Abstract
Despite enormous developments in medicine, infective endocarditis (IE) remains an ongoing issue for physicians due to increased morbidity and persistently high mortality. Our goal was to assess clinical outcomes in patients with IE and identify determinants of in-hospital mortality. Material and methods: The analysis was retrospective, single-centered, and comprised 270 patients diagnosed with IE from 2005 to 2021 (median age 65 (51–74), male 177 (65.6%). Native IE (NVIE) was observed in 180 (66.7%), prosthetic IE (PVIE) in 88 (33.6%), and cardiac device-related IE (CDRIE) in 2 (0.7%), with non-survivors having much higher rates. Healthcare-associated IE (HAIE) was 72 (26.7%), Staphylococci were the most prevalent pathogen, and the proportion of Gram-negative bacteria (GNB) non-HACEK was significantly greater in non-survivors than survivors (11 (15%) vs. 9 (4.5%), p = 0.004). Overall, 54 (20%) patients underwent early surgery, with a significant difference between dead and alive patients (3 (4.5%) vs. 51 (25.1%, p = 0.000). The overall in-hospital mortality rate was 24.8% (67). Logistic regression was conducted on the total sample (n = 270) for the period 2005–2021, as well as the sub-periods 2005–2015 (n = 119) and 2016–2021 (n = 151), to identify any differences in the trend of IE. For the overall group, the presence of septic shock (OR-83.1; 95% CI (17.0–405.2), p = 0.000) and acute heart failure (OR—24.6; 95% CI (9.2–65.0), p = 0.000) increased the risk of mortality. Early surgery (OR-0.03, 95% CI (0.01–0.16), p = 0.000) and a low Charlson comorbidity index (OR-0.85, 95% CI (0.74–0.98, p = 0.026) also lower this risk. Between 2005 and 2015, the presence of septic shock (OR 76.5, 95% CI 7.11–823.4, p = 0.000), acute heart failure (OR-11.5, 95% CI 2.9–46.3, p = 0.001), and chronic heart failure (OR-1.3, 95% CI 1.1–1.8, p = 0.022) enhanced the likelihood of a fatal outcome. Low Charlson index comorbidity (CCI) lowered the risk (OR-0.7, 95% CI 0.5–0.95, p = 0.026). For the period 2016–2021, the variable with the major influence for the model is the failure to perform early surgery in indicated patients (OR-240, 95% CI 23.2—2483, p = 0.000) followed by a complication of acute heart failure (OR-72.2, 95% CI 7.5–693.6. p = 0.000), septic shock (OR-17.4, 95% CI 2.0–150.8, p = 0.010), previous stroke (OR-9.2, 95% CI 1.4–59.4, p = 0.020) and low ejection fraction (OR-1.1, 95% CI 1.0–1.2, p = 0.004). Conclusions: Knowing the predictors of mortality would change the therapeutic approach to be more aggressive, improving the short- and long-term prognosis of IE patients.
1. Introduction
Although IE was described 350 years ago [1], it continues to provide a significant challenge to doctors for a variety of reasons. IE is a developing illness, and despite the availability of advanced imaging and microbiological tools, identification is frequently challenging and delayed. The global incidence of IE has increased to 13/100,000 [2]. In recent decades, improvements in medical and surgical treatment have had no effect on death or major complication rates [2,3]. Prevention is critical for reducing morbidity in IE. Maintaining proper oral hygiene and dental health, as well as following current recommendations for dental care and other procedures and manipulations, are critical for preventing IE.
The mortality rate for IE remains high, ranging from 15 to 30% [2,3,4], and is linked to the disease’s shifting characteristics. Patients are older than sixty years and have numerous comorbidities. A high Charlson Comorbidity Index (>3), diabetes, renal failure, prosthetic valve IE (PVIE), and hemodialysis all predict a bad prognosis [4,5,6,7,8]. The prevalence of PVIE has increased in recent decades [2,9,10]. Novel types of IE, such as indwelling device-related IE and post-TAVI (transcatheter aortic valve implantation) IE, are becoming more common as medical science and technology develop. They are difficult to treat and have a poor prognosis because they occur in older individuals with severe comorbidities and frequently require surgery [11,12,13,14,15].
