Identification of Bioactive Substances Derived from the Probiotic-Induced Bioconversion of Lagerstroemia speciosa Pers. Leaf Extract That Have Beneficial Effects on Diabetes and Obesity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Culture
2.2. L. plantarum CBT-LP3 Mediated Bioconversion of BLE
2.3. Cell Lines
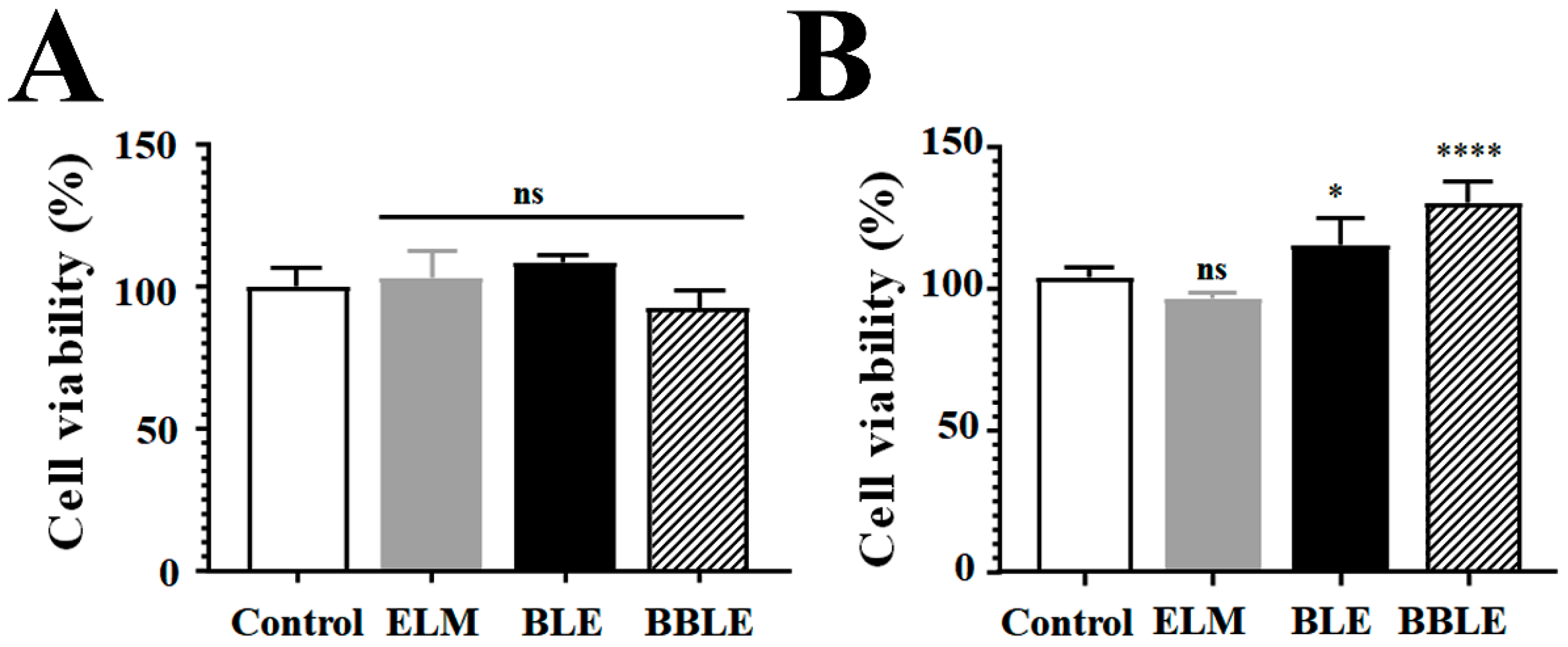
2.4. Determination of Cell Viability
2.5. Differentiation of 3T3 L1 Cells
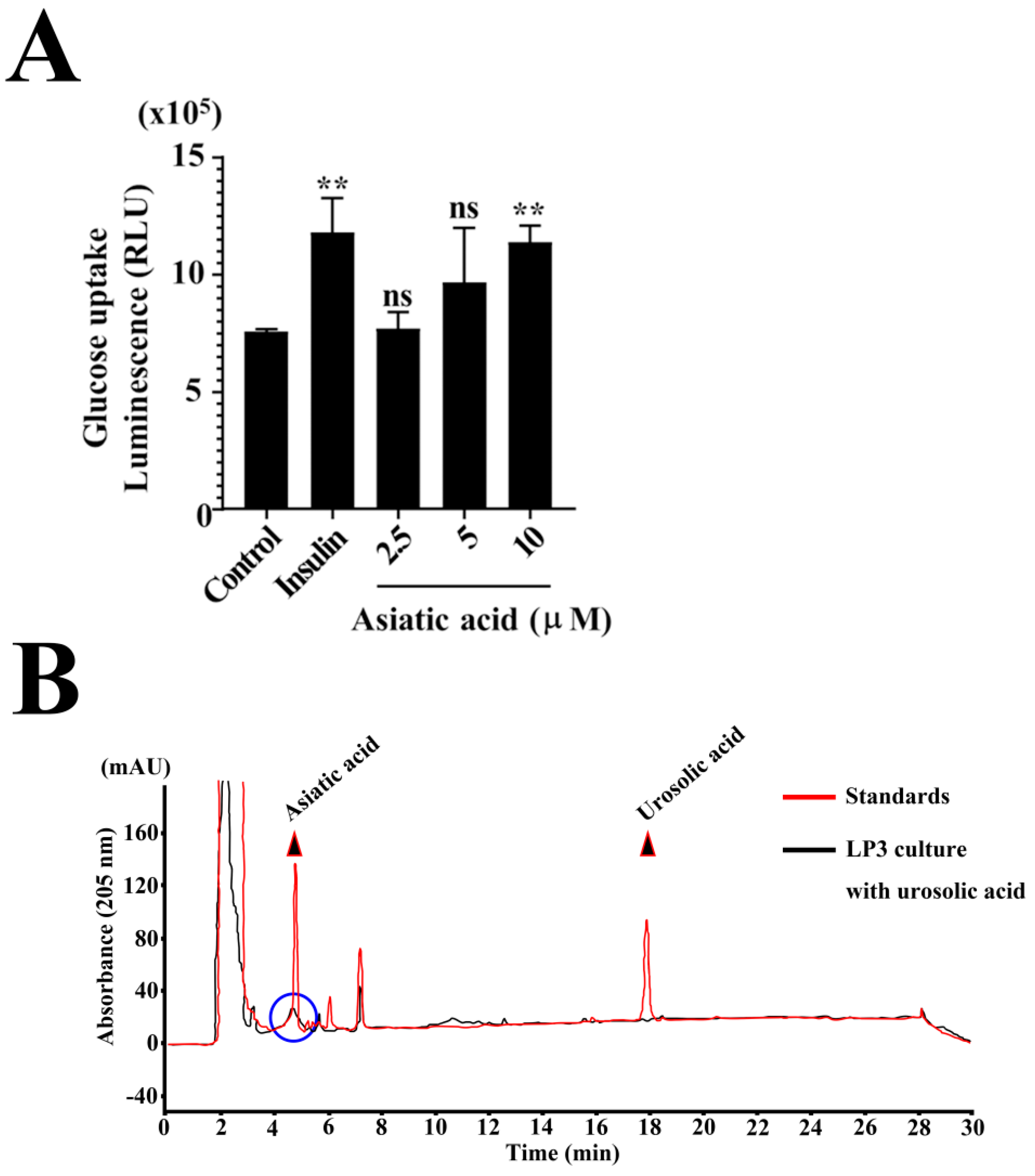
2.6. Glucose Uptake Assay in 3T3-L1 Adipocytes
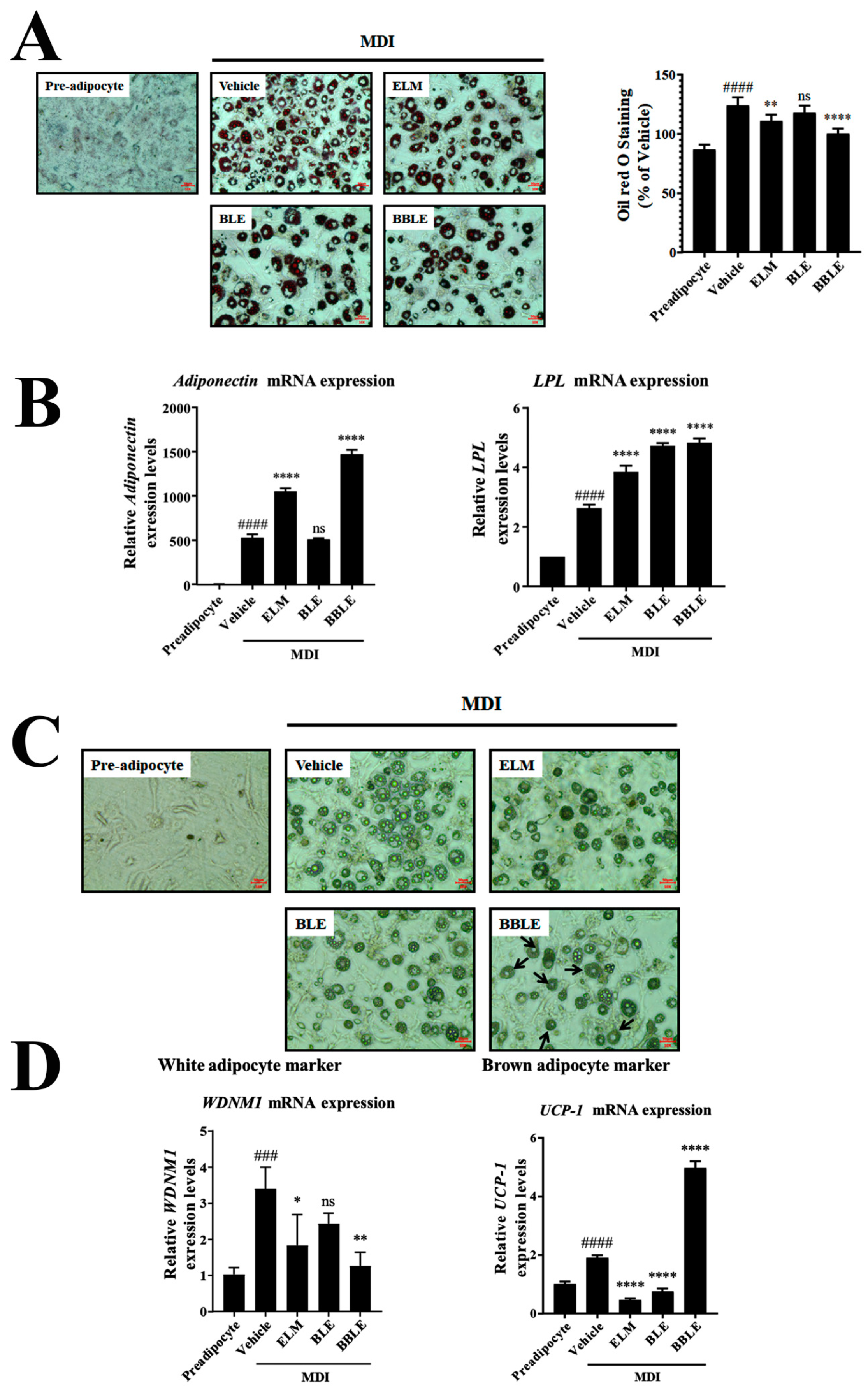
2.7. Oil Red O Staining
2.8. Analysis of the Expression of Signaling-Related Genes Using qPCR
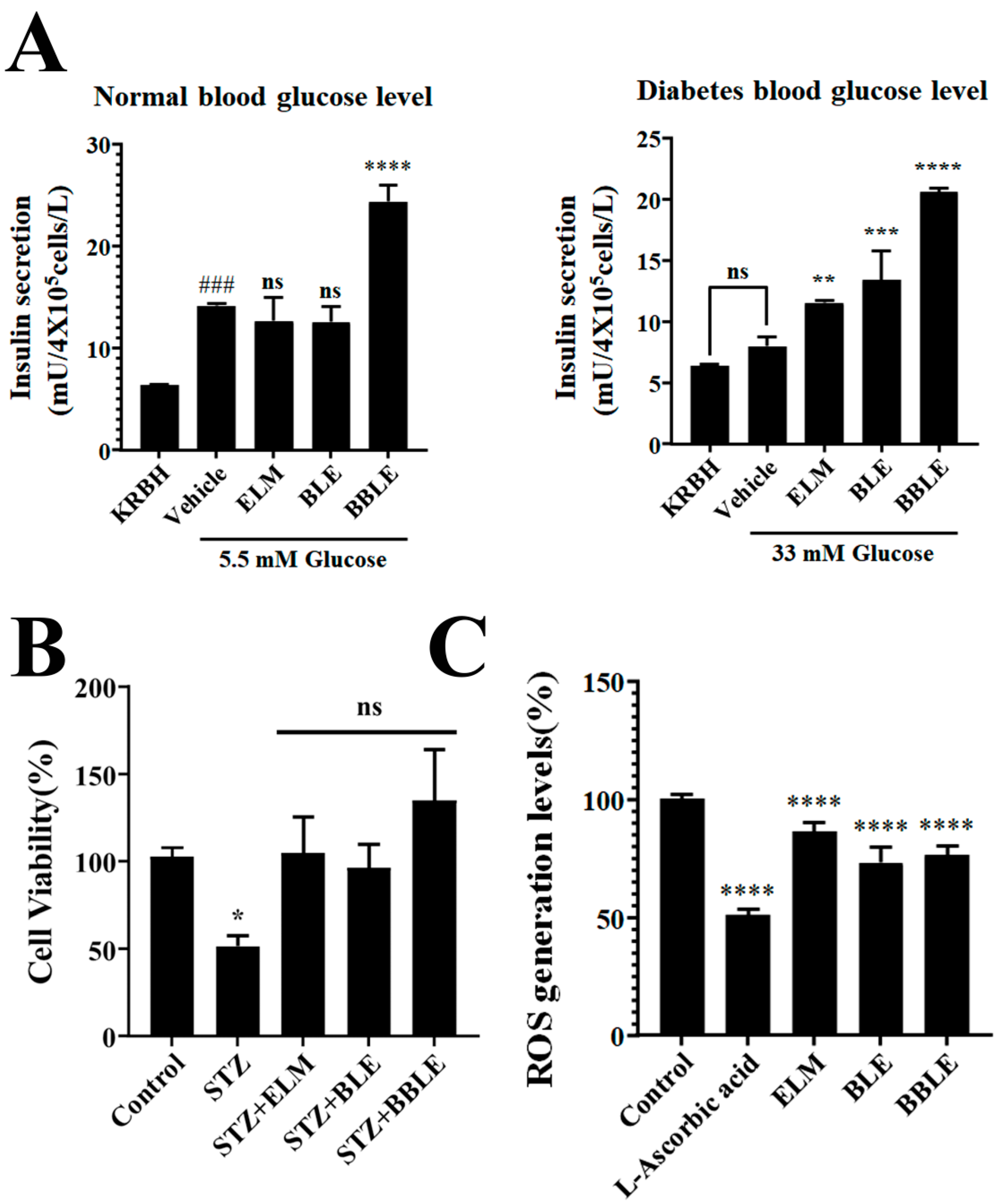
2.9. Insulin Secretion Assay
2.10. Reactive Oxygen Species (ROS) Scavenging Assay
2.11. Metabolomic Profiling
2.11.1. Metabolomic Analysis
2.11.2. Metabolomic Data Analysis
2.12. Chromatography for the Quantification of the Ursolic Acid and Asiatic Acid Concentrations
2.13. Statistical Analysis
3. Results
3.1. Effect of BLE with or without L. plantarum CBT-LP3-Mediated Bioconversion on the Viability of Each Cell Line
3.2. Effect of L. plantarum CBT-LP3-Bioconverted Banaba Leaf Extract on Glucose Uptake
3.3. Effect of L. plantarum CBT-LP3-Mediated Bioconversion on the Adipocyte Browning Stimulated by BLE
3.4. Effects of L. plantarum CBT-LP3-Mediated Bioconversion on the Stimulation of Insulin Secretion and STZ Recovery by Banaba Leaf Extract
3.5. Metabolomic Profiling of L. plantarum CBT-LP3-BBLE
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Quisumbing, E. Medicinal Plants of Phlippines; Katha Publishing: Quezon City, PH, USA, 1978; p. 640. [Google Scholar]
- Mishra, Y.; Khan, M.S.Y.; Zafar, R.; Agarwal, S.S. Hypoglycemic activity of leaves of Lagerstroemia speciosa (L.) Pers. Indian J. Pharmacol. 1990, 22, 174–176. [Google Scholar]
- Priya, T.T.; Sabu, M.C.; Jolly, C.I. Free radical scavenging and anti-inflammatory properties of Lagerstroemia speciosa (L). Inflammopharmacology 2008, 16, 182–187. [Google Scholar] [CrossRef]
