A Novel C-Terminal Truncated Bacteriocin Found by Comparison between Leuconostoc mesenteroides 406 and 213M0 Isolated from Mongolian Traditional Fermented Milk, Airag
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Culture Conditions
2.2. Antibacterial Activity Assay
2.3. Sequential Measurement of Bacterial Growth and Bacteriocin Production
2.4. Antibacterial Spectral Assay
2.5. PCR and DNA Sequencing to Close Plasmid Gaps
2.6. Plasmid Curing
2.7. Bacteriocin Purification
2.8. N-Terminal Amino Acid Sequencing
2.9. Mass Spectrometry Analysis
3. Results
3.1. Comparison of Bacterial Growth and Bacteriocin Production
3.2. Comparison of Antibacterial Spectra
3.3. DNA Sequencing and Plasmid Mapping
3.4. Homology Analysis of Mesentericin Y105–B105-Related Genes
3.5. Plasmid Curing
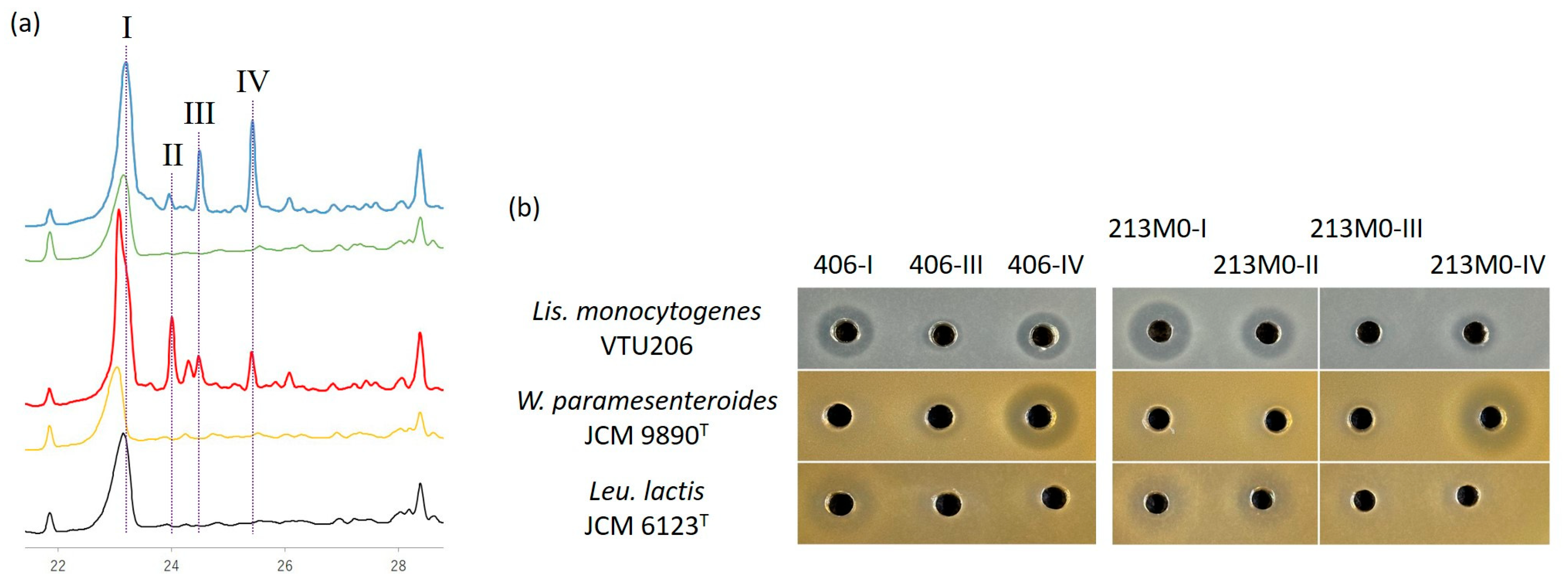
3.6. Purification of Bacteriocins
3.7. Identification of Purified Bacteriocins
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Buchanan, R.L.; Gorris, L.G.M.; Hayman, M.M.; Jackson, T.C.; Whiting, R.C. A review of Listeria Monocytogenes: An update on outbreaks, virulence, dose-response, ecology, and risk assessments. Food Control 2017, 75, 1–13. [Google Scholar] [CrossRef]
- Román, S.; Sánchez-Siles, L.M.; Siegrist, M. The importance of food naturalness for consumers: Results of a systematic review. Trends Food Sci. Technol. 2017, 67, 44–57. [Google Scholar] [CrossRef]
- Alvarez-Sieiro, P.; Montalbán-López, M.; Mu, D.; Kuipers, O.P. Bacteriocins of lactic acid bacteria: Extending the family. Appl. Microbiol. Biotechnol. 2016, 100, 2939–2951. [Google Scholar] [CrossRef] [PubMed]
- Silva, C.C.G.; Silva, S.P.M.; Ribeiro, S.C. Application of bacteriocins and protective cultures in dairy food preservation. Front. Microbiol. 2018, 9, 594. [Google Scholar] [CrossRef] [PubMed]
- Stiles, M.E. Bacteriocins produced by Leuconostoc species. J. Dairy Sci. 1994, 77, 2718–2724. [Google Scholar] [CrossRef] [PubMed]
- Orberg, P.K.; Sandine, W.E. Common occurrence of plasmid DNA and vancomycin resistance in Leuconostoc spp. Appl. Environ. Microbiol. 1984, 48, 1129–1133. [Google Scholar] [CrossRef]
