Calorie Restriction Decreases Competitive Fitness in Saccharomyces cerevisiae Following Heat Stress
Abstract
:1. Introduction
2. Materials and Methods
2.1. Media Preparation
2.2. Microorganisms
2.3. Determination of the Lag Phase’s Duration and Doubling Times
2.4. Application of Heat Stress
2.5. RNAseq and Transcriptome Data Analysis
2.6. Statistical Analysis
3. Results
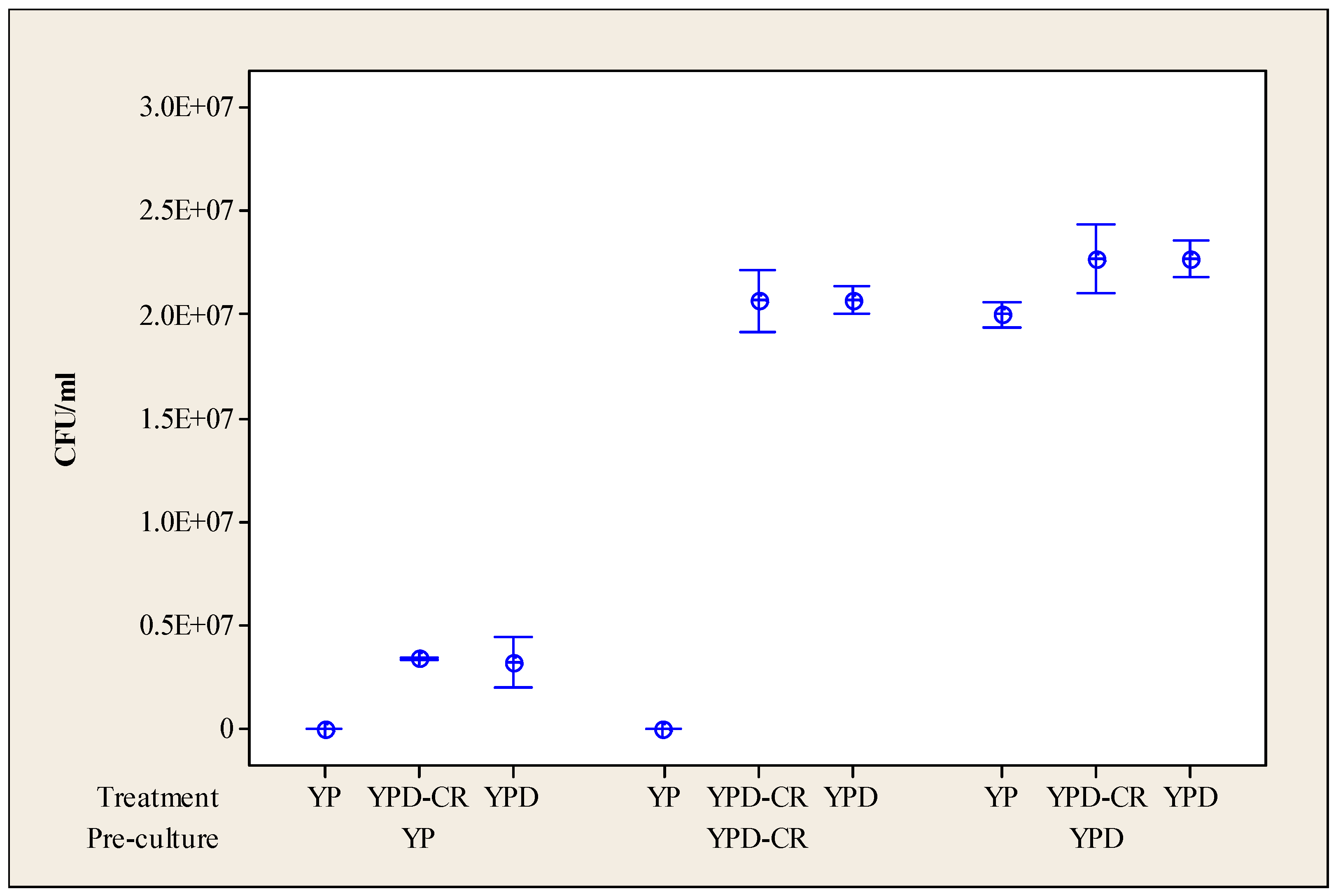
3.1. Impact of Calorie Restriction on Subsequent Culturability
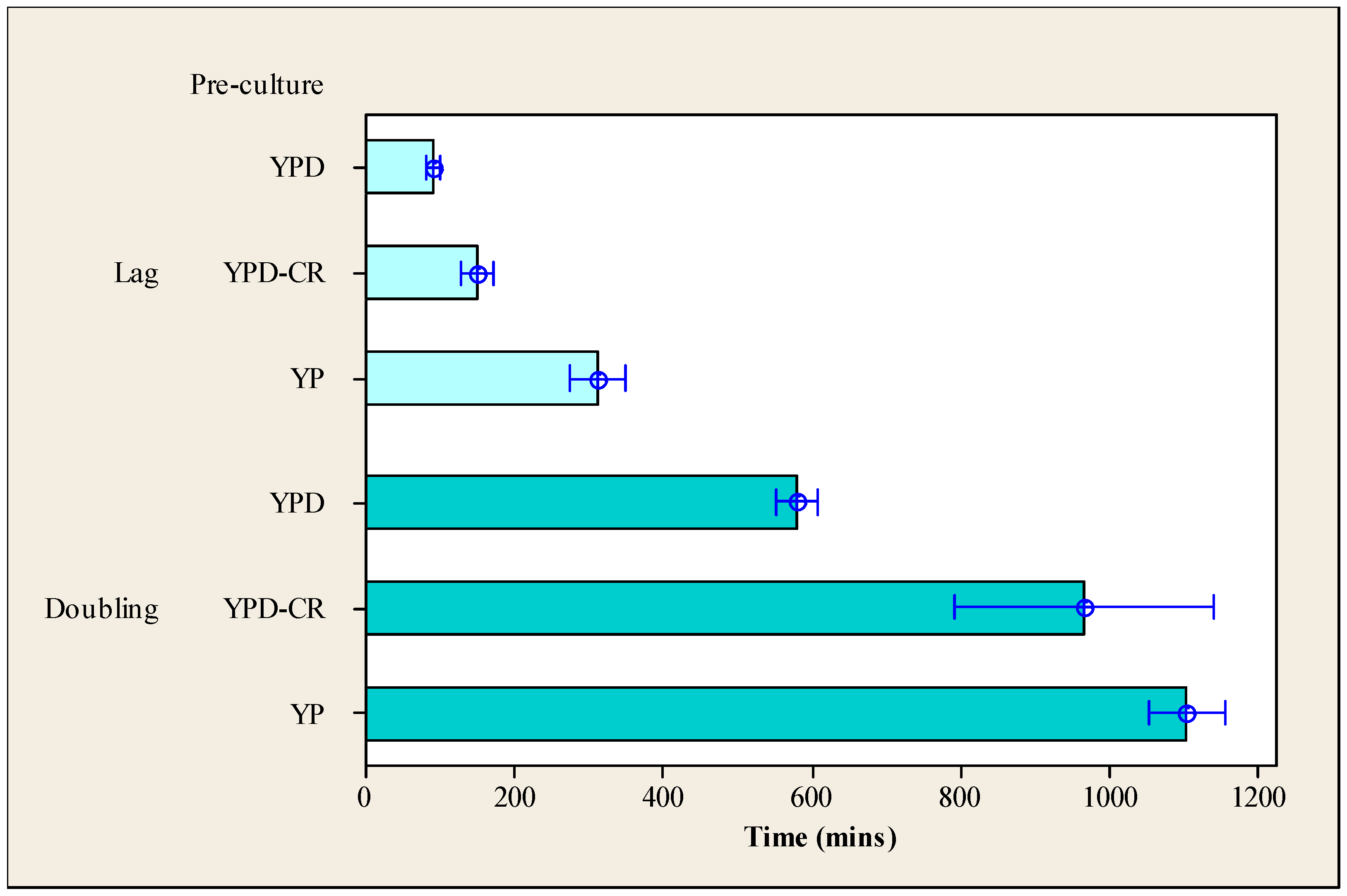
3.2. Impact of Calorie Restriction on the Subsequent Lag Phase and Doubling Time
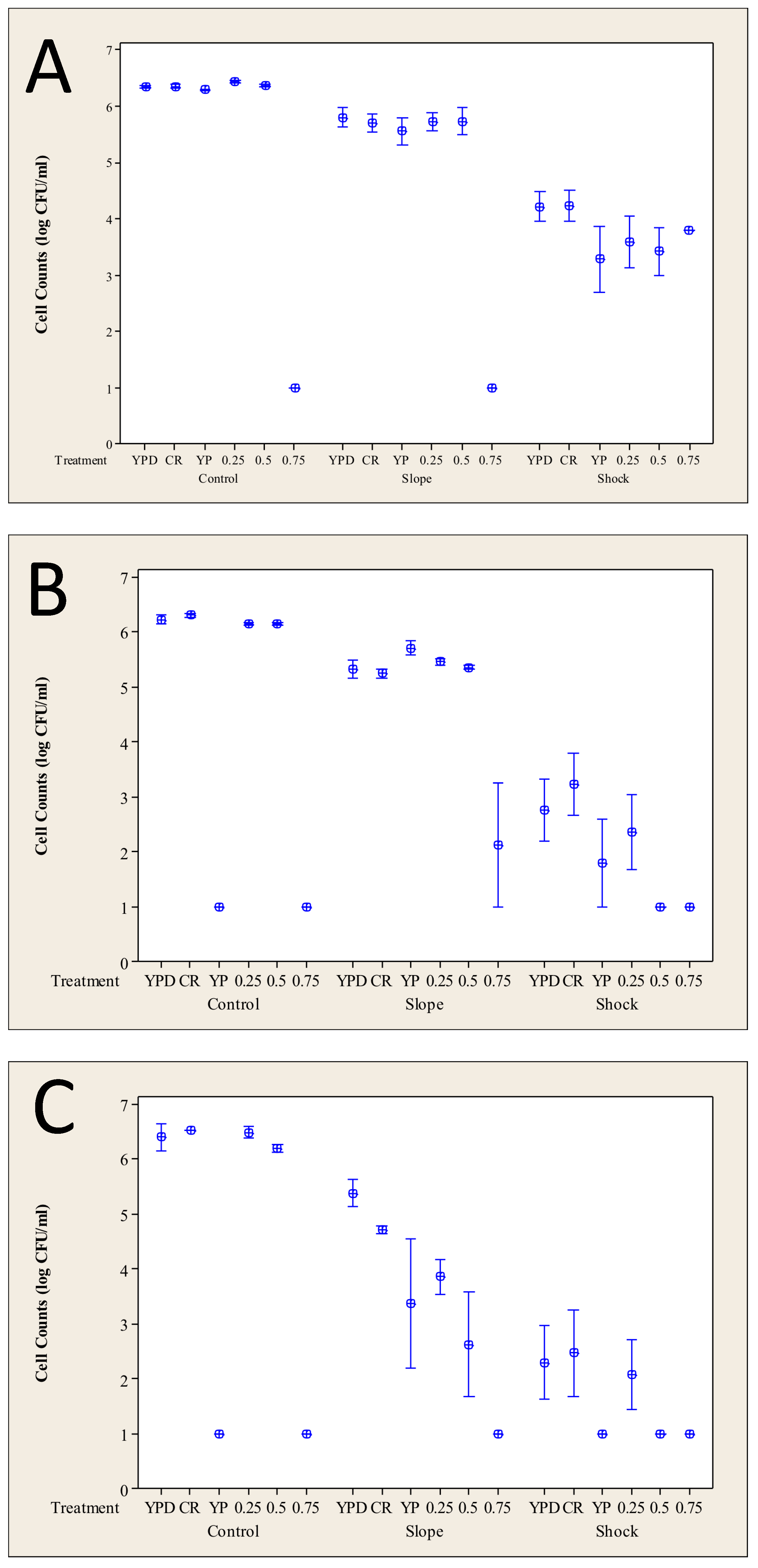
3.3. Impact of Calorie Restriction on the Heat Stress Response
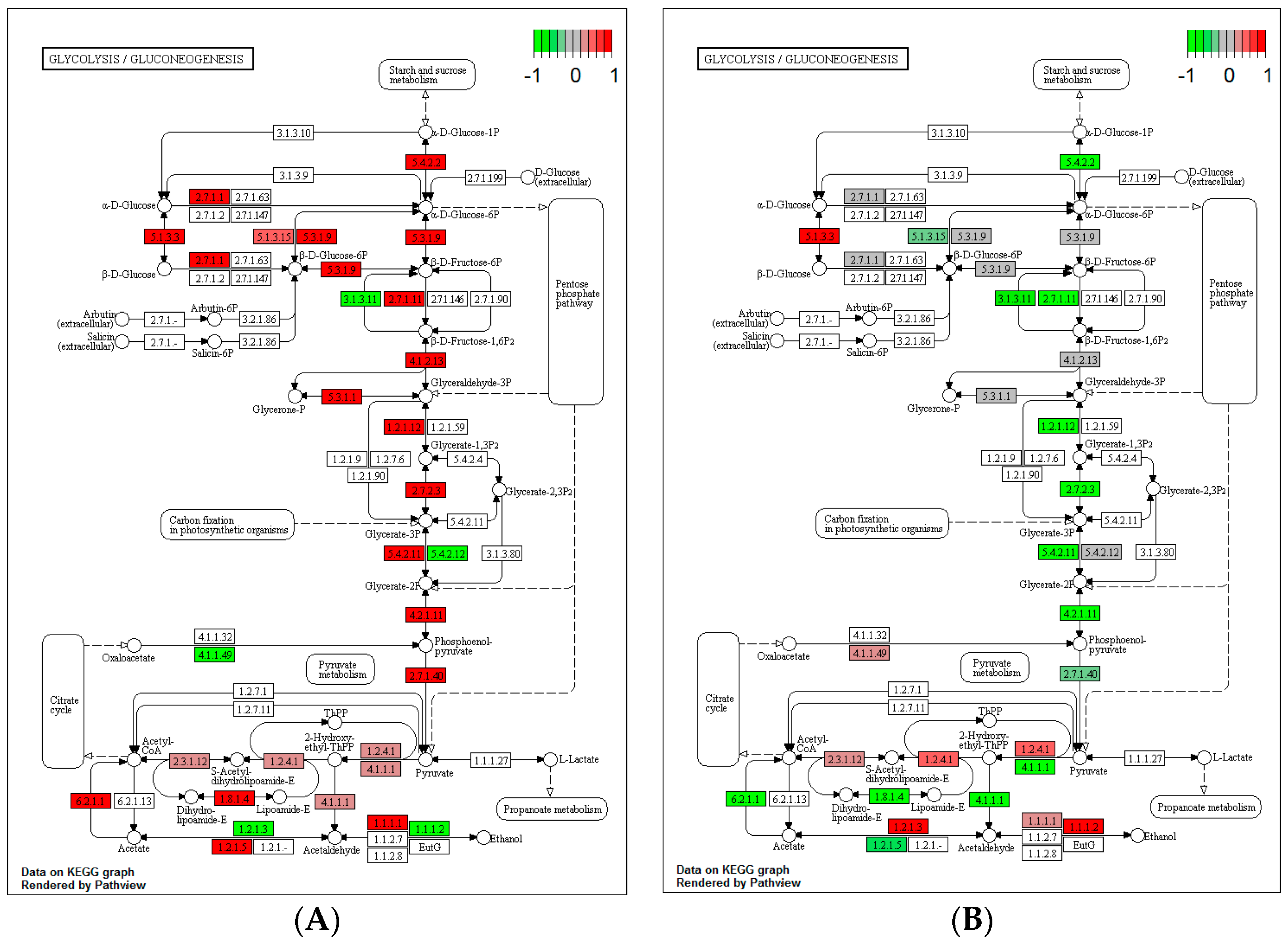
3.4. Impact of Heat Stress on Gene Expression
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yu, R.; Nielsen, J. Yeast systems biology in understanding principles of physiology underlying complex human diseases. Curr. Opin. Biotechnol. 2020, 63, 63–69. [Google Scholar] [CrossRef]
- Gershon, H.; Gershon, D. The budding yeast, Saccharomyces cerevisiae, as a model for aging research: A critical review. Mech. Ageing Dev. 2000, 120, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Huberts, D.H.; Gonzalez, J.; Lee, S.S.; Litsios, A.; Hubmann, G.; Wit, E.C.; Heinemann, M. Calorie restriction does not elicit a robust extension of replicative lifespan in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 2014, 111, 11727–11731. [Google Scholar] [CrossRef]
- Lin, S.J.; Kaeberlein, M.; Andalis, A.A.; Sturtz, L.A.; Defossez, P.A.; Culotta, V.C.; Fink, G.R.; Guarente, L. Calorie restriction extends Saccharomyces cerevisiae lifespan by increasing respiration. Nature 2002, 418, 344–348. [Google Scholar] [CrossRef] [PubMed]
