AoRan1 Is Involved in Regulating Conidiation, Stress Resistance, Secondary Metabolism, and Pathogenicity in Arthrobotrys oligospora
Abstract
:1. Introduction
2. Materials and Methods
2.1. Strains and Culture Conditions
2.2. Phylogenetic Analysis of AoRan1
2.3. Deletion of Aoran1
2.4. Comparison of Hyphal Growth and Sporulation
2.5. Comparison of Mycelial Growth under Different External Pressures
2.6. Comparison of Pathogenicity and Observation of Trap Morphology
2.7. Staining Analysis
2.8. RT-qPCR Analysis
2.9. Metabolomics Analysis
2.10. Statistical Analysis
3. Results
3.1. Sequence Analysis of AoRan1
3.2. Verification of Positive Transformants
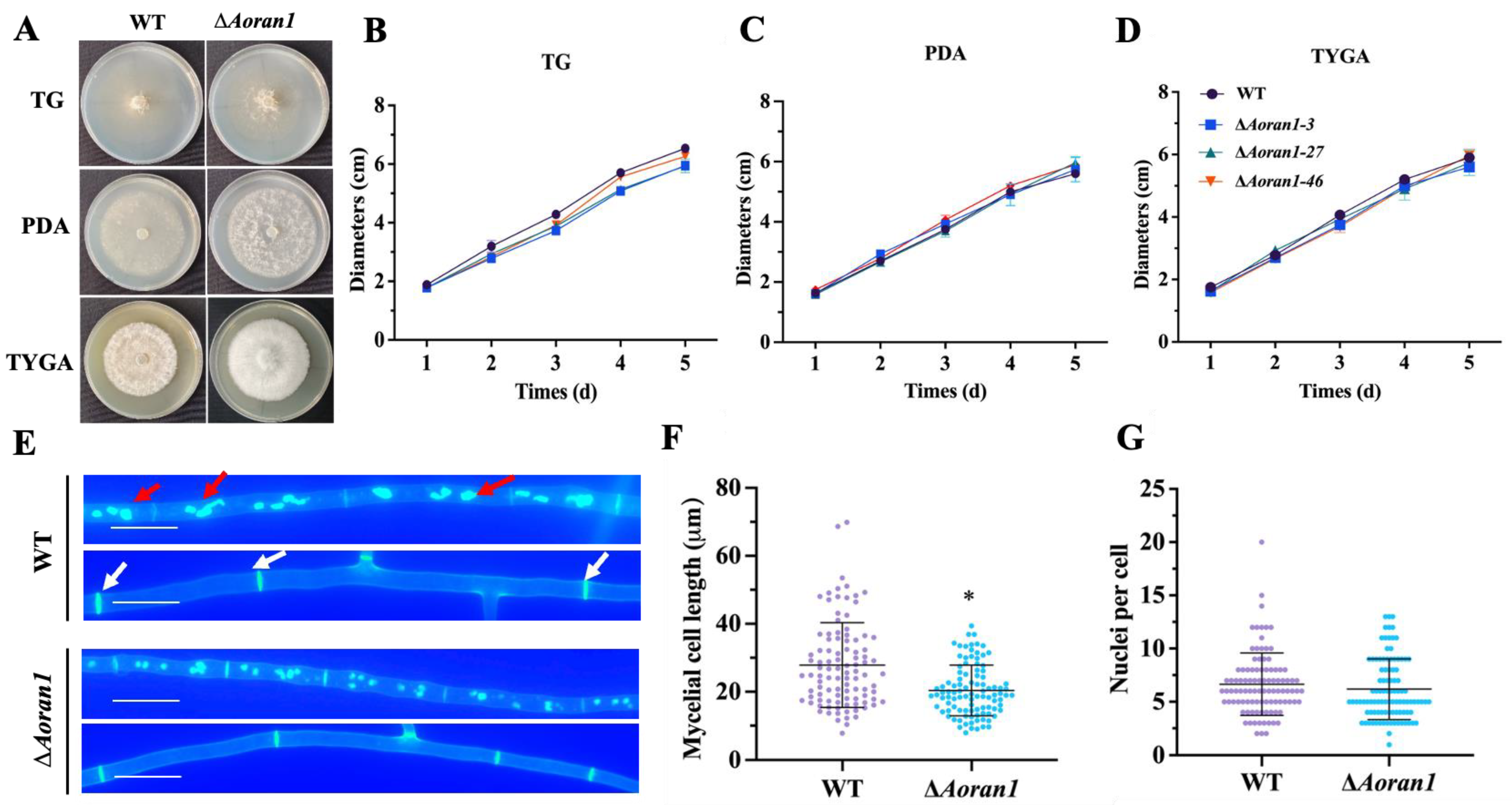
3.3. Comparison of Growth Rates between WT and ∆Aoran1 Mutant Strains
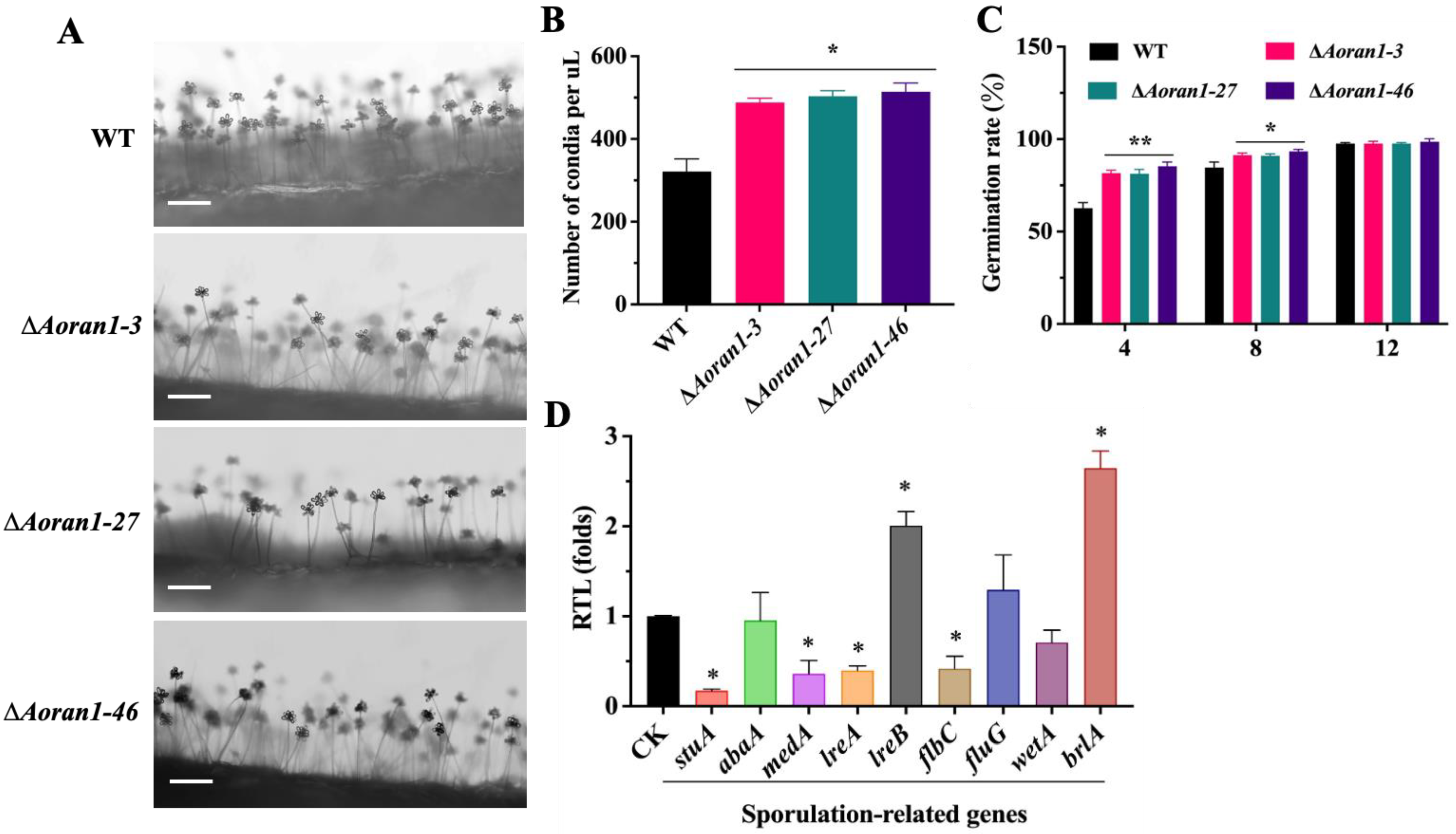
3.4. AoRan1 Plays a Vital Role in Conidiation
3.5. AoRan1 Is Critical to Trap Formation and Pathogenicity
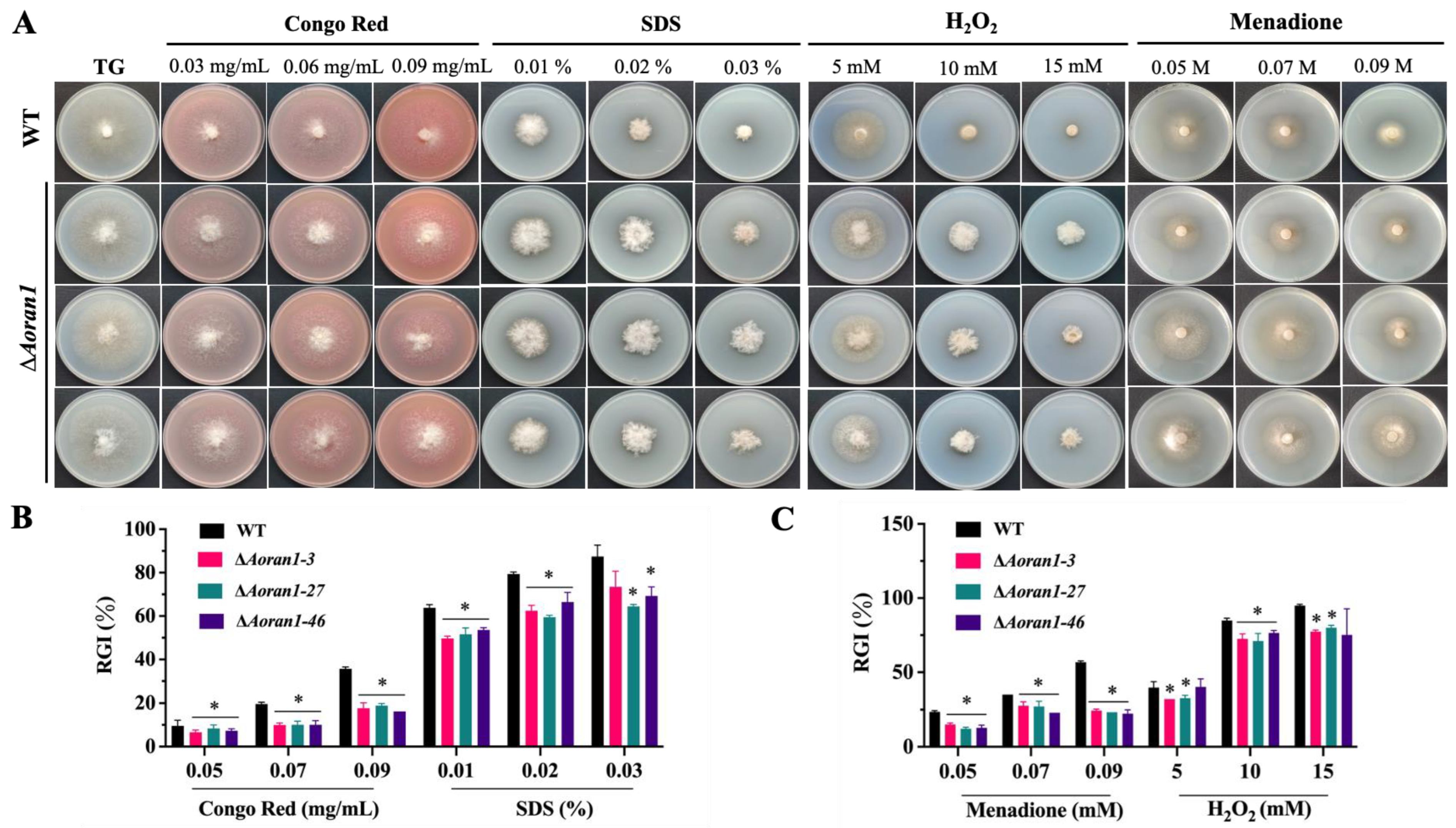
3.6. AoRan1 Is Involved in Stress Response
3.7. AoRan1 Regulates LD Accumulation and Autophagic Level
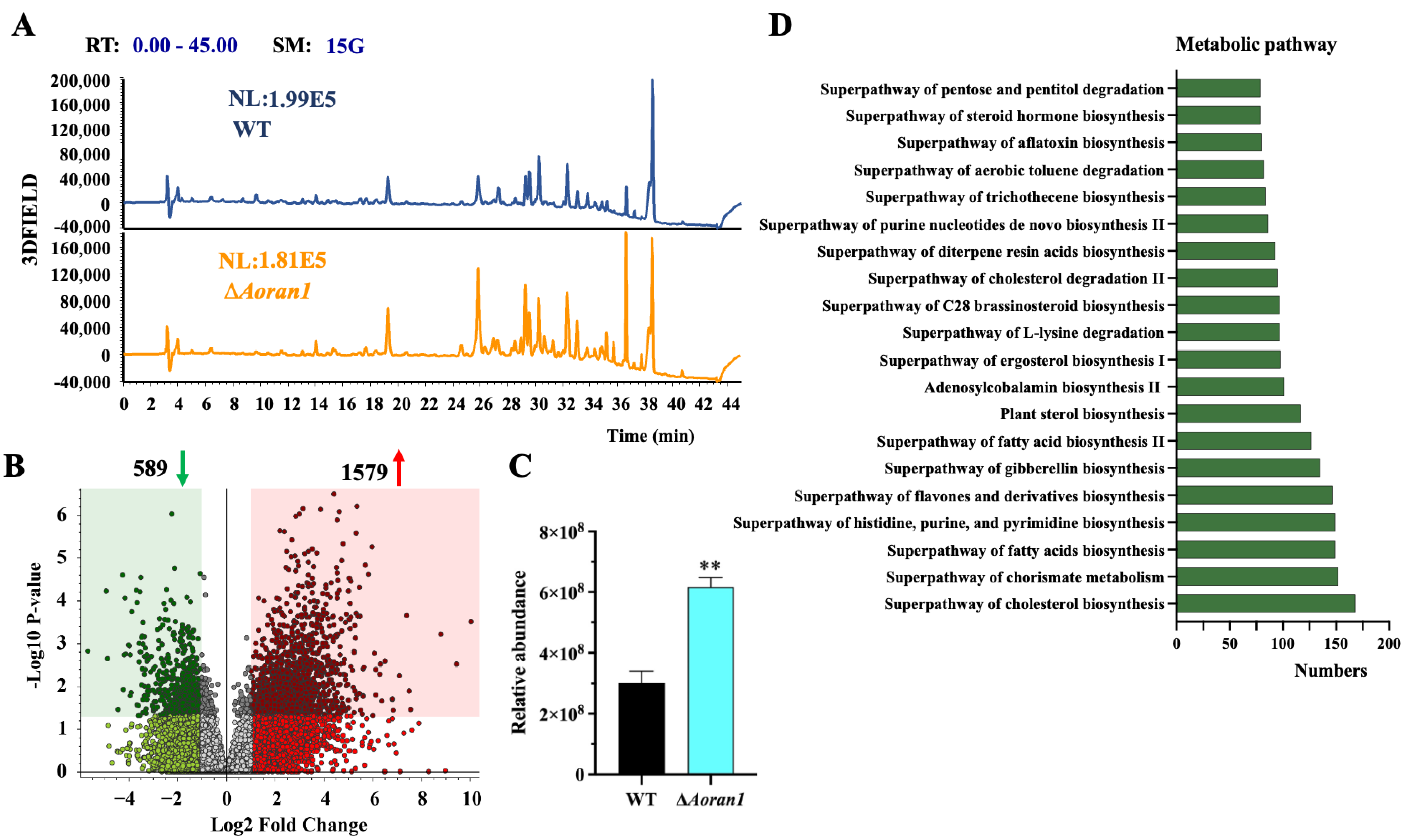
3.8. AoRan1 Contributes to Secondary Metabolism
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abad, P.; Gouzy, J.; Aury, J.-M.; Castagnone-Sereno, P.; Danchin, E.G.J.; Deleury, E.; Perfus-Barbeoch, L.; Anthouard, V.; Artiguenave, F.; Blok, V.C.; et al. Genome sequence of the metazoan plant-parasitic nematode Meloidogyne incognita. Nat. Biotechnol. 2008, 26, 909–915. [Google Scholar] [CrossRef] [PubMed]
- Coyne, D.L.; Cortada, L.; Dalzell, J.J.; Claudius-Cole, A.O.; Haukeland, S.; Luambano, N.; Talwana, H. Plant-parasitic nematodes and food security in sub-Saharan Africa. Annu. Rev. Phytopathol. 2018, 25, 381–403. [Google Scholar] [CrossRef]
- Tzean, Y.; Chou, T.-H.; Hsiao, C.-C.; Shu, P.-Y.; Walton, J.D.; Tzean, S.-S. Cloning and characterization of cuticle-degrading serine protease from nematode-trapping fungus Arthrobotrys musiformis. Mycoscience 2016, 57, 136–143. [Google Scholar] [CrossRef]