- Hastings, J.W.; Sailer, M.; Johnson, K.; Roy, K.L.; Vederas, J.C.; Stiles, M.E. Characterization of leucocin A-UAL 187 and cloning of the bacteriocin gene from Leuconostoc Gelidum. J. Bacteriol. 1991, 173, 7491–7500. [Google Scholar] [CrossRef] [PubMed]
- Blom, H.; Katla, T.; Holck, A.; Sletten, K.; Axelsson, L.; Holo, H. Characterization, production, and purification of leucocin H, a two-peptide bacteriocin from Leuconostoc MF215B. Curr. Microbiol. 1999, 39, 43–48. [Google Scholar] [CrossRef]
- Masuda, Y.; Ono, H.; Kitagawa, H.; Ito, H.; Mu, F.; Sawa, N.; Zendo, T.; Sonomoto, K. Identification and characterization of leucocyclicin Q, a novel cyclic bacteriocin produced by Leuconostoc mesenteroides TK41401. Appl. Environ. Microbiol. 2011, 77, 8164–8170. [Google Scholar] [CrossRef]
- Shi, F.; Wang, Y.; Li, Y.; Wang, X. Mode of action of leucocin K7 produced by Leuconostoc mesenteroides K7 against Listeria monocytogenes and its potential in milk preservation. Biotechnol. Lett. 2016, 38, 1551–1557. [Google Scholar] [CrossRef]
- Hwang, I.C.; Oh, J.K.; Kim, S.H.; Oh, S.; Kang, D.K. Isolation and characterization of an anti-listerial bacteriocin from Leuconostoc lactis SD501. Korean J. Food Sci. Anim. Resour. 2018, 38, 1008–1018. [Google Scholar] [CrossRef] [PubMed]
- Lahiri, D.; Chakraborti, S.; Jasu, A.; Nag, M.; Dutta, B.; Dash, S.; Ray, R.R. Production and purification of bacteriocin from Leuconostoc lactis SM 2 strain. Biocatal. Agric. Biotechnol. 2020, 30, 101845. [Google Scholar] [CrossRef]
- Ahn, H.; Lee, D.; Lee, S.; Lee, K.G. Isolation and characterisation of the bacteriocin-producing Leuconostoc citreum HW02 from malts. Inst. J. Food Sci. Technol. 2023, 58, 83–93. [Google Scholar] [CrossRef]
- Papathanasopoulos, M.A.; Krier, F.; Revol-Junelles, A.M.; Lefebvre, G.; Le Caer, J.P.; von Holy, A.; Hastings, J.W. Multiple bacteriocin production by Leuconostoc mesenteroides TA33a and other Leuconostoc/Weissella strains. Curr. Microbiol. 1997, 35, 331–335. [Google Scholar] [CrossRef] [PubMed]
