Distribution Characteristics of Nitrogen-Cycling Microorganisms in Deep-Sea Surface Sediments of Western South China Sea
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling Site Description and Sample Collection
2.2. Environmental Parameter Analyses
2.3. DNA Extraction and Metagenomic Sequencing
2.4. Quantitative Analysis of Nitrogen-Cycling Functional Genes
2.5. Statistical Analysis
3. Results
3.1. Sediment Environmental Properties
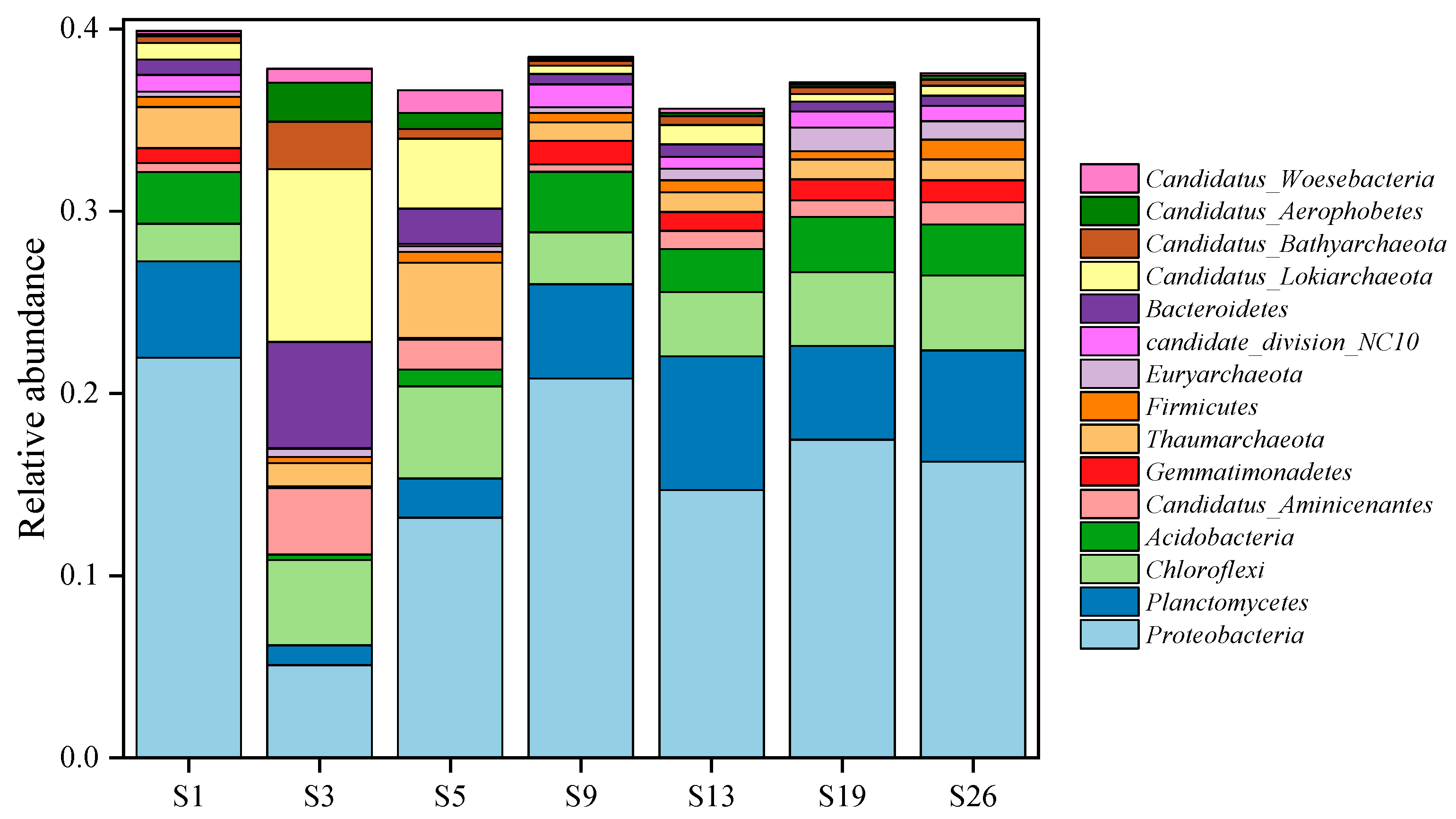
3.2. Microbial Community Composition in Surface Sediments of Western South China Sea
3.2.1. Phylum Level
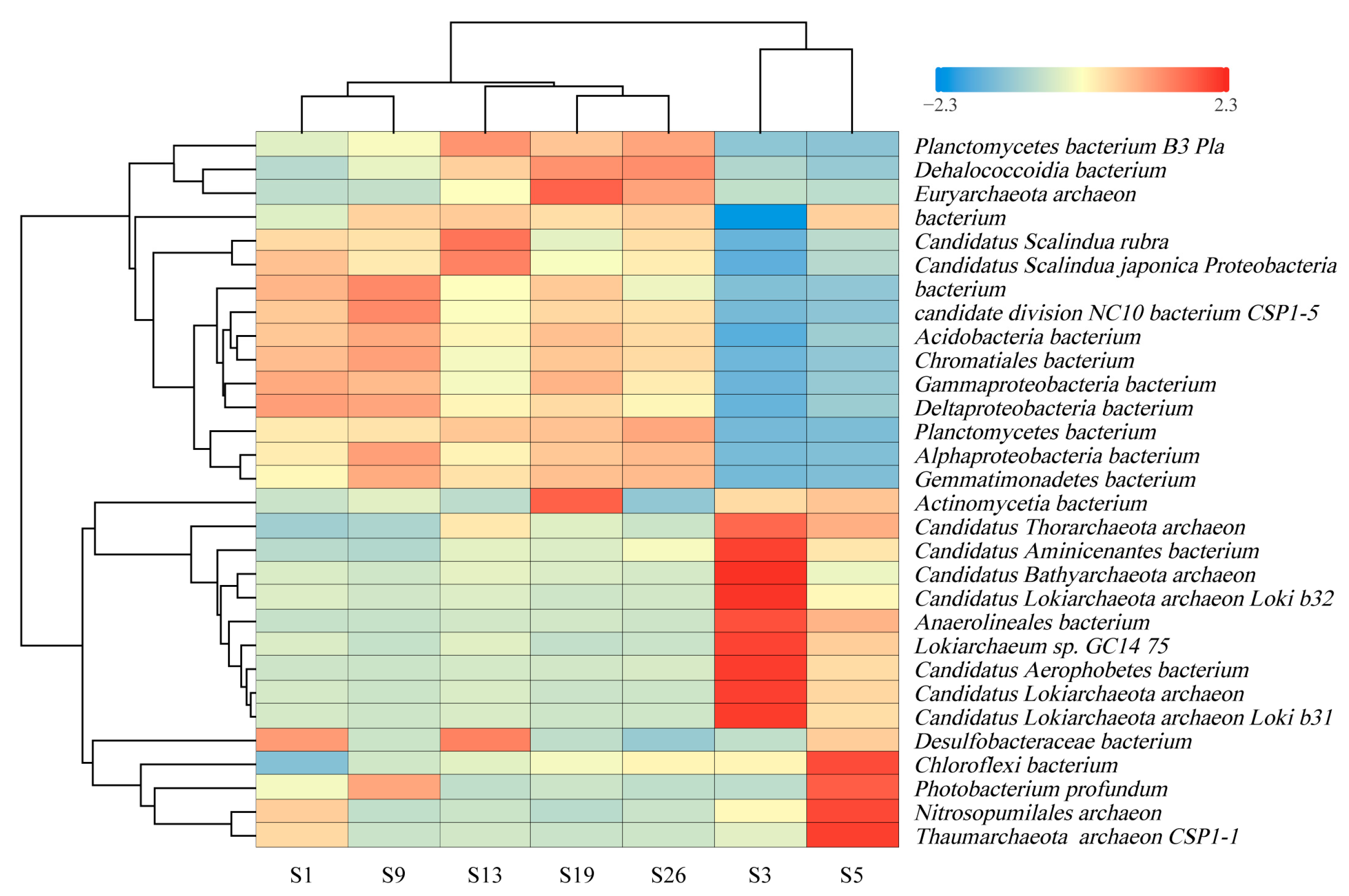
3.2.2. Species Level
3.3. Nitrogen-Cycling Genes Abundance in Deep-Sea Surface Sediments of Western South China Sea
3.3.1. Nitrogen Fixation
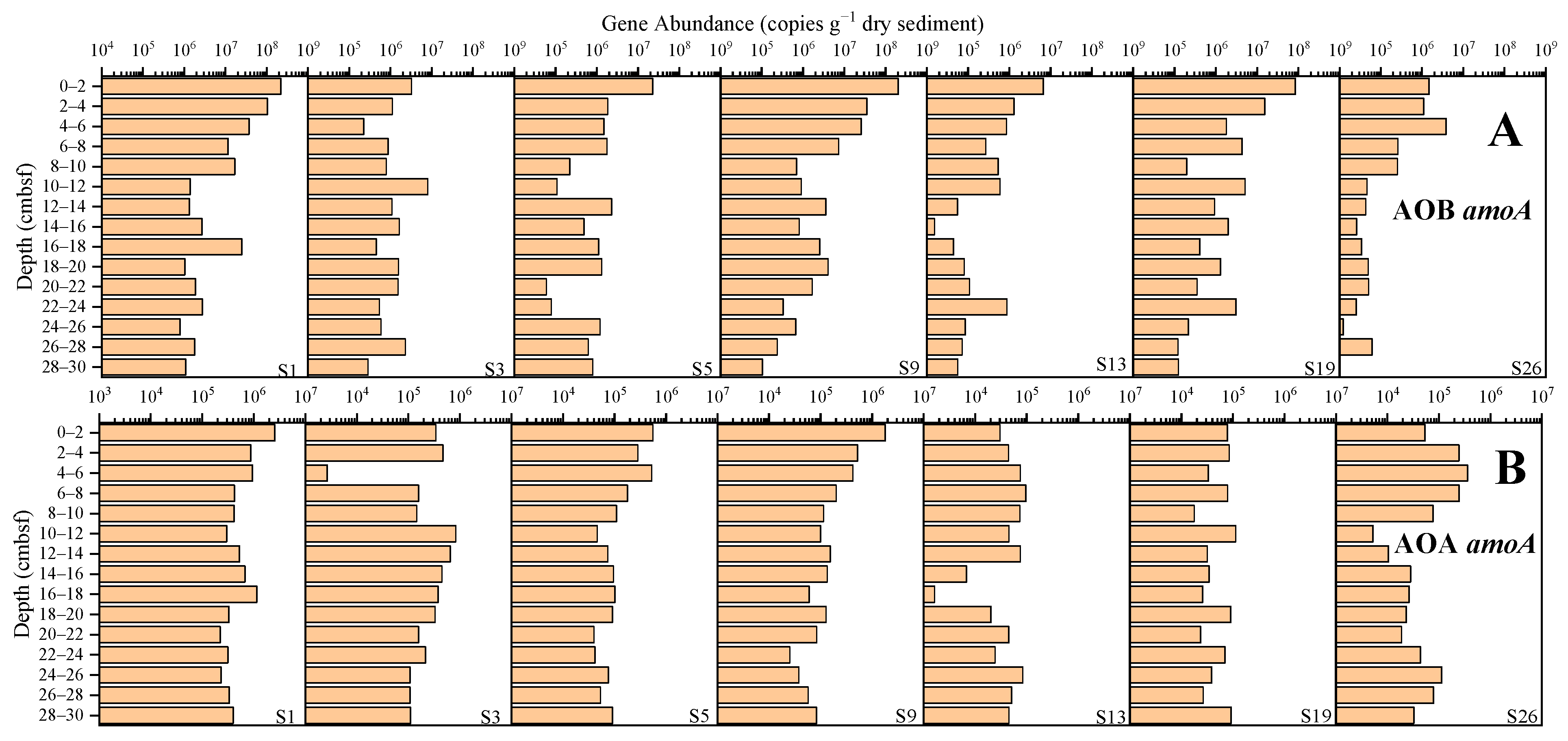
3.3.2. Nitrification
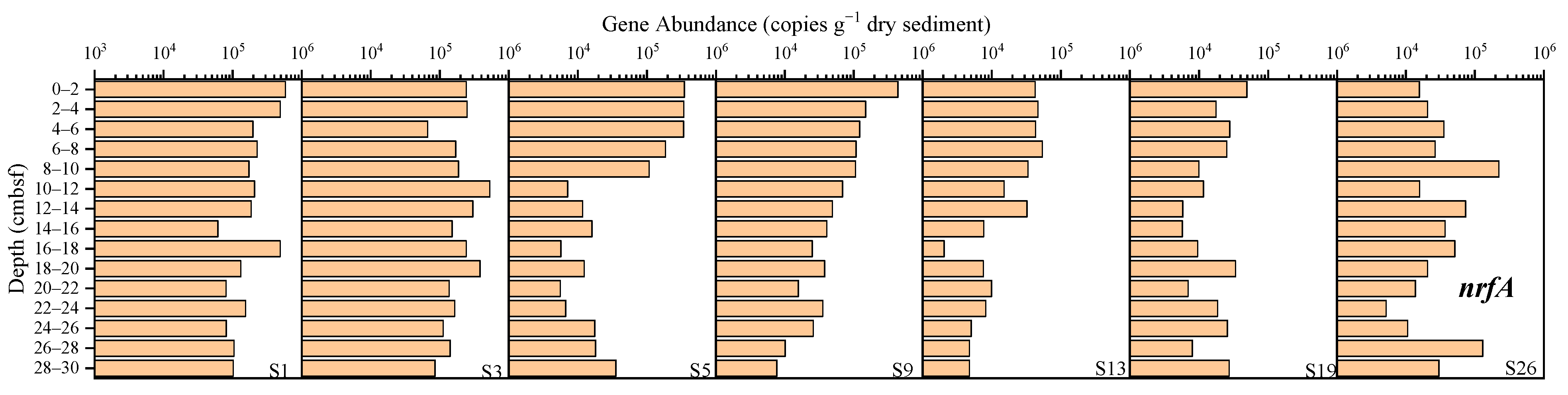
3.3.3. Dissimilatory Nitrate Reduction to Ammonium (DNRA)
3.3.4. Denitrification
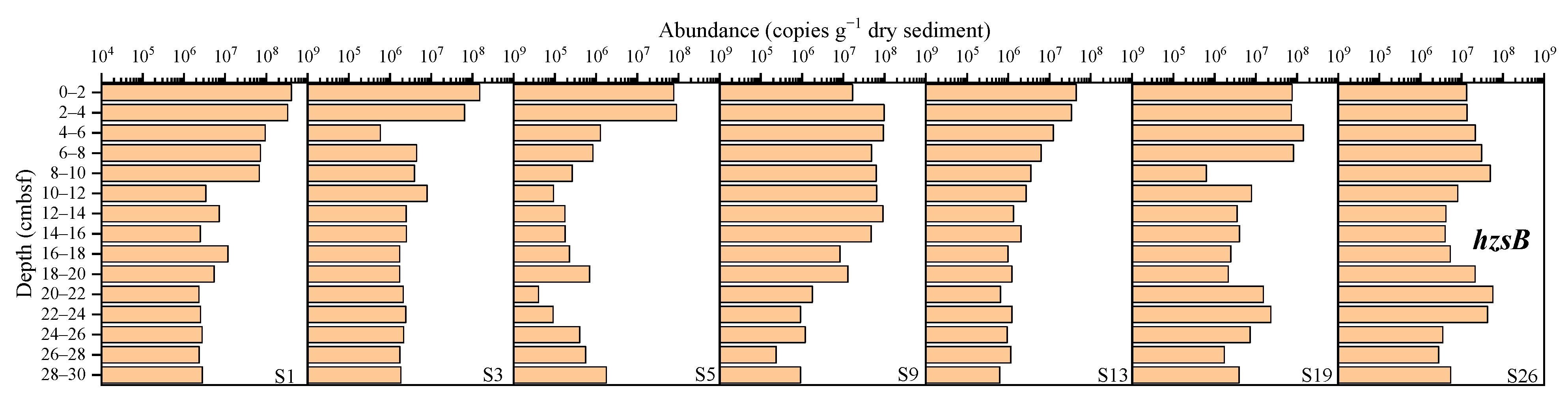
3.3.5. Anaerobic Ammonium-Oxidizing Bacteria (Anammox)
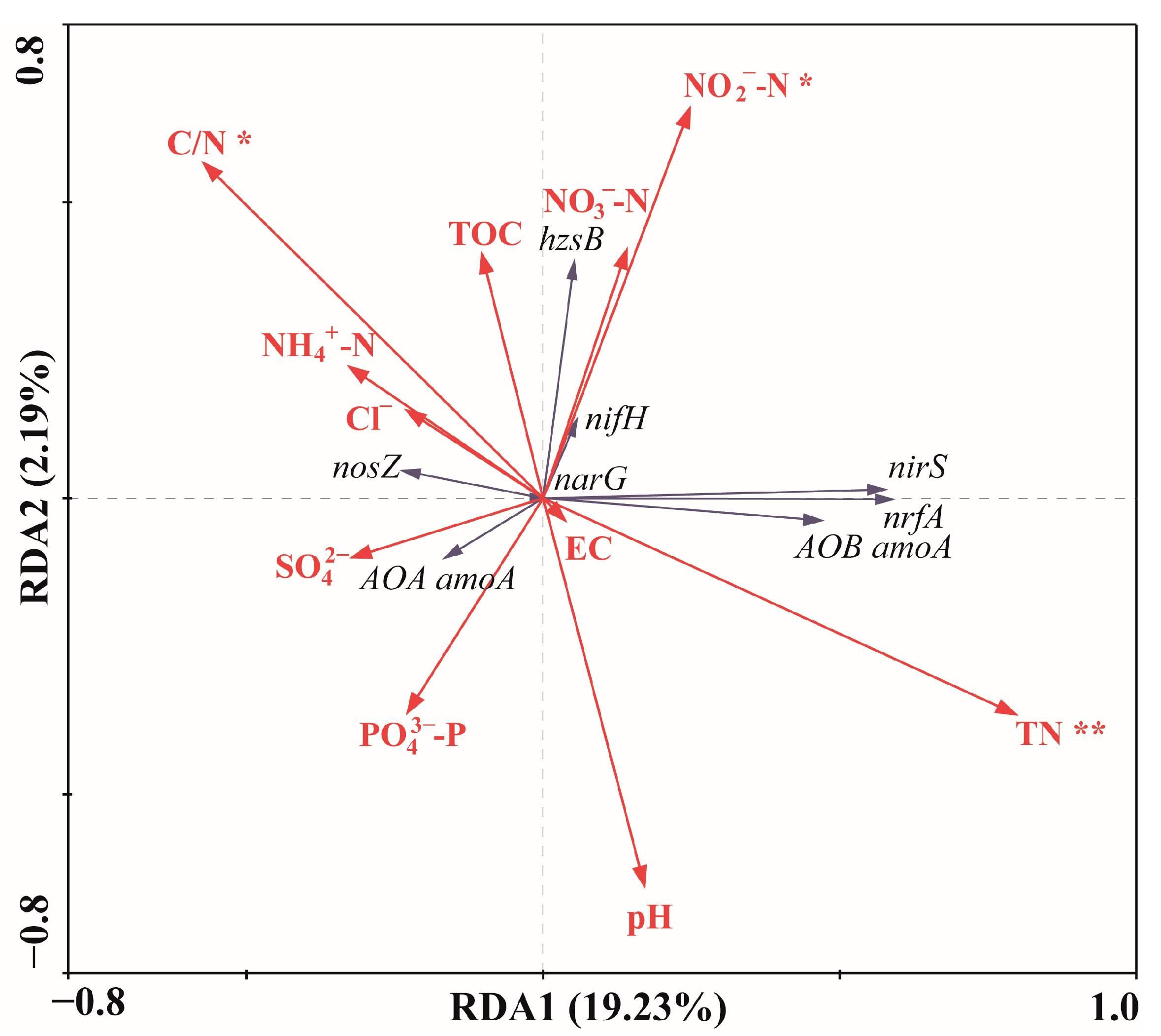
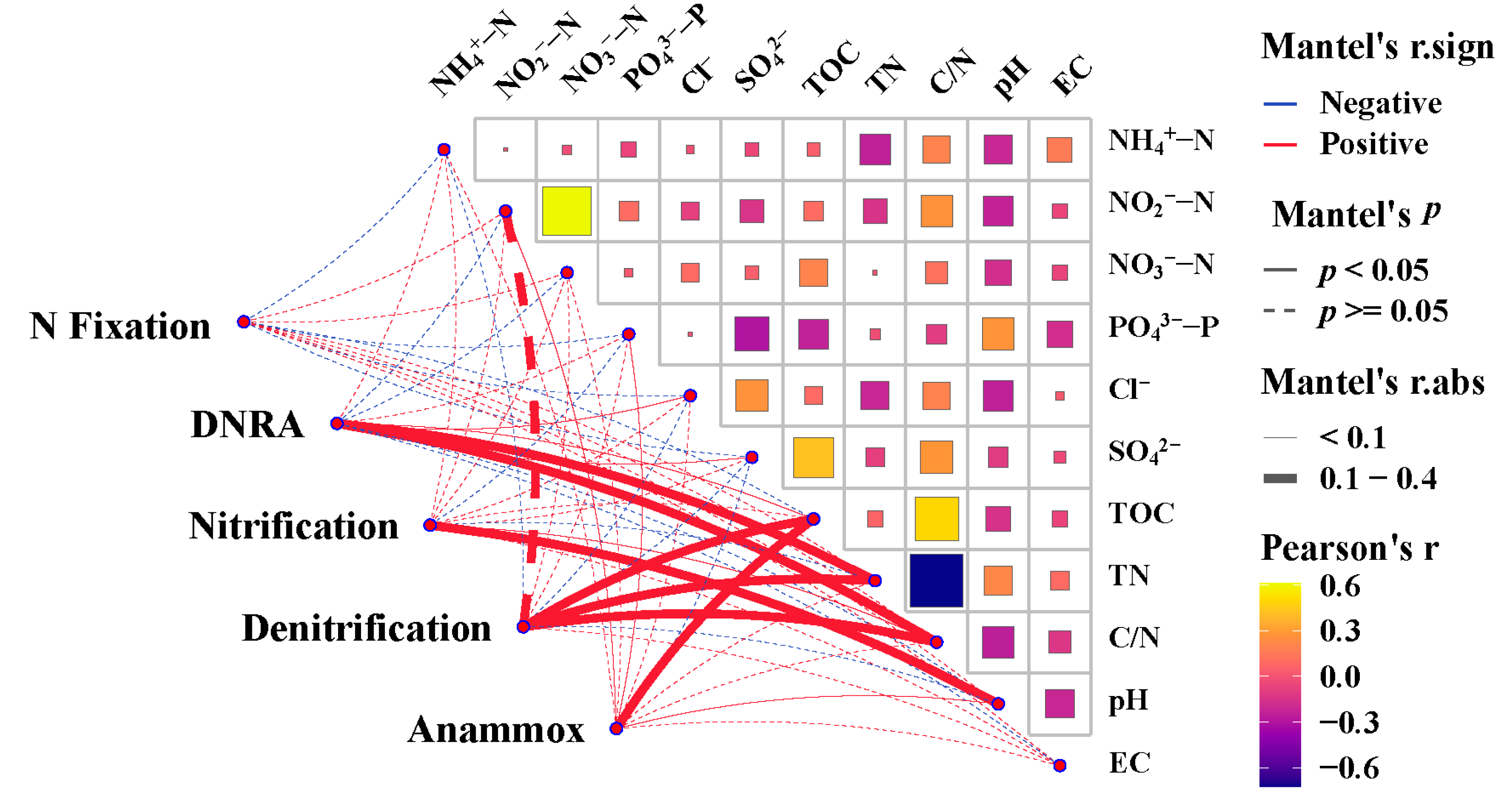
3.4. Effects of Environmental Parameters on the Abundance of Nitrogen-Cycling Genes in the Surface Sediments of the Western South China Sea
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arrigo, K.R. Marine microorganisms and global nutrient cycles. Nature 2005, 437, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Jetten, M.S.M. The microbial nitrogen cycle. Environ. Microbiol. 2008, 10, 2903–2909. [Google Scholar] [CrossRef]
- Gruber, N.; Galloway, J.N. An earth-system perspective of the global nitrogen cycle. Nature 2008, 451, 293–296. [Google Scholar] [CrossRef] [PubMed]
- Voss, M.; Bange, H.W.; Dippner, J.W.; Middelburg, J.J.; Montoya, J.P.; Ward, B. The marine nitrogen cycle: Recent discoveries, uncertainties and the potential relevance of climate change. Philos. Trans. R. Soc. B 2013, 368, 20130121. [Google Scholar] [CrossRef] [PubMed]
- Zehr, J.P.; Kudela, R.M. Nitrogen cycle of the open ocean: From genes to ecosystems. Annu. Rev. Mar. Sci. 2010, 3, 197–225. [Google Scholar] [CrossRef]