- Khan, M.T.; Lampronti, I.; Martello, D.; Bianchi, N.; Jabbar, S.; Choudhuri, M.S.; Datta, B.K.; Gambari, R. Identification of pyrogallol as an antiproliferative compound present in extracts from the medicinal plant Emblica officinalis: Effects on in vitro cell growth of human tumor cell lines. Int. J. Oncol. 2002, 21, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Unno, T.; Ushitani, M.; Hayashi, K.; Kakuda, T. Antiobesity activity of extracts from Lagerstroemia speciosa L. leaves on female KK-Ay mice. J. Nutr. Sci. Vitaminol. 1999, 45, 791–795. [Google Scholar] [CrossRef] [PubMed]
- Kakuda, T.; Sakane, I.; Takihara, T.; Ozaki, Y.; Takeuchi, H.; Kuroyanagi, M. Hypoglycemic effect of extracts from Lagerstroemia speciosa L. leaves in genetically diabetic KK-AY mice. Biosci. Biotechnol. Biochem. 1996, 60, 204–208. [Google Scholar] [CrossRef]
- Alkahtani, S.; Hasnain, M.S.; Algamdy, H.; Aljarba, N.H.; AlKahtane, A. Acute and sub-acute oral toxicity Lagerstroemia speciosa in Sprague-Dawley rats. Saudi J. Biol. Sci. 2022, 29, 1585–1591. [Google Scholar] [CrossRef]
- Tabish, S.A. Is Diabetes Becoming the Biggest Epidemic of the Twenty-first Century? Int. J. Health Sci. 2007, 1, V–VIII. [Google Scholar]
- Ramtahal, R.; Khan, C.; Maharaj-Khan, K.; Nallamothu, S.; Hinds, A.; Dhanoo, A.; Yeh, H.-C.; Hill-Briggs, F.; Lazo, M. Prevalence of self-reported sleep duration and sleep habits in type 2 diabetes patients in South Trinidad. J. Epidemiol. Glob. Health 2015, 5, S35–S43. [Google Scholar] [CrossRef] [PubMed]
- Lone, S.; Lone, K.; Khan, S.; Pampori, R.A. Assessment of metabolic syndrome in Kashmiri population with type 2 diabetes employing the standard criteria’s given by WHO, NCEPATP III and IDF. J. Epidemiol. Glob. Health 2017, 7, 235–239. [Google Scholar] [CrossRef]
- Mahanta, T.G.; Joshi, R.; Mahanta, B.N.; Xavier, D. Prevalence of modifiable cardiovascular risk factors among tea garden and general population in Dibrugarh, Assam, India. J. Epidemiol. Glob. Health 2013, 3, 147–156. [Google Scholar] [CrossRef]
- Alkhatib, A.; Tsang, C.; Tiss, A.; Bahorun, T.; Arefanian, H.; Barake, R.; Khadir, A.; Tuomilehto, J. Functional Foods and Lifestyle Approaches for Diabetes Prevention and Management. Nutrients 2017, 9, 1310. [Google Scholar] [CrossRef] [PubMed]
