Endophytic Fungi: A Treasure Trove of Antifungal Metabolites
Abstract
:1. Introduction
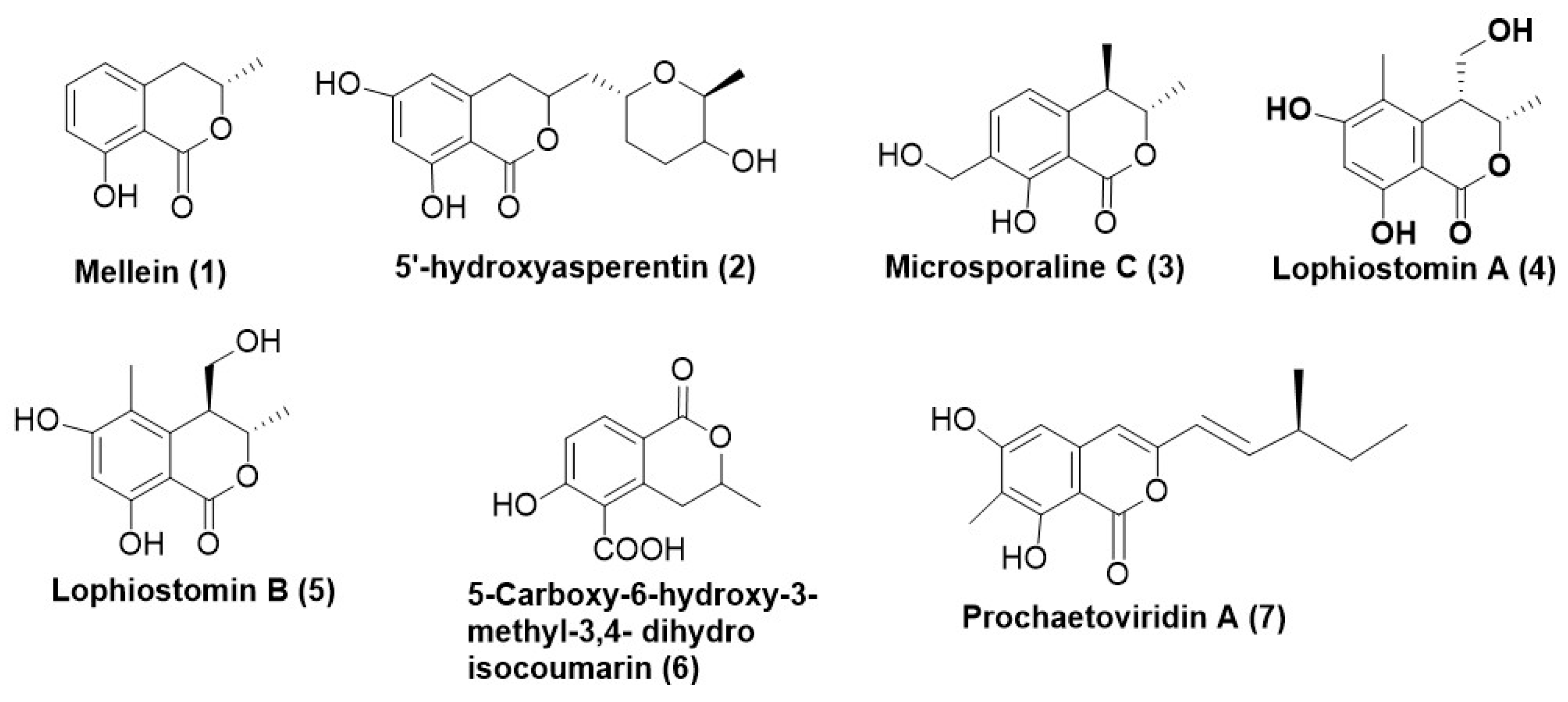
2. Coumarins
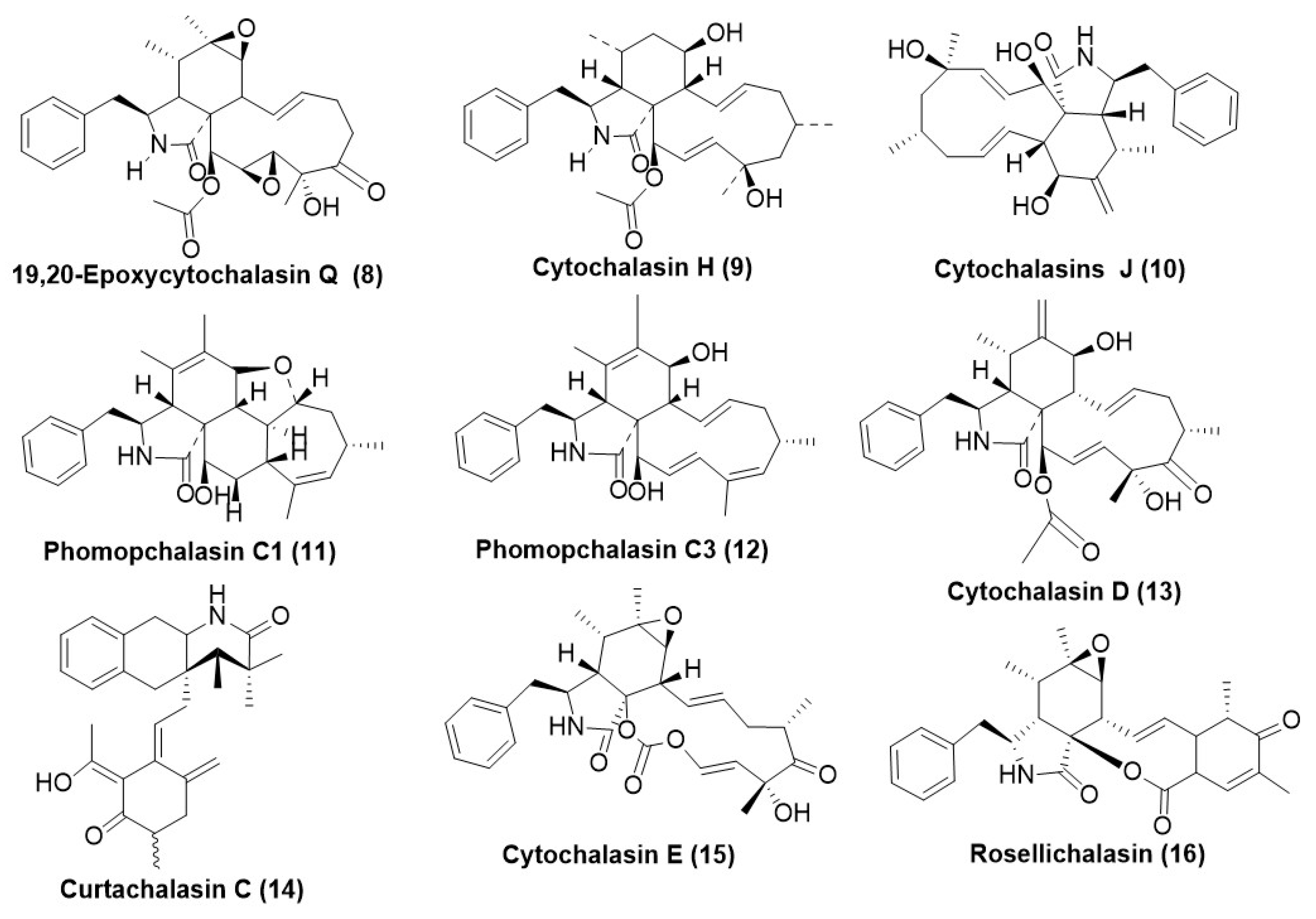
3. Cytochalasins (Aka Cytochalasans)
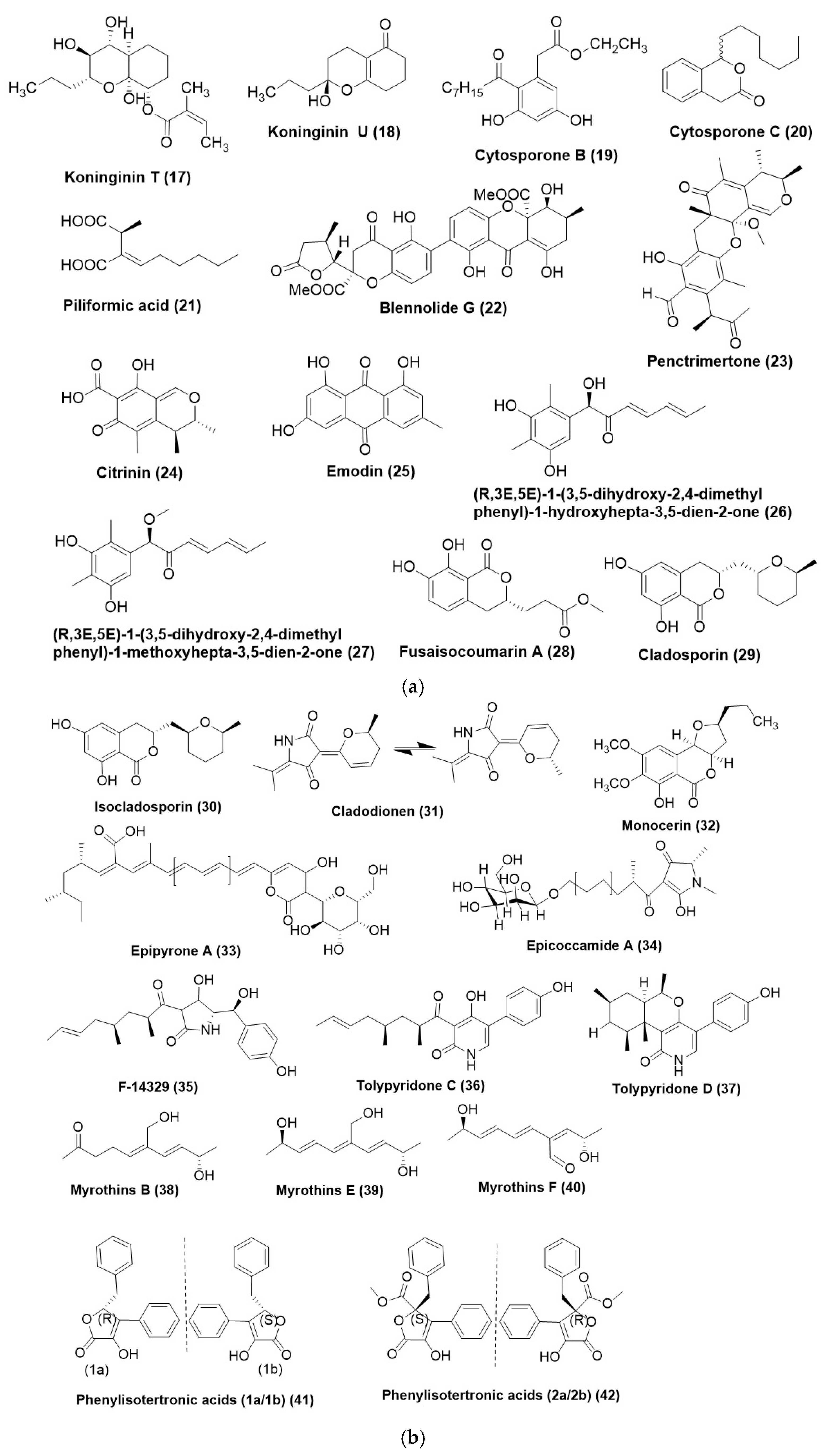
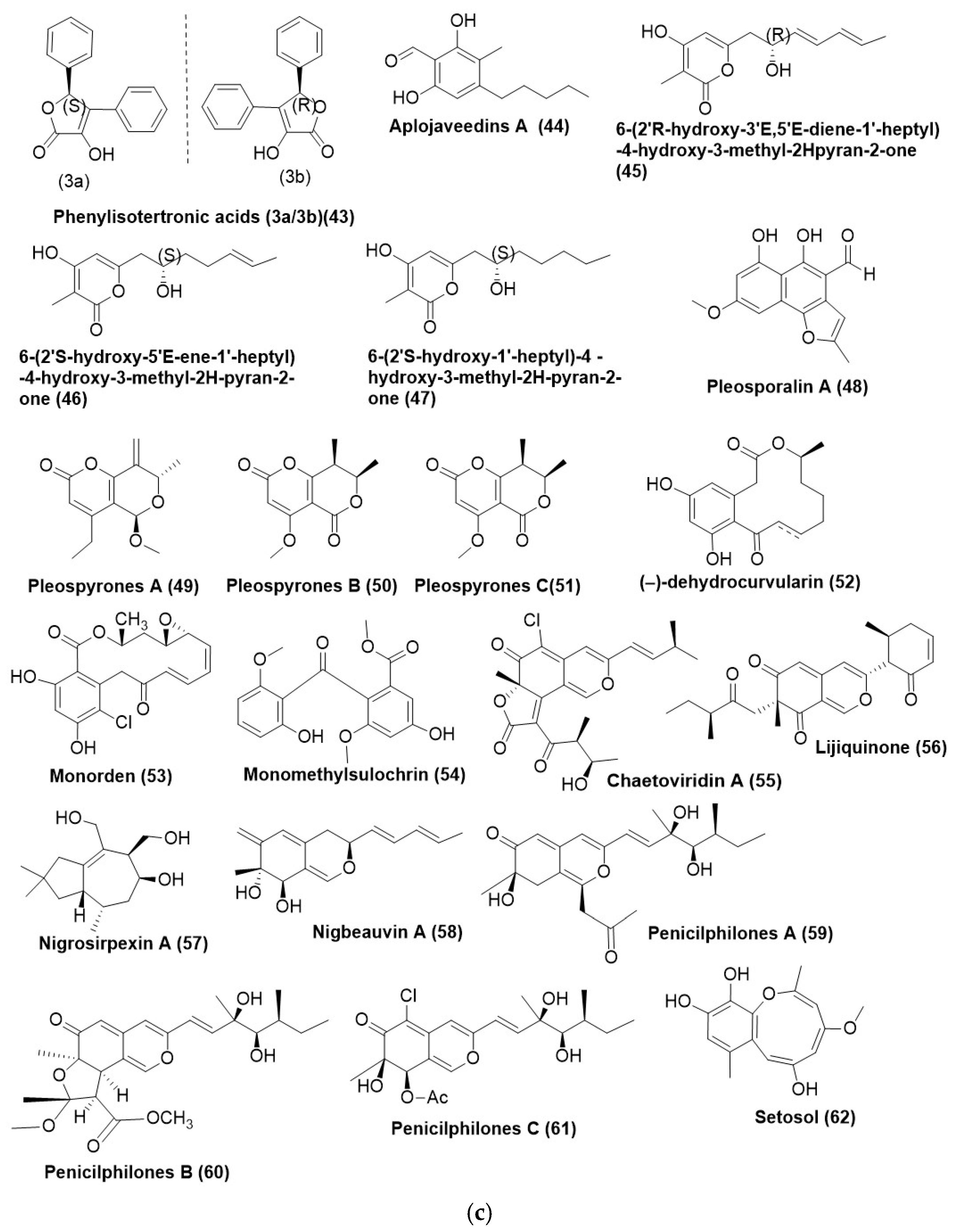
4. Polyketides
5. Xanthones
6. Terpenes and Terpenoids
7. Other Miscellaneous Compounds
7.1. Acids and Their Derivatives
7.2. Furans and Their Derivatives
7.3. Benzene and Derivatives
7.4. Heterocyclic Compounds
7.5. Ketones and Their Derivatives
7.6. Macrocyclic Lactones/Macrolides
7.7. Peptides and Their Derivatives
7.8. Prenylated Amino Acids
7.9. Quinones
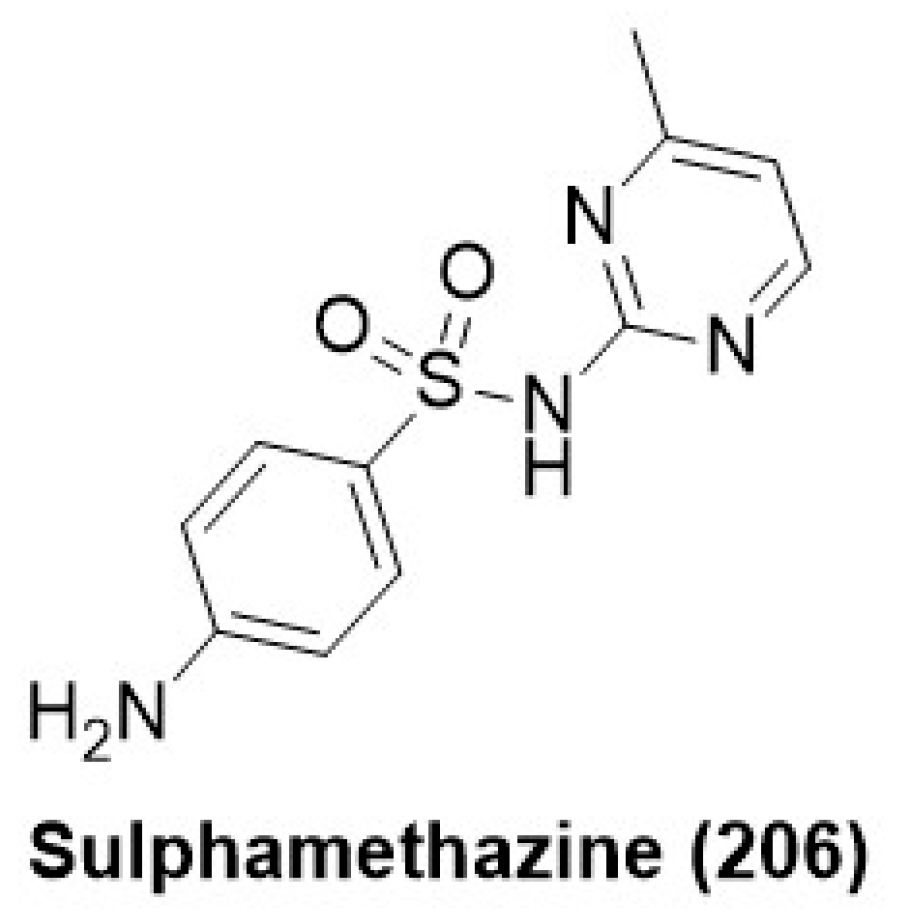
7.10. Sulphonamide Derivatives
8. Volatile Organic Compounds (VOCs) as Antifungals
9. Methods Used for Activation of Silent Biosynthetic Genes
9.1. Epigenetic Modification
9.2. The Co-Culture Strategy
9.3. OSMAC
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Saxena, S.; Chhibber, M.; Singh, I.P. Fungal bioactive compounds in pharmaceutical research and development. Curr. Bioact. Compd. 2019, 15, 211–231. [Google Scholar] [CrossRef]
- Hirai, K.; Minato, N. Effects of gliotoxin on mitochondrial functions and glutathione metabolism in Candida albicans. Biochem. Biophys. Res. Commun. 2008, 367, 34–38. [Google Scholar]
- Park, H.S.; Jun, C.D.; Jeon, B.H.; Kim, S. Dehydrogliotoxin selectively induces apoptosis in HL-60 cells by activating c-Jun N-terminal kinase/stress-activated protein kinase. Biochem. Biophys. Res. Commun. 2001, 286, 16–23. [Google Scholar]
- Wang, P.; Lou, H.; Zhang, T.; Fan, Z. Design and synthesis of bis(methylthio)gliotoxin and its analogues: Selective antifungal agents against Cryptococcus neoformans. Bioorg. Med. Chem. Lett. 2011, 21, 2095–2098. [Google Scholar]
- Brentani, R.R. Griseofulvin as an Antifungal Drug: A Historical Perspective. J. Am. Acad. Dermatol. 1986, 15, 814–821. [Google Scholar]
- Wang, H.; Yan, J.; Zhang, Y. Biological Control of Plant Diseases: Griseofulvin as a Potential Fungicide. Pestic. Biochem. Physiol. 2010, 98, 239–243. [Google Scholar]
- Riveiro, M.E.; Kimpe, N.D.; Moglioni, A.G.; Vázquez, R.S.; Monczor, F.; Shayo, C.; Davio, C. Coumarins: Old Compounds with Novel Promising Therapeutic Perspectives. Curr. Med. Chem. 2010, 17, 1325–1338. [Google Scholar] [CrossRef]
- Correia, J.; Borges, A.; Simões, M.; Simões, L.C. Beyond Penicillin: The Potential of Filamentous Fungi for Drug Discovery in the Age of Antibiotic Resistance. Antibiotics 2023, 12, 1250. [Google Scholar] [CrossRef]
- Morales-Sánchez, V.; Díaz, C.E.; Trujillo, E.; Olmeda, S.A.; Valcarcel, F.; Muñoz, R.; Andrés, M.F.; González-Coloma, A. Bioactive Metabolites from the Endophytic Fungus Aspergillus sp. SPH2. J. Fungi 2021, 7, 109. [Google Scholar] [CrossRef]
- Yehia, R.S.; Osman, G.H.; Assaggaf, H.; Salem, R.; Mohamed, M.S. Isolation of potential antimicrobial metabolites from en-dophytic fungus Cladosporium cladosporioides from endemic plant Zygophyllum mandavillei. S. Afr. J. Bot. 2020, 134, 296–302. [Google Scholar] [CrossRef]
- Liao, G.; Wu, P.; Liu, Z.; Xue, J.; Li, H.; Wei, X. 2 H-pyranone and isocoumarin derivatives from the endophytic fungus Pes-talotiopsis microspora SC3082 derived from Scaevola taccada (Gaertn.) Roxb. Nat. Prod. Res. 2021, 35, 3644–3651. [Google Scholar] [CrossRef]
- Mao, Z.; Xue, M.; Gu, G.; Wang, W.; Li, D.; Lai, D.; Zhou, L. Lophiostomin A–D: New 3,4-dihydroisocoumarin derivatives from the endophytic fungus Lophiostoma sp. Sigrf10. RSC Adv. 2020, 10, 6985–6991. [Google Scholar] [CrossRef]
- Chapla, V.M.; Zeraik, M.L.; Cafeu, M.C.; Silva, G.H.; Cavalheiro, A.J.; Bolzani, V.S.; Young, M.; Pfenning, L.H.; Araujo, A.R. Griseofulvin, diketopiperazines and cytochalasins from endophytic fungi Colletotrichum crassipes and Xylaria sp., and their antifungal, antioxidant and anticholinesterase activities. J. Braz. Chem. Soc. 2018, 29, 1707–1713. [Google Scholar] [CrossRef]
- Yan, W.; Cao, L.L.; Zhang, Y.Y.; Zhao, R.; Zhao, S.S.; Khan, B.; Ye, Y.H. New metabolites from endophytic fungus Chaetomium globosum CDW7. Molecules 2018, 23, 2873. [Google Scholar] [CrossRef]
- Scherlach, K.; Boettger, D.; Remme, N.; Hertweck, C. The chemistry and biology of cytochalasans. Nat. Prod. Rep. 2010, 27, 869–886. [Google Scholar] [CrossRef]
- Otteskog, P.; Wanger, L.; Sundqvist, K.-G. Cytochalasins distinguish by their action resting human T lymphocytes from acti-vated T-cell blasts. Exp. Cell Res. 1983, 144, 443–454. [Google Scholar] [CrossRef]
- Torralba, S.; Raudaskoski, M.; Pedregosa, A.M.; Laborda, F. Effect of cytochalasin A on apical growth, actin cytoskeleton organization and enzyme secretion in Aspergillus nidulans. Microbiology 1998, 144, 45–53. [Google Scholar] [CrossRef]
- Zhang, Y.; Cai, J.; Huang, L.; Xu, Z.; Yang, X.; Li, J.; Zhu, X. Improving the productivity of 19,20-epoxy-cytochalasin Q in Xylaria sp. sof11 with culture condition optimization. Prep. Biochem. Biotechnol. 2016, 46, 461–466. [Google Scholar] [CrossRef]
- Prasad, R.; Goffeau, A. Yeast ATP-binding cassette transporters conferring multidrug resistance. Annu. Rev. Microbiol. 2012, 66, 39–63. [Google Scholar] [CrossRef]