- Pajares, S.; Ramos, R. Processes and microorganisms involved in the marine nitrogen cycle: Knowledge and gaps. Front. Mar. Sci. 2019, 6, 739. [Google Scholar] [CrossRef]
- Hutchins, D.A.; Capone, D.C. The marine nitrogen cycle: New developments and global change. Nat. Rev. Microbiol. 2022, 20, 401–444. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.; Wang, X.T.; Subhas, A.V.; Pavia, F.J.; Adkins, J.F.; Berelson, W.M. Depth profiles of suspended carbon and nitrogen along a North Pacific transect: Concentrations, isotopes, and ratios. Limnol. Oceanogr. 2022, 67, 247–260. [Google Scholar] [CrossRef]
- Xu, H.M.; Song, G.D.; Yang, S.Q.; Zhu, R.S.; Zhang, G.L.; Liu, S.M. Benthic nitrogen cycling in the deep ocean of the Kuroshio Extension region. Front. Mar. Sci. 2022, 9, 997810. [Google Scholar] [CrossRef]
- Lin, G.M.; Huang, J.; Lu, J.G.; Su, M.; Hu, B.Q.; Lin, X.B. Geochemical and microbial insights into vertical distributions of genetic potential of N-cycling processes in deep-sea sediments. Ecol. Indic. 2021, 125, 107461. [Google Scholar] [CrossRef]
- Nunoura, T.; Nishizawa, M.; Kikuchi, T.; Tsubouchi, T.; Hirai, M.; Koide, O.; Miyazaki, J.; Hirayama, H.; Koba, K.; Takai, K. Molecular biological and isotopic biogeochemical prognoses of the nitrification-driven dynamic microbial nitrogen cycle in hadopelagic sediments. Environ. Microbiol. 2013, 15, 3087–3107. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Hannisdal, B.; Mogollon, J.M.; Jorgensen, S.L. Nitrifier abundance and diversity peak at deep redox transition zones. Sci. Rep. 2019, 9, 8633. [Google Scholar] [CrossRef] [PubMed]
- Risgaard-Petersen, N.; Meyer, R.L.; Schmid, M.; Jetten, M.S.M.; Enrich-Prast, A.; Rysgaard, S.; Revsbech, N.P. Anaerobic ammonium oxidation in an estuarine sediment. Aquat. Microb. Ecol. 2004, 36, 293–304. [Google Scholar] [CrossRef]
- Brin, L.D.; Giblin, A.E.; Rich, J.J. Environmental controls of anammox and denitrification in southern New England estuarine and shelf sediments. Limnol. Oceanogr. 2014, 59, 851–860. [Google Scholar] [CrossRef]
- Kits, K.D.; Sedlacek, C.J.; Lebedeva, E.V.; Han, P.; Bulaev, A.; Pjevac, P.; Daebeler, A.; Romano, S.; Albertsen, M.; Stein, L.Y.; et al. Kinetic analysis of a complete nitrifier reveals an oligotrophic lifestyle. Nature 2017, 549, 269–272. [Google Scholar] [CrossRef]