- Sawa, N.; Okamura, K.; Zendo, T.; Himeno, K.; Nakayama, J.; Sonomoto, K. Identification and characterization of novel multiple bacteriocins produced by Leuconostoc pseudomesenteroides QU 15. J. Appl. Microbiol. 2010, 109, 282–291. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.S.; Wu, H.C.; Kuo, C.Y.; Chen, Y.W.; Ho, S.; Yanagida, F. Leucocin C-607, a novel bacteriocin from the multiple-bacteriocin-producing Leuconostoc pseudomesenteroides 607 isolated from persimmon. Probiotics Antimicrob. Proteins 2018, 10, 148–156. [Google Scholar] [CrossRef]
- Wulijideligen; Asahina, T.; Hara, K.; Arakawa, K.; Nakano, H.; Miyamoto, T. Production of bacteriocin by Leuconostoc mesenteroides 406 isolated from Mongolian fermented mare’s milk, airag. Anim. Sci. J. 2012, 83, 704–711. [Google Scholar] [CrossRef]
- Arakawa, K.; Yoshida, S.; Aikawa, H.; Hano, C.; Bolormaa, T.; Burenjargal, S.; Miyamoto, T. Production of a bacteriocin-like inhibitory substance by Leuconostoc mesenteroides subsp. dextranicum 213M0 isolated from Mongolian fermented mare milk, airag. Anim. Sci. J. 2016, 87, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Morita, H.; Toh, H.; Oshima, K.; Nakano, A.; Hano, C.; Yoshida, S.; Nguyen, T.T.T.; Tashiro, K.; Arakawa, K.; Miyamoto, T. Draft genome sequence of Leuconostoc mesenteroides 406 isolated from the traditional fermented mare milk airag in Tuv Aimag, Mongolia. Genome Announc. 2016, 4, e00166-16. [Google Scholar] [CrossRef]
- Morita, H.; Toh, H.; Oshima, K.; Nakano, A.; Hano, C.; Yoshida, S.; Bolormaa, T.; Burenjargal, S.; Nguyen, C.T.K.; Tashiro, K.; et al. Draft genome sequence of Leuconostoc mesenteroides 213M0, isolated from traditional fermented mare milk airag in Bulgan Aimag, Mongolia. Genome Announc. 2016, 4, e00178-16. [Google Scholar] [CrossRef] [PubMed]
- Héchard, Y.; Derijard, B.; Letellier, F.; Cenatiempo, Y. Characterization and purification of mesentericin Y105, an anti-Listeria bacteriocin from Leuconostoc mesenteroides. J. Gen. Microbiol. 1992, 138, 2725–2731. [Google Scholar] [CrossRef]
- Fremaux, C.; Héchard, Y.; Cenatiempo, Y. Mesentericin Y105 gene clusters in Leuconostoc mesenteroides Y105. Microbiology 1995, 141, 1637–1645. [Google Scholar] [CrossRef] [PubMed]