- Prasad, R.; Rawal, M.K.; Shah, A.H.; Sundaram, S. Antifungals: Mechanism of Action and Drug Resistance. In Fungi: Biology and Applications; Kavanagh, K., Ed.; Springer: Cham, Switzerland, 2015; pp. 345–360. [Google Scholar] [CrossRef]
- Carvalho, C.R.; Ferreira-D’Silva, A.; Wedge, D.E.; Cantrell, C.L.; Rosa, L.H. Antifungal activities of cytochalasins produced by Diaporthe miriciae, an endophytic fungus associated with tropical medicinal plants. Can. J Microbiol. 2018, 64, 835–843. [Google Scholar] [CrossRef]
- Huang, G.; Lin, W.; Li, H.; Tang, Q.; Hu, Z.; Huang, H.; Deng, X.; Xu, Q. Pentacyclic cytochalasins and their derivatives from the endophytic fungus Phomopsis sp. xz-18. Molecules 2021, 26, 6505. [Google Scholar] [CrossRef]
- Elias, L.M.; Fortkamp, D.; Sartori, S.B.; Ferreira, M.C.; Gomes, L.H.; Azevedo, J.L.; Montoya, Q.V.; Rodrigues, A.; Ferreira, A.G.; Lira, S.P. The potential of compounds isolated from Xylaria spp. as antifungal agents against anthracnose. Braz. J. Micrbiol. 2018, 49, 840–847. [Google Scholar] [CrossRef]
- Wang, W.X.; Lei, X.; Ai, H.L.; Bai, X.; Li, J.; He, J.; Li, Z.H.; Zheng, Y.S.; Feng, T.; Liu, J.K. Cytochalasans from the endophytic fungus Xylaria cf. curta with resistance reversal activity against fluconazole-resistant Candida albicans. Org. Lett. 2019, 21, 1108–1111. [Google Scholar] [CrossRef]
- Somboon, P.; Soontorngun, N. An actin depolymerizing agent 19, 20-epoxycytochalasin Q of Xylaria sp. BCC 1067 enhanced antifungal action of azole drugs through ROS-mediated cell death in yeast. Microbiol. Res. 2021, 243, 126646. [Google Scholar] [CrossRef]
- Huang, X.; Zhou, D.; Liang, Y.; Liu, X.; Cao, F.; Qin, Y.; Mo, T.; Xu, Z.; Li, J.; Yang, R. Cytochalasins from endophytic Diaporthe sp. GDG-118. Nat. Prod. Res. 2021, 35, 3396–3403. [Google Scholar] [CrossRef]
- Qin, J.; Lyu, A.; Zhang, Q.H.; Yang, L.; Zhang, J.; Wu, M.D.; Li, G.Q. Strain identification and metabolites isolation of Aspergillus capensis CanS-34A from Brassica napus. Mol. Biol. Rep. 2019, 46, 3451–3460. [Google Scholar] [CrossRef]
- Yuzawa, S.; Backman, T.W.H.; Keasling, J.D.; Katz, L. Synthetic biology of polyketide synthases. J. Ind. Microbiol. Biotechnol. 2018, 45, 621–633. [Google Scholar] [CrossRef]
- Cecilia, M.-A.A.; Liliana, S.; Oscar, Z. It only takes one to do many jobs: Amphotericin B as antifungal and immunomodulatory drug. Front. Microbiol. 2012, 3, 286. [Google Scholar] [CrossRef]
- Grover, N.D. Echinocandins: A ray of hope in antifungal drug therapy. Indian J. Pharmacol. 2010, 42, 9–11. [Google Scholar] [CrossRef]
- Bartlett, D.W.; Clough, J.M.; Godwin, J.R.; Hall, A.A.; Hamer, M.; Parr-Dobrzanski, B. The strobilurin fungicides. Pest Manag. Sci. 2002, 58, 649–662. [Google Scholar] [CrossRef]
- Rajamanikyam, M.; Vadlapudi, V.; Amanchy, R.; Upadhyayula, S.M. Endophytic Fungi as Novel Resources of natural Ther-apeutics. Braz. Arch. Biol. Technol. 2017, 60, e17160542. [Google Scholar] [CrossRef]
- Biasetto, C.R.; Somensi, A.; Sordi, R.; Chapla, V.M.; Ebrahimi, S.N.; Silva, G.H.; Teles, H.L.; Bolzani, V.D.S.; Young, M.C.M.; Pfenning, L.H.; et al. The new koninginins TU from Phomopsis stipata, an endophytic fungus isolated from Styrax camporum Pohl. Phytochem. Lett. 2020, 36, 106–110. [Google Scholar] [CrossRef]
- Yin, C.; Liu, H.; Shan, Y.; Gupta, V.K.; Jiang, Y.; Zhang, W.; Tan, H.; Gong, L. Cytosporone B, as a biological preservative: Purification, Fungicidal activity and Mechanism of Action against Geotrichum citriaurantii. Biomolecules 2019, 9, 125. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.Q.; Du, S.T.; Xiao, J.; Wang, D.C.; Han, W.B.; Zhang, Q.; Gao, J.M. Isolation and characterization of antifungal me-tabolites from the Melia azedarach-associated fungus Diaporthe eucalyptorum. J. Agric. Food Chem. 2020, 68, 2418–2425. [Google Scholar] [CrossRef] [PubMed]
- da Silva, P.H.; Souza, M.P.D.; Bianco, E.A.; da Silva, S.R.; Soares, L.N.; Costa, E.V.; Silva, F.; Barison, A.; Forim, M.R.; Cass, Q.B.; et al. Antifungal polyketides and other compounds from Amazonian endophytic Talaromyces fungi. J. Braz. Chem. Soc. 2018, 29, 622–630. [Google Scholar] [CrossRef]
- Li, H.T.; Duan, R.T.; Liu, T.; Yang, R.N.; Wang, J.P.; Liu, S.X.; Yang, Y.B.; Zhou, H.; Ding, Z.T. Penctrimertone, a bioactive citrinin dimer from the endophytic fungus Penicillium sp. T2-11. Fitoterapia 2020, 146, 104711. [Google Scholar] [CrossRef]
- Luo, H.; Qing, Z.; Deng, Y.; Deng, Z.; Tang, X.A.; Feng, B.; Lin, W. Two polyketides produced by endophytic Penicillium citrinum DBR-9 from medicinal plant Stephania kwangsiensis and their antifungal activity against plant pathogenic fungi. Nat. Prod. Commun. 2019, 14, 20219910398. [Google Scholar] [CrossRef]
- Ding, Z.; Wang, X.; Kong, F.D.; Huang, H.M.; Zhao, Y.N.; Liu, M.; Wang, Z.P.; Han, J. Overexpression of global regulator talae1 leads to the discovery of new antifungal polyketides from endophytic fungus Trichoderma afroharzianum. Front. Microbiol. 2020, 11, 622785. [Google Scholar] [CrossRef]
- Ebrahim, W.; Özkaya, F.C.; Ebada, S.S. Antifungal metabolites from endophytic fungus Fusarium verticillioides strain WF18. S. Afr. J. Bot. 2020, 133, 40–44. [Google Scholar] [CrossRef]