- Hoshino, T.; Toki, T.; Ijiri, A.; Morono, Y.; Machiyama, H.; Ashi, J.; Okamura, K.; Inagaki, F. Atribacteria from the subseafloor sedimentary biosphere disperse to the hydrosphere through submarine mud volcanoes. Front. Microbiol. 2017, 8, 01135. [Google Scholar] [CrossRef]
- Ruff, S.E.; Felden, J.; Gruber-Vodicka, H.R.; Marcon, Y.; Knittel, K.; Ramette, A.; Boetius, A. In situ development of a methanotrophic microbiome in deep-sea sediments. ISME J. 2019, 13, 197–213. [Google Scholar] [CrossRef]
- Chakraborty, A.; Ruff, S.E.; Dong, X.; Ellefson, E.D.; Li, C.; Brooks, J.M.; Mcbee, J.; Bernard, B.B.; Hubert, C.R.J. Hydrocarbon seepage in the deep seabed links subsurface and seafloor biospheres. Proc. Natl. Acad. Sci. USA 2020, 117, 11029–11037. [Google Scholar] [CrossRef] [PubMed]
- Kong, Y.; Lei, H.Y.; Zhang, Z.L.; Cheng, W.D.; Wang, B.; Pan, F.L.; Huang, F.F.; Huang, F.L.; Li, W.Q. Depth profiles of geochemical features, geochemical activities and biodiversity of microbial communities in marine sediments from the Shenhu area, the northern South China Sea. Sci. Total Environ. 2021, 779, 146233. [Google Scholar] [CrossRef]
- Zhao, R.; Mogollon, J.M.; Abby, S.S.; Schleper, C.; Biddle, J.F.; Roerdink, D.L.; Thorseth, I.H.; Jorgensen, S.L. Geochemical transition zone powering microbial growth in subsurface sediments. Proc. Natl. Acad. Sci. USA 2020, 117, 32617–32626. [Google Scholar] [CrossRef]
- Schauberger, C.; Middelboe, M.; Larsen, M.; Peoples, L.M.; Bartlett, D.H.; Kirpekar, F.; Rowden, A.A.; Wenzhofer, F.; Thamdrup, B.; Glud, R.N. Spatial variability of prokaryotic and viral abundances in the Kermadec and Atacama Trench regions. Limnol. Oceanogr. 2021, 66, 2095–2109. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.L.; Zhou, Y.H.; Lei, Z.Y.; Liu, G.L.; Hua, Y.M.; Zhou, W.B.; Wan, X.Q.; Zhu, D.W.; Zhao, J.W. Effects of Potamogeton crispus decline in the rhizosphere on the abundance of anammox bacteria and nirS denitrifying bacteria. Environ. Pollut. 2020, 260, 114018. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, X.C.; Fang, W.K.; Cheng, Y.; Cai, H.; Zhang, S.Q. Impact of different types of anthropogenic pollution on bacterial community and metabolic genes in urban river sediments. Sci. Total Environ. 2021, 793, 148475. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.S.; Wang, B.; Luo, Y.M.; Li, H.L.; Zhang, J.J.; Jin, H.Y.; Chen, J.F. Changes of carbon to nitrogen ratio in particulate organic matter in the marine mesopelagic zone: A case from the South China Sea. Mar. Chem. 2021, 231, 103930. [Google Scholar] [CrossRef]
- Pachiadaki, M.G.; Yakimov, M.M.; LaCono, V.; Leadbetter, E.; Edgcomb, V. Unveiling microbial activities along the halocline of Thetis, a deep-sea hypersaline anoxic basin. ISME J. 2014, 8, 2478–2489. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.Y.; Rattray, J.E.; Campbell, D.C.; Webb, J.; Chakraborty, A.; Adebayo, O.; Matthews, S.; Li, C.; Fowler, M.; Morrison, N.M.; et al. Thermogenic hydrocarbon biodegradation by diverse depth-stratified microbial populations at a Scotian Basin cold seep. Nat. Commun. 2020, 11, 5825. [Google Scholar] [CrossRef]
- Hollingsworth, A.L.; Jones, D.O.B.; Young, R. Spatial variability of abyssal nitrifying microbes in the North-Eastern Clarion-Clipperton Zone. Front. Mar. Sci. 2021, 8, 663420. [Google Scholar] [CrossRef]
- Ahmad, H.A.; Guo, B.B.; Zhuang, X.M.; Zhao, Y.Y.; Ahmad, S.; Lee, T.; Zhu, J.G.; Dong, Y.L.; Ni, S.Q. A twilight for the complete nitrogen removal via synergistic partial-denitrification, anammox, and DNRA process. Npj Clean Water 2021, 4, 31. [Google Scholar] [CrossRef]
- Martino, A.; Rhodes, M.E.; Leon-Zayas, R.; Valente, I.E.; Biddle, J.F.; House, C.H. Microbial Diversity in Sub-Seafloor Sediments from the Costa Rica Margin. Geosciences 2019, 9, 218. [Google Scholar] [CrossRef]
- Li, Z.X.; Pan, D.; Wei, G.S.; Pi, W.L.; Zhang, C.W.; Wang, J.H.; Peng, Y.Y.; Zhang, L.; Wang, Y.; Hubert, C.R.J.; et al. Deep sea sediments associated with cold seeps are a subsurface reservoir of viral diversity. ISME J. 2021, 15, 2366–2378. [Google Scholar] [CrossRef]
- More, K.D.; Wuchter, C.; Irigoien, X.; Tierney, J.E.; Giosan, L.; Grice, K.; Coolen, M.J.L. Subseafloor Archaea reflect 139 kyrs of paleodepositional changes in the northern Red Sea. Geobiology 2021, 19, 162–172. [Google Scholar] [CrossRef] [PubMed]