- Maslanka, R.; Kwolek-Mirek, M.; Zadrag-Tecza, R. Consequences of calorie restriction and calorie excess for the physiological parameters of the yeast Saccharomyces cerevisiae cells. FEMS Yeast Res. 2017, 17, fox087. [Google Scholar] [CrossRef]
- Das, S.K.; Balasubramanian, P.; Weerasekara, Y.K. Nutrition modulation of human aging: The calorie restriction paradigm. Mol. Cell. Endocrinol. 2017, 455, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Heilbronn, L.K.; Ravussin, E. Calorie restriction and aging: Review of the literature and implications for studies in humans. Am. J. Clin. Nutr. 2003, 78, 361–369. [Google Scholar] [CrossRef]
- Uno, M.; Honjoh, S.; Matsuda, M.; Hoshikawa, H.; Kishimoto, S.; Yamamoto, T.; Ebisuya, M.; Yamamoto, T.; Matsumoto, K.; Nishida, E. A fasting-responsive signaling pathway that extends life span in C. elegans. Cell Rep. 2013, 3, 79–91. [Google Scholar] [CrossRef]
- Hursting, S.D.; Nunez, N.P.; Varticovski, L.; Vinson, C. The obesity-cancer link: Lessons learned from a fatless mouse. Cancer Res. 2007, 67, 2391–2393. [Google Scholar] [CrossRef]
- Mattison, J.A.; Lane, M.A.; Roth, G.S.; Ingram, D.K. Calorie restriction in rhesus monkeys. Exp. Gerontol. 2003, 38, 35–46. [Google Scholar] [CrossRef]
- Francois, J.; Parrou, J.L. Reserve carbohydrates metabolism in the yeast Saccharomyces cerevisiae. FEMS Microbiol. Rev. 2001, 25, 125–145. [Google Scholar] [CrossRef]
- Goldberg, A.A.; Bourque, S.D.; Kyryakov, P.; Gregg, C.; Boukh-Viner, T.; Beach, A.; Burstein, M.T.; Machkalyan, G.; Richard, V.; Rampersad, S.; et al. Effect of calorie restriction on the metabolic history of chronologically aging yeast. Exp. Gerontol. 2009, 44, 555–571. [Google Scholar] [CrossRef]