- Ahmad, G.; Khan, A.; Khan, A.A.; Ali, A.; Mohhamad, H.I. Biological control: A novel strategy for the control of the plant parasitic nematodes. Antonie Van Leeuwenhoek 2021, 114, 885–912. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Tian, J.; Xiang, M.; Liu, X. How carnivorous fungi use three-celled constricting rings to trap nematodes. Protein Cell 2012, 3, 325–328. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, E.; An, Z.; Liu, X. Evolution of nematode-trapping cells of predatory fungi of the orbiliaceae based on evidence from rRNA-Encoding DNA and multiprotein sequences. Proc. Natl. Acad. Sci. USA 2007, 104, 8379–8384. [Google Scholar] [CrossRef]
- Larsen, M. Prospects for controlling animal parasitic nematodes by predacious micro fungi. Parasitology 2000, 120, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Kerry, B.R. Rhizosphere interactions and the exploitation of microbial agents for the biological control of plant-parasitic nematodes. Annu. Rev. Phytopathol. 2000, 38, 423–441. [Google Scholar] [CrossRef]
- Jiang, X.; Xiang, M.; Liu, X. Nematode-trapping fungi. Microbiol. Spectr. 2017, 5, FUNK-0022-2016. [Google Scholar] [CrossRef]
- Wang, S.; Liu, X. Tools and basic procedures of gene manipulation in nematode-trapping fungi. Mycology 2023, 10, 75–90. [Google Scholar] [CrossRef]
- Yang, J.; Wang, L.; Ji, X.; Feng, Y.; Li, X.; Zou, C.; Xu, J.; Ren, Y.; Mi, Q.; Wu, J.; et al. Genomic and proteomic analyses of the fungus Arthrobotrys oligospora provide insights into nematode-trap formation. PLoS Pathog. 2011, 7, e1002179. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Hoffmann, D.S.; Wang, M.; Schuhmacher, L.; Stroe, M.C.; Schreckenberger, B.; Elstner, M.; Fischer, R. GprC of the nematode-trapping fungus Arthrobotrys flagrans activates mitochondria and reprograms fungal cells for nematode hunting. Nat. Microbiol. 2024, 9, 1752–1763. [Google Scholar] [CrossRef]
- Lin, H.C.; de Ulzurrun, G.V.; Chen, S.A.; Yang, C.T.; Tay, R.J.; Iizuka, T.; Huang, T.Y.; Kuo, C.Y.; Gonçalves, A.P.; Lin, S.Y.; et al. Key processes required for the different stages of fungal carnivory by a nematode-trapping fungus. PLoS Biol. 2023, 21, e3002400. [Google Scholar] [CrossRef]
