Plants with Antimicrobial Activity against Escherichia coli, a Meta-Analysis for Green Veterinary Pharmacology Applications
Abstract
1. Introduction
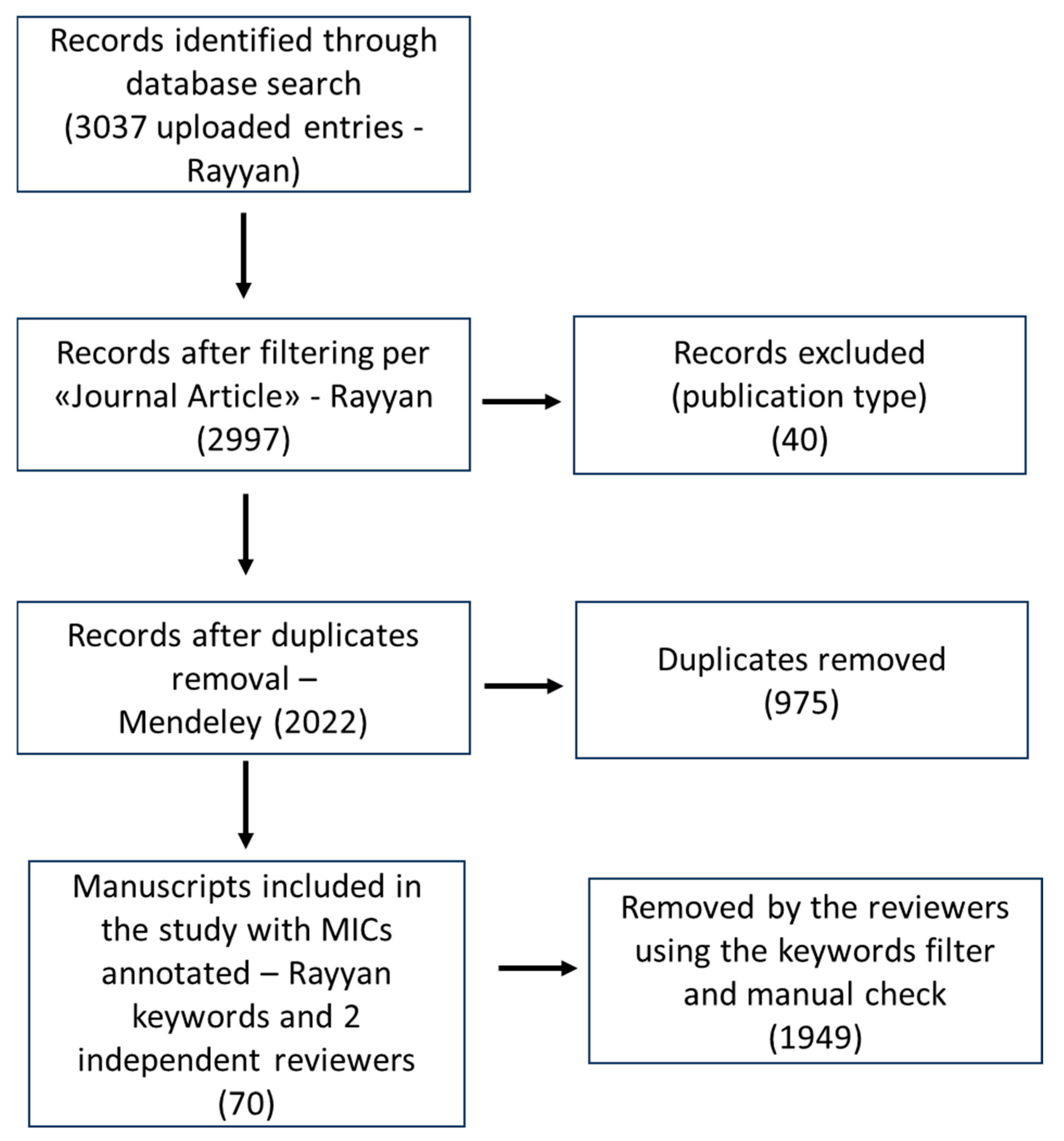
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gruel, G.; Sellin, A.; Riveiro, H.; Pot, M.; Breurec, S.; Guyomard-Rabenirina, S.; Talarmin, A.; Ferdinand, S. Antimicrobial Use and Resistance in Escherichia coli from Healthy Food-Producing Animals in Guadeloupe. BMC Vet. Res. 2021, 17, 116. [Google Scholar] [CrossRef] [PubMed]
- Aijaz, M.; Ahmad, M.; Ansari, M.A.; Ahmad, S. Antimicrobial Resistance in a Globalized World: Current Challenges and Future Perspectives. Int. J. Pharm. Drug Des. 2023, 1, 7–22. [Google Scholar]
- Prestinaci, F.; Pezzotti, P.; Pantosti, A. Antimicrobial Resistance: A Global Multifaceted Phenomenon. Pathog. Glob. Health 2015, 109, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.K.; Hussein, S.; Qurbani, K.; Ibrahim, R.H.; Fareeq, A.; Mahmood, K.A.; Mohamed, M.G. Antimicrobial Resistance: Impacts, Challenges, and Future Prospects. J. Med. Surg. Public Health 2024, 2, 100081. [Google Scholar] [CrossRef]
- Piras, C.; Greco, V.; Gugliandolo, E.; Soggiu, A.; Tilocca, B.; Bonizzi, L.; Zecconi, A.; Cramer, R.; Britti, D.; Urbani, A. Raw Cow Milk Bacterial Consortium as Bioindicator of Circulating Anti-Microbial Resistance (AMR). Animals 2020, 10, 2378. [Google Scholar] [CrossRef]
- Piras, C.; Soggiu, A.; Greco, V.; Martino, P.A.; Del Chierico, F.; Putignani, L.; Urbani, A.; Nally, J.E.; Bonizzi, L.; Roncada, P. Mechanisms of Antibiotic Resistance to Enrofloxacin in Uropathogenic Escherichia coli in Dog. J. Proteom. 2015, 127, 365–376. [Google Scholar] [CrossRef]
- Oppedisano, F.; De Fazio, R.; Gugliandolo, E.; Crupi, R.; Palma, E.; Abbas Raza, S.H.; Tilocca, B.; Merola, C.; Piras, C.; Britti, D. Mediterranean Plants with Antimicrobial Activity against Staphylococcus aureus, a Meta-Analysis for Green Veterinary Pharmacology Applications. Microorganisms 2023, 11, 2264. [Google Scholar] [CrossRef]
- Piras, C.; Di Ciccio, P.A.; Soggiu, A.; Greco, V.; Tilocca, B.; Costanzo, N.; Ceniti, C.; Urbani, A.; Bonizzi, L.; Ianieri, A.S. Aureus Biofilm Protein Expression Linked to Antimicrobial Resistance: A Proteomic Study. Animals 2021, 11, 966. [Google Scholar] [CrossRef]
- Persad, A.K.; Lejeune, J.T. Animal Reservoirs of Shiga Toxin-Producing Escherichia coli. In Enterohemorrhagic Escherichia coli Other Shiga Toxin-Producing E. coli; Wiley: New York, NY, USA, 2015; pp. 211–230. [Google Scholar]
- Pokharel, P.; Dhakal, S.; Dozois, C.M. The Diversity of Escherichia coli Pathotypes and Vaccination Strategies against This Versatile Bacterial Pathogen. Microorganisms 2023, 11, 344. [Google Scholar] [CrossRef]