- Martin, B.; Mattson, M.P.; Maudsley, S. Caloric restriction and intermittent fasting: Two potential diets for successful brain aging. Ageing Res. Rev. 2006, 5, 332–353. [Google Scholar] [CrossRef] [PubMed]
- Barnosky, A.R.; Hoddy, K.K.; Unterman, T.G.; Varady, K.A. Intermittent fasting vs daily calorie restriction for type 2 diabetes prevention: A review of human findings. Transl. Res. 2014, 164, 302–311. [Google Scholar] [CrossRef] [PubMed]
- Schuller, D.; Alves, H.; Dequin, S.; Casal, M. Ecological survey of Saccharomyces cerevisiae strains from vineyards in the Vinho Verde Region of Portugal. FEMS Microbiol. Ecol. 2005, 51, 167–177. [Google Scholar] [CrossRef]
- Davey, H.M.; Cross, E.J.M.; Davey, C.L.; Gkargkas, K.; Delneri, D.; Hoyle, D.C.; Oliver, S.G.; Kell, D.B.; Griffith, G.W. Genome-wide analysis of longevity in nutrient-deprived Saccharomyces cerevisiae reveals importance of recycling in maintaining cell viability. Environ. Microbiol. 2012, 14, 1249–1260. [Google Scholar] [CrossRef] [PubMed]
- Perez-Samper, G.; Cerulus, B.; Jariani, A.; Vermeersch, L.; Barrajón Simancas, N.; Bisschops, M.M.; van den Brink, J.; Solis-Escalante, D.; Gallone, B.; De Maeyer, D. The crabtree effect shapes the Saccharomyces cerevisiae lag phase during the switch between different carbon sources. MBio 2018, 9, e01331-18. [Google Scholar] [CrossRef] [PubMed]
- Carey, J.R.; Liedo, P.; Harshman, L.; Zhang, Y.; Müller, H.G.; Partridge, L.; Wang, J.L. Life history response of Mediterranean fruit flies to dietary restriction. Aging Cell 2002, 1, 140–148. [Google Scholar] [CrossRef]