Healthcare-associated IE (HAIE) accounts for almost one-third of all cases and has been driven by medical advancements, improved treatment, and a higher life expectancy of patients. It is often associated with a poor outcome [16,17]. The microbiological spectrum of IE has shifted, with staphylococci and enterococci predominating, posing challenges for treatment [10].
Complications, such as acute or worsening heart failure, septic shock, acute neurological events, acute renal failure related to valve dysfunction, embolism, and/or persistent infection, are the most important predictors of mortality [18]. These are also the main indications for early surgical intervention.
Early risk assessment for complications and death is crucial. The timely identification of high-risk patients would shift the therapeutic approach to a more aggressive one, particularly when deciding on surgical therapy. Early surgery is associated with reduced in-hospital mortality and improved long-term prognosis for IE patients [9,10]. Knowing the predictors of death and complications can help us make better treatment options.
The characteristics of IE vary according to the country’s geographical and socioeconomic status. Randomized prospective studies are difficult to undertake because the condition is relatively rare. For certain regions and nations, information is mostly collected via retrospective and single-center examinations.
There has been a shortage of information on the predictors of in-hospital mortality in Bulgaria over the past few decades. We looked at the determinants of in-hospital death in patients with IE over the course of 17 years.
2. Material and Method
This is a retrospective, single-center study, including 270 patients diagnosed with IE according to the modified Duke criteria, treated at the University Hospital “St Georgi” in the city of Plovdiv, Bulgaria for the period January 2005–December 2021. Patients were separated into two sub-periods: before (2005–2015, n = 119) and after (2016–2021, n = 151) the most recent current IE guidelines (2015) to find differences in the disease progression. The incidence has been growing during the last six years. We observed an increase in cases of prosthetic IE, healthcare-associated IE, increased age, and improved diagnosis. These findings are consistent with current IE data provided by the Global Burden of Disease 2019.
The hospital’s capacity is 1500 beds, and the Cardiology Clinic is a reference center for treating IE for a large part of southern Bulgaria. The medical records of treated patients with codes I33, I38, and I39 for the described period were used. Variables studied included demographics, risk group, presence of predisposing heart disease, comorbidities, Charlson Comorbidity Index (CCI) [19], entry gate, predictors of transient bacteremia, clinical, echocardiographic findings, causative organisms, complications, and clinical outcome.
2.1. Definition and Classification of IE
The diagnosis was defined as definite or possible IE according to the modified Duke criteria [20]. Surgical treatment of IE was defined as early if surgery was performed during antibiotic treatment. Valvular involvement in IE was determined on the basis of echocardiographic findings, other imaging studies, cardiac surgery, or, in some cases, clinical presentation. IE was classified by mode of acquisition as community-associated IE (CAIE), healthcare-associated IE (HAIE), and intravenous drug-associated IE (IDUIE). These categories are mutually exclusive. IE was defined as HAIE according to the following criteria: occurrence of IE > 48 h after hospital admission or within 6 months after hospital discharge for ≥2 days; IE developed within 6 months after a significant invasive procedure performed during hospitalization or in an outpatient setting; extensive outpatient healthcare contacts, defined as receiving wound care or intravenous treatment within 1 month before the onset of IE; or stay in a clinic-home for similar care [12,13,14,15]. IE occurring on a prosthetic valve within 12 months of surgery is defined as prosthetic valve early endocarditis (PVIE) and is classified as HAIE. Patients with a recent (within 1 month) or longer history of intravenous drug use were classified as IDUIE. Patients with no medical history and no history of injecting drug use were classified as CAIE. IE following dental treatment was considered to be CAIE if there was no other healthcare contact. The presence of septic emboli and an extracardiac focus of infection was defined as a focus of infection detected by imaging or on the basis of typical clinical presentation. Complications were diagnosed according to established diagnostic criteria and recommendations.
2.2. Statistical Methods
Quantitative data are presented as arithmetic mean ± standard deviation (mean ± SD) or median and interquartile range (25–75 percentiles) according to the type of distribution of the variables (Kolmogorov–Smirnov test). Categorical variables were summarized using absolute (n) and relative (%) magnitudes. A Mann–Whitney test for independent samples was used to compare quantitative variables between two groups. A Z-test was used to compare the relative shares of categorical variables between the studied groups. Logistic regression was performed to determine the simultaneous influence of significant independent variables identified in the univariate analysis to predict belonging to one of two mutually exclusive categories (alive/dead) of the dependent variable output. To determine whether there were differences in the distribution of survival between patients who underwent early surgery and those who did not, a log-rank test was performed. A p-value < 0.05 (two-tailed test) was considered statistically significant for all tests. A statistical analysis was performed using SPSS, version 26.0 (IBM Corp., Binghamton, NY, USA).