- Dayem, M.A.; Fleury, Y.; Devilliers, G.; Chaboisseau, E.; Girard, R.; Nicolas, P.; Delfour, A. The putative immunity protein of the gram-positive bacteria Leuconostoc mesenteroides is preferentially located in the cytoplasm compartment. FEMS Microbiol. Lett. 1996, 138, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Biet, F.; Berjeaud, J.M.; Worobo, R.W.; Cenatiempo, Y.; Fremaux, C. Heterologous expression of the bacteriocin mesentericin Y105 using the dedicated transport system and the general secretion pathway. Microbiology 1998, 144, 2845–2854. [Google Scholar] [CrossRef]
- Héchard, Y.; Berjeaud, J.M.; Cenatiempo, Y. Characterization of the mesB gene and expression of bacteriocins by Leuconostoc mesenteroides Y105. Curr. Microbiol. 1999, 39, 265–269. [Google Scholar] [CrossRef]
- Aucher, W.; Simonet, V.; Fremaux, C.; Dalet, K.; Simon, L.; Cenatiempo, Y.; Frère, J.; Berjeaud, J.M. Differences in mesentericin secretion systems from two Leuconostoc strains. FEMS Microbiol. Lett. 2004, 232, 15–22. [Google Scholar] [CrossRef]
- Mok, J.S.; Miyamoto, T.; Kataoka, K. Properties of antibacterial substance produced by wild Lactobacillus strain IMC-1 from Inner Mongolian cheese. Anim. Sci. Technol. 1998, 69, 768–777. [Google Scholar]
- Tagg, J.R.; McGiven, A.R. Assay system for bacteriocins. Appl. Microbiol. 1971, 21, 943. [Google Scholar] [CrossRef] [PubMed]
- Klaenhammer, T. Genetics of bacteriocins produced by lactic acid bacteria. FEMS Microbiol. Rev. 1993, 12, 39–85. [Google Scholar] [CrossRef]
- Revol-Junelles, A.M.; Mathis, R.; Krier, F.; Fleury, Y.; Delfour, A.; Lefebvre, G. Leuconostoc mesenteroides subsp. mesenteroides FR52 synthesizes two distinct bacteriocins. Lett. Appl. Microbiol. 1996, 23, 120–124. [Google Scholar]
- Castano, S.; Desbat, B.; Delfour, A.; Dumas, J.M.; da Silva, A.; Dufourcq, J. Study of structure and orientation of mesentericin Y105, a bacteriocin from Gram-positive Leuconostoc mesenteroides, and its Trp-substituted analogues in phospholipid environments. Biochim. Biophys. Acta 2005, 1668, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Fimland, G.; Blingsmo, O.R.; Sletten, K.; Jung, G.; Nes, I.F.; Nissen-Meyer, J. New biologically active hybrid bacteriocins constructed by combining regions from various pediocin-like bacteriocins: The C-terminal region is important for determining specifity. Appl. Environ. Microbiol. 1996, 62, 3313–3318. [Google Scholar] [CrossRef] [PubMed]