- Techtman, S.M.; Mahmoudi, N.; Whitt, K.T.; Campa, M.F.; Fortney, J.L.; Joyner, D.C.; Hazen, T.C. Comparison of Thaumarchaeotal populations from four deep sea basins. FEMS Microbiol. Ecol. 2017, 93, fix128. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.X.; Cai, Z.R.; Yao, Y.J.; Huang, Q.R.; Li, Z.Y. Tectonic dynamics of the Zhongjiannan Basin in the western South China Sea since the late Miocene. Front. Earth Sci. 2023, 10, 996267. [Google Scholar] [CrossRef]
- Chen, J.X.; Song, H.B.; Guan, Y.X.; Yang, S.X.; Pinheiro, L.M.; Bai, Y.; Liu, B.R.; Geng, M.H. Morphologies, classification and genesis of pockmarks, mud volcanoes and associated fluid escape features in the northern Zhongjiannan Basin, South China Sea. Deep-Sea Res. Part II 2015, 122, 106–117. [Google Scholar] [CrossRef]
- Geng, M.H.; Song, H.B.; Guan, Y.X.; Chen, J.X.; Zhang, R.W.; Zhang, B.J.; Zhang, X.D. Sill-related seafloor domes in the Zhongjiannan Basin, western South China Sea. Mar. Petrol. Geol. 2020, 122, 104669. [Google Scholar] [CrossRef]
- Zhang, G.X.; Wang, X.J.; Li, L.; Sun, L.Y.; Guo, Y.Q.; Lu, Y.T.; Li, W.; Wang, Z.Q.; Qian, J.; Yang, T.T.; et al. Gas hydrate accumulation related to pockmarks and faults in the Zhongjiannan Basin, South China Sea. Front. Earth Sci. 2022, 10, 902469. [Google Scholar] [CrossRef]
- Corinaldesi, C.; Dell’Anno, A.; Danovaro, R. Viral infections stimulate the metabolism and shape prokaryotic assemblages in submarine mud volcanoes. ISME J. 2012, 6, 1250–1259. [Google Scholar] [CrossRef]
- Nickel, J.C.; di Primio, R.; Kallmeyer, J.; Hammer, O.; Horsfield, B.; Stoddart, D.; Brunstad, H.; Mangelsdorf, K. Tracing the origin of thermogenic hydrocarbon signals in pockmarks from the southwestern Barents Sea. Org. Geochem. 2013, 63, 73–84. [Google Scholar] [CrossRef]
- Begmatov, S.; Savvichev, A.S.; Kadnikov, V.V.; Beletsky, A.V.; Rusanov, I.I.; Klyuvitkin, A.A.; Novichkova, E.A.; Mardanov, A.V.; Pimenov, N.V.; Ravin, N.V. Microbial communities involved in methane, sulfur, and nitrogen cycling in the sediments of the Barents Sea. Microorganisms 2021, 9, 2362. [Google Scholar] [CrossRef]