3. Results
Of the 270 patients, 205 (75.9%) had definite IE, with 133 (65%) having two major criteria and 72 (35%) having one major plus three minor criteria. There were 65 (24.1%) cases of possible IE, 62 (95%) with one major and one minor criterion, and three with three minor criteria. The overall mortality rate was 24.8% (67). Table 1 shows the baseline characteristics of the patients. The median age was 65 (51–74) years, with non-survivors being significantly older than survivors (67 (53–75) vs. 62 (44–73) years, p = 0.003). In the whole sample, there were 177 men (65.6%), with no difference between survivors and non-survivors. The majority of patients (180/66.7%) had native valve IE, whereas 88 (33.6%) had prosthetic valve IE. We found CDRIE only in the non-survivor group (2 (3%), p = 0.013) in patients with pacemaker, VVI mode (single chamber). There was no significant difference between deceased and surviving patients in terms of risk group, predisposing cardiac conditions, port of entry, or type of IE based on mode of acquisition. The most common congenital heart condition is bicuspid aortic valves and mitral valve prolapsus (7.1%), with two patients (0.7%) having repaired Tetralogy of Fallo. According to current guidelines (1998, 2007, 2015), they were all not eligible for antibiotic prophylaxis.

Table 1.
Baseline characteristics.
Patients had a wide range of comorbidities, the most common of which were arterial hypertension 171 (63.3%), chronic heart failure 124 (45.9%), previous cardiac surgery 95 (35.2%), chronic renal failure 70 (25.9%), coronary artery disease 64 (23.7%), diabetes 51 (18.9%), atrial fibrillation 49 (18.1%), and more. We discovered a significant difference with more cases in the deceased than in those who survived CCI (4 (3–6) vs. 3 (1–5), p < 0.0001), chronic renal disease (26 (38.8%) vs. 44 (24.7%), p = 0.006), atrial fibrillation (18 (26.9%) vs. 31 (15.3%), p = 0.033), and prior stroke (15 (22.4%) vs. 25 (12.3%), p = 0.044). The most prevalent symptoms were fever 263 (97.4%), anemia 248 (92.5%), and a heart murmur 178 (66.2%). The number of cases with splenomegaly 49 (8.1%) and rash 14 (5.5%) decreased dramatically. The deceased group had significantly fewer febrile cases (63 (94%) versus 200 (98.5%), p = 0.045). In terms of complications, we found that there were significantly more cases of acute heart failure (57 (85.1%) vs. 71 (35%), p < 0.0001), septic shock (20 (29.9%) vs. 3 (1.5%), p < 0.0001), and worsening kidney function (36 (53.7%) vs. 75 (36.9%), p = 0.015) in those who died compared to those who survived. Early surgery was performed in 54 (20%) patients overall, but less frequently in non-survivors than in survivors (3 (4.5%) vs. 51 (25.1%), p < 0.0001) (Table 2).

Table 2.
Comorbidity, clinical symptoms, and complications.
The echocardiographic data are presented in Supplementary Table S1. Transesophageal echocardiography (TOE) was performed more frequently in survivors than in deceased patients (86 (42.4%) vs. 11 (16.4%), p = 0.000). We found that AV-TV involvement was significantly more common in the deceased group (3 (4.5%) vs. 1 (0.5%, p = 0.019), and ejection fraction was significantly lower in the same group (55 (51–66) vs. 62 (55–68), p = 0.001).
Hemoculture results were negative in 111 (41.1%), and the most common pathogens were staphylococci in 89 (33%). Enterococci were more prevalent (25 (9.3%)) than streptococci (21 (7.8%)). GNB non-HACEK (Hemophilus species, Actinobacillus, Cardiobacterium, Eikenella, or Kingella) were significantly more frequent in the deceased group (10 (14.9%) vs. 9 (4.5%), p = 0.004), especially for Escherichia coli (5 (7.4%) vs. 4 (2.0%), p = 0.030) and Serratia marcescens (3 (4.5%) vs. 1 (0.5%), p = 0.019) (Supplementary Table S2).