- Yang, S.-C.; Lin, C.-H.; Aljuffali, I.A.; Fang, J.-Y. Current Pathogenic Escherichia coli Foodborne Outbreak Cases and Therapy Development. Arch. Microbiol. 2017, 199, 811–825. [Google Scholar] [CrossRef]
- Zhang, S.; Liao, X.; Ding, T.; Ahn, J. Role of β-Lactamase Inhibitors as Potentiators in Antimicrobial Chemotherapy Targeting Gram-Negative Bacteria. Antibiotics 2024, 13, 260. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control (ECDC); European Food Safety Authority (EFSA); European Medicines Agency (EMA). Antimicrobial Consumption and Resistance in Bacteria from Humans and Food-producing Animals: Fourth Joint Inter-agency Report on Integrated Analysis of Antimicrobial Agent Consumption and Occurrence of Antimicrobial Resistance in Bacteria from Humans and food-producing animals in the EU/EEA JIACRA IV—2019−2021. EFSA J. 2024, 22, e8589. [Google Scholar]
- Piras, C.; Tilocca, B.; Castagna, F.; Roncada, P.; Britti, D.; Palma, E. Plants with Antimicrobial Activity Growing in Italy: A Pathogen-Driven Systematic Review for Green Veterinary Pharmacology Applications. Antibiotics 2022, 11, 919. [Google Scholar] [CrossRef] [PubMed]
- Zu, K.; Zhang, C.; Chen, F.; Zhang, Z.; Ahmad, S.; Nabi, G. Latitudinal Gradients of Angiosperm Plant Diversity and Phylogenetic Structure in China’s Nature Reserves. Glob. Ecol. Conserv. 2023, 42, e02403. [Google Scholar] [CrossRef]
- Yao, Z.; Xin, Y.; Yang, L.; Zhao, L.; Ali, A. Precipitation and Temperature Regulate Species Diversity, Plant Coverage and Aboveground Biomass through Opposing Mechanisms in Large-Scale Grasslands. Front. Plant Sci. 2022, 13, 999636. [Google Scholar] [CrossRef] [PubMed]
- Winkler, M.; Lamprecht, A.; Steinbauer, K.; Hülber, K.; Theurillat, J.; Breiner, F.; Choler, P.; Ertl, S.; Gutiérrez Girón, A.; Rossi, G. The Rich Sides of Mountain Summits–a Pan-European View on Aspect Preferences of Alpine Plants. J. Biogeogr. 2016, 43, 2261–2273. [Google Scholar] [CrossRef]
- Anthelme, F.; Mato, M.W.; Maley, J. Elevation and Local Refuges Ensure Persistence of Mountain Specific Vegetation in the Nigerien Sahara. J. Arid Environ. 2008, 72, 2232–2242. [Google Scholar] [CrossRef]
- Bolpagni, R.; Laini, A.; Stanzani, C.; Chiarucci, A. Aquatic Plant Diversity in Italy: Distribution, Drivers and Strategic Conservation Actions. Front. Plant Sci. 2018, 9, 116. [Google Scholar] [CrossRef]
- Selvi, F.; Campetella, G.; Canullo, R.; Chelli, S.; Domina, G.; Farris, E.; Gasperini, C.; Rosati, L.; Wellstein, C.; Carrari, E. The Italian Endemic Forest Plants. Plant Ecol. Evol. 2023, 156, 29–45. [Google Scholar] [CrossRef]
- Greco, V.; Piras, C.; Pieroni, L.; Urbani, A. Direct Assessment of Plasma/Serum Sample Quality for Proteomics Biomarker Investigation. In Serum/Plasma Proteomics. Methods in Molecular Biology; Humana Press: New York, NY, USA, 2017; pp. 3–21. [Google Scholar]
