Wu, Y. Short-term changes in dietary fat levels and starch sources affect weight management, glucose and lipid metabolism, and gut microbiota in adult cats. J. Anim. Sci. 2023, 101, skad276. [Google Scholar] [CrossRef] [PubMed]
- Freitas, R.; Vasques, A.C.J.; Fernandes, G.D.R.; Ribeiro, F.B.; Solar, I.; Barbosa, M.G.; Pititto, B.A.; Geloneze, B.; Ferreira, S.R.G. Associations of Blautia Genus With Early-Life Events and Later Phenotype in the NutriHS. Front. Cell Infect. Microbiol. 2022, 12, 838750. [Google Scholar] [CrossRef] [PubMed]
- Radjabzadeh, D.; Boer, C.G.; Beth, S.A.; van der Wal, P.; Kiefte-De Jong, J.C.; Jansen, M.A.E.; Konstantinov, S.R.; Peppelenbosch, M.P.; Hays, J.P.; Jaddoe, V.W.V.; et al. Diversity, compositional and functional differences between gut microbiota of children and adults. Sci. Rep. 2020, 10, 1040. [Google Scholar] [CrossRef]
- Hassan, N.E.; El Shebini, S.M.; El-Masry, S.A.; Ahmed, N.H.; Kamal, A.N.; Ismail, A.S.; Alian, K.M.; Mostafa, M.I.; Selim, M.; Afify, M.A.S. Brief overview of dietary intake, some types of gut microbiota, metabolic markers and research opportunities in sample of Egyptian women. Sci. Rep. 2022, 12, 17291. [Google Scholar] [CrossRef]
- Kusnadi, Y.; Saleh, M.I.; Ali, Z.; Hermansyah, H.; Murti, K.; Hafy, Z.; Yuristo, N.S.E. Firmicutes/Bacteroidetes Ratio of Gut Microbiota and Its Relationships with Clinical Parameters of Type 2 Diabetes Mellitus: A Systematic Review. Open Access Maced. J. Med. Sci. 2023, 11, 67–72. [Google Scholar] [CrossRef]
- Magne, F.; Gotteland, M.; Gauthier, L.; Zazueta, A.; Pesoa, S.; Navarrete, P.; Balamurugan, R. The Firmicutes/Bacteroidetes Ratio: A Relevant Marker of Gut Dysbiosis in Obese Patients? Nutrients 2020, 12, 1474. [Google Scholar] [CrossRef] [PubMed]
- Burakova, I.; Smirnova, Y.; Gryaznova, M.; Syromyatnikov, M.; Chizhkov, P.; Popov, E.; Popov, V. The Effect of Short-Term Consumption of Lactic Acid Bacteria on the Gut Microbiota in Obese People. Nutrients 2022, 14, 3384. [Google Scholar] [CrossRef]
- Mao, K.; Gao, J.; Wang, X.; Li, X.; Geng, S.; Zhang, T.; Sadiq, F.A.; Sang, Y. Bifidobacterium animalis subsp. lactis BB-12 Has Effect Against Obesity by Regulating Gut Microbiota in Two Phases in Human Microbiota-Associated Rats. Front. Nutr. 2022, 8, 811619. [Google Scholar] [CrossRef]
- Hitch, T.C.A.; Riedel, T.; Oren, A.; Overmann, J.; Lawley, T.D.; Clavel, T. Automated analysis of genomic sequences facilitates high-throughput and comprehensive description of bacteria. ISME Commun. 2021, 1, 16. [Google Scholar] [CrossRef] [PubMed]
- Paek, J.; Shin, Y.; Kook, J.K.; Chang, Y.H. Blautia argi sp. nov., a new anaerobic bacterium isolated from dog faeces. Int. J. Syst. Evol. Microbiol. 2019, 69, 33–38. [Google Scholar] [CrossRef]
- Lagkouvardos, I.; Pukall, R.; Abt, B.; Foesel, B.U.; Meier-Kolthoff, J.P.; Kumar, N.; Bresciani, A.; Martínez, I.; Just, S.; Ziegler, C.; et al. The Mouse Intestinal Bacterial Collection (miBC) provides host-specific insight into cultured diversity and functional potential of the gut microbiota. Nat. Microbiol. 2016, 1, 16131. [Google Scholar] [CrossRef]
- Liu, C.; Du, M.X.; Abuduaini, R.; Yu, H.Y.; Li, D.H.; Wang, Y.J.; Zhou, N.; Jiang, M.Z.; Niu, P.X.; Han, S.S.; et al. Correction: Enlightening the taxonomy darkness of human gut microbiomes with a cultured biobank. Microbiome 2022, 10, 163. [Google Scholar] [CrossRef]