- Johnsen, L.; Fimland, G.; Eijsink, V.; Nissen-Meyer, J. Engineering increased stability in the antimicrobial peptide pediocin PA-1. Appl. Environ. Microbiol. 2000, 66, 4798–4802. [Google Scholar] [CrossRef]
- Kuniyoshi, T.M.; O’Connor, P.M.; Lawton, E.; Thapa, D.; Mesa-Pereira, B.; Abulu, S.; Hill, C.; Ross, R.P.; Oliveira, R.P.S.; Cotter, P.D. An oxidation resistant pediocin PA-1 derivative and penocin A display effective anti-Listeria activity in a model human gut environment. Gut Microbes 2022, 14, 2004071. [Google Scholar] [CrossRef]
- Campanero, C.; Muñoz-Atienza, E.; Diep, D.B.; Feito, J.; Arbulu, S.; Del Campo, R.; Nes, I.F.; Hernández, P.E.; Herranz, C.; Cintas, L.M. Biochemical, genetic and transcriptional characterization of multibacteriocin production by the anti-pneumococcal dairy strain Streptococcus infantarius LP90. PLoS ONE 2020, 15, e0229417. [Google Scholar] [CrossRef] [PubMed]
- de Paula, A.T.; Jeronymo-Ceneviva, A.B.; Silva, L.F.; Todorov, S.D.; de Melo Franco, B.D.G.; Choiset, Y.; Haertlé, T.; Chobert, J.M.; Dousset, X.; Penna, A.L.B. Leuconostoc mesenteroides SJRP55: A bacteriocinogenic strain isolated from Brazilian water buffalo mozzarella cheese. Probiotics Antimicrob. Proteins 2014, 6, 186–197. [Google Scholar] [CrossRef]
- Dündar, H.; Salih, B.; Bozoğlu, F. Purification and characterization of a bacteriocin from an oenological strain of Leuconostoc mesenteroides subsp. cremoris. Prep. Biochem. Biotechnol. 2016, 46, 354–359. [Google Scholar] [CrossRef]
- Xiraphi, N.; Georgalaki, M.; Rantsiou, K.; Cocolin, L.; Tsakalidou, E.; Drosinos, E.H. Purification and characterization of a bacteriocin produced by Leuconostoc mesenteroides E131. Meat Sci. 2008, 80, 194–203. [Google Scholar] [CrossRef]
- Vassiliadis, G.; Peduzzi, J.; Zirah, S.; Thomas, X.; Rebuffat, S.; Destoumieux-Garzón, D. Insight into siderophore-carrying peptide biosynthesis: Enterobactin is a precursor for microcin E492 posttranslational modification. Antimicrob. Agents Chemother. 2007, 51, 3546–3553. [Google Scholar] [CrossRef]