- Piras, C.; Soggiu, A.; Bonizzi, L.; Greco, V.; Ricchi, M.; Arrigoni, N.; Bassols, A.; Urbani, A.; Roncada, P. Identification of Immunoreactive Proteins of Mycobacterium avium subsp. Paratuberculosis. Proteomics 2015, 15, 813–823. [Google Scholar] [CrossRef]
- Leporatti, M.L.; Impieri, M. Ethnobotanical Notes about Some Uses of Medicinal Plants in Alto Tirreno Cosentino Area (Calabria, Southern Italy). J. Ethnobiol. Ethnomed. 2007, 3, 34. [Google Scholar] [CrossRef] [PubMed]
- Badalamenti, N.; Modica, A.; Ilardi, V.; Bruno, M.; Maresca, V.; Zanfardino, A.; Di Napoli, M.; Castagliuolo, G.; Varcamonti, M.; Basile, A. Daucus Carota Subsp. Maximus (Desf.) Ball from Pantelleria, Sicily (Italy): Isolation of Essential Oils and Evaluation of Their Bioactivity. Nat. Prod. Res. 2021, 36, 5842–5847. [Google Scholar] [CrossRef]
- Zucca, P.; Pintus, M.; Manzo, G.; Nieddu, M.; Steri, D.; Rinaldi, A.C. Antimicrobial, Antioxidant and Anti-Tyrosinase Properties of Extracts of the Mediterranean Parasitic Plant Cytinus hypocistis. BMC Res. Notes 2015, 8, 562. [Google Scholar] [CrossRef] [PubMed]
- Miceli, N.; Cavò, E.; Ragusa, S.; Cacciola, F.; Dugo, P.; Mondello, L.; Marino, A.; Cincotta, F.; Condurso, C.; Taviano, M.F. Phytochemical Characterization and Biological Activities of a Hydroalcoholic Extract Obtained from the Aerial Parts of Matthiola incana (L.) R.Br. Subsp. Incana (Brassicaceae) Growing Wild in Sicily (Italy). Chem. Biodivers. 2019, 16, e1800677. [Google Scholar] [CrossRef]
- Garzoli, S.; Turchetti, G.; Giacomello, P.; Tiezzi, A.; Masci, V.L.; Ovidi, E. Liquid and Vapour Phase of Lavandin (Lavandula × Intermedia) Essential Oil: Chemical Composition and Antimicrobial Activity. Molecules 2019, 24, 2701. [Google Scholar] [CrossRef]
- Caputo, L.; Nazzaro, F.; Souza, L.F.; Aliberti, L.; De Martino, L.; Fratianni, F.; Coppola, R.; De Feo, V. Laurus Nobilis: Composition of Essential Oil and Its Biological Activities. Molecules 2017, 22, 930. [Google Scholar] [CrossRef] [PubMed]
- Astaf’eva, O.V.; Sukhenko, L.T. Comparative Analysis of Antibacterial Properties and Chemical Composition of Glycyrrhiza glabra L. from Astrakhan Region (Russia) and Calabria Region (Italy). Bull. Exp. Biol. Med. 2014, 156, 829–832. [Google Scholar] [CrossRef]
- Fratianni, F.; Coppola, R.; Nazzaro, F. Phenolic Composition and Antimicrobial and Antiquorum Sensing Activity of an Ethanolic Extract of Peels from the Apple Cultivar Annurca. J. Med. Food 2011, 14, 957–963. [Google Scholar] [CrossRef]
- Sadeghi, Z.; Yang, J.L.; Venditti, A.; Moridi Farimani, M. A Review of the Phytochemistry, Ethnopharmacology and Biological Activities of Teucrium Genus (Germander). Nat. Prod. Res. 2021, 36, 5647–5664. [Google Scholar] [CrossRef]