- Kaneuchi, C.; Benno, Y.; Mitsuoka, T. Clostridium coccoides, a New Species from the Feces of Mice. Int. J. Syst. Evol. Microbiol. 1976, 26, 482–486. [Google Scholar] [CrossRef]
- Kim, J.-S.; Park, J.-E.; Lee, K.C.; Choi, S.-H.; Oh, B.S.; Yu, S.Y.; Eom, M.K.; Kang, S.W.; Han, K.-I.; Suh, M.K.; et al. Blautia faecicola sp. nov., isolated from faeces from a healthy human. Int. J. Syst. Evol. Microbiol. 2020, 70, 2059–2065. [Google Scholar] [CrossRef]
- Park, S.K.; Kim, M.S.; Bae, J.W. Blautia faecis sp. nov., isolated from human faeces. Int. J. Syst. Evol. Microbiol. 2013, 63, 599–603. [Google Scholar] [CrossRef]
- Afrizal, A.; Hitch, T.C.A.; Viehof, A.; Treichel, N.; Riedel, T.; Abt, B.; Buhl, E.M.; Kohlheyer, D.; Overmann, J.; Clavel, T. Anaerobic single-cell dispensing facilitates the cultivation of human gut bacteria. Environ. Microbiol. 2022, 24, 3861–3881. [Google Scholar] [CrossRef]
- Furuya, H.; Ide, Y.; Hamamoto, M.; Asanuma, N.; Hino, T. Isolation of a novel bacterium, Blautia glucerasei sp. nov., hydrolyzing plant glucosylceramide to ceramide. Arch. Microbiol. 2010, 192, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Holdeman, L.V.; Moore, W.E.C. New Genus, Coprococcus, Twelve New Species, and Emended Descriptions of Four Previously Described Species of Bacteria from Human Feces. Int. J. Syst. Evol. Microbiol. 1974, 24, 260–277. [Google Scholar] [CrossRef]
- Shin, N.R.; Kang, W.; Tak, E.J.; Hyun, D.W.; Kim, P.S.; Kim, H.S.; Lee, J.Y.; Sung, H.; Whon, T.W.; Bae, J.W. Blautia hominis sp. nov., isolated from human faeces. Int. J. Syst. Evol. Microbiol. 2018, 68, 1059–1064. [Google Scholar] [CrossRef] [PubMed]
- Bernalier, A.; Willems, A.; Leclerc, M.; Rochet, V.; Collins, M.D. Ruminococcus hydrogenotrophicus sp. nov., a new H2/CO2-utilizing acetogenic bacterium isolated from human feces. Arch. Microbiol. 1996, 166, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.J.; Abdugheni, R.; Liu, C.; Zhou, N.; You, X.; Liu, S.J. Blautia intestinalis sp. nov., isolated from human feces. Int. J. Syst. Evol. Microbiol. 2021, 71, 005005. [Google Scholar] [CrossRef]
- Lu, L.F.; Yang, Y.; Chai, L.J.; Lu, Z.M.; Zhang, L.Q.; Qin, H.; Yang, P.; Xu, Z.H.; Shen, C.H. Blautia liquoris sp. nov., isolated from the mud in a fermentation cellar used for the production of Chinese strong-flavour liquor. Int. J. Syst. Evol. Microbiol. 2021, 71, 005041. [Google Scholar] [CrossRef]
- Simmering, R.; Taras, D.; Schwiertz, A.; Le Blay, G.; Gruhl, B.; Lawson, P.A.; Collins, M.D.; Blaut, M. Ruminococcus luti sp. nov., isolated from a human faecal sample. Syst. Appl. Microbiol. 2002, 25, 189–193. [Google Scholar] [CrossRef]
- Moore, W.E.C.; Johnson, J.L.; Holdeman, L.V. Emendation of Bacteroidaceae and Butyrivibrio and Descriptions of Desulfomonas gen. nov. and Ten New Species in the Genera Desulfomonas, Butyrivibrio, Eubacterium, Clostridium, and Ruminococcus. Int. J. Syst. Evol. Microbiol. 1976, 26, 238–252. [Google Scholar] [CrossRef]
- Lawson, P.A.; Finegold, S.M. Reclassification of Ruminococcus obeum as Blautia obeum comb. nov. Int. J. Syst. Evol. Microbiol. 2015, 65, 789–793. [Google Scholar] [CrossRef]
- Miura, T.; Shimamura, M.; Yamazoe, A.; Kawasaki, H. Blautia parvula sp. nov., isolated from Japanese faecal samples. Int. J. Syst. Evol. Microbiol. 2023, 73, 005871. [Google Scholar] [CrossRef] [PubMed]
- Maturana, J.L.; Cárdenas, J.P. Insights on the Evolutionary Genomics of the Blautia Genus: Potential New Species and Genetic Content Among Lineages. Front. Microbiol. 2021, 12, 660920. [Google Scholar] [CrossRef] [PubMed]