- Park, G.; Servin, J.A.; Turner, G.E.; Altamirano, L.; Colot, H.V.; Collopy, P.; Litvinkova, L.; Li, L.; Jones, C.A.; Diala, F.-G.; et al. Global analysis of serine-threonine protein kinase genes in Neurospora crassa. Eukaryot. Cell 2011, 10, 1553–1564. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; McLeod, M. Molecular mimicry in development: Identification of Ste11+ as a substrate and Mei3+ as a pseudosubstrate inhibitor of Ran1+ kinase. Cell 1996, 87, 869–880. [Google Scholar] [CrossRef]
- Goldsmith, E.J.; Akella, R.; Min, X.; Zhou, T.; Humphreys, J.M. Substrate and docking interactions in serine/threonine protein kinases. Chem. Rev. 2007, 107, 5065–5081. [Google Scholar] [CrossRef]
- Burgess, A.; Vigneron, S.; Brioudes, E.; Labbé, J.-C.; Lorca, T.; Castro, A. Loss of human greatwall results in G2 arrest and multiple mitotic defects due to deregulation of the cyclin B-Cdc2/PP2A balance. Proc. Natl. Acad. Sci. USA 2010, 107, 12564–12569. [Google Scholar] [CrossRef] [PubMed]
- Herzig, S.; Neumann, J. Effects of serine/threonine protein phosphatases on ion channels in excitable membranes. Physiol. Rev. 2000, 80, 173–210. [Google Scholar] [CrossRef]
- Lorca, T.; Bernis, C.; Vigneron, S.; Burgess, A.; Brioudes, E.; Labbé, J.-C.; Castro, A. Constant regulation of both the MPF amplification loop and the greatwall-PP2A pathway is required for metaphase II arrest and correct entry into the first embryonic cell cycle. J. Cell Sci. 2010, 123, 2281–2291. [Google Scholar] [CrossRef]
- Cipak, L.; Hyppa, R.W.; Smith, G.R.; Gregan, J. ATP analog-sensitive Pat1 protein kinase for synchronous fission yeast meiosis at physiological temperature. Cell Cycle 2012, 11, 1626–1633. [Google Scholar] [CrossRef]
- Bhola, T.; Kapuy, O.; Vinod, P.K. Computational modelling of meiotic entry and commitment. Sci. Rep. 2018, 8, 180. [Google Scholar] [CrossRef]
- Kitamura, K.; Katayama, S.; Dhut, S.; Sato, M.; Watanabe, Y.; Yamamoto, M.; Toda, T. Phosphorylation of Mei2 and Ste11 by Pat1 kinase inhibits sexual differentiation via ubiquitin proteolysis and 14-3-3 protein in fission yeast. Dev. Cell 2001, 1, 389–399. [Google Scholar] [CrossRef] [PubMed]