- Perkins, C.; Siddiqui, S.; Puri, M.; Demain, A.L. Biotechnological applications of microbial bioconversions. Crit. Rev. Biotechnol. 2016, 36, 1050–1065. [Google Scholar] [CrossRef] [PubMed]
- Hegazy, M.E.; Mohamed, T.A.; ElShamy, A.I.; Mohamed, A.E.; Mahalel, U.A.; Reda, E.H.; Shaheen, A.M.; Tawfik, W.A.; Shahat, A.A.; Shams, K.A.; et al. Microbial biotransformation as a tool for drug development based on natural products from mevalonic acid pathway: A review. J. Adv. Res. 2015, 6, 17–33. [Google Scholar] [CrossRef]
- Liu, Y.; Hui, X.; Ibrahim, S.A.; Huang, W. Increasing antiradical activity of polyphenols from lotus seed epicarp by probiotic bacteria bioconversion. Molecules 2018, 23, 2667. [Google Scholar] [CrossRef]
- Bae, E.A.; Kim, N.Y.; Han, M.J.; Choo, M.K.; Kim, D.H. Transformation of ginsenosides to compound K (IH-901) by lactic acid bacteria of human intestine. J. Microbiol. Biotechnol. 2003, 13, 9–14. [Google Scholar]
- Jung, J.; Jang, H.J.; Eom, S.J.; Choi, N.S.; Lee, N.K.; Paik, H.D. Fermentation of red ginseng extract by the probiotic Lactobacillus plantarum KCCM 11613P: Ginsenoside conversion and antioxidant effects. J. Ginseng Res. 2019, 43, 20–26. [Google Scholar] [CrossRef]
- Park, H.; Kim, H.S.; Eom, S.J.; Kim, K.T.; Paik, H.D. Antioxidative and anticanceric activities of Magnolia (Magnolia denudata) flower petal extract fermented by Pediococcus acidilactici KCCM 11614. Molecules 2015, 20, 12154–12165. [Google Scholar] [CrossRef] [PubMed]
- Pervaiz, I.; Ahmad, S.; Madni, M.A.; Ahmad, H.; Khaliq, F.H. Microbial biotransformation: A tool for drug designing. Appl. Biochem. Microbiol. 2013, 49, 435–449. [Google Scholar] [CrossRef]
- Lee, Y.; Yoon, Y.; Choi, K.H. Probiotics-Mediated Bioconversion and Periodontitis. Food Sci. Anim. Resour. 2021, 41, 905–922. [Google Scholar] [CrossRef]
- Seddik, H.A.; Bendali, F.; Gancel, F.; Fliss, I.; Spano, G.; Drider, D. Lactobacillus plantarum and its probiotic and food potentialities. Probiotics Antimicrob. Proteins 2017, 9, 111–122. [Google Scholar]
- Lee, K.; Lee, Y. Effect of Lactobacillus plantarum as a starter on the food quality and microbiota of kimchi. Food Sci. Biotechnol. 2010, 19, 641–646. [Google Scholar] [CrossRef]