- Pimenov, N.V.; Ulyanova, M.O.; Kanapatsky, T.A.; Veslopolova, E.F.; Sigalevich, P.A.; Sivkov, V.V. Microbially mediated methane and sulfur cycling in pockmark sediments of the Gdansk Basin, Baltic Sea. Geo-Mar. Lett. 2010, 30, 439–448. [Google Scholar] [CrossRef]
- Nickel, J.C.; di Primio, R.; Mangelsdorf, K.; Stoddart, D.; Kallmeyer, J. Characterization of microbial activity in pockmark fields of the SW-Barents Sea. Mar. Geol. 2012, 332, 152–162. [Google Scholar] [CrossRef]
- Carrier, V.; Svenning, M.M.; Grundger, F.; Niemann, H.; Dessandier, P.A.; Panieri, G.; Kalenitchenko, D. The impact of methane on microbial communities at marine Arctic gas hydrate bearing sediment. Front. Microbiol. 2020, 11, 01932. [Google Scholar] [CrossRef]
- Yu, T.T.; Li, M.; Niu, M.Y.; Fan, X.B.; Liang, W.Y.; Wang, F.P. Difference of nitrogen-cycling microbes between shallow bay and deep-sea sediments in the South China Sea. Appl. Microbiol. Biot. 2018, 102, 447–459. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, B.B.; Boetius, A. Feast and famine—Microbial life in the deep-sea bed. Nat. Rev. Microbiol. 2007, 5, 770–781. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.L.; Li, D.; Mi, T.Z.; Zhao, J.; Liu, C.G.; Sun, C.J.; Zhen, Y. Characteristics of the archaeal and bacterial communities in core sediments from Southern Yap Trench via in situ sampling by the manned submersible Jiaolong. Sci. Total Environ. 2020, 703, 134884. [Google Scholar] [CrossRef]
- Wu, H.P.; Hao, B.B.; Cai, Y.P.; Liu, G.H.; Xing, W. Effects of submerged vegetation on sediment nitrogen-cycling bacterial communities in Honghu Lake (China). Sci. Total Environ. 2021, 755, 142541. [Google Scholar] [CrossRef] [PubMed]
- Rong, L.L.; Zhao, L.F.; Zhao, L.C.; Cheng, Z.P.; Yao, Y.M.; Yuan, C.L.; Wang, L.; Sun, H.W. LDPE microplastics affect soil microbial communities and nitrogen cycling. Sci. Total Environ. 2021, 773, 145640. [Google Scholar] [CrossRef]
- Chang, Y.K.; Yin, G.Y.; Hou, L.J.; Liu, M.; Zheng, Y.L.; Han, P.; Dong, H.P.; Liang, X.; Gao, D.Z.; Liu, C. Nitrogen removal processes coupled with nitrification in coastal sediments off the north East China Sea. J. Soil Sediments 2021, 21, 3289–3299. [Google Scholar] [CrossRef]