- Nurse, P. Mutants of the fission yeast Schizosaccharomyces pombe which alter the shift between cell proliferation and sporulation. Mol. Gen. Genet. 1985, 198, 497–502. [Google Scholar] [CrossRef]
- Watanabe, Y.; Yamamoto, M.S. Pombe Mei2+ encodes an RNA-binding protein essential for premeiotic DNA synthesis and meiosis I, which cooperates with a novel RNA species meiRNA. Cell 1994, 78, 487–498. [Google Scholar] [CrossRef]
- Beach, D.; Rodgers, L.; Gould, J. RAN1 + controls the transition from mitotic division to meiosis in fission yeast. Curr. Genet. 1985, 10, 297–311. [Google Scholar] [CrossRef]
- Madhani, H.D.; Fink, G.R. Combinatorial control required for the specificity of yeast MAPK signaling. Science 1997, 275, 1314–1317. [Google Scholar] [CrossRef]
- Gustin, M.C.; Albertyn, J.; Alexander, M.; Davenport, K. MAP kinase pathways in the yeast Saccharomyces cerevisiae. Microbiol. Mol. Biol. Rev. 1998, 62, 1264–1300. [Google Scholar] [CrossRef]
- Li, D.; Qin, L.; Wang, Y.; Xie, Q.; Li, N.; Wang, S.; Yuan, J. AflSte20 regulates morphogenesis, stress response, and aflatoxin biosynthesis of Aspergillus flavus. Toxins 2019, 11, 73. [Google Scholar] [CrossRef] [PubMed]
- Pulido, V.; Rodríguez-Peña, J.M.; Alonso, G.; Sanz, A.B.; Arroyo, J.; García, R. mRNA decapping activator Pat1 is required for efficient yeast adaptive transcriptional responses via the cell wall integrity MAPK pathway. J. Mol. Biol. 2024, 436, 1685700. [Google Scholar] [CrossRef]
- Xie, M.; Bai, N.; Yang, X.; Liu, Y.; Zhang, K.-Q.; Yang, J. Fus3 regulates asexual development and trap morphogenesis in the nematode-trapping fungus Arthrobotrys oligospora. iScience 2023, 26, 107404. [Google Scholar] [CrossRef]
- Xie, M.; Ma, N.; Bai, N.; Yang, L.; Yang, X.; Zhang, K.-Q.; Yang, J. PKC-SWI6 signaling regulates asexual development, cell wall integrity, stress response, and lifestyle transition in the nematode-trapping fungus Arthrobotrys oligospora. Sci. China Life Sci. 2022, 65, 2455–2471. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [PubMed]