- Marco, M.L.; Heeney, D.; Binda, S.; Cifelli, C.J.; Cotter, P.D.; Foligné, B.; Gänzle, M.; Kort, R.; Pasin, G.; Pihlanto, A.; et al. Health benefits of fermented foods: Microbiota and beyond. Curr. Opin. Biotechnol. 2017, 44, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Zhao, C.; Pu, X.; Li, T.; Shi, X.; Wang, B.; Cheng, W. Flavor and functional analysis of lactobacillus plantarum fermented apricot juice. Fermentation 2022, 8, 533. [Google Scholar] [CrossRef]
- Yang, X.; Hong, J.; Wang, L.; Cai, C.; Mo, H.; Wang, J.; Fang, X.; Liao, Z. Effect of lactic acid bacteria fermentation on plant-based products. Fermentation 2024, 10, 48. [Google Scholar] [CrossRef]
- Kong, B.M.; Park, M.J.; Min, J.W.; Kim, H.B.; Kim, S.H.; Kim, S.Y.; Yang, D.C. Physico-chemical characteristics of white, fermented and red ginseng extracts. J. Ginseing Res. 2008, 32, 238. [Google Scholar]
- Kim, H.S.; Ham, J.S. Antioxidative ability of lactic acid bacteria. Korean J. Food Sci. Ani. Resour. 2003, 23, 186. [Google Scholar]
- Sekine, K.M.; Yoida, T.; Saito, M.; Kuboyama, M.; Kawashima, T.; Hashimoto, Y. A new morphologically characterized cell wall preparation (whole peptidoglycan) from Bifidobacterium infantis with a higher efficacy on the regression of an established tumor in mice. Cancer Res. 1985, 4, 1300. [Google Scholar]
- Jeon, B.S.; Park, J.W.; Kim, B.K.; Kim, H.K.; Jung, T.S.; Hahm, J.R.; Kim, D.R.; Cho, Y.S.; Cha, J.Y. Fermented mushroom milk supplemented dietary fiber prevents the onset of obesity and hypertriglyceridemia in Otsuka Long-Evans Tokushima Fatty (OLETF) rats. Diabetes Obes. Metab. 2005, 7, 709. [Google Scholar] [CrossRef]
- An, B.C.; Ryu, Y.; Yoon, Y.S.; Choi, O.; Park, H.J.; Kim, T.Y.; Kim, S.I.; Kim, B.K.; Chung, M.J. Colorectal cancer therapy using a Pediococcus pentosaceus SL4 drug delivery system secreting lactic acid bacteria-derived protein p8. Mol. Cells 2019, 42, 755–762. [Google Scholar]
- Vishwanath, D.; Srinivasan, H.; Patil, M.S.; Seetarama, S.; Agrawal, S.K.; Dixit, M.N.; Dhar, K. Novel method to differentiate 3T3 L1 cells in vitro to produce highly sensitive adipocytes for a GLUT4 mediated glucose uptake using fluorescent glucose analog. J. Cell Commun. Signal 2013, 7, 129–140. [Google Scholar] [CrossRef]