- Pan, F.; El-Kashef, D.H.; Kalscheuer, R.; Mueller, W.E.; Lee, J.; Feldbruegge, M.; Mandi, A.; Kurtan, T.; Liu, Z.; Wu, W.; et al. Cladosins L-O, new hybrid polyketides from the endophytic fungus Cladosporium sphaerospermum WBS017. Eur. J. Med. Chem. 2020, 191, 112159. [Google Scholar] [CrossRef]
- d’Errico, G.; Aloj, V.; Flematti, G.R.; Sivasithamparam, K.; Worth, C.M.; Lombardi, N.; Ritieni, A.; Marra, R.; Lorito, M.; Vinale, F. Metabolites of a Drechslera sp. endophyte with potential as biocontrol and bioremediation agent. Nat. Prod. Res. 2021, 35, 4508–4516. [Google Scholar] [CrossRef] [PubMed]
- Harwoko, H.; Lee, J.; Hartmann, R.; Mándi, A.; Kurtán, T.; Müller, W.E.; Feldbrügge, M.; Kalscheuer, R.; Ancheeva, E.; Daletos, G.; et al. Azacoccones F-H, new flavipin-derived alkaloids from an endophytic fungus Epicoccum nigrum MK214079. Fitoterapia 2020, 146, 104698. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.Y.; Zhong, Y.; Yu, Y.; Shi, D.F.; Huang, H.Y.; Tang, X.L.; Wang, Y.H.; Chen, G.D.; Zhang, H.P.; Liu, C.L.; et al. 4-Hydroxypyridones from Heterologous Expression and Cultivation of the Native Host. J. Nat. Prod. 2020, 83, 3338–3346. [Google Scholar] [CrossRef] [PubMed]
- Xiong, D.S.; Yang, Y.B.; Hu, B.Y.; Miao, C.P.; Wang, Y.L.; Zou, J.M.; Li, L.; Li, Y.Q.; Luo, X.D.; Zhao, L.X. Myrothins A-F from Endophytic Fungus Myrothecium sp. BS-31 harboured in Panax notoginseng. Chem. Biodivers. 2021, 18, e2000964. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.G.; Li, J.J.; Chen, S.M.; Mou, L.M.; Zou, J.; Wang, C.X.; Chen, G.D.; Qin, S.Y.; Yao, X.S.; Gao, H. Phenylisotertronic acids from the TCM endophytic fungus Phyllosticta sp. Fitoterapia 2018, 124, 86–91. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, L.; Kalscheuer, R.; Liu, Z.; Proksch, P. Antifungal polyketide derivatives from the endophytic fungus Aplo-sporella javeedii. Bioorg. Med. Chem. 2020, 28, 115456. [Google Scholar] [CrossRef]
- Zhao, T.; Xu, L.L.; Zhang, Y.; Lin, Z.H.; Xia, T.; Yang, D.F.; Chen, Y.M.; Yang, X.L. Three new α-pyrone derivatives from the plant endophytic fungus Penicillium ochrochloronthe and their antibacterial, antifungal, and cytotoxic activities. J. Asian Nat. Prod. Res. 2019, 21, 851–858. [Google Scholar] [CrossRef]
- Li, G.; Xu, K.; Chen, W.-Q.; Guo, Z.-H.; Liu, Y.-T.; Qiao, Y.-N.; Sun, Y.; Sun, G.; Peng, X.-P.; Lou, H.-X. Heptaketides from the endophytic fungus Pleosporales sp. F46 and their antifungal and cytotoxic activities. RSC Adv. 2019, 9, 12913–12920. [Google Scholar] [CrossRef]
- Lai, D.; Mao, Z.; Zhou, Z.; Zhao, S.; Xue, M.; Dai, J.; Zhou, L.; Li, D. New chlamydosporol derivatives from the endophytic fungus Pleosporales sp. Sigrf05 and their cytotoxic and antimicrobial activities. Sci. Rep. 2020, 10, 8193. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, L.; Zhu, G.; Zuo, M.; Gong, Q.; He, W.; Li, M.; Yuan, C.; Hao, X.; Zhu, W. New phenylpyridone derivatives from the Penicillium sumatrense GZWMJZ-313, a fungal endophyte of Garcinia multiflora. Chin. Chem. Lett. 2019, 30, 431–434. [Google Scholar] [CrossRef]
- Nguyen, H.T.; Choi, S.; Kim, S.; Lee, J.H.; Park, A.R.; Yu, N.H.; Yoon, H.; Bae, C.H.; Yeo, J.H.; Choi, G.J.; et al. The Hsp90 inhibitor, monorden, is a promising lead compound for the development of novel fungicides. Front. Plant Sci. 2020, 11, 371. [Google Scholar] [CrossRef]
- Zhang, H.; An, Z.; Zhou, F. Secondary Metabolites from Rhizopus sp., an Endophytic Fungus in Astragalus membranaceus and Preliminary Evaluation of Inhibition of Plant Pathogen Activity. Chem. Nat. Compd. 2020, 56, 366–369. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, Y.; Luo, H.; Xue, Z. Azaphilones: Diverse Structures and Spectrum of Biological Activities. Nat. Prod. Rep. 2019, 36, 1324–1336. [Google Scholar]
- Arunpanichlert, J.; Rukachaisirikul, V.; Sukpondma, Y.; Phongpaichit, S.; Tewtrakul, S.; Rungjindamai, N.; Sakayaroj, J. Azaphilone and Isocoumarin Derivatives from the Endophytic Fungus Penicillium sclerotiorum PSU-A13. Chem. Chem. Pharm. Bull. 2010, 58, 1033–1036. [Google Scholar] [CrossRef]
- Xu, J.; Shao, M.; Wang, Q.; Wang, L. The Antifungal Activity of Azaphilone Pigments from the Marine-Derived Fungus Peni-cillium sclerotiorum. Mar. Drugs 2015, 13, 1024–1034. [Google Scholar] [CrossRef]
- Sun, W.; Li, X. The Chemistry of Azaphilones: Michael Addition Reactions and Biological Implications. Org. Biomol. Chem. 2021, 19, 12–20. [Google Scholar] [CrossRef]
- Cain, J.W.; Miller, K.I.; Kalaitzis, J.A.; Chau, R.; Neilan, B.A. Genome mining of a fungal endophyte of Taxus yunnanensis (Chinese yew) leads to the discovery of a novel azaphilone polyketide, lijiquinone. Microb. Biotechnol. 2020, 13, 1415–1427. [Google Scholar] [CrossRef]
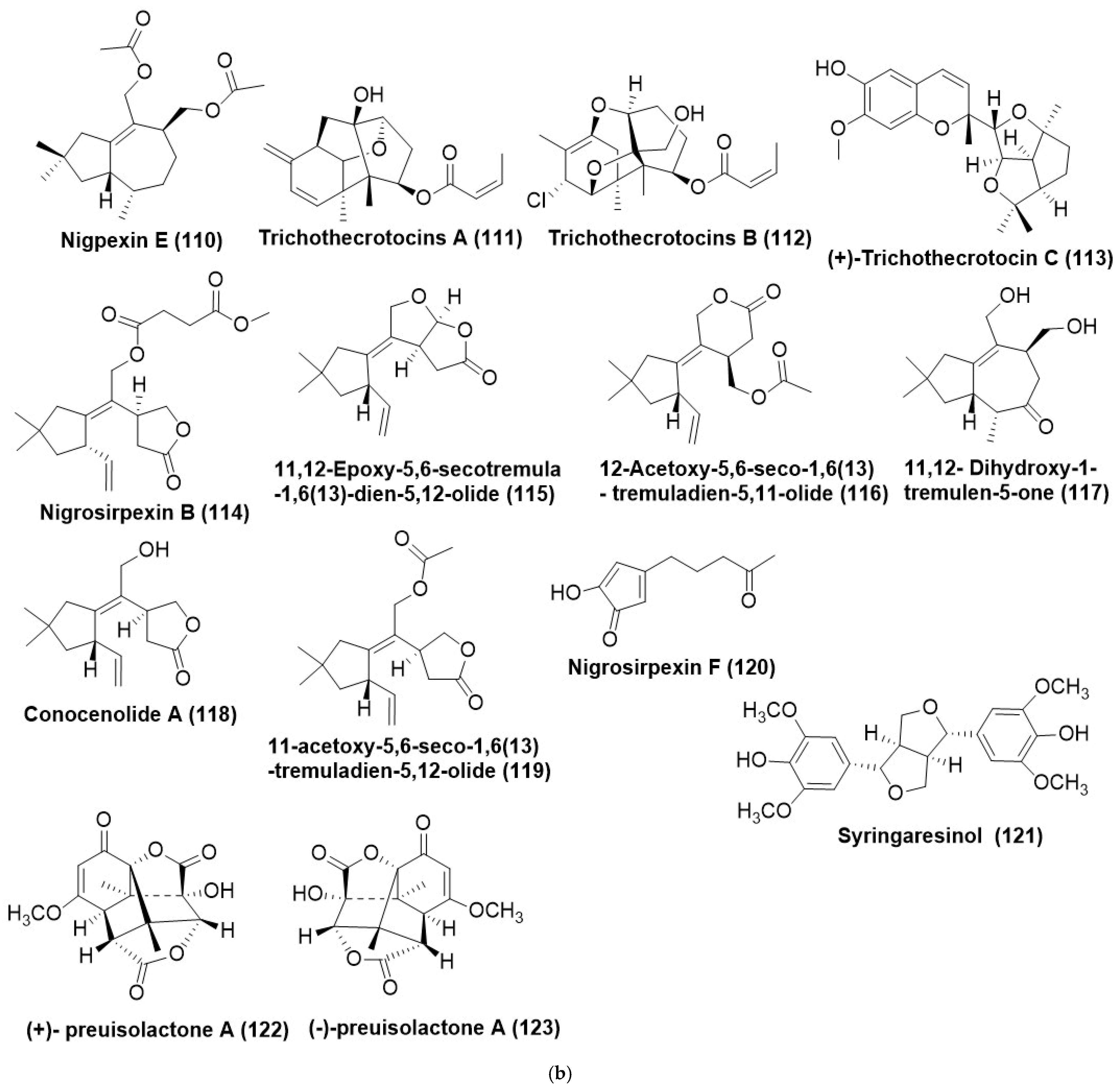
- Zhou, Q.Y.; Yang, X.Q.; Zhang, Z.X.; Wang, B.Y.; Hu, M.; Yang, Y.B.; Zhou, H.; Ding, Z.T. New azaphilones and tremulane sesquiterpene from endophytic Nigrospora oryzae cocultured with Irpex lacteus. Fitoterapia 2018, 130, 26–30. [Google Scholar] [CrossRef]