- Colot, H.V.; Park, G.; Turner, G.E.; Ringelberg, C.; Crew, C.M.; Litvinkova, L.; Weiss, R.L.; Borkovich, K.A.; Dunlap, J.C. A high-throughput gene knockout procedure for Neurospora reveals functions for multiple transcription factors. Proc. Natl. Acad. Sci. USA 2006, 103, 10352–10357. [Google Scholar] [CrossRef]
- Zhu, Y.; Yang, X.; Bai, N.; Liu, Q.; Yang, J. AoRab7A interacts with AoVps35 and AoVps41 to regulate vacuole assembly, trap formation, conidiation, and functions of proteasomes and ribosomes in Arthrobotrys oligospora. Microbiol. Res. 2024, 280, 127573. [Google Scholar] [CrossRef]
- Tunlid, A.; Ahman, J.; Oliver, R.P. Transformation of the nematode-trapping fungus Arthrobotrys oligospora. FEMS Microbiol. Lett. 1999, 173, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Liu, Y.; Duan, S.; Bai, N.; Zhu, M.; Yang, J. Cellular communication and fusion regulate cell fusion, trap morphogenesis, conidiation, and secondary metabolism in Arthrobotrys oligospora. Microbiol. Res. 2024, 278, 127516. [Google Scholar] [CrossRef]
- Zhao, X.; Fan, Y.; Xiang, M.; Kang, S.; Wang, S.; Liu, X. DdaCrz1, a C2H2-Type transcription factor, regulates growth, conidiation, and stress resistance in the nematode-trapping fungus Drechslerella dactyloides. J. Fungi 2022, 8, 750. [Google Scholar] [CrossRef]
- Chen, Y.; Gao, Y.; Zhang, K.; Zou, C. Autophagy is required for trap formation in the nematode-trapping fungus Arthrobotrys oligospora. Environ. Microbiol. Rep. 2013, 5, 511–517. [Google Scholar] [CrossRef]
- Yang, L.; Li, X.; Ma, Y.; Zhu, M.; Zhang, K.-Q.; Yang, J. The Arf-GAPs, AoAge1 and AoAge2, regulate diverse cellular processes, conidiation, trap formation, and secondary metabolism in Arthrobotrys oligospora. Microbiol. Res. 2024, 285, 127779. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Bai, N.; Duan, S.; Shen, Y.; Zhu, L.; Yang, J. Characterizing the role of AosfgA and AofluG in mycelial and conidial development in Arthrobotrys oligospora and their role in secondary metabolism. Microorganisms 2024, 12, 615. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Teng, L.L.; Song, T.Y.; Xu, Z.F.; Liu, X.; Dai, R.; Chen, Y.H.; Li, S.H.; Zhang, K.Q.; Niu, X.M. Selected mutations revealed intermediates and key precursors in the biosynthesis of polyketide-terpenoid hybrid sesquiterpenyl epoxy-cyclohexenoids. Org. Lett. 2017, 19, 3923–3926. [Google Scholar] [CrossRef]
- Zhou, J.; Wu, Q.-F.; Li, S.-H.; Yan, J.-X.; Wu, L.; Cheng, Q.-Y.; He, Z.-Q.; Yue, X.-T.; Zhang, K.-Q.; Zhang, L.-L.; et al. The multifaceted gene 275 embedded in the PKS-PTS gene cluster was involved in the regulation of arthrobotrisin biosynthesis, TCA Cycle, and septa formation in nematode-trapping fungus Arthrobotrys oligospora. J. Fungi 2022, 8, 1261. [Google Scholar] [CrossRef] [PubMed]
- He, Z.-Q.; Tan, J.-L.; Li, N.; Zhang, H.-X.; Chen, Y.-H.; Wang, L.-J.; Zhang, K.-Q.; Niu, X.-M. Sesquiterpenyl epoxy-cyclohexenoids and their signaling functions in nematode-trapping fungus Arthrobotrys oligospora. J. Agric. Food Chem. 2019, 67, 13061–13072. [Google Scholar] [CrossRef]
- Andersson, K.-M.; Kumar, D.; Bentzer, J.; Friman, E.; Ahrén, D.; Tunlid, A. Interspecific and host-related gene expression patterns in nematode-trapping fungi. BMC Genom. 2014, 15, 968. [Google Scholar] [CrossRef]
- Zhang, D.; Zhu, X.; Sun, F.; Zhang, K.; Niu, S.; Huang, X. The roles of actin cytoskeleton and actin-associated protein Crn1p in trap formation of Arthrobotrys oligospora. Res. Microbiol. 2017, 168, 655–663. [Google Scholar] [CrossRef]
- McLeod, M.; Shor, B.; Caporaso, A.; Wang, W.; Chen, H.; Hu, L. Cpc2, a fission yeast homologue of mammalian RACK1 protein, interacts with Ran1 (Pat1) kinase to regulate cell cycle progression and meiotic development. Mol. Cell. Biol. 2000, 20, 4016–4027. [Google Scholar] [CrossRef]
- Liu, X.; Miao, Q.; Zhou, Z.; Lu, S.; Li, J. Identification of three novel conidiogenesis-related genes in the nematode-trapping fungus Arthrobotrys oligospora. Pathogens 2022, 11, 717. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, H.; Tsukahara, K.; Kanaoka, Y.; Jinno, S.; Okayama, H. Isolation of a mammalian homologue of a fission yeast differentiation regulator. Mol. Cell. Biol. 1999, 19, 3829–3841. [Google Scholar] [CrossRef]