- Miceli, N.; Filocamo, A.; Ragusa, S.; Cacciola, F.; Dugo, P.; Mondello, L.; Celano, M.; Maggisano, V.; Taviano, M.F. Chemical Characterization and Biological Activities of Phenolic-Rich Fraction from Cauline Leaves of Isatis tinctoria L. (Brassicaceae) Growing in Sicily, Italy. Chem. Biodivers. 2017, 14, e1700073. [Google Scholar] [CrossRef]
- Fratianni, F.; Riccardi, R.; Spigno, P.; Ombra, M.N.; Cozzolino, A.; Tremonte, P.; Coppola, R.; Nazzaro, F. Biochemical Characterization and Antimicrobial and Antifungal Activity of Two Endemic Varieties of Garlic (Allium sativum L.) of the Campania Region, Southern Italy. J. Med. Food 2016, 19, 686–691. [Google Scholar] [CrossRef] [PubMed]
- Mancini, E.; Senatore, F.; Del Monte, D.; De Martino, L.; Grulova, D.; Scognamiglio, M.; Snoussi, M.; De Feo, V. Studies on Chemical Composition, Antimicrobial and Antioxidant Activities of Five Thymus vulgaris L. Essential Oils. Molecules 2015, 20, 12016–12028. [Google Scholar] [CrossRef] [PubMed]
- Gelmini, F.; Squillace, P.; Testa, C.; Sparacino, A.C.; Angioletti, S.; Beretta, G. GC-MS Characterisation and Biological Activity of Essential Oils from Different Vegetative Organs of Plectranthus barbatus and Plectranthus caninus Cultivated in North Italy. Nat. Prod. Res. 2015, 29, 993–998. [Google Scholar] [CrossRef]
- Carlo Tenore, G.; Troisi, J.; Di Fiore, R.; Basile, A.; Novellino, E. Chemical Composition, Antioxidant and Antimicrobial Properties of Rapa Catozza Napoletana (Brassica rapa L. Var. Rapa DC.) Seed Meal, a Promising Protein Source of Campania Region (Southern Italy) Horticultural Germplasm. J. Sci. Food Agric. 2012, 92, 1716–1724. [Google Scholar] [CrossRef]
- Cottiglia, F.; Loy, G.; Garau, D.; Floris, C.; Casu, M.; Pompei, R.; Bonsignore, L. Antimicrobial Evaluation of Coumarins and Flavonoids from the Stems of Daphne Gnidium L. Phytomedicine 2001, 8, 302–305. [Google Scholar] [CrossRef] [PubMed]
- Loy, G.; Cottiglia, F.; Garau, D.; Deidda, D.; Pompei, R.; Bonsignore, L. Chemical Composition and Cytotoxic and Antimicrobial Activity of Calycotome villosa (Poiret) Link Leaves. Farmaco 2001, 56, 433–436. [Google Scholar] [CrossRef]
- Mazzanti, G.; Battinelli, L.; Salvatore, G. Antimicrobial Properties of the Linalol-Rich Essential Oil of Hyssopos officinalis L. var Decumbens (Lamiaceae). Flavour Fragr. J. 1998, 13, 289–294. [Google Scholar] [CrossRef]
- Tuberoso, C.I.; Kowalczyk, A.; Coroneo, V.; Russo, M.T.; Dessì, S.; Cabras, P. Chemical Composition and Antioxidant, Antimicrobial, and Antifungal Activities of the Essential Oil of Achillea ligustica All. J. Agric. Food Chem. 2005, 53, 10148–10153. [Google Scholar] [CrossRef]
- Romeo, F.V.; Fabroni, S.; Ballistreri, G.; Muccilli, S.; Spina, A.; Rapisarda, P. Characterization and Antimicrobial Activity of Alkaloid Extracts from Seeds of Different Genotypes of Lupinus Spp. Sustainability 2018, 10, 788. [Google Scholar] [CrossRef]