- Wan, X.; Saris, P.E.J.; Takala, T.M. Genetic characterization and expression of leucocin B, a class IId bacteriocin from Leuconostoc carnosum 4010. Res. Microbiol. 2015, 166, 494–503. [Google Scholar] [CrossRef]
- Morisset, D.; Frère, J. Heterologous expression of bacteriocins using the mesentericin Y105 dedicated transport system by Leuconostoc mesenteroides. Biochimie 2002, 84, 569–576. [Google Scholar] [CrossRef] [PubMed]
- Eicher, C.; Coulon, J.; Favier, M.; Alexandre, H.; Reguant, C.; Grandvalet, C. Citrate metabolism in lactic acid bacteria: Is there a beneficial effect for Oenococcus oeni in wine? Front. Microbiol. 2024, 14, 1283220. [Google Scholar] [CrossRef] [PubMed]
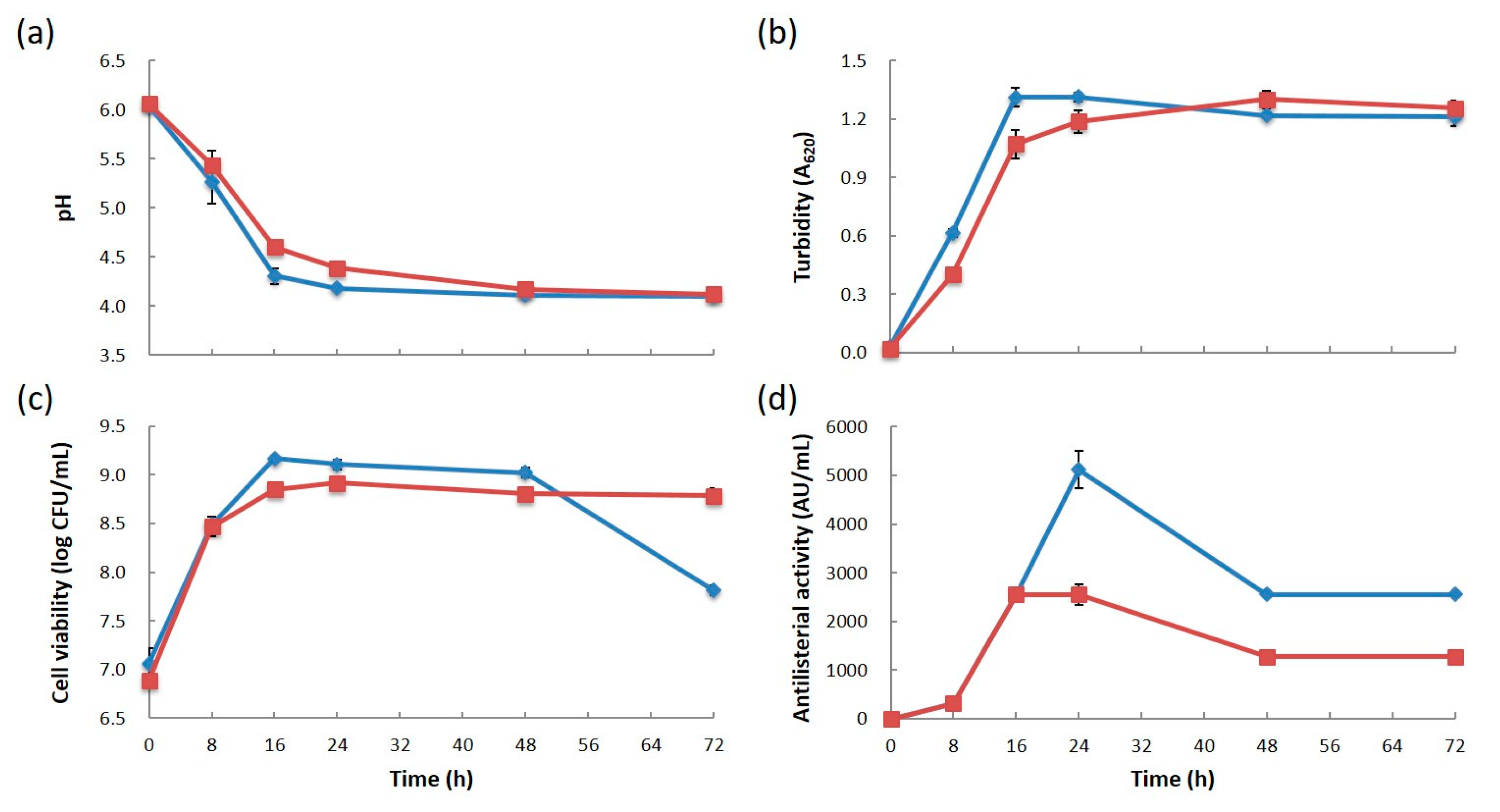

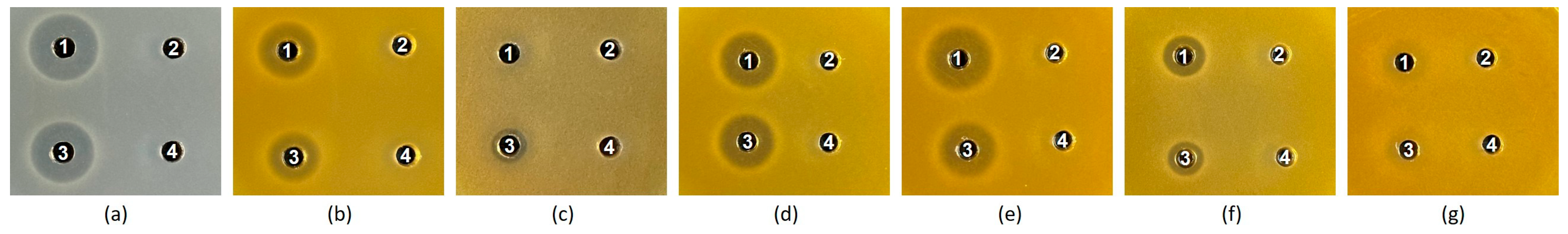
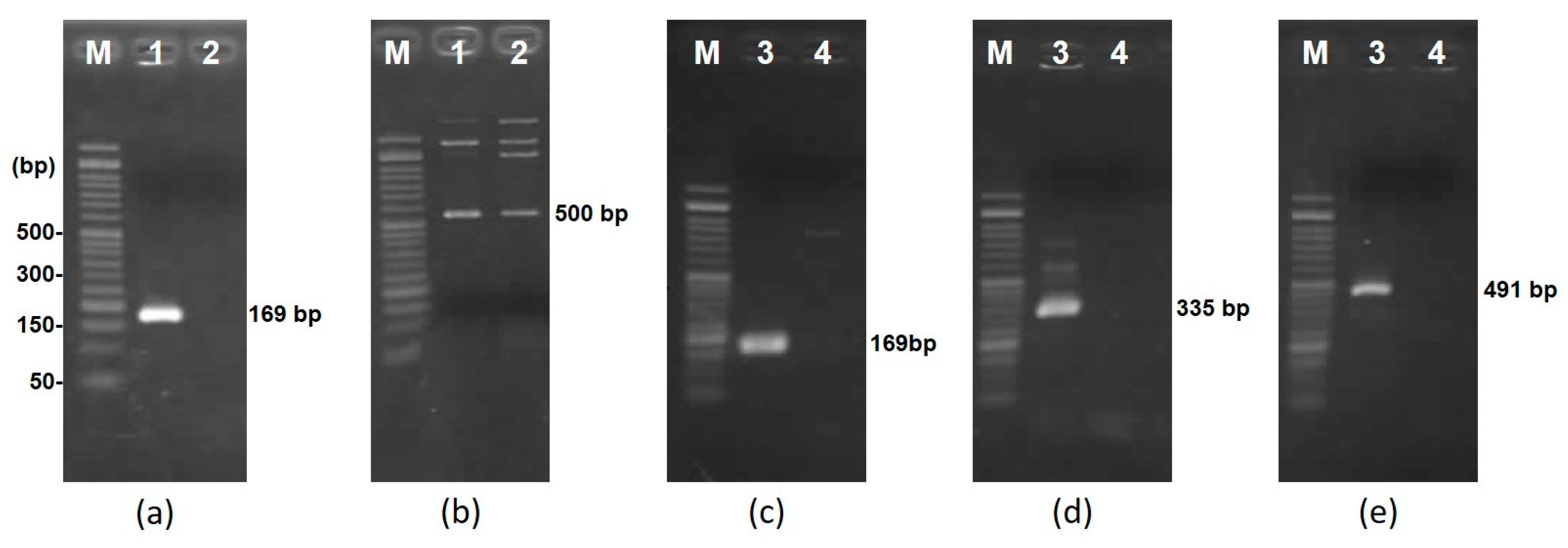
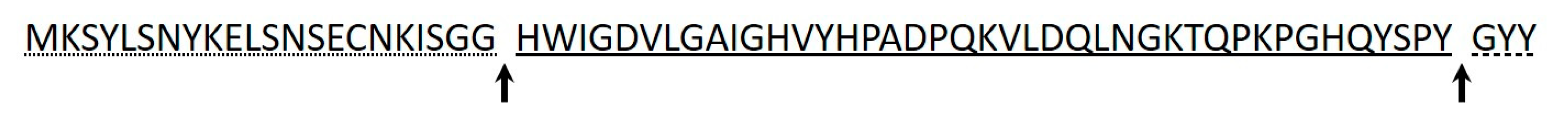

| Tested Bacterium | Culture Condition 2 | Antibacterial Activity (AU/mL) 3 | ||
|---|---|---|---|---|
| Species | Strain 1 | Strain 406 | Strain 213M0 | |
| Listeria monocytogenes | VTU 206 | 37 °C, TYLG | 4969 ± 99.1 | 2695 ± 63.1 |
| Listeria monocytogenes | JCM 7671 | 37 °C, TYLG | 3093 ± 111.6 | 2560 ± 105.2 |
| Listeria monocytogenes | JCM 7672 | 37 °C, TYLG | 3328 ± 85.9 | 3072 ± 97.1 |
| Listeria monocytogenes | JCM 7673 | 37 °C, TYLG | 3584 ± 70.1 | 2944 ± 105.2 |
| Listeria monocytogenes | JCM7674 | 37 °C, TYLG | 5546 ± 188.4 | 3584 ± 105.2 |
| Listeria monocytogenes | JCM 7675 | 37 °C, TYLG | 2560 ± 0.0 | 2560 ± 0.0 |
| Listeria monocytogenes | JCM 7679 | 37 °C, TYLG | 5120 ± 0.0 | 5120 ± 0.0 |
| Listeria monocytogenes | JCM 7680 | 37 °C, TYLG | 1706 ± 37.0 | 2560 ± 0.0 |
| Listeria ivanovii subsp. ivanovii | JCM 7681T | 37 °C, TYLG | 15,360 ± 280.4 | 9386 ± 104.5 |
| Enterococcus faecalis | JCM 5803T | 37 °C, MRS | 724 ± 13.6 | 600 ± 6.8 |
| Enterococcus faecium | JCM 5804T | 37 °C, MRS | - | - |
| Lactococcus cremoris subsp. cremoris | NBRC 100676T | 30 °C, MRS | - | - |
| Lactococcus lactis subsp. lactis | NBRC 100933T | 30 °C, MRS | - | - |
| Lactococcus lactis subsp. lactis | IFO 12007 | 30 °C, MRS | - | - |
| Lactococcus lactis subsp. lactis | NIAI 527 | 30 °C, MRS | - | - |
| Lactococcus lactis subsp. lactis | NIAI N-7 | 30 °C, MRS | - | - |