- Egel, R.; Egel-Mitani, M. Premeiotic DNA synthesis in fission yeast. Exp. Cell Res. 1974, 88, 127–134. [Google Scholar] [CrossRef]
- Chen, M.; Yang, H.-Y.; Cao, Y.-R.; Hui, Q.-Q.; Fan, H.-F.; Zhang, C.-C.; Han, J.-J.; Guo, Z.-Y.; Xu, J.; Zhang, K.-Q.; et al. Functional characterization of core regulatory genes involved in sporulation of the nematophagous fungus Purpureocillium lavendulum. mSphere 2020, 5, e00932-20. [Google Scholar] [CrossRef] [PubMed]
- Bai, N.; Xie, M.; Liu, Q.; Zhu, Y.; Yang, X.; Zhang, K.-Q.; Yang, J. AoMedA has a complex regulatory relationship with AoBrlA, AoAbaA, and AoWetA in conidiation, trap formation, and secondary metabolism in the nematode-trapping fungus Arthrobotrys oligospora. Appl. Environ. Microbiol. 2023, 89, e00983-23. [Google Scholar] [CrossRef]
- Twumasi-Boateng, K.; Yu, Y.; Chen, D.; Gravelat, F.N.; Nierman, W.C.; Sheppard, D.C. Transcriptional profiling identifies a role for BrlA in the response to nitrogen fepletion and for StuA in the regulation of secondary metabolite clusters in Aspergillus fumigatus. Eukaryot. Cell 2009, 8, 104–115. [Google Scholar] [CrossRef] [PubMed]
- Fischer, M.S.; Glass, N.L. Communicate and fuse: How filamentous fungi establish and maintain an interconnected mycelial network. Front. Microbiol. 2019, 10, 619. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.-A.; Lin, H.-C.; Schroeder, F.C.; Hsueh, Y.-P. Prey sensing and response in a nematode-trapping fungus is governed by the MAPK pheromone response pathway. Genetics 2021, 217, iyaa008. [Google Scholar] [CrossRef]
- Yamamoto, T.G.; Chikashige, Y.; Ozoe, F.; Kawamukai, M.; Hiraoka, Y. Activation of the pheromone-responsive MAP kinase drives haploid cells to undergo ectopic meiosis with normal telomere clustering and sister chromatid segregation in fission yeast. J. Cell Sci. 2004, 117, 3875–3886. [Google Scholar] [CrossRef]
- Pinan-Lucarré, B.; Balguerie, A.; Clavé, C. Accelerated cell death in Podospora autophagy mutants. Eukaryot. Cell 2005, 4, 1765–1774. [Google Scholar] [CrossRef]