Predictors of in-hospital mortality from univariate analysis are summarized in Table 3.

Table 3.
Predictors of in-hospital mortality—p-value from univariate analysis.
Logistic regression was performed on the entire sample (n = 270) for the period 2005–2021 to determine the simultaneous influence of significant independent variables identified in the univariate analysis to predict belonging to one of two mutually exclusive categories (alive/dead) of the dependent variable output. The logistic regression model was statistically significant, χ2(4) = 138.07, p < 0.0001. The model explained 59.4% (Nagelkerke R2) of the variation in outcome and correctly classified 86.3% of cases. The presence of septic shock increased the odds of death by a factor of 83.1 and the complication of acute HF by a factor of 24. Early surgery and low CCI reduce this risk (Table 4).

Table 4.
Logistic regression for periods.
Logistic regression was performed on the sample (n = 119), covering the period 2005–2015, to determine the concurrent influence of significant independent variables identified in the univariate analysis to predict belonging to one of two mutually exclusive categories (alive/dead) of the dependent variable outcome. The logistic regression model was statistically significant, χ2(4) = 52.16, p < 0.0001. The model explained 52.4% (Nagelkerke R2) of the variation in outcome and correctly classified 86.6% of the cases. The probability of a fatal outcome is increased by the presence of (1) septic shock by a factor of 76.5; (2) acute heart failure complications by a factor of 11.5; and (3) to a lesser chronic heart failure extent by a comorbidity. A low CCI reduces this risk (Table 4).