| Lactococcus lactis subsp. lactis | JCM 7638 | 30 °C, MRS | - | - |
| Lactococcus lactis subsp. hordniae | NBRC 100931T | 30 °C, MRS | - | - |
| Leuconostoc mesenteroides subsp. cremoris | NBRC 107766T | 30 °C, MRS | 72 ± 2.5 | 32 ± 1.3 |
| Leuconostoc mesenteroides subsp. dextranicum | NBRC 100495T | 30 °C, MRS | 471 ± 11.2 | 235 ± 5.5 |
| Leuconostoc mesenteroides subsp. mesenteroides | NBRC 100496T | 30 °C, MRS | - | - |
| Leuconostoc lactis | JCM 6123T | 30 °C, MRS | 20 ± 0.0 | 40 ± 1.4 |
| Pediococcus acidilactici | JCM 8797T | 30 °C, MRS | - | - |
| Pediococcus parvulus | JCM 5889T | 30 °C, MRS | - | - |
| Pediococcus pentosaceus | JCM 5885 | 37 °C, MRS | - | - |
| Pediococcus pentosaceus | JCM 5890T | 37 °C, MRS | - | - |
| Streptococcus thermophilus | JCM 17834T | 37 °C, MRS | - | - |
| Streptococcus thermophilus | JCM 20026 | 37 °C, MRS | - | - |
| Weissella cibaria | JCM 12495T | 30 °C, MRS | 297 ± 8.6 | 114 ± 6.1 |
| Weissella confusa | JCM 1093T | 30 °C, MRS | - | - |
| Weissella paramesenteroides | JCM 9890T | 30 °C, MRS | 1768 ± 30.3 | 522 ± 8.4 |
| Weissella viridescens | JCM 1174T | 37 °C, MRS | - | - |
| Primer Name | Sequence (5′–3′) | Target |
|---|---|---|
| 406-33-F1 | CCCAATACACCTTTACCACCAC | Contig-33 (pLM406A) in strain 406 |
| 406-33-R1 | CTTGGATTGTGGGAACAAGA | |
| 406-40-F1 | AGAAACTGCCCGTGATGGAAAC | Contig-40 (pLM406B) in strain 406 |
| 406-40-R1 | GCTGGTGTTGGATTGTCTTTGCT | |
| 213-26-F4 | AGCGGTTGCTATAACGGCTA | Contig-26 (pLM213M0A) in strain 213M0 |
| 213-26-R4 | GCTTCAAATGACGACTGCAA | |
| 213-26-F5 | CGAGCTTTAAAGGGTGCTGAAAAAT | Contig-26 (pLM213M0A) in strain 213M0 |
| 213-26-R5 | CGCTACTGAATTTCTTGTCAAGGTTGT | |
| 213-30-F1 | TTAGTCCGTGAGCGGTTTATGAGAG | Contig-30 (pLM213M0B) in strain 213M0 |
| 213-30-R1 | AATCAAGAAAGGAGCTGTGATGACG | |
| 213-48-F2 | TTGCGCTAATCGGTCAATGG | Contig-48 (pLM213M0C) in strain 213M0 |
| 213-48-R2 | GTGACCGACCGTAGGGAGACTTTAT | |
| mesY-F | ACCAAAATCCATTTCCACCA | Structural gene (mesY) of mesentericin Y105 |
| mesY-R | TCTGTGGAAGCATATCAGCAA |
| Plasmid | Length (bp) | GC (%) | ORFs | Accession No. |
|---|---|---|---|---|
| pLM406A | 32,775 | 34.2 | 42 | LC832860 |
| pLM406B | 23,705 | 39.4 | 24 | LC832861 |