- Diass, K.; Merzouki, M.; Elfazazi, K.; Azzouzi, H.; Challioui, A.; Azzaoui, K.; Hammouti, B.; Touzani, R.; Depeint, F.; Ayerdi Gotor, A.; et al. Essential Oil of Lavandula Officinalis: Chemical Composition and Antibacterial Activities. Plants 2023, 12, 1571. [Google Scholar] [CrossRef]
- Villa-Ruano, N.; Pacheco-Hernández, Y.; Rubio-Rosas, E.; Ruiz-González, N.; Cruz-Duran, R.; Lozoya-Gloria, E.; Zurita-Vásquez, G.; Franco-Monsreal, J. Alkaloid Profile, Antibacterial and Allelopathic Activities of Lupinus jaimehintoniana B.L. Turner (Fabaceae). Arch. Biol. Sci. 2012, 64, 1065–1072. [Google Scholar] [CrossRef]
- Soulaimani, B.; Abbad, I.; Varoni, E.; Iriti, M.; Mezrioui, N.-E.; Hassani, L.; Abbad, A. Optimization of Antibacterial Activity of Essential Oil Mixture Obtained from Three Medicinal Plants: Evaluation of Synergism with Conventional Antibiotics and Nanoemulsion Effectiveness. S. Afr. J. Bot. 2022, 151, 900–908. [Google Scholar] [CrossRef]
- Rota, C.; Carraminana, J.J.; Burillo, J.; Herrera, A. In Vitro Antimicrobial Activity of Essential Oils from Aromatic Plants against Selected Foodborne Pathogens. J. Food Prot. 2004, 67, 1252–1256. [Google Scholar] [CrossRef]
- Fahimi, S.; Hajimehdipoor, H.; Shabanpoor, H.; Bagheri, F.; Shekarchi, M. Synergic Antibacterial Activity of Some Essential Oils from Lamiaceae. Res. J. Pharmacogn. 2015, 2, 23–29. [Google Scholar]
- El-Kased, R.F.; El-Kersh, D.M. GC-MS Profiling of Naturally Extracted Essential Oils: Antimicrobial and Beverage Preservative Actions. Life 2022, 12, 1587. [Google Scholar] [CrossRef]
- Eliuz, E.A.E.; Ayas, D.; Goksen, G. In Vitro Phototoxicity and Antimicrobial Activity of Volatile Oil Obtained from Some Aromatic Plants. J. Essent. Oil Bear. Plants 2017, 20, 758–768. [Google Scholar] [CrossRef]
- Luchesi, L.A.; Paulus, D.; Busso, C.; Frata, M.T.; De Oliveira, P.J.B. Chemical Composition and Antibacterial Activity of Essential oils. Rev. Bras. Plantas Med. 2019, 21, 50–59. [Google Scholar]
- Elemike, E.E.; Onwudiwe, D.C.; Ekennia, A.C.; Katata-Seru, L. Biosynthesis, Characterization, and Antimicrobial Effect of Silver Nanoparticles Obtained Using Lavandula x Intermedia. Res. Chem. Intermed. 2017, 43, 1383–1394. [Google Scholar] [CrossRef]
- Adaszyńska-Skwirzyńska, M.; Zych, S.; Bucław, M.; Majewska, D.; Dzięcioł, M.; Szczerbińska, D. Evaluation of the Antibacterial Activity of Gentamicin in Combination with Essential Oils Isolated from Different Cultivars and Morphological Parts of Lavender (Lavandula angustifolia Mill.) against Selected Bacterial Strains. Molecules 2023, 28, 5781. [Google Scholar] [CrossRef]
- Guo, F.; Chen, Q.; Liang, Q.; Zhang, M.; Chen, W.; Chen, H.; Yun, Y.; Zhong, Q.; Chen, W. Antimicrobial Activity and Proposed Action Mechanism of Linalool against Pseudomonas fluorescens. Front. Microbiol. 2021, 12, 562094. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, K.; Zhang, K.; Zhang, J.; Fu, J.; Li, J.; Wang, G.; Qiu, Z.; Wang, X.; Li, J. Antibacterial Activity of Cinnamomum Camphora Essential Oil on Escherichia coli during Planktonic Growth and Biofilm Formation. Front. Microbiol. 2020, 11, 561002. [Google Scholar] [CrossRef]