- Zhou, L.; Yang, Y.; Wang, X.; Liu, S.; Shang, W.; Yuan, G.; Li, F.; Tang, J.; Chen, M.; Chen, J. Berberine stimulates glucose transport through a mechanism distinct from insulin. Metabolism. 2007, 56, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Zacarías, J.L.; Castro-Muñozledo, F.; Kuri-Harcuch, W. Quantitation of adipose conversion and triglycerides by staining intracytoplasmic lipids with Oil red O. Histochemistry 1992, 97, 493–497. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Grinberg, Y.Y.; Dibbern, M.E.; Levasseur, V.A.; Kraig, R.P. Insulin-like growth factor-1 abrogates microglial oxidative stress and TNF-alpha responses to spreading depression. J. Neurochem. 2013, 126, 662–672. [Google Scholar] [CrossRef]
- Mok, J.-H.; Joo, M.; Duong, V.-A.; Cho, S.; Park, J.-M.; Eom, Y.-S.; Song, T.-H.; Lim, H.-J.; Lee, H. Proteomic and metabolomic analyses of maggots in porcine corpses for post-mortem interval estimation. Appl. Sci. 2021, 11, 7885. [Google Scholar] [CrossRef]
- Xue, J.; Domingo-Almenara, X.; Guijas, C.; Palermo, A.; Rinschen, M.M.; Isbell, J.; Benton, H.P.; Siuzdak, G. Enhanced in-source fragmentation annotation enables novel data independent acquisition and autonomous METLIN molecular identification. Anal. Chem. 2020, 92, 6051–6059. [Google Scholar] [CrossRef]
- Pang, Z.; Chong, J.; Zhou, G.; de Lima Morais, D.A.; Chang, L.; Barrette, M.; Gauthier, C.; Jacques, P.-É.; Li, S.; Xia, J. MetaboAnalyst 5.0: Narrowing the Gap between Raw Spectra and Functional Insights. Nucleic Acids Res. 2021, 49, W388–W396. [Google Scholar] [CrossRef]
- Züllig, T.; Zandl-Lang, M.; Trötzmüller, M.; Hartler, J.; Plecko, B.; Köfeler, H.C. A metabolomics workflow for analyzing complex biological samples using a combined method of untargeted and target-list based approaches. Metabolites 2020, 10, 342. [Google Scholar] [CrossRef]
- Liu, F.; Kim, J.; Li, Y.; Liu, X.; Li, J.; Chen, X. An extract of Lagerstroemia speciosa L. has insulin-like glucose uptake-stimulatory and adipocyte differentiation-inhibitory activities in 3T3-L1 cells. J. Nutr. 2001, 131, 2242–2247. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.K.; Paik, H.D. Bioconversion using lactic acid bacteria: Ginsenosides, GABA, and phenolic compounds. J. Microbiol. Biotechnol. 2017, 27, 869–877. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.K.; Lee, B.; Kim, C.Y. Natural extracts that stimulate adipocyte browning and their underlying mechanisms. Antioxidants 2021, 10, 308. [Google Scholar] [CrossRef]