- Zhang, Z.X.; Yang, X.Q.; Zhou, Q.Y.; Wang, B.Y.; Hu, M.; Yang, Y.B.; Zhou, H.; Ding, Z.T. New azaphilones from Nigrospora oryzae co-cultured with Beauveria bassiana. Molecules 2018, 23, 1816. [Google Scholar] [CrossRef]
- Feng, Z.-Y.; Huang, P.-Z.; Jiang, S.-J.; Shen, S.-J.; Chen, J.-J. Three new highly oxygenated azaphilones from an endophytic fungus Penicillium sp. LZUC-S1. Tetrahedron Lett. 2022, 113, 154260. [Google Scholar] [CrossRef]
- Chen, H.L.; Zhao, W.T.; Liu, Q.P.; Chen, H.Y.; Zhao, W.; Yang, D.F.; Yang, X.L. (±)-Preisomide: A new alkaloid featuring a rare naturally occurring tetrahydro-2H-1,2-oxazin skeleton from an endophytic fungus Preussia isomera by using OSMAC strategy. Fitoterapia 2020, 141, 104475. [Google Scholar] [CrossRef] [PubMed]
- Pinto, M.M.M.; Sousa, M.E.; Nascimento, M.S.J. Xanthone Derivatives: New Insights in Biological Activities. Curr. Med. Chem. 2005, 12, 2517–2538. [Google Scholar] [CrossRef] [PubMed]
- Karunanidhi, A.; Thomas, R.; Chithra, S. Antifungal potential of xanthones: A review. J. Pharmacogn. Phytochem. 2019, 8, 507–514. [Google Scholar]
- Cao, H.-Y.; Yi, C.; Sun, S.-F.; Li, Y.; Liu, Y.-B. Anti-inflammatory Dimeric Tetrahydroxanthones from an Endophytic Muy-ocopron laterale. J. Nat. Prod. 2022, 85, 148–161. [Google Scholar] [CrossRef]
- Asomadu, R.O.; Ezeorba, T.P.C.; Ezike, T.C.; Uzoechina, J.O. Exploring the antioxidant potential of endophytic fungi: A review on methods for extraction and quantification of total antioxidant capacity (TAC). 3 Biotech 2024, 14, 127. [Google Scholar] [CrossRef]
- Henry, T.A.; Zincke, T. Early isolation and identification of gentisin from Gentiana lutea. J. Chem. Res. 1821, 10, 23–30. [Google Scholar]
- Kamal, A.; Zambonelli, A. Isolation and structural elucidation of tajixanthone from Aspergillus stellatus. J. Nat. Prod. 1979, 33, 123–128. [Google Scholar]
- Lamb, D.C.; Kelly, D.E.; White, T.C.; Kelly, S.L. The antifungal azole drugs are inhibitors of the P450 enzyme sterol 14α-demethylase (CYP51) from Candida albicans. J. Chemother. 1998, 10, 435–441. [Google Scholar]
- Fotie, J.; Bohle, D.S. Pharmacological and Biological Activities of Xanthones. Anti-Infect. Agents Med. Chem. 2006, 5, 15–31. [Google Scholar] [CrossRef]
- Nes, W.D. Biosynthesis of cholesterol and other sterols. Chem. Rev. 2011, 111, 6423–6451. [Google Scholar] [CrossRef]
- Lupetti, A.; Danesi, R.; Campa, M.; Tacca, M.D.; Kelly, S. Molecular basis of resistance to azole antifungals. Trends Mol. Med. 2002, 8, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Nishimoto, A.T.; Sharma, C.; Rogers, P.D. Molecular and genetic basis of azole antifungal resistance in the opportunistic pathogenic fungus Candida albicans. J. Antimicrob. Chemother. 2020, 75, 257–270. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Sun, F.; Li, G.; Wang, C.; Zhang, Y.; Wu, C.; Zhang, C.; Sun, Y.; Wu, S.; Zhang, Y.; et al. New xanthones with anti-agricultural fungal pathogen activities from the endophytic fungus Diaporthe goulteri L17. J. Agric. Food Chem. 2021, 69, 11216–11224. [Google Scholar] [CrossRef] [PubMed]
- Miao, C.; Wang, J.; Huang, R.; Liu, S.; Zheng, K.; Liu, C.; Lu, Y.; Wu, S. Antifungal xanthones produced by the endophytic fungus Paraconionthyrium sp. YM 311593. Folia Microbiol. 2020, 65, 567–572. [Google Scholar] [CrossRef] [PubMed]
- Paduch, R.; Kandefer-Szerszeń, M.; Trytek, M.; Fiedurek, J. Terpenes: Substances useful in human healthcare. Arch. Immunol. Ther. Exp. 2007, 55, 315–327. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Khan, A.; Yousuf, S.; Khan, L.A.; Manzoor, N. Probing the Potential of Eugenol as an Antifungal Agent: Inhibition of Ergosterol Biosynthesis and Membrane Integrity in Candida albicans. Med. Mycol. 2010, 48, 21–26. [Google Scholar] [CrossRef]
- Kim, K.J.; Park, H.J. Anti-Candida albicans activity of limonene, thymol, and carvacrol. Mycobiology 2012, 40, 310–315. [Google Scholar] [CrossRef]
- Chen, H.Y.; Liu, T.K.; Shi, Q.; Yang, X.L. Sesquiterpenoids and diterpenes with antimicrobial activity from Leptosphaeria sp. XL026, an endophytic fungus in Panax notoginseng. Fitoterapia 2019, 137, 104243. [Google Scholar] [CrossRef]
- Shi, X.S.; Meng, L.H.; Li, X.M.; Li, X.; Wang, D.J.; Li, H.L.; Zhou, X.W.; Wang, B.G. Trichocadinins B-G: Anti-microbial Cadinane sesquiterpenes from Trichoderma virens QA-8, an endophytic fungus obtained from the medicinal plant Artemisia argyi. J. Nat. Prod. 2019, 82, 2470–2476. [Google Scholar] [CrossRef]
- Chapla, V.M.; Honório, A.E.; Gubiani, J.R.; Vilela, A.F.; Young, M.C.; Cardoso, C.L.; Pavan, F.R.; Cicarelli, R.M.; Ferreira, P.M.P.; Bolzani, V.D.S.; et al. Acetylcholinesterase inhibition and antifungal activity of cyclohexanoids from the endophytic fungus Saccharicola sp. Phytochem. Lett. 2020, 39, 116–123. [Google Scholar] [CrossRef]