- Guyot, S.; Ferret, E.; Gervais, P. Responses of Saccharomyces cerevisiae to thermal stress. Biotechnol. Bioeng. 2005, 92, 403–409. [Google Scholar] [CrossRef]
- Guyot, S.; Gervais, P.; Young, M.; Winckler, P.; Dumont, J.; Davey, H.M. Surviving the heat: Heterogeneity of response in Saccharomyces cerevisiae provides insight into thermal damage to the membrane. Environ. Microbiol. 2015, 17, 2982–2992. [Google Scholar] [CrossRef]
- Binai, N.A.; Bisschops, M.M.; van Breukelen, B.; Mohammed, S.; Loeff, L.; Pronk, J.T.; Heck, A.J.; Daran-Lapujade, P.; Slijper, M. Proteome adaptation of Saccharomyces cerevisiae to severe calorie restriction in Retentostat cultures. J. Proteome Res. 2014, 13, 3542–3553. [Google Scholar] [CrossRef] [PubMed]
- Boender, L.G.; Almering, M.J.; Dijk, M.; van Maris, A.J.; de Winde, J.H.; Pronk, J.T.; Daran-Lapujade, P. Extreme calorie restriction and energy source starvation in Saccharomyces cerevisiae represent distinct physiological states. Biochim. Biophys. Acta 2011, 1813, 2133–2144. [Google Scholar] [CrossRef]
- Gervais, P.; Martínez de Marañón, I. Effect of the Kinetics of Temperature-Variation on Saccharomyces-Cerevisiae Viability and Permeability. Biochim. Biophys. Acta (BBA)-Biomembr. 1995, 1235, 52–56. [Google Scholar] [CrossRef]
- Robinson, J.T.; Thorvaldsdóttir, H.; Turner, D.; Mesirov, J.P. igv.js: An embeddable JavaScript implementation of the Integrative Genomics Viewer (IGV). Bioinformatics 2023, 39, btac830. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M.; Goto, S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Postgate, J.R. Viability measurements and the survival of microbes under minimum stress. Adv. Microb. Physiol. 1967, 1, 1–23. [Google Scholar]