- Aguirre, F.; Brown, A.; Cho, N.H.; Dahlquist, G.; Dodd, S.; Dunning, T.; Hirst, M.; Hwang, C.; Magliano, D.; Patterson, C.; et al. IDF Diabetes Atlas, 6th ed.; International Diabetes Federation: Brussels, Belgium, 2013; pp. 22–27. [Google Scholar]
- Nahdi, A.M.T.A.; John, A.; Raza, H. Elucidation of molecular mechanisms of streptozotocin-induced oxidative stress, apoptosis, and mitochondrial dysfunction in Rin-5F pancreatic β-cells. Oxid. Med. Cell Longev. 2017, 2017, 7054272. [Google Scholar] [CrossRef] [PubMed]
- Kellogg, J.J.; Todd, D.A.; Egan, J.M.; Raja, H.A.; Oberlies, N.H.; Kvalheim, O.M.; Cech, N.B. Biochemometrics for natural products research: Comparison of data analysis approaches and application to identification of bioactive compounds. J. Nat. Prod. 2017, 79, 376–386. [Google Scholar] [CrossRef] [PubMed]
- Jayakumar, K.S.; Sajan, J.S.; Aswati Nair, R.; Padmesh Pillai, P.; Deepu, S.; Padmaja, R.; Agarwal, A.; Pandurangan, A.G. Corosolic acid content and SSR markers in Lagerstroemia speciosa (L.) Pers.: A comparative analysis among populations across the Southern Western Ghats of India. Phytochemistry 2014, 106, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Nazaruk, J.; Borzym-Kluczyk, M. The role of triterpenes in the management of diabetes mellitus and its complications. Phytochem. Rev. 2015, 14, 675–690. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.-O.; Lee, S.U.; Yuk, H.J.; Jang, H.-J.; Lee, J.-W.; Kwon, E.-B.; Paik, J.-H.; Choi, S.; Nizar, A.; Bach, T.T.; et al. Metabolomics approach to identify the active substances influencing the antidiabetic activity of Lagerstroemia species. J. Funct. Foods 2020, 64, 103684. [Google Scholar] [CrossRef]
- Artasensi, A.; Mazzolari, A.; Pedretti, A.; Vistoli, G.; Fumagalli, L. Obesity and type 2 diabetes: Adiposopathy as a triggering factor and therapeutic options. Molecules 2023, 28, 3094. [Google Scholar] [CrossRef] [PubMed]