| pLM213M0A | 41,975 | 33.5 | 51 | LC832857 |
| pLM213M0B | 11,669 | 33.8 | 14 | LC832858 |
| pLM213M0C | 6449 | 30.2 | 8 | LC832859 |
| Producer | Fraction | N-Terminal Sequence | MS [M + H]+ | Identification |
|---|---|---|---|---|
| Leuconostoc mesenteroides 406 | 406-I | KYYGNGVH(X)TKSG- | 3869 | Mesentericin Y105 |
| 406-III | KGVLGWLSMASSA- | 3463 | Oxidized mesentericin B105 | |
| 406-IV | KGVLGWL- | 3447 | Mesentericin B105 | |
| Leuconostoc mesenteroides 213M0 | 213M0-I | KYYGNGVH(X)TKSG- | 3869 | Mesentericin Y105 |
| 213M0-II | HWIGDVLGAIGHV- | 4564 | Mesentericin M 1 | |
| 213M0-III | - | 3463 | Oxidized mesentericin B105 | |
| 213M0-IV | KGVLGWL- | 3447 | Mesentericin B105 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hasiqimuge; Hano, C.; Arakawa, K.; Yoshida, S.; Zhao, J.; Toh, H.; Morita, H.; Miyamoto, T. A Novel C-Terminal Truncated Bacteriocin Found by Comparison between Leuconostoc mesenteroides 406 and 213M0 Isolated from Mongolian Traditional Fermented Milk, Airag. Microorganisms 2024, 12, 1781. https://doi.org/10.3390/microorganisms12091781
Hasiqimuge, Hano C, Arakawa K, Yoshida S, Zhao J, Toh H, Morita H, Miyamoto T. A Novel C-Terminal Truncated Bacteriocin Found by Comparison between Leuconostoc mesenteroides 406 and 213M0 Isolated from Mongolian Traditional Fermented Milk, Airag. Microorganisms. 2024; 12(9):1781. https://doi.org/10.3390/microorganisms12091781
Chicago/Turabian StyleHasiqimuge, Chihiro Hano, Kensuke Arakawa, Saki Yoshida, Junliang Zhao, Hidehiro Toh, Hidetoshi Morita, and Taku Miyamoto. 2024. "A Novel C-Terminal Truncated Bacteriocin Found by Comparison between Leuconostoc mesenteroides 406 and 213M0 Isolated from Mongolian Traditional Fermented Milk, Airag" Microorganisms 12, no. 9: 1781. https://doi.org/10.3390/microorganisms12091781
APA StyleHasiqimuge, Hano, C., Arakawa, K., Yoshida, S., Zhao, J., Toh, H., Morita, H., & Miyamoto, T. (2024). A Novel C-Terminal Truncated Bacteriocin Found by Comparison between Leuconostoc mesenteroides 406 and 213M0 Isolated from Mongolian Traditional Fermented Milk, Airag. Microorganisms, 12(9), 1781. https://doi.org/10.3390/microorganisms12091781