- Yeh, R.-Y.; Shiu, Y.-L.; Shei, S.-C.; Cheng, S.-C.; Huang, S.-Y.; Lin, J.-C.; Liu, C.-H. Evaluation of the Antibacterial Activity of Leaf and Twig Extracts of Stout Camphor Tree, Cinnamomum kanehirae, and the Effects on Immunity and Disease Resistance of White shrimp, Litopenaeus vannamei. Fish Shellfish Immunol. 2009, 27, 26–32. [Google Scholar] [CrossRef]
- Duda-Madej, A.; Viscardi, S.; Grabarczyk, M.; Topola, E.; Kozłowska, J.; Mączka, W.; Wińska, K. Is Camphor the Future in Supporting Therapy for Skin Infections? Pharmaceuticals 2024, 17, 715. [Google Scholar] [CrossRef]
- Trombetta, D.; Castelli, F.; Sarpietro, M.G.; Venuti, V.; Cristani, M.; Daniele, C.; Saija, A.; Mazzanti, G.; Bisignano, G. Mechanisms of Antibacterial Action of Three Monoterpenes. Antimicrob. Agents Chemother. 2005, 49, 2474–2478. [Google Scholar] [CrossRef] [PubMed]
- Basavegowda, N.; Baek, K.-H. Combination Strategies of Different Antimicrobials: An Efficient and Alternative Tool for Pathogen Inactivation. Biomedicines 2022, 10, 2219. [Google Scholar] [CrossRef] [PubMed]
- Khayyat, S. Thermal, Photo-Oxidation and Antimicrobial Studies of Linalyl Acetate as a Major Ingredient of Lavender Essential Oil. Arab. J. Chem. 2020, 13, 1575–1581. [Google Scholar] [CrossRef]
- Salvatori, E.S.; Morgan, L.V.; Ferrarini, S.; Zilli, G.A.L.; Rosina, A.; Almeida, M.O.P.; Hackbart, H.C.S.; Rezende, R.S.; Albeny-Simões, D.; Oliveira, J.V. Anti-Inflammatory and Antimicrobial Effects of Eucalyptus Spp. Essential Oils: A Potential Valuable Use for an Industry Byproduct. Evid. Based Complement. Altern. Med. 2023, 2023, 2582698. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Singh, S.; Bond, J.; Singh, A.; Rustagi, A. Evaluation of Antibacterial Properties of Essential Oils from Clove and Eucalyptus. Evaluation 2014, 7, 291–294. [Google Scholar]
- Fatimazahra, M.; Jamila, C.; Achraf, A.; Maaghloud, F.E.; Nour-eddine, C.; Mohamed, D. Eucalyptol from Rosmarinus officinalis L. as an Antioxidant and Antibacterial Agent against Poultry-Isolated Bacterial Strains: In Vitro and in Silico Study. Chem. Africa 2024, 7, 1865–1876. [Google Scholar] [CrossRef]
- Salehi, B.; Upadhyay, S.; Erdogan Orhan, I.; Kumar Jugran, A.; Jayaweera, S.L.D.; Dias, D.A.; Sharopov, F.; Taheri, Y.; Martins, N.; Baghalpour, N. Therapeutic Potential of α-and β-Pinene: A Miracle Gift of Nature. Biomolecules 2019, 9, 738. [Google Scholar] [CrossRef]
- Bhatt, P.; Negi, P.S. Antioxidant and Antibacterial Activities in the Leaf Extracts of Indian Borage (Plectranthus amboinicus). Food Nutr. Sci. 2012, 3, 146–152. [Google Scholar]