- Corb Aron, R.A.; Abid, A.; Vesa, C.M.; Nechifor, A.C.; Behl, T.; Ghitea, T.C.; Munteanu, M.A.; Fratila, O.; Andronie-Cioara, F.L.; Toma, M.M.; et al. Recognizing the benefits of pre-/probiotics in metabolic syndrome and type 2 diabetes mellitus considering the influence of Akkermansia muciniphila as a key gut bacterium. Microorganisms 2021, 9, 618. [Google Scholar] [CrossRef]
- Zhang, X.; Miao, Q.; Pan, C.; Yin, J.; Wang, L.; Qu, L.; Yin, Y.; Wei, Y. Research advances in probiotic fermentation of Chinese herbal medicines. iMeta 2023, 2, e93. [Google Scholar] [CrossRef]
- Moisan, A.; Lee, Y.K.; Zhang, J.D.; Hudak, C.S.; Meyer, C.A.; Prummer, M.; Zoffmann, S.; Truong, H.H.; Ebeling, M.; Kiialainen, A.; et al. White-to-brown metabolic conversion of human adipocytes by JAK inhibition. Nat. Cell Biol. 2015, 17, 57–67. [Google Scholar] [CrossRef]
- Shakya, S.; Danshiitsoodol, N.; Noda, M.; Sugiyama, M. Transcriptional profiling of geniposide bioconversion into genipin during gardenia fructus extract fermentation by Lactobacillus (Lactiplantibacillus) plantarum SN13T. Biosci. Microbiota Food Health 2024, 43, 120–127. [Google Scholar] [CrossRef]
- Herman, A.; Herman, A.P. biological activity of fermented plant extracts for potential dermal applications. Pharmaceutics 2023, 15, 2775. [Google Scholar] [CrossRef] [PubMed]
- Park, C.; Lee, J.S. Banaba: The natural remedy as antidiabetic drug. Biomed. Res. 2011, 22, 127–131. [Google Scholar]
- Yeates, R.A. Artemotil Artecef. Curr. Opin. Investig. Drugs 2002, 3, 545–549. [Google Scholar]
- Caliber, M.; Saad, F. Testosterone therapy for prevention and reversal of type 2 diabetes in men with low testosterone. Curr. Opin. Pharmacol. 2021, 58, 83–89. [Google Scholar] [CrossRef]
- Kumari, K.; Kumar, R.; Memon, A.; Kumari, B.; Tehrim, M.; Kumari, P.; Shehryar, M.; Islam, H.; Islam, R.; Khatri, M.; et al. Treatment with testosterone therapy in type 2 diabetic hypogonadal adult males: A systematic review and meta-analysis. Clin. Pract. 2023, 13, 454–469. [Google Scholar] [CrossRef]
- Fookes, C. List of Common Antihistamines + Uses & Side Effects. 2022. Available online: https://www.drugs.com/ (accessed on 7 August 2024).