Logistic regression was performed on the sample (n = 151) spanning the period 2016–2021 to determine the concurrent influence of significant independent variables identified using univariate analysis to predict belonging to one of two mutually exclusive categories (alive/dead) of the dependent variable output. The logistic regression model was statistically significant, χ2(5) = 118.47, p < 0.0001. The model explained 80.9% (Nagelkerke R2) of the variation in the outcome and correctly classified 94.0% of the cases. The most influential variable for the model (B = 5.48) was not performing early surgery in indicated patients, which increased the probability of death by 240-fold, followed by the complications of HF (B = 4.28) and septic shock (2.86) with an increased risk by 72.40 times and by 17.41, respectively. Reduced ejection fraction and previous stroke also increased the odds of death by a factor of 1.11 and 9.2, respectively (Table 4).
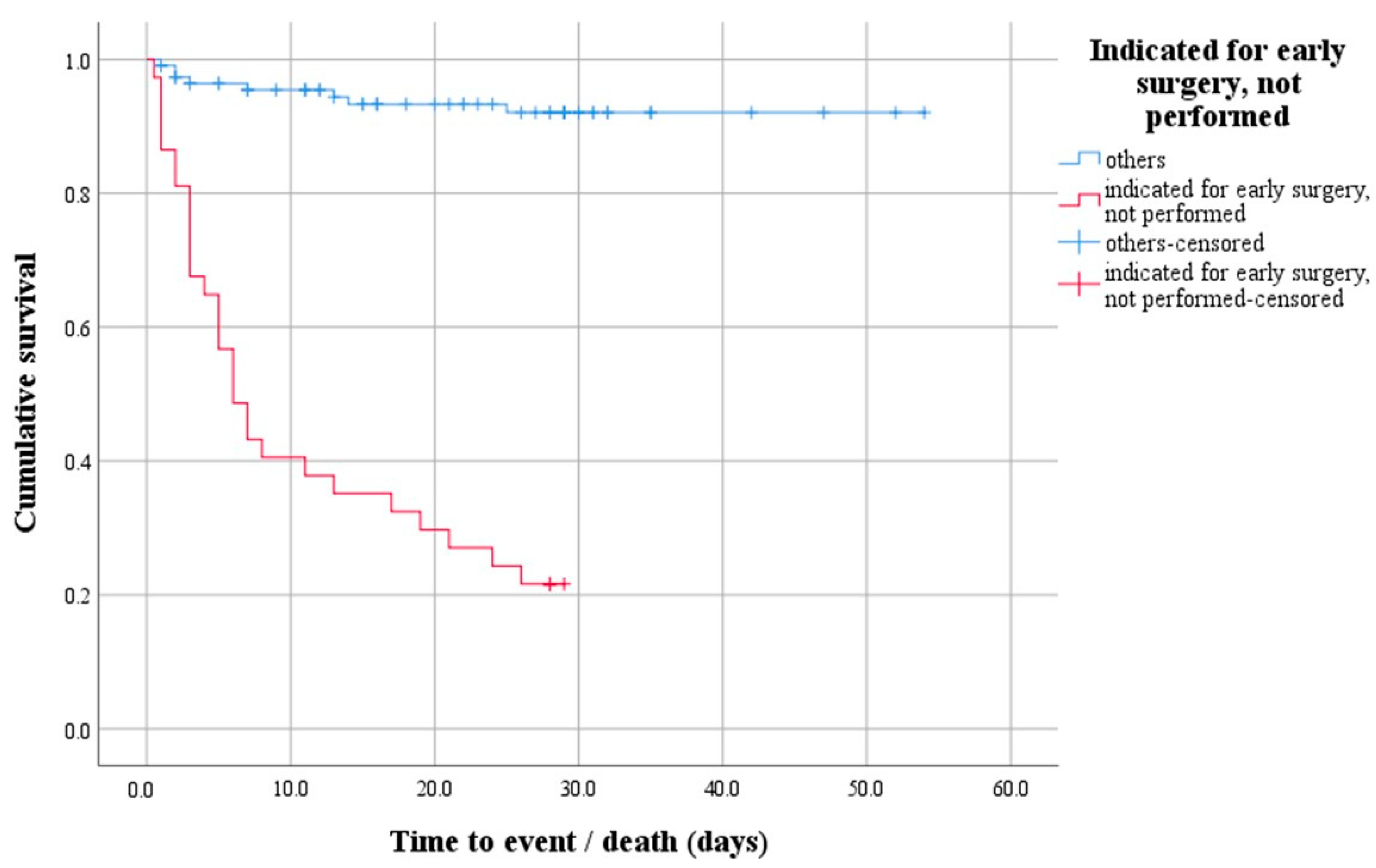
A survival analysis with a log-rank test was performed to determine whether there was a difference in the distribution of survival between patients indicated for early surgery that was not performed and all others. The test result is statistically significant, i.e., the survival distribution between the two groups was different—χ2(1) = 91.47, p < 0.0001 (Figure 1). Descriptively, the outcome is presented by the median time to event (death), with the estimated time to death being 6 days after hospitalization for the group of patients indicated for early surgery that was not performed.