- Rodrigues, F.F.G.; Boligon, A.A.; Menezes, I.R.A.; Galvão-Rodrigues, F.F.; Salazas, G.J.T.; Nonato, C.F.A.; Braga, N.T.T.M.; Correia, F.M.A.; Caldas, G.F.R.; Coutinho, H.D.M. Hplc/Dad, Antibacterial and Antioxidant Activities of Plectranthus Species (Lamiaceae) Combined with the Chemometric Calculations. Molecules 2021, 26, 7665. [Google Scholar] [CrossRef] [PubMed]
- Santos, F.A.V.; Serra, C.G.; Bezerra, R.J.A.C.; Figueredo, F.G.; Matias, F.F.; Menezes, I.R.A.; Costa, J.G.M.; Coutinho, H.D.M. Antibacterial Activity of Plectranthus amboinicus Lour (Lamiaceae) Essential Oil against Streptococcus mutans. Eur. J. Integr. Med. 2016, 8, 293–297. [Google Scholar] [CrossRef]
- Dahham, S.S.; Tabana, Y.M.; Iqbal, M.A.; Ahamed, M.B.K.; Ezzat, M.O.; Majid, A.S.A.; Majid, A.M.S.A. The Anticancer, Antioxidant and Antimicrobial Properties of the Sesquiterpene β-Caryophyllene from the Essential Oil of Aquilaria crassna. Molecules 2015, 20, 11808–11829. [Google Scholar] [CrossRef] [PubMed]
- Moo, C.-L.; Yang, S.-K.; Osman, M.-A.; Yuswan, M.H.; Loh, J.-Y.; Lim, W.-M.; Swee-Hua-Erin, L.I.M.; Lai, K.-S. Antibacterial Activity and Mode of Action of β-Caryophyllene on Bacillus Cereus. Polish J. Microbiol. 2020, 69, 49–54. [Google Scholar] [CrossRef]
- Dickson, K.; Scott, C.; White, H.; Zhou, J.; Kelly, M.; Lehmann, C. Antibacterial and Analgesic Properties of Beta-Caryophyllene in a Murine Urinary Tract Infection Model. Molecules 2023, 28, 4144. [Google Scholar] [CrossRef] [PubMed]
- Swamy, M.K.; Akhtar, M.S.; Sinniah, U.R. Antimicrobial Properties of Plant Essential Oils against Human Pathogens and Their Mode of Action: An Updated Review. Evid. Based Complement. Altern. 2016, 2016, 3012462. [Google Scholar] [CrossRef] [PubMed]
- Montanari, R.M.; Barbosa, L.C.A.; Demuner, A.J.; Silva, C.J.; Carvalho, L.S.; Andrade, N.J. Chemical Composition and Antibacterial Activity of Essential Oils from Verbenaceae Species: Alternative Sources of (E)-Caryophyllene and Germacrene-D. Quim. Nova 2011, 34, 1550–1555. [Google Scholar] [CrossRef]
- Bajalan, I.; Rouzbahani, R.; Pirbalouti, A.G.; Maggi, F. Variation in Chemical Composition and Antibacterial Activity of the Essential Oil of Wild Populations of Phlomis olivieri. Chem. Biodivers. 2017, 14, e1600444. [Google Scholar] [CrossRef]
- Lampart-Szczapa, E.; Siger, A.; Trojanowska, K.; Nogala-Kalucka, M.; Malecka, M.; Pacholek, B. Chemical Composition and Antibacterial Activities of Lupin Seeds Extracts. Nahr. Food 2003, 47, 286–290. [Google Scholar] [CrossRef]
- Abdel-Shafi, S.; El-Nemr, M.; Enan, G.; Osman, A.; Sitohy, B.; Sitohy, M. Isolation and Characterization of Antibacterial Conglutinins from Lupine Seeds. Molecules 2022, 28, 35. [Google Scholar] [CrossRef] [PubMed]
- Erdemoglu, N.; Ozkan, S.; Tosun, F. Alkaloid Profile and Antimicrobial Activity of Lupinus angustifolius L. Alkaloid Extract. Phytochem. Rev. 2007, 6, 197–201. [Google Scholar] [CrossRef]