- Shi, Y.; Zhang, X.Y.; Wang, Z.C.; Xu, Z.W.; He, C.G.; Sheng, L.X.; Liu, H.Y.; Wang, Z.Q. Shift in nitrogen transformation in peatland soil by nitrogen inputs. Sci. Total Environ. 2021, 764, 142924. [Google Scholar] [CrossRef]
- Chen, C.; Yin, G.Y.; Hou, L.J.; Liu, M.; Jiang, Y.H.; Zheng, D.S.; Gao, D.Z.; Liu, C.; Zheng, Y.L.; Han, P. Effects of sulfamethoxazole on coupling of nitrogen removal with nitrification in Yangtze Estuary sediments. Environ. Pollut. 2021, 271, 116382. [Google Scholar] [CrossRef]
- Huang, F.J.; Lin, X.B.A.; Hu, W.F.; Zeng, F.; He, L.; Yin, K.D. Nitrogen cycling processes in sediments of the Pearl River Estuary: Spatial variations, controlling factors, and environmental implications. Catena 2021, 206, 105545. [Google Scholar] [CrossRef]
- Wu, J.P.; Hong, Y.G.; Chang, X.Y.; Jiao, L.J.; Li, Y.B.; Liu, X.H.; Xie, H.T.; Gu, J.D. Unexpectedly high diversity of anammox bacteria detected in deep-sea surface sediments of the South China Sea. FEMS Microbiol. Ecol. 2019, 95, fiz013. [Google Scholar] [CrossRef] [PubMed]
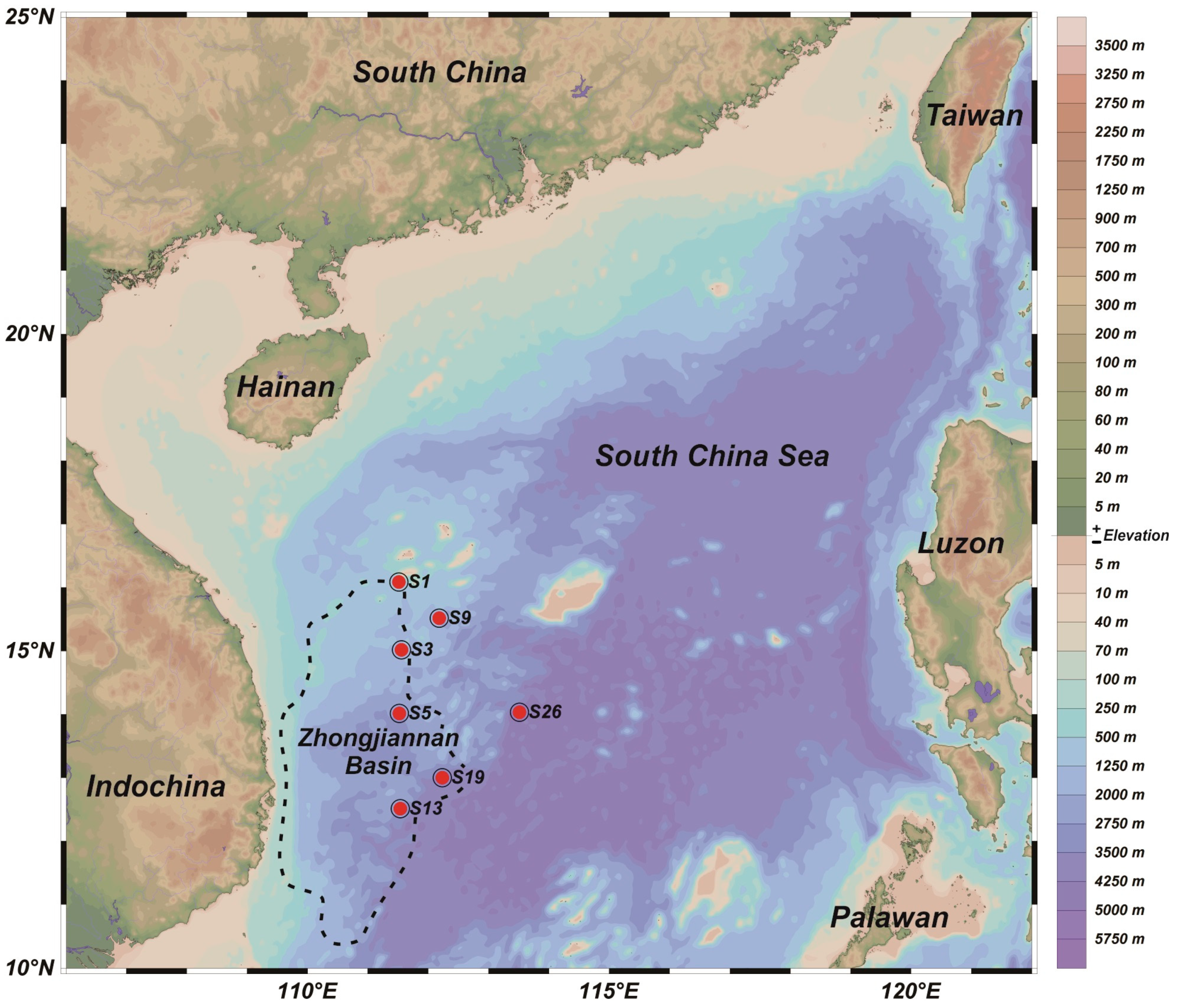


| Site | Latitude | Longitude | Water Depth (m) |
|---|---|---|---|
| S1 | 16.09° N | 111.52° E | 1179 |
| S3 | 15.02° N | 111.56° E | 1395 |
| S5 | 14.02° N | 111.52° E | 2855 |
| S9 | 15.51° N | 112.19° E | 1889 |
| S13 | 12.51° N | 111.54° E | 2522 |
| S19 | 13.00° N | 112.24° E | 3563 |
| S26 | 14.03° N | 113.51° E | 4150 |
| Site | NH4+-N | NO2−-N | NO3−-N | PO43−-P | Cl− | SO42− | TOC | TN | C/N | pH | EC |
|---|---|---|---|---|---|---|---|---|---|---|---|
| μmol L−1 | μmol L−1 | μmol L−1 | μmol L−1 | mmol L−1 | mmol L−1 | % | % | mS cm−1 | |||
| S1 (n = 15) | 142.32 ± 49.67 ab | 4.80 ± 1.98 ab | 5.50 ± 6.44 a | 427.45 ± 192.48 a | 1545.34 ± 281.11 bc | 70.30 ± 13.67 cd | 7.49 ± 0.26 bc | 4.51 ± 0.07 b | 1.67 ± 0.53 c | 7.69 ± 0.09 b | 31.38 ± 1.32 ab |
| S3 (n = 15) | 95.68 ± 48.14 b | 4.21 ± 2.59 ab | 5.53 ± 2.40 a | 258.31 ± 130.21 b | 1390.74 ± 213.52 c | 73.81 ± 24.18 cd | 5.65 ± 0.12 d | 6.12 ± 0.18 a | 0.96 ± 0.18 c | 7.77 ± 0.10 a | 30.77 ± 1.41 b |
| S5 (n = 15) | 264.85 ± 145.06 a | 8.92 ± 3.08 a | 3.37 ± 3.09 a | 374.52 ± 214.95 a | 1262.35 ± 425.48 c | 57.74 ± 25.29 d | 2.66 ± 0.14 e | 0.67 ± 0.02 d | 4.04 ± 1.64 b | 7.70 ± 0.07 b | 31.58 ± 1.43 ab |