- Huang, R.; Jiang, B.G.; Li, X.N.; Wang, Y.T.; Liu, S.S.; Zheng, K.X.; He, J.; Wu, S.H. Polyoxygenated cyclohexenoids with promising α-glycosidase inhibitory activity produced by Phomopsis sp. YE3250, an endophytic fungus derived from Paeonia delavayi. J. Agric. Food Chem. 2018, 66, 1140–1146. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Tao, Y.; Tao, X.; Su, Q.; Cai, J.; Qin, C.; Ding, W.; Li, C. Sesquiterpenes with phytopathogenic fungi inhibitory activities from fungus Trichoderma virens from Litchi chinensis Sonn. J. Agric. Food Chem. 2019, 67, 10646–10652. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.; Zhao, S.; Gu, C.; Tian, K.; Wang, Z.; Liu, F.; Ye, Y. Antifungal meroterpenes and dioxolanone derivatives from plant-associated endophytic fungus Phyllosticta sp. WGHL2. Fitoterapia 2021, 148, 104778. [Google Scholar] [CrossRef] [PubMed]
- Ai, H.L.; Shi, B.B.; Li, W.; He, J.; Li, Z.H.; Feng, T.; Liu, J.K. Bipolarithizole A, an antifungal phenylthiazole-sativene mero-sesquiterpenoid from the potato endophytic fungus Bipolaris eleusines. Org. Chem. Front. 2022, 9, 1814–1819. [Google Scholar] [CrossRef]
- Wu, Y.M.; Zhou, Q.Y.; Yang, X.Q.; Luo, Y.J.; Qian, J.J.; Liu, S.X.; Yang, Y.B.; Ding, Z.T. Induction of anti-phytopathogenic metabolite and squalene production and phytotoxin elimination by adjustment of the mode of fermentation in cocultures of phytopathogenic Nigrospora oryzae and Irpex lacteus. J. Agric. Food Chem. 2019, 67, 11877–11882. [Google Scholar] [CrossRef]
- Wang, D.L.; Yang, X.Q.; Shi, W.Z.; Cen, R.H.; Yang, Y.B.; Ding, Z.T. The selective anti-fungal metabolites from Irpex lacteus and applications in the chemical interaction of Gastrodia elata, Armillaria sp., and endophytes. Fitoterapia 2021, 155, 105035. [Google Scholar] [CrossRef]
- Yang, H.X.; Ai, H.L.; Feng, T.; Wang, W.X.; Wu, B.; Zheng, Y.S.; Sun, H.; He, J.; Li, Z.H.; Liu, J.K. Trichothecrotocins A–C, anti-phytopathogenic agents from potato endophytic fungus Trichothecium crotocinigenum. Org. Lett. 2018, 20, 8069–8072. [Google Scholar] [CrossRef]
- Shi, L.J.; Wu, Y.M.; Yang, X.Q.; Xu, T.T.; Yang, S.; Wang, X.Y.; Yang, Y.B.; Ding, Z.T. The co-cultured Nigrospora oryzae and Colletotrichum gloeosporioides, Irpex lacteus, and the plant host Dendrobium officinale bidirectionally regulate the production of phytotoxins by anti-phytopathogenic metabolites. J. Nat. Prod. 2020, 83, 1374–1382. [Google Scholar] [CrossRef]
- Weber, H.A.; Gloer, J.B. The preussomerins: Novel antifungal metabolites from the coprophilous fungus Preussia isomera Cain. J. Org. Chem. 1991, 56, 4355–4360. [Google Scholar] [CrossRef]
- Siless, G.E.; Gallardo, G.L.; Rodriguez, M.A.; Rincón, Y.A.; Godeas, A.M.; Cabrera, G.M. Metabolites from the dark septate endophyte Drechslera sp. evaluation by LC/MS and principal component analysis of culture extracts with histone deacetylase inhibitors. Chem. Biodivers. 2018, 15, e1800133. [Google Scholar] [CrossRef]
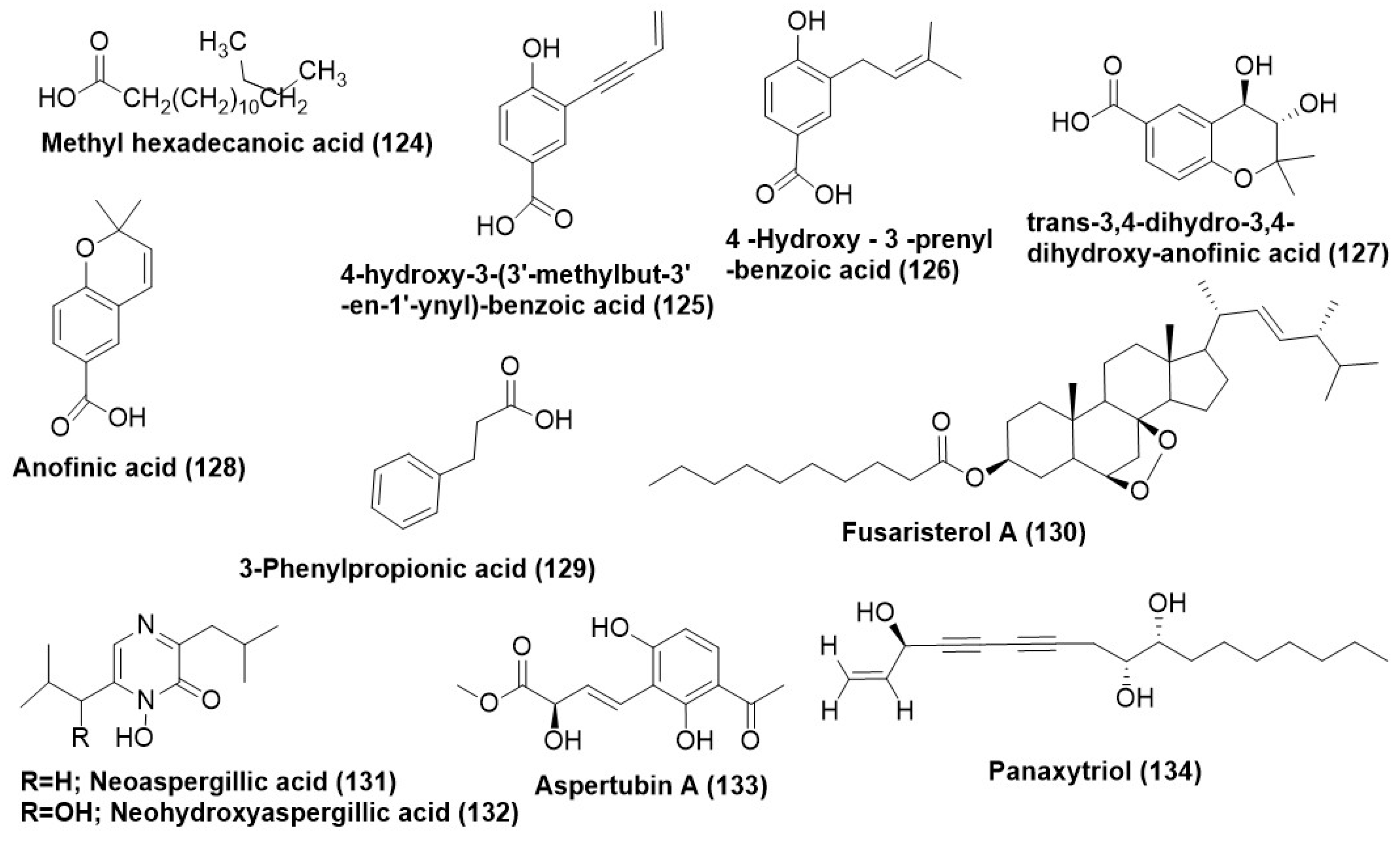
- Ibrahim, S.R.M.; Mohamed, G.A.; Al Haidari, R.A.; El-Kholy, A.A.E.S.; Asfour, H.Z.; Zayed, M.F. Fusaristerol A: A new cytotoxic and antifungal ergosterol fatty acid ester from the endophytic fungus Fusarium sp. associated with Mentha longifolia roots. Pharmacogn. Mag. 2018, 14, 308–313. [Google Scholar] [CrossRef]
- Wu, Y.M.; Yang, X.Q.; Zhao, T.D.; Shi, W.Z.; Sun, L.J.; Cen, R.H.; Yang, Y.B.; Ding, Z.T. Antifeedant and antifungal activities of metabolites isolated from the co-culture of endophytic fungus Aspergillus tubingensis S1120 with red ginseng. Chem. Biodivers. 2021, 19, e2100608. [Google Scholar] [CrossRef]
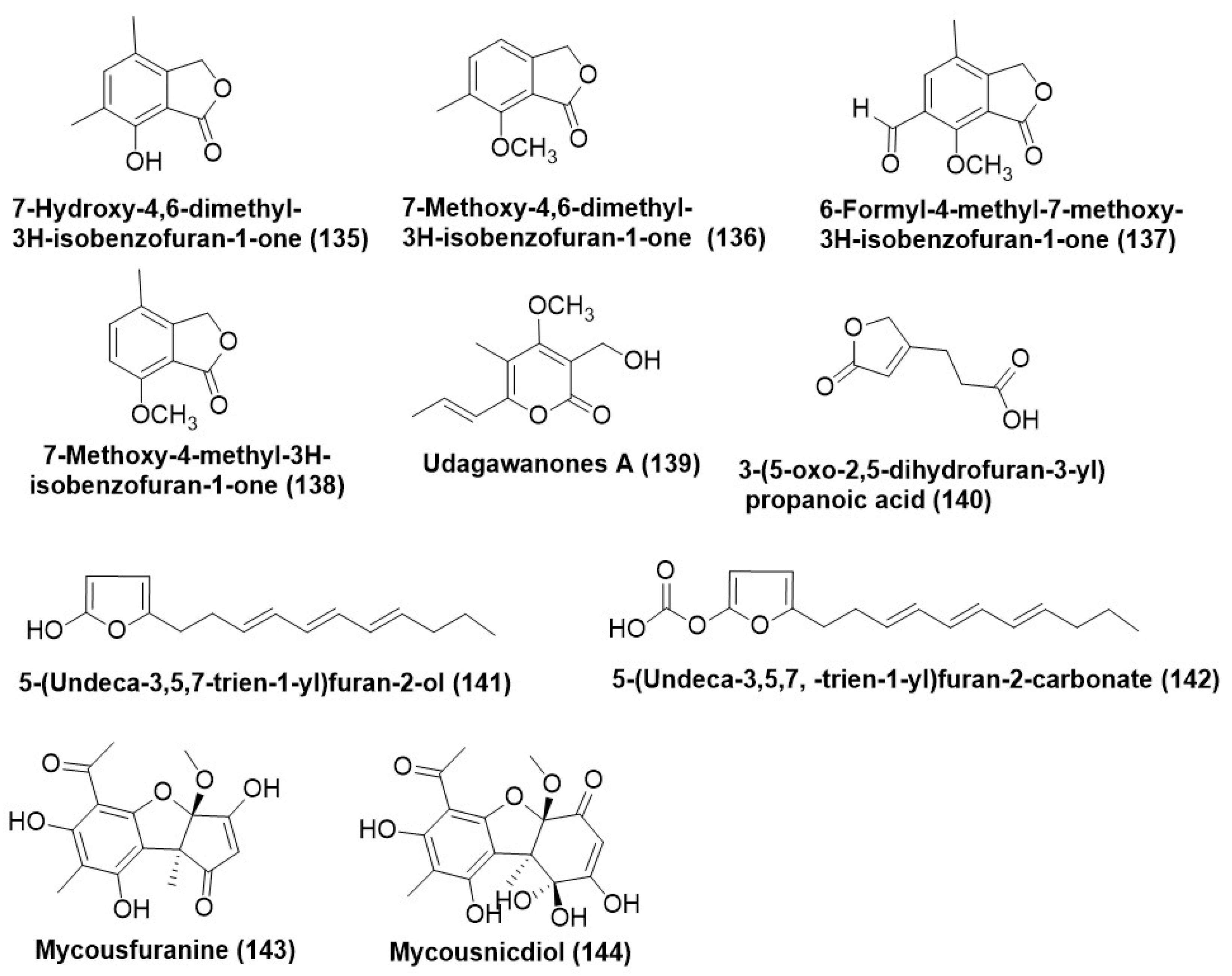
- Sánchez-Fernández, R.E.; Sánchez-Fuentes, R.; Rangel-Sánchez, H.; Hernández-Ortega, S.; López-Cortés, J.G.; Mací-as-Rubalcava, M.L. Antifungal and antioomycete activities and modes of action of isobenzofuranones isolated from the en-dophytic fungus Hypoxylon anthochroum strain Gseg1. Pestic. Biochem. Physiol. 2020, 169, 104670. [Google Scholar] [CrossRef] [PubMed]
- Macabeo, A.P.G.; Cruz, A.J.C.; Narmani, A.; Arzanlou, M.; Babai-Ahari, A.; Pilapil, L.A.E.; Garcia, K.Y.M.; Huch, V.; Stadler, M. Tetrasubstituted α-pyrone derivatives from the endophytic fungus, Neurospora udagawae. Phytochem. Lett. 2020, 35, 147–151. [Google Scholar] [CrossRef]
- Yang, X.F.; Wang, N.N.; Kang, Y.F.; Ma, Y.M. A new furan derivative from an endophytic Aspergillus tubingensis of Decaisnea insignis (Griff.) Hook.f. & Thomson. Nat. Prod. Res. 2019, 33, 2777–2783. [Google Scholar] [CrossRef]
- Wu, X.; Pang, X.J.; Xu, L.L.; Zhao, T.; Long, X.Y.; Zhang, Q.Y.; Qin, H.L.; Yang, D.F.; Yang, X.L. Two new alkylated furan derivatives with antifungal and antibacterial activities from the plant endophytic fungus Emericella sp. Nat. Prod. Res. 2018, 32, 2625–2631. [Google Scholar] [CrossRef]
- de Oliveira, D.M.; Pereira, C.B.; Mendes, G.; Junker, J.; Kolloff, M.; Rosa, L.H.; Rosa, C.A.; Alves, T.M.; Zani, C.L.; Johann, S.; et al. Two new usnic acid derivatives from the endophytic fungus Mycosphaerella sp. Z. Naturforsch. C 2018, 73, 449–455. [Google Scholar] [CrossRef]
- De Amorim, M.R.; Hilário, F.; dos Santos Junior, F.M.; Junior, J.M.B.; Bauab, T.M.; Araújo, A.R.; Carlos, I.Z.; Vilegas, W.; Dos Santos, L.C. New benzaldehyde and benzopyran compounds from the endophytic fungus Paraphaeosphaeria sp. F03 and their antimicrobial and cytotoxic activities. Planta Med. 2019, 85, 957–964. [Google Scholar] [CrossRef]