- Sun, Y.; Zang, Z.; Zhong, L.; Wu, M.; Su, Q.; Gao, X.; Zan, W.; Lin, D.; Zhao, Y.; Zhang, Z. Identification of adiponectin receptor agonist utilizing a fluorescence polarization based high throughput assay. PLoS ONE 2013, 8, e63354. [Google Scholar]
- Barceloux, D.G. Medical Toxicology of Drug Abuse: Synthesized Chemicals and Psychoactive Plants; John Wiley & Sons: Hoboken, NJ, USA, 2013; pp. 255–262. [Google Scholar]
- Ramachandran, V.; Saravanan, R.; Senthilraja, P. Antidiabetic and antihyperlipidemic activity of asiatic acid in diabetic rats, role of HMG CoA: In vivo and in silico approaches. Phytomedicine 2014, 21, 225–232. [Google Scholar] [CrossRef]


| Metabolite | FC | Up or Down Regulation |
|---|---|---|
| 3-[(Methoxycarbonyl)amino]-2,2,3-trimethylbutanoic acid | 22.31 | UP |
| Butanethiol | 16.528 | UP |
| 11-Deoxocucurbitacin I | 8.9384 | UP |
| Propapyriogenin A2 | 5.9063 | UP |
| Artemotil | 4.4698 | UP |
| Testosterone decanoate | 4.1097 | UP |
| Clocinizine | 4.0083 | UP |
| Matairesinol | 3.9018 | UP |
| DL-Malic acid | 3.8591 | UP |
| Asiatic acid | 3.8478 | UP |
| 5-[2-(3-Furyl)ethyl]-8a-(hydroxymethyl)-5,6-dimethyl-3,4,4a,5,6,7,8,8a-octahydro-1-naphthalenecarboxylic acid | 3.8229 | UP |
| (4Z)-9,11-Dihydroxy-1,1,4,7a-tetramethyl-10-(3-methylbutanoyl)-1,1a,2,3,6,7,7a,8,13a,13b-decahydrocyclopropa [9,10] cyclodeca [1,2-b]chromene-12-carbaldehyde | 3.6902 | UP |
| 6-Hydroxycaproic acid | 3.5304 | UP |
| Prostaglandin B1 | 3.3927 | UP |
| 15,16-DiHODE | 3.3456 | UP |
| Fenfluramine | 3.2537 | UP |
| (6Z)-3,6,10-Trimethyl-3a,5,8,10,11,11a-hexahydrocyclodeca[b]furan-2,9(3H,4H)-dione | 3.2519 | UP |
| 3,7,9-Trihydroxy-6H-[1] benzofuro [3,2-c]chromen-6-one | 3.0903 | UP |
| NP-003039 | 3.0869 | UP |
| (15Z)-9,12,13-Trihydroxy-15-octadecenoic acid | 2.8871 | UP |
| 13(S)-HOTrE | 2.6243 | UP |
| NP-020283 | 2.6179 | UP |
| Asn-Val | 2.3794 | UP |
| UNII:V9Q7UUZ15U | 2.354 | UP |
| 1,1-Diethyl-3-oxo-2-triazanolate | 2.3058 | UP |
| Methylmalonic acid | 2.2318 | UP |
| 4-Nitrophenol | 2.0699 | UP |
| Carglumic acid | 0.45971 | DOWN |
| Isoniazid | 0.42035 | DOWN |
| (S)-ACPA | 0.38543 | DOWN |
| Felbamate | 0.28071 | DOWN |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
An, B.C.; Kwak, S.H.; Ahn, J.Y.; Won, H.Y.; Kim, T.H.; Ryu, Y.; Chung, M.J. Identification of Bioactive Substances Derived from the Probiotic-Induced Bioconversion of Lagerstroemia speciosa Pers. Leaf Extract That Have Beneficial Effects on Diabetes and Obesity. Microorganisms 2024, 12, 1848. https://doi.org/10.3390/microorganisms12091848
An BC, Kwak SH, Ahn JY, Won HY, Kim TH, Ryu Y, Chung MJ. Identification of Bioactive Substances Derived from the Probiotic-Induced Bioconversion of Lagerstroemia speciosa Pers. Leaf Extract That Have Beneficial Effects on Diabetes and Obesity. Microorganisms. 2024; 12(9):1848. https://doi.org/10.3390/microorganisms12091848
Chicago/Turabian StyleAn, Byung Chull, Sang Hee Kwak, Jun Young Ahn, Hye Yeon Won, Tae Hoon Kim, Yongku Ryu, and Myung Jun Chung. 2024. "Identification of Bioactive Substances Derived from the Probiotic-Induced Bioconversion of Lagerstroemia speciosa Pers. Leaf Extract That Have Beneficial Effects on Diabetes and Obesity" Microorganisms 12, no. 9: 1848. https://doi.org/10.3390/microorganisms12091848