- Postgate, J.R. Viable counts and viability. Meth. Microbiol. 1969, 1, 611–628. [Google Scholar]
- Davey, H.M. Life, Death, and In-Between: Meanings and Methods in Microbiology. Appl. Environ. Microbiol. 2011, 77, 5571–5576. [Google Scholar] [CrossRef]
- Davey, H.M.; Kell, D.B.; Weichart, D.H.; Kaprelyants, A.S. Estimation of microbial viability using flow cytometry. In Current Protocols in Cytometry (Supplement 29); Robinson, J.P., Ed.; Wiley: New York, NY, USA, 2004; pp. 11.13.11–11.13.21. [Google Scholar]
- Martínez de Marañón, I.; Chaudanson, N.; Joly, N.; Gervais, P. Slow heat rate increases yeast thermotolerance by maintaining plasma membrane integrity. Biotechnol. Bioeng. 1999, 65, 176–181. [Google Scholar] [CrossRef]
- Mason, C.A.; Hamer, G.; Bryers, J.D. The Death and Lysis of Microorganisms in Environmental Processes. FEMS Microbiol. Lett. 1986, 39, 373–401. [Google Scholar] [CrossRef]
- Nioh, I.; Furusaka, C. Growth of Bacteria in Heat-Killed Cell Suspensions of Same Bacteria. J. Gen. Appl. Microbiol. 1968, 14, 373–385. [Google Scholar] [CrossRef]
- Reddy, N.M.S.; Rao, B.S.; Murthy, M.S.S. Liquid Holding Recovery in Stationary and Log Phase Cultures of Diploid Yeast Exposed to Gamma and Alpha Radiations. Radiat. Environ. Biophys. 1976, 13, 167–175. [Google Scholar] [CrossRef]
- Postmus, J.; Canelas, A.B.; Bouwman, J.; Bakker, B.M.; van Gulik, W.; de Mattos, M.J.; Brul, S.; Smits, G.J. Quantitative analysis of the high temperature-induced glycolytic flux increase in Saccharomyces cerevisiae reveals dominant metabolic regulation. J. Biol. Chem. 2008, 283, 23524–23532. [Google Scholar] [CrossRef] [PubMed]