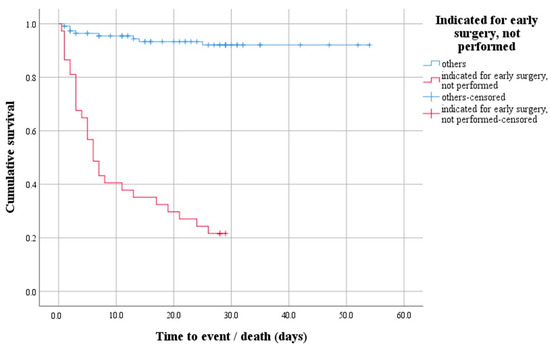
Figure 1.
Survival function demonstrating the difference in survival distribution between patients indicated for early surgery that was not performed and all others.
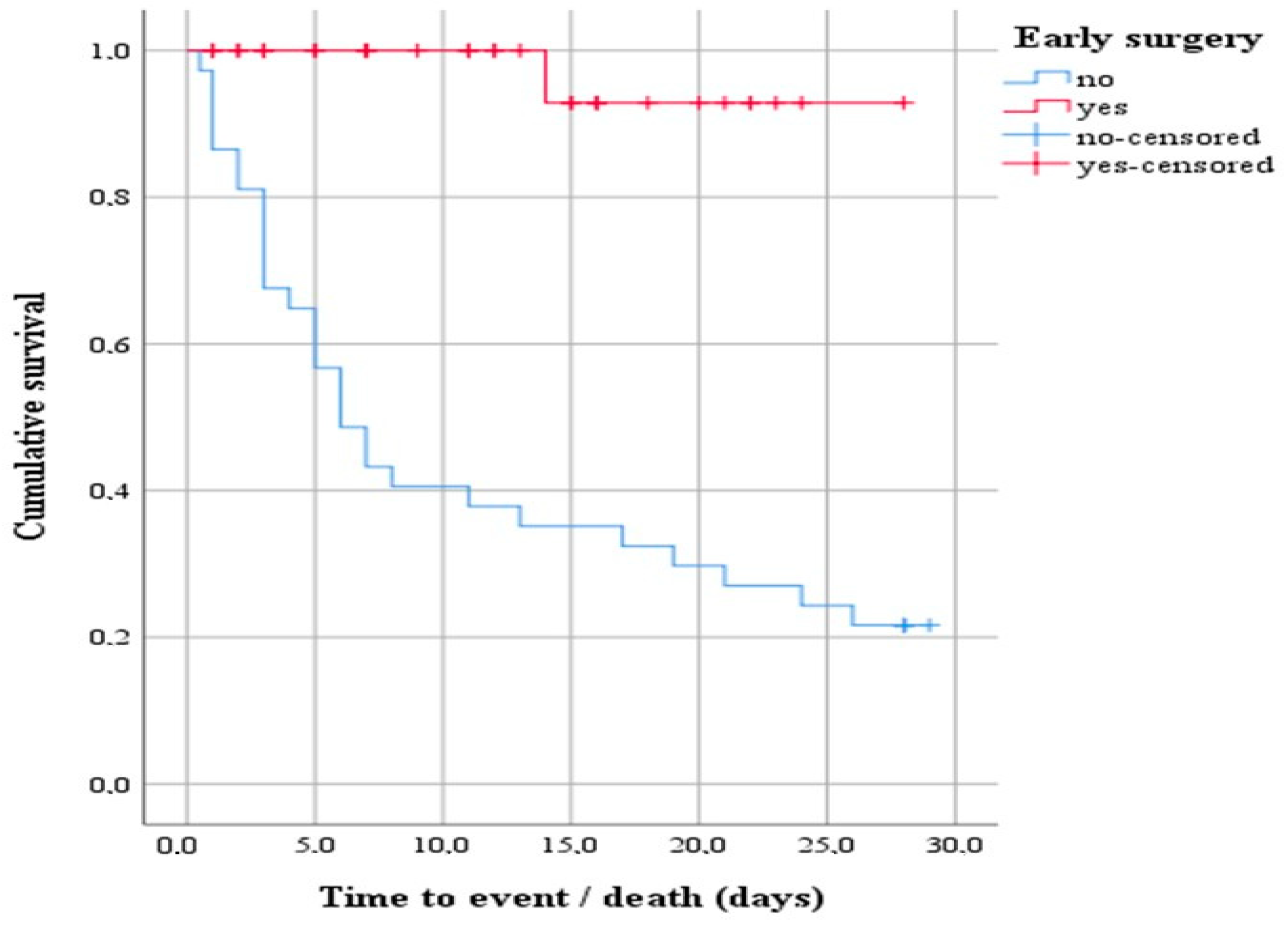
A subsequent survival analysis was performed with a log-rank test to determine whether there was a difference in the distribution of survival between patients indicated for early surgery and those who did not. The result of the test was statistically significant, i.e., the survival distribution between the two groups was different—χ2(1) = 25.20, p < 0.0001 (Figure 2). Descriptively, the outcome is presented by the median time to event (death), with the estimated time to death being 6 days after hospitalization for the group of patients indicated for early surgery that was not performed. In addition, the 75% percentile of the data showed survival 3 days from hospitalization.
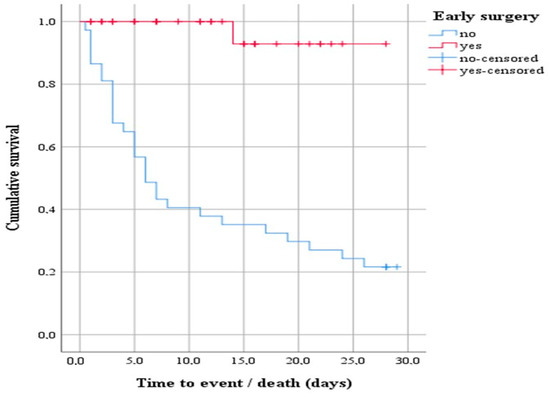
Figure 2.
Survival function showing the difference in the distribution of survival between patients indicated for early surgery that underwent early surgery and those that did not.
4. Discussion
Our sample had an overall in-hospital mortality rate for IE of 24.4%. These results are similar to those published in the literature, which ranges from 15 to 30% [2,3,21,22]. Patients in our country are generally health insured and have access to quality healthcare. The results of the univariate analysis related to increased mortality (Table 3) can be stated as follows:
- Patient characteristics include age, high CCI > 3, chronic renal disease, prior stroke, and atrial fibrillation.