| Plant | Total Records | Records Included |
|---|---|---|
| Daucus carota subsp. Maximus | 121 | 1 |
| Cytinus hypocistis | 1 | 0 |
| Matthiola incana | 4 | 0 |
| Lavandula spp. | 48 | 8 |
| Laurus nobilis | 70 | 14 |
| Glycyrrhiza glabra L. | 17 | 0 |
| Malus domestica var. Annurca | 2 | 0 |
| Teucrium spp. | 67 | 12 |
| Isatis tinctoria L. | 3 | 1 |
| Allium sativum L. | 381 | 31 |
| Thymus vulgaris L. | 66 | 16 |
| Plectranthus barbatus and Plectranthus caninus | 11 | 3 |
| Brassica rapa | 49 | 1 |
| Daphne gnidium L. | 3 | 1 |
| Calycotome villosa | 2 | 0 |
| Hyssopos officinalis L. | 1 | 0 |
| Achillea ligustica | 1 | 0 |
| Lupinus spp. | 53 | 3 |
| Plant | Average MIC mg/mL | Five Most Abundant Compounds | References |
|---|---|---|---|
| Lavandula spp. | 0.144 | Linalool | Essential Oil of Lavandula officinalis: Chemical Composition and Antibacterial Activities [42] |
| Camphor | |||
| Linalyl acetate | |||
| Eucalyptol | |||
| 4-Terpinenol | |||
| Plectranthus spp. | 0.26 | 1-octen-3-ol | GC–MS characterisation and biological activity of essential oils from different vegetative organs of Plectranthus barbatus and Plectranthus caninus cultivated in north Italy [35] |
| anethol | |||
| b-caryophyllene | |||
| terpinen-4-ol | |||
| D germacrene | |||
| Lupinus jaimehintoniana | 0.140 | Lupanine | Alkaloid Profile, Antibacterial And Allelopathic Activities Of Lupinus jaimehintoniana B.L. Turner (Fabaceae). [43] |
| 5,6-dehydrolupanine | |||
| d-thermopsine | |||
| Sparteine | |||
| Nuttalline |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Fazio, R.; Oppedisano, F.; Caioni, G.; Tilocca, B.; Piras, C.; Britti, D. Plants with Antimicrobial Activity against Escherichia coli, a Meta-Analysis for Green Veterinary Pharmacology Applications. Microorganisms 2024, 12, 1784. https://doi.org/10.3390/microorganisms12091784
De Fazio R, Oppedisano F, Caioni G, Tilocca B, Piras C, Britti D. Plants with Antimicrobial Activity against Escherichia coli, a Meta-Analysis for Green Veterinary Pharmacology Applications. Microorganisms. 2024; 12(9):1784. https://doi.org/10.3390/microorganisms12091784
Chicago/Turabian StyleDe Fazio, Rosario, Francesca Oppedisano, Giulia Caioni, Bruno Tilocca, Cristian Piras, and Domenico Britti. 2024. "Plants with Antimicrobial Activity against Escherichia coli, a Meta-Analysis for Green Veterinary Pharmacology Applications" Microorganisms 12, no. 9: 1784. https://doi.org/10.3390/microorganisms12091784
APA StyleDe Fazio, R., Oppedisano, F., Caioni, G., Tilocca, B., Piras, C., & Britti, D. (2024). Plants with Antimicrobial Activity against Escherichia coli, a Meta-Analysis for Green Veterinary Pharmacology Applications. Microorganisms, 12(9), 1784. https://doi.org/10.3390/microorganisms12091784