- Mensonides, F.I.; Hellingwerf, K.J.; de Mattos, M.J.; Brul, S. Multiphasic adaptation of the transcriptome of Saccharomyces cerevisiae to heat stress. Food Res. Int. 2013, 54, 1103–1112. [Google Scholar] [CrossRef]
- Monod, J. The Growth of Bacterial Cultures. Annu. Rev. Microbiol. 1949, 3, 371–394. [Google Scholar] [CrossRef]
- Stratford, M.; Steels, H.; Nebe-von-Caron, G.; Avery, S.V.; Novodvorska, M.; Archer, D.B. Population heterogeneity and dynamics in starter culture and lag phase adaptation of the spoilage yeast Zygosaccharomyces bailii to weak acid preservatives. Int. J. Food Microbiol. 2014, 181, 40–47. [Google Scholar] [CrossRef]
- Solopova, A.; van Gestel, J.; Weissing, F.J.; Bachmann, H.; Teusink, B.; Kok, J.; Kuipers, O.P. Bet-hedging during bacterial diauxic shift. Proc. Natl. Acad. Sci. USA 2014, 111, 7427–7432. [Google Scholar] [CrossRef]
- Johnston, G.C.; Singer, R.A. Ribosomal Precursor RNA-Metabolism and Cell Division in the Yeast Saccharomyces cerevisiae. Mol. Gen. Genet. 1980, 178, 357–360. [Google Scholar] [CrossRef]
- Paris, S.; Pringle, J.R. Saccharomyces cerevisiae—Heat and Gluculase Sensitivities of Starved Cells. Ann. Inst. Pasteur Mic. 1983, B134, 379–385. [Google Scholar]
- Harris, N.; MacLean, M.; Hatzianthis, K.; Panaretou, B.; Piper, P.W. Increasing Saccharomyces cerevisiae stress resistance, through the overactivation of the heat shock response resulting from defects in the Hsp90 chaperone, does not extend replicative life span but can be associated with slower chronological ageing of nondividing cells. Mol. Genet. Genom. 2001, 265, 258–263. [Google Scholar] [CrossRef]
- Walsh, R.M.; Martin, P.A. Growth of Saccharomyces cerevisiae and Saccharomyces uvarum in a Temperature-Gradient Incubator. J. Inst. Brew. 1977, 83, 169–172. [Google Scholar] [CrossRef]