- Complications include acute heart failure, renal failure, and septic shock.
- Echocardiographic findings include low EF (%), significant tricuspid regurgitation, and bivalve IE with aortic and tricuspid valve involvement.
- Microbiological characteristics of non-HACEK Gram-negative bacteria, including Escherichia coli and Serratia marcescens.
- Failure to undertake early surgery when required.
Gram-negative bacteria non-HACEK (Hemophilus species, Actinobacillus, Cardiobacterium, Eikenella, or Kingella) were significantly more prevalent in the deceased group, particularly Escherichia coli and Serratia marcescens. Marco Falcone et al. reported Escherichia coli to be the most common cause of GNB-IE [23]. GNB non-HASEK-IE is a rare infection that causes substantial in-hospital mortality and is defined by its presence in elderly patients with severe comorbidities, nosocomial acquisition, and a poor outcome [24,25].
Acute heart failure and septic shock were significant predictors of in-hospital death, although early surgery and a low CCI were protective. Acute cardiac failure is the most prevalent consequence of IE and the primary reason for early surgery [9,10]. The incidence of cardiac failure in IE has been reported to be between 19 and 75% [26,27]. This complication is most typically caused by leaflet perforation or rupture, mitral chordal rupture, valve dehiscence in PVIE, and, less frequently, intracardiac fistula, valve obstruction, or myocardial infarction due to embolization [10]. The intensity of presentation is determined by previous cardiac function and associated comorbidities. Heart failure in IE is a well-established independent predictor of in-hospital and one-year death. Surgery remained the only effective treatment associated with increased survival [26,27,28].
Septic shock is a life-threatening consequence of IE that affects roughly 5–12% of patients [2,3,29,30,31,32]. It is a widely recognized independent predictor of in-hospital death. S. aureus and Gram-negative bacteria, nosocomial acquisition, severe renal failure, diabetes mellitus, and central nervous system embolism all increase the risk of septic shock [29]. Approximately two-thirds of individuals with IE who develop septic shock die. Septic shock is the most common reason for early surgery in cases of persistent infection. There is compelling evidence that early surgical intervention lowers in-hospital and 1-year mortality in these patients [29].
Comorbidities are an essential part of the IE patient profile and a predictor of disease prognosis. We used the Charlson Comorbidity Index (CCI) [19], which is the most commonly accepted assessment of the prognostic impact of many chronic conditions. Our results are consistent with prior research on the CCI as an independent prognostic factor in IE [33,34,35].
The independent predictors of death identified in our investigation are consistent with the literature. The most generally documented predictors of death include heart failure, age > 70 years [2,3,31,36], septic shock [30,37,38], and a high CCI [2,39]. Other studies have identified PVIE, Staphylococcus aureus IE, cerebrovascular sequelae, and paravalvular abscess as independent predictors of in-hospital death [3,21,31,36,40].
Surgical intervention in IE is increasingly recommended by American and European guidelines for complicated infective endocarditis [10]. The number of patients receiving surgical treatment ranges from 20% to 70%, depending on the country and availability of cardiac surgical resources [41]. In our analysis, early surgery was performed in 20% of cases, which is consistent with the Denmark study’s 22.5% [42] and relatively low when compared to 45–62% in other countries [2,3,21,40]. Early surgery is a protective indicator, and failure to perform early surgery when indicated is a strong predictor of in-hospital death [2]. Data from the literature show that almost half of patients with IE are indicated for early surgery, but more than half of them do not receive it. The reasons are various. EURO-ENDO reports the following reasons for not performing early surgery—58.2%; death before surgery—22.5%; patient refusal—18.8%; neurological complications—11.2%; lack of cardiac surgery in the medical facility—6.2%; other—20.7% [2]. Our data demonstrate that early surgery improves survival in IE. We discovered that performing early surgery was an independent predictor of decreasing in-hospital mortality, whereas failing to conduct surgery when recommended was a substantial predictor of death in IE.
5. Limitation
This retrospective analysis used data from a single center’s clinical database. Another restriction is the long study period, as well as changing guidelines and evidence, particularly about early surgery, the use of novel imaging techniques for diagnosis, such as 18F-fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG PET/CT), and new antibiotic molecules. Technological developments in echocardiography have an impact on echocardiographic data. Despite these limitations, our study is the only one of its kind in Bulgaria in recent decades, and it followed a significant number of patients for a long time (17 years).