- Rieu-Lesme, F.; Morvan, B.; Collins, M.D.; Fonty, G.; Willems, A. A new H2/CO2-using acetogenic bacterium from the rumen: Description of Ruminococcus schinkii sp. nov. FEMS Microbiol. Lett. 1996, 140, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Park, S.K.; Kim, M.S.; Roh, S.W.; Bae, J.W. Blautia stercoris sp. nov., isolated from human faeces. Int. J. Syst. Evol. Microbiol. 2012, 62, 776–779. [Google Scholar] [CrossRef]


| Refs. | Inducer | Effect on Blautia | Status | FB Ratio | Method Used | Treatment Period | Country | Blautia Related Findings |
|---|---|---|---|---|---|---|---|---|
| Jie et al. 2021 [54] | dietary and exercise | (−) | Good | nd | metagenomic shotgun sequencing | 24 weeks | China | B. wexlerae was the strongest predictors for weight loss when present in high abundance at baseline. |
| Cuevas-Sierra et al. [55] | Medium protein & low-fat diets | (−) | Good | nd | 16S rRNA, V3–V4 region sequencing | 16 weeks | Spain | Blautia was negatively correlated with BMI loss percentage in women on a low-fat diet. |
| Gómez-Pérez et al. 2022 [42] | Mediterranean diet and exercise | (−) | Good | Reduced | 16S rRNA, V2–V9 region sequencing | 48 weeks | Spain | Decrease in Blautia may be associated with the development of non-alcoholic fatty liver disease. |
| Yuan et al. 2022 [58] | improved ketogenic diet | (+) | Bad | Reduced | 16S rRNA, V3–V4 region sequencing | 12 weeks | China | Decreased the abundance of Blautia and enhanced weight loss in obese individuals. |
| Wang et al. 2023 [59] | hypocaloric balanced diet | (+) | Bad | Reduced | 16S rRNA, V3–V4 region sequencing | 12 weeks | China | Diet led to significant weight loss and changes in the gut microbiota of obese individuals, including a decrease in the abundance of Blautia. |
| Medawar et al. 2021 [60] | eating habits and Roux-en-Y gastric bypass | (−) | Good | nd | 16S rRNA, V3–V4 region sequencing | NA | Germany | Blautia abundance correlated with healthier eating behavior, but this was reduced with Roux-en-Y gastric bypass. |
| Wang et al. 2023 [56] | improved ketogenic diet and exercise | (−) | Good | nd | metagenomic sequencing | 12 weeks | China | B. obeum was positively associated with VFA, while a negative association was observed with B. producta, B. hansenii, B. wexlerae, and Blautia sp. CAG257. |
| Pataky et al. 2016 [57] | hypocaloric hyperproteic diet | (−) | Good | nd | shotgun metagenomics | 3 weeks | Switzerland | Blautia was negatively associated with changes in the body fat mass. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chanda, W.; Jiang, H.; Liu, S.-J. The Ambiguous Correlation of Blautia with Obesity: A Systematic Review. Microorganisms 2024, 12, 1768. https://doi.org/10.3390/microorganisms12091768
Chanda W, Jiang H, Liu S-J. The Ambiguous Correlation of Blautia with Obesity: A Systematic Review. Microorganisms. 2024; 12(9):1768. https://doi.org/10.3390/microorganisms12091768
Chicago/Turabian StyleChanda, Warren, He Jiang, and Shuang-Jiang Liu. 2024. "The Ambiguous Correlation of Blautia with Obesity: A Systematic Review" Microorganisms 12, no. 9: 1768. https://doi.org/10.3390/microorganisms12091768
APA StyleChanda, W., Jiang, H., & Liu, S.-J. (2024). The Ambiguous Correlation of Blautia with Obesity: A Systematic Review. Microorganisms, 12(9), 1768. https://doi.org/10.3390/microorganisms12091768