- Liu, P.; Zhang, D.; Shi, R.; Yang, Z.; Zhao, F.; Tian, Y. Antimicrobial potential of endophytic fungi from Astragalus chinensis. 3 Biotech 2019, 9, 405. [Google Scholar] [CrossRef]
- Khan, B.; Zhao, S.; Wang, Z.; Ye, Y.; Ahmed, R.N.; Yan, W. Eremophilane sesquiterpenes and benzene derivatives from the endophyte Microdiplodia sp. WGHS5. Chem. Biodivers. 2021, 18, e2000949. [Google Scholar] [CrossRef]
- Ma, Y.M.; Qiao, K.; Kong, Y.; Li, M.Y.; Guo, L.X.; Miao, Z.; Fan, C. A new isoquinoline alkaloid from an endophytic fungus R22 of Nerium indicum. Nat. Prod. Res. 2018, 32, 2375–2381. [Google Scholar] [CrossRef]
- Zhang, P.; Li, X.; Yuan, X.L.; Du, Y.M.; Wang, B.G.; Zhang, Z.F. Antifungal prenylated diphenyl ethers from Arthrinium arundinis, an endophytic fungus isolated from the leaves of tobacco (Nicotiana tabacum L.). Molecules 2018, 23, 3179. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Dan, W.-J.; Li, Y.-X.; Peng, G.-R.; Zheng, A.-L.; Gao, J.M. Antifungal metabolites from Alternaria atrans: An endophytic fungus is Psidium guajava. Nat. Prod. Commun. 2019, 14. [Google Scholar] [CrossRef]
- Zhu, J.; Li, Z.; Lu, H.; Liu, S.; Ding, W.; Li, J.; Xiong, Y.; Li, C. New diphenyl ethers from a fungus Epicoccum sorghinum L28 and their antifungal activity against phytopathogens. Bioorg. Chem. 2021, 115, 105232. [Google Scholar] [CrossRef]
- Ibrahim, S.R.; Mohamed, G.A.; Al Haidari, R.A.; Zayed, M.F.; El-Kholy, A.A.; Elkhayat, E.S.; Ross, S.A. Fusarithioamide B, a new benzamide derivative from the endophytic fungus Fusarium chlamydosporium with potent cytotoxic and antimicrobial activities. Bioorg. Med. Chem. 2018, 26, 786–790. [Google Scholar] [CrossRef]
- Tanney, J.B.; Renaud, J.B.; Miller, J.D.; McMullin, D.R. New 1, 3-benzodioxin-4-ones from Synnemapestaloides ericacearum sp. nov., a biosynthetic link to remarkable compounds within the Xylariales. PLoS ONE 2018, 13, e0198321. [Google Scholar] [CrossRef] [PubMed]
- Tanapichatsakul, C.; Pansanit, A.; Monggoot, S.; Brooks, S.; Prachya, S.; Kittakoop, P.; Panuwet, P.; Pripdeevech, P. Antifungal activity of 8-methoxynaphthalen-1-ol isolated from the endophytic fungus Diatrype palmicola MFLUCC 17-0313 against the plant pathogenic fungus Athelia rolfsii on tomatoes. Peer J. 2020, 8, e9103. [Google Scholar] [CrossRef] [PubMed]
- Harwoko, H.; Daletos, G.; Stuhldreier, F.; Lee, J.; Wesselborg, S.; Feldbrügge, M.; Müller, W.E.; Kalscheuer, R.; Ancheeva, E.; Proksch, P. Dithiodiketopiperazine derivatives from endophytic fungi Trichoderma harzianum and Epicoccum nigrum. Nat. Prod. Res. 2021, 35, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Guo, D.L.; Liu, G.H.; Fu, X.; Gu, Y.C.; Ding, L.S.; Zhou, Y. Antifungal halogenated cyclopentenones from the en-dophytic fungus Saccharicola bicolor of Bergenia purpurascens by the one strain-many compounds strategy. J. Agric. Food Chem. 2019, 68, 185–192. [Google Scholar] [CrossRef]
- Barthélemy, M.; Elie, N.; Genta-Jouve, G.; Stien, D.; Touboul, D.; Eparvier, V. Identification of antagonistic compounds between the palm tree Xylariale endophytic fungi and the phytopathogen Fusarium oxysporum. J. Agric. Food Chem. 2021, 69, 10893–10906. [Google Scholar] [CrossRef]
- Abdou, R.; Shabana, S.; Rateb, M.E.; Terezin, E. Bioactive prenylated tryptophan analogue from an endophyte of Centaurea stoebe. Nat. Prod. Res. 2020, 34, 503–510. [Google Scholar] [CrossRef] [PubMed]
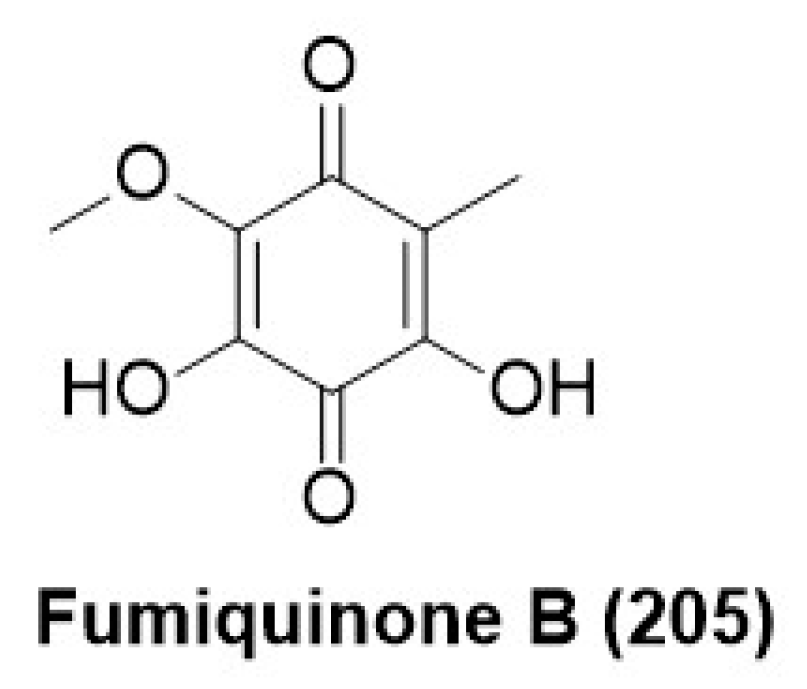
- Grigoletto, D.F.; Correia, A.M.L.; Abraham, W.R.; Rodrigues, A.; Assis, M.A.; Ferreira, A.G.; Massaroli, M.; de Lira, S.P. Sec-ondary metabolites produced by endophytic fungi: Novel antifungal activity of fumiquinone B. Acta Sci. Biol. Sci. 2019, 41, 48785. [Google Scholar] [CrossRef]
- Chowdhary, K.; Kaushik, N. UPLC–MS and dereplication-based identification of metabolites in antifungal extracts of fungal endophytes. Proc. Natl. Acad. Sci. India B—Biol. Sci. 2019, 89, 1379–1387. [Google Scholar] [CrossRef]
- Morath, S.U.; Hung, R.; Bennett, J.W. Fungal volatile organic compounds: A review with emphasis on their biotechnological potential. Fungal Biol. Rev. 2012, 26, 73–83. [Google Scholar] [CrossRef]
- Kaddes, A.; Fauconnier, M.; Sassı, K.; Nasraoui, B.; Jijakli, M.H. Endophytic fungal volatile compounds as solution for sus-tainable agriculture. Molecules 2019, 24, 1065. [Google Scholar] [CrossRef]
- Farh, M.E.; Jeon, J. Roles of Fungal Volatiles from Perspective of Distinct Lifestyles in Filamentous Fungi. Plant Pathol. J. 2020, 36, 193–203. [Google Scholar] [CrossRef]
- Yuan, Z.-L.; Chen, Y.-C.; Xu, B.-G.; Zhang, C.-L. Current perspectives on the volatile-producing fungal endophytes. Crit. Rev. Biotechnol. 2012, 32, 363–373. [Google Scholar] [CrossRef]
- Tilocca, B.; Cao, A.; Migheli, Q. Scent of a Killer: Microbial volatilome and its role in the biological control of plant pathogens. Front. Microbiol. 2020, 11, 41. [Google Scholar] [CrossRef]
- Zhao, X.; Zhou, J.; Tian, R.; Liu, Y. Microbial volatile organic compounds: Antifungal mechanisms, applications and challenges. Front. Microbiol. 2022, 13, 922450. [Google Scholar] [CrossRef]
- Yan, D.H.; Song, X.; Li, H.; Luo, T.; Dou, G.; Strobel, G. Antifungal activities of volatile secondary metabolites of four Diaporthe strains isolated from Catharanthus roseus. J. Fungi 2018, 4, 65. [Google Scholar] [CrossRef]