| S9 (n = 15) | 108.27 ± 29.61 b | 7.26 ± 13.87 ab | 9.60 ± 11.95 a | 459.66 ± 114.04 a | 1870.85 ± 392.73 ab | 75.53 ± 21.51 c | 6.88 ± 0.25 cd | 1.10 ± 0.01 cd | 6.28 ± 2.30 a | 7.70 ± 0.13 ab | 27.33 ± 3.69 c |
| S1 (n = 15) | 292.07 ± 254.87 a | 3.43 ± 1.85 b | 3.13 ± 1.72 a | 400.53 ± 90.73 a | 1817.42 ± 461.37 ab | 73.60 ± 17.23 cd | 8.62 ± 0.09 a | 1.39 ± 0.02 c | 6.32 ± 0.93 a | 7.70 ± 0.09 ab | 30.94 ± 1.45 b |
| S19 (n = 15) | 227.79 ± 144.58 ab | 5.28 ± 2.41 ab | 4.29 ± 2.15 a | 164.42 ± 47.57 bc | 1583.26 ± 1094.12 bc | 120.71 ± 24.13 a | 10.20 ± 0.15 a | 1.61 ± 0.02 c | 6.43 ± 1.09 a | 7.70 ± 0.07 ab | 30.30 ± 1.45 b |
| S26 (n = 15) | 267.21 ± 364.55 a | 6.90 ± 5.78 ab | 9.99 ± 24.99 a | 150.70 ± 60.02 c | 2181.61 ± 269.16 a | 93.74 ± 24.83 b | 6.51 ± 0.14 cd | 1.19 ± 0.02 cd | 5.52 ± 1.19 a | 7.48 ± 0.06 c | 32.78 ± 1.02 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, X.; Chen, H.; Jiang, K.; Zhang, B.; Li, R.; Zhu, X.; Sun, L.; Ng, Z.L.; Su, M. Distribution Characteristics of Nitrogen-Cycling Microorganisms in Deep-Sea Surface Sediments of Western South China Sea. Microorganisms 2024, 12, 1901. https://doi.org/10.3390/microorganisms12091901
Yin X, Chen H, Jiang K, Zhang B, Li R, Zhu X, Sun L, Ng ZL, Su M. Distribution Characteristics of Nitrogen-Cycling Microorganisms in Deep-Sea Surface Sediments of Western South China Sea. Microorganisms. 2024; 12(9):1901. https://doi.org/10.3390/microorganisms12091901
Chicago/Turabian StyleYin, Xingjia, Hui Chen, Kaixi Jiang, Boda Zhang, Ruohong Li, Xinzhe Zhu, Lianpeng Sun, Zhi Lin Ng, and Ming Su. 2024. "Distribution Characteristics of Nitrogen-Cycling Microorganisms in Deep-Sea Surface Sediments of Western South China Sea" Microorganisms 12, no. 9: 1901. https://doi.org/10.3390/microorganisms12091901
APA StyleYin, X., Chen, H., Jiang, K., Zhang, B., Li, R., Zhu, X., Sun, L., Ng, Z. L., & Su, M. (2024). Distribution Characteristics of Nitrogen-Cycling Microorganisms in Deep-Sea Surface Sediments of Western South China Sea. Microorganisms, 12(9), 1901. https://doi.org/10.3390/microorganisms12091901