- Woo, J.M.; Yang, K.M.; Kim, S.U.; Blank, L.M.; Park, J.B. High temperature stimulates acetic acid accumulation and enhances the growth inhibition and ethanol production by Saccharomyces cerevisiae under fermenting conditions. Appl. Microbiol. Biot. 2014, 98, 6085–6094. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, N.; Maeda, Y.; Ikeda, A.; Sakurai, H. Regulation of thermotolerance by stress-induced transcription factors in Saccharomyces cerevisiae. Eukaryot. Cell 2008, 7, 783–790. [Google Scholar] [CrossRef] [PubMed]
- Verghese, J.; Abrams, J.; Wang, Y.Y.; Morano, K.A. Biology of the Heat Shock Response and Protein Chaperones: Budding Yeast (Saccharomyces cerevisiae) as a Model System. Microbiol. Mol. Biol. Rev. 2012, 76, 115–158. [Google Scholar] [CrossRef] [PubMed]
- Wauters, R.; Britton, S.J.; Verstrepen, K.J. Old yeasts, young beer—The industrial relevance of yeast chronological life span. Yeast 2021, 38, 339–351. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hill, L.; Guyot, S.; Bertheau, L.; Davey, H. Calorie Restriction Decreases Competitive Fitness in Saccharomyces cerevisiae Following Heat Stress. Microorganisms 2024, 12, 1838. https://doi.org/10.3390/microorganisms12091838
Hill L, Guyot S, Bertheau L, Davey H. Calorie Restriction Decreases Competitive Fitness in Saccharomyces cerevisiae Following Heat Stress. Microorganisms. 2024; 12(9):1838. https://doi.org/10.3390/microorganisms12091838
Chicago/Turabian StyleHill, Lucy, Stéphane Guyot, Lucie Bertheau, and Hazel Davey. 2024. "Calorie Restriction Decreases Competitive Fitness in Saccharomyces cerevisiae Following Heat Stress" Microorganisms 12, no. 9: 1838. https://doi.org/10.3390/microorganisms12091838