- Kikuma, T.; Ohneda, M.; Arioka, M.; Kitamoto, K. Functional analysis of the ATG8 homologue AoAtg8 and role of autophagy in differentiation and germination in Aspergillus oryzae. Eukaryot. Cell 2006, 5, 1328–1336. [Google Scholar] [CrossRef]
- Deng, Y.Z.; Ramos-Pamplona, M.; Naqvi, N.I. Autophagy-assisted glycogen catabolism regulates asexual differentiation in Magnaporthe oryzae. Autophagy 2009, 5, 33–43. [Google Scholar] [CrossRef]
- Li, X.; Wu, Y.; Liu, Z.; Zhang, C. The function and transcriptome analysis of a bZIP transcription factor CgAP1 in Colletotrichum gloeosporioides. Microbiol. Res. 2017, 197, 39–48. [Google Scholar] [CrossRef]
- Wei, L.-X.; Zhang, H.-X.; Tan, J.-L.; Chu, Y.-S.; Li, N.; Xue, H.-X.; Wang, Y.-L.; Niu, X.-M.; Zhang, Y.; Zhang, K.-Q. Arthrobotrisins A–C, oligosporons from the nematode-trapping fungus Arthrobotrys oligospora. J. Nat. Prod. 2011, 74, 1526–1530. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Hu, X.; Pop, M.; Wernet, N.; Kirschhöfer, F.; Brenner-Weiß, G.; Keller, J.; Bunzel, M.; Fischer, R. Fatal attraction of Caenorhabditis elegans to predatory fungi through 6-methyl-salicylic acid. Nat. Commun. 2021, 12, 5462. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duan, S.; Liu, Q.; Shen, Y.; Zhu, L.; Yuan, H.; Yang, J. AoRan1 Is Involved in Regulating Conidiation, Stress Resistance, Secondary Metabolism, and Pathogenicity in Arthrobotrys oligospora. Microorganisms 2024, 12, 1853. https://doi.org/10.3390/microorganisms12091853
Duan S, Liu Q, Shen Y, Zhu L, Yuan H, Yang J. AoRan1 Is Involved in Regulating Conidiation, Stress Resistance, Secondary Metabolism, and Pathogenicity in Arthrobotrys oligospora. Microorganisms. 2024; 12(9):1853. https://doi.org/10.3390/microorganisms12091853
Chicago/Turabian StyleDuan, Shipeng, Qianqian Liu, Yanmei Shen, Lirong Zhu, Hui Yuan, and Jinkui Yang. 2024. "AoRan1 Is Involved in Regulating Conidiation, Stress Resistance, Secondary Metabolism, and Pathogenicity in Arthrobotrys oligospora" Microorganisms 12, no. 9: 1853. https://doi.org/10.3390/microorganisms12091853