- Yeh, C.C.; Wang, C.J.; Chen, Y.J.; Tsai, S.H.; Chung, W.H. Potential of a volatile producing endophytic fungus Nodulisporium species, PDL-005 for the control of Penicillium digitatum. Biol. Control 2021, 152, 104459. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, D.; Liu, J.; Pan, H.; Qin, J.; Zhang, Y. Antifungal effects of volatile organic compounds from the endophytic fungus Cryptosporiopsis ericae Cc-HG-7 isolated from Coptis chinensis Franch. Biocontrol Sci. Technol. 2018, 28, 496–508. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, Y.; Cai, J.; Liu, X.; Huang, G. Antifungal activity of volatile compounds generated by endophytic fungi Saro-cladium brachiariae HND5 against Fusarium oxysporum f. sp. cubense. PLoS ONE 2021, 16, e0260747. [Google Scholar] [CrossRef] [PubMed]
- Khruengsai, S.; Pripdeevech, P.; Tanapichatsakul, C.; Srisuwannapa, C.; D’Souza, P.E.; Panuwet, P. Antifungal properties of volatile organic compounds produced by Daldinia eschscholtzii MFLUCC 19-0493 isolated from Barleria prionitis leaves against Colletotrichum acutatum and its post-harvest infections on strawberry fruits. Peer J. 2021, 9, e11242. [Google Scholar] [CrossRef] [PubMed]
- Saxena, S.; Strobel, G.A. Marvellous Muscodor spp.: Update on their biology and applications. Microb. Ecol. 2021, 82, 5–20. [Google Scholar] [CrossRef] [PubMed]
- Pena, L.C.; Jungklaus, G.H.; Savi, D.C.; Ferriera-Maba, L.; Servienski, A.; Maia, B.H.; Annies, V.; Galli-Terasawa, L.V.; Gleinke, C.; Kava, V. Muscodor brasiliensis sp. nov. produces volatile organic compounds with activity against Penicillium digitatum. Microbiol. Res. 2019, 221, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Khruengsai, S.; Pripdeevech, P.; D’Souza, P.E.; Panuwet, P. Biofumigation activities of volatile compounds from two Tricho-derma afroharzianum strains against Fusarium infections in fresh chilies. J. Sci. Food. Agric. 2021, 101, 5861–5871. [Google Scholar] [CrossRef] [PubMed]
- Sdiri, Y.; Lopes, T.; Rodrigues, N.; Silva, K.; Rodrigues, I.; Pereira, J.A.; Baptista, P. Biocontrol ability and production of volatile organic compounds as potential mechanisms of action of Olive endophytes against Colletotrichum acutatum. Microorganisms 2022, 10, 571. [Google Scholar] [CrossRef]
- Deshmukh, S.K.; Dufossé, L.; Chhipa, H.; Saxena, S.; Mahajan, G.B.; Gupta, M.K. Fungal endophytes: A potential source of antibacterial compounds. J. Fungi 2022, 8, 164. [Google Scholar] [CrossRef]
- Guo, B.; Wang, Y.; Sun, X.; Tang, K. Bioactive natural products from endophytes: A review. Appl. Biochem. Microbiol. 2008, 44, 136–142. [Google Scholar] [CrossRef]
- Mozsik, L.; Lacovelli, R.; Bovenberg, R.A.L.; Driessen, A.J.M. Transcriptional Activation of Biosynthetic Gene Clusters in Filamentous Fungi. Front. Bioeng. Biotechnol. 2022, 10, 901037. [Google Scholar] [CrossRef] [PubMed]
- Guzmán-Chávez, F.; Zwahlen, R.D.; Bovenberg, R.; Driessen, A. Engineering of the filamentous fungus Penicillium chrysogenum as cell factory for natural products. Front. Microb. 2018, 9, 2768. [Google Scholar] [CrossRef] [PubMed]
- Aghcheh, R.K.; Kubicek, C.P. Epigenetics as an emerging tool for improvement of fungal strains used in biotechnology. Appl. Microbiol. Biotechnol. 2015, 99, 6167–6181. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.Z.; Liang, B.W.; Li, X.F.; Liu, Q. Induced production of new diterpenoids in the fungus Penicillium funiculosum. Nat. Prod. Commun. 2014, 9, 607–608. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Zhuang, Z.; Zhang, F.; Song, F.; Zhong, H.; Ran, F.; Yu, S.; Xu, G.; Lan, F.; Wang, S. Inhibition of aflatoxin metabolism and growth of Aspergillus flavus in liquid culture by a DNA methylation inhibitor. Food Addit. Contam. Part A 2014, 31, 554–563. [Google Scholar]
- Yang, X.; Huang, L.; Ruan, X.L. Epigenetic modifiers alter the secondary metabolite composition of a plant endophytic fungus, Pestalotiopsis crassiuscula obtained from the leaves of Fragaria chiloensis. J. Asian Nat. Prod. Res. 2014, 16, 412–417. [Google Scholar] [CrossRef]
- Honda, S.; Lewis, Z.A.; Shimada, K.; Fischle, W.; Sack, R.; Selker, E.U. Heterochromatin protein 1 forms dis-tinct complexes to direct histone deacetylation and DNA methylation. Nat. Struct. Mol. Biol. 2012, 19, 471–477. [Google Scholar] [CrossRef]
- Hur, J.Y.; Eunju, J.; Kim, Y.C.; Lee, C.R. Strategies of natural products discovery by unlocking cryptic biosynthetic gene clusters in fungi. Separations 2023, 10, 333. [Google Scholar] [CrossRef]
- El-Hawary, S.S.; Sayed, A.M.; Mohammed, R.; Hassan, H.M.; Zaki, M.A.; Rateb, M.E.; Mohammed, T.A.; Amin, E.; Abdel-mohsen, U.R. Epigenetic modifiers induce bioactive phenolic metabolites in the marine-derived fungus Penicillium brevicom-pactum. Mar. Drugs 2018, 16, 253. [Google Scholar] [CrossRef]
- Pillay, L.C.; Nekati, L.; Makhwitine, P.J.; Ndlovu, S.I. Epigenetic activation of silent biosynthetic gene clusters in endophytic fungi using small molecular modifiers. Front. Microbiol. 2022, 13, 815008. [Google Scholar] [CrossRef]
- Xue, M.; Hou, X.; Fu, J.; Zhang, J.; Wang, J.; Zhao, Z.; Xu, D.; Lai, D.; Zhou, L. Recent advances in search of bioactive secondary metabolites from fungi triggered by chemical epigenetic modifiers. J. Fungi 2023, 9, 172. [Google Scholar] [CrossRef] [PubMed]
- Goers, L.; Freemont, P.; Polizzi, K.M. Co-culture systems and technologies: Taking synthetic biology to the next level. J. R. Soc. Interface 2014, 11, 20140065. [Google Scholar] [CrossRef]
- Alanzi, A.; Elhawary, E.A.; Ashour, M.L.; Moussa, A.Y. Aspergillus co-cultures: A recent insight into their secondary metab-olites and microbial interactions. Arch. Pharm. Res. 2023, 46, 273–298. [Google Scholar] [CrossRef] [PubMed]
- König, C.C.; Scherlach, K.; Schroeckh, V.; Horn, F.; Nietzsche, S.; Brakhage, A.A. Bacterium induces cryptic meroterpenoid pathway in the pathogenic fungus Aspergillus fumigatus. Chembiochem 2013, 14, 938–942. [Google Scholar] [CrossRef] [PubMed]
- Nicault, M.; Zaiter, A.; Dumarcay, S.; Chiambault, P.; Gelhaye, E.; Leblond, P.; Bontemps, C. Elicitation of antimicrobial active compounds by Streptomyces-fungus co-cultures. Microorganisms 2021, 9, 178. [Google Scholar] [CrossRef] [PubMed]
- Pan, R.; Bai, X.; Chen, J.; Zhang, H.; Wang, H. Exploring structural diversity of microbe secondary metabolites using OSMAC strategy: A literature review. Front. Microbiol. 2019, 10, 294. [Google Scholar] [CrossRef]
- Brzonkalik, K.; Hümmer, D.; Syldatk, C.; Neumann, A. Influence of pH and carbon to nitrogen ratio on mycotoxin production by Alternaria alternata in submerged cultivation. AMB Express 2012, 2, 28. [Google Scholar] [CrossRef]
- Gakuubi, M.M.; Munusamy, M.; Liang, Z.X.; Ng, S.B. Fungal Endophytes: A Promising Frontier for Discovery of Novel Bio-active Compounds. J. Fungi 2021, 7, 786. [Google Scholar] [CrossRef]
- Dinarvand, M.; Rezaee, M.; Masomian, M.; Jazayeri, S.D.; Zareian, M.; Abbasi, S.; Ariff, A.B. Effect of C/N ratio and media optimization through response surface methodology on simultaneous productions of intra- and extracellular inulinase and invertase from Aspergillus niger ATCC 20611. Biomed Res. Int. 2013, 2013, 508968. [Google Scholar] [CrossRef]
- Wu, G.; Sun, X.; Yu, G.; Wang, W.; Zhu, T.; Gu, Q.; Li, D. Cladosins A-E, hybrid polyketides from a deep-sea-derived fungus, Cladosporium sphaerospermum. J. Nat. Prod. 2014, 77, 270–275. [Google Scholar] [CrossRef]
- Liao, Y.; Wei, Z.H.; Bai, L.; Deng, Z.; Zhong, J.J. Effect of fermentation temperature on validamycin A production by Strep-tomyces hygroscopicus 5008. J. Biotechnol. 2009, 142, 271–274. [Google Scholar] [CrossRef] [PubMed]
- Onaka, H. Novel antibiotic screening methods to awaken silent or cryptic secondary metabolic pathways in actinomycetes. J. Antibiot. 2017, 70, 865–870. [Google Scholar] [CrossRef] [PubMed]
- Halaby, T.; Boots, H.; Vermeulen, A.; van der Ven, A.; Beguin, H.; van Hooff, H.; Jacobs, J. Phaeohyphomycosis caused by Alternaria infectoria in a renal transplant recipient. J. Clin. Microbiol. 2001, 39, 1952–1955. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, C.; Montero, J.; Alio, J.L.; Abad, J.L.; Ruiz-Moreno, J.M.; Colom, F. Rapid molecular diagnosis of posttraumatic keratitis and endophthalmitis caused by Alternaria infectoria. J. Clin. Microbiol. 2003, 41, 3358–3360. [Google Scholar] [CrossRef] [PubMed]
- Sexton, A.C.; Howlett, B.J. Parallels in fungal pathogenesis on plant and animal hosts. Eukaryot. Cell 2006, 5, 1941–1949. [Google Scholar] [CrossRef]
- Palmore, T.N.; Shea, Y.R.; Childs, R.W.; Sherry, R.M.; Walsh, T.J. Fusarium proliferatum soft tissue infection at the site of a puncture by a plant: Recovery, isolation, and direct molecular identification. J. Clin. Microbiol. 2010, 48, 338–342. [Google Scholar] [CrossRef]
- Shivaprakash, M.R.; Appannanavar, S.B.; Dhaliwal, M.; Gupta, A.; Gupta, S.; Gupta, A.; and Chakrabarti, A. Colletotrichum truncatum: An unusual pathogen causing mycotic keratitis and endophthalmitis. J. Clin. Microbiol. 2011, 49, 2894–2898. [Google Scholar] [CrossRef]
- Sandoval-Denis, M.; Sutton, D.A.; Martin-Vicente, A.; Cano-Lira, J.F.; Wiederhold, N.; Guarro, J.; Gene, J. Cladosporium species recovered from clinical samples in the United States. J. Clin. Microbiol. 2015, 53, 2990–3000. [Google Scholar] [CrossRef]
- Kim, J.-S.; Yoon, S.-J.; Park, Y.-J.; Kim, S.-Y.; Ryu, C.-M. Crossing the kingdom border: Human diseases caused by plant path-ogens. Environ. Microbiol. 2020, 22, 2485–2495. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saxena, S.; Dufossé, L.; Deshmukh, S.K.; Chhipa, H.; Gupta, M.K. Endophytic Fungi: A Treasure Trove of Antifungal Metabolites. Microorganisms 2024, 12, 1903. https://doi.org/10.3390/microorganisms12091903
Saxena S, Dufossé L, Deshmukh SK, Chhipa H, Gupta MK. Endophytic Fungi: A Treasure Trove of Antifungal Metabolites. Microorganisms. 2024; 12(9):1903. https://doi.org/10.3390/microorganisms12091903
Chicago/Turabian StyleSaxena, Sanjai, Laurent Dufossé, Sunil K. Deshmukh, Hemraj Chhipa, and Manish Kumar Gupta. 2024. "Endophytic Fungi: A Treasure Trove of Antifungal Metabolites" Microorganisms 12, no. 9: 1903. https://doi.org/10.3390/microorganisms12091903