6. Conclusions
Acute cardiac failure and septic shock are independent predictors of in-hospital mortality. A low Charlson comorbidity index and timely surgery improved survival. Knowing the predictors of mortality would shift the therapeutic approach to be more aggressive, improving the short- and long-term prognosis of patients with IE.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/microorganisms12091919/s1, Table S1. Echocardiographic results. Table S2. Microbiological agent.
Author Contributions
Conceptualization, B.D.-Y., F.N. and M.T.; Methodology, B.D.-Y., F.N., R.R. and M.T.; Validation, R.R.; Formal analysis, R.R.; Investigation, B.D.-Y.; Data curation, B.D.-Y.; Writing—original draft, B.D.-Y.; Writing—review & editing, B.D.-Y.; Visualization, R.R.; Supervision, M.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study was conducted with the consent of the Local Ethics Committee (decision #2/09.03.2023) and in accordance with the principles of the Declaration of Helsinki.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Geller, S.A. Infective endoarditis: A history of the development of its undestanding. Autops. Case Rep. 2013, 3, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Habib, G.; Erba, P.A.; Iung, B.; Donal, E.; Cosyns, B.; Laroche, C.; Popescu, B.A.; Prendergast, B.; Tornos, P.; Sadeghpour, A.; et al. Clinical presentation, aetiology and outcome of infective endocarditis. Results of the ESC-EORP EURO-ENDO (European infective endocarditis) registry: A prospective cohort study. Eur. Heart J. 2019, 40, 3222–3232. [Google Scholar] [CrossRef] [PubMed]
- Murdoch, D.R. Clinical Presentation, Etiology, and Outcome of Infective Endocarditis in the 21st Century. Arch. Intern. Med. 2009, 169, 463. [Google Scholar] [CrossRef]
- Armiñanzas, C.; Fariñas-Alvarez, C.; Zarauza, J.; Muñoz, P.; González Ramallo, V.; Martínez Sellés, M.; Meda, J.M.M.; Pericás, J.M.; Goenaga, M.Á.; Burgos, G.O.; et al. Role of age and comorbidities in mortality of patients with infective endocarditis. Eur. J. Intern. Med. 2019, 64, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Pericàs, J.M.; Llopis, J.; Jiménez-Exposito, M.J.; Kourany, W.M.; Almirante, B.; Carosi, G.; Durante-Mangoni, E.; Fortes, C.Q.; Giannitsioti, E.; Lerakis, S.; et al. Infective Endocarditis in Patients on Chronic Hemodialysis. J. Am. Coll. Cardiol. 2021, 77, 1629–1640. [Google Scholar] [CrossRef]
- Duval, X.; Alla, F.; Doco-Lecompte, T.; Le Moing, V.; Delahaye, F.; Mainardi, J.L.; Plesiat, P.; Célard, M.; Hoen, B.; Leport, C. Diabetes mellitus and infective endocarditis: The insulin factor in patient morbidity and mortality. Eur. Heart J. 2006, 28, 59–64. [Google Scholar] [CrossRef]
- Chu, V.H.; Cabell, C.H.; Benjamin, D.K.; Kuniholm, E.F.; Fowler, V.G.; Engemann, J.; Sexton, D.J.; Corey, G.R.; Wang, A. Early Predictors of In-Hospital Death in Infective Endocarditis. Circulation 2004, 109, 1745–1749. [Google Scholar] [CrossRef]
- Menchi-Elanzi, M.; Ramos-Rincón, J.M.; Merino-Lucas, E.; Reus-Bañuls, S.; Torrús-Tendero, D.; Clíment-Paya, V.; Boix, V.; Portilla-Sogorb, J. Infective endocarditis in elderly and very elderly patients. Aging Clin. Exp. Res. 2020, 32, 1383–1388. [Google Scholar] [CrossRef] [PubMed]
- Habib, G.; Lancellotti, P.; Antunes, M.J.; Bongiorni, M.G.; Casalta, J.P.; Del Zotti, F.; Dulgheru, R.; El Khoury, G.; Erba, P.A.; Iung, B.; et al. 2015 ESC Guidelines for the management of infective endocarditis: The Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur. Heart J. 2015, 36, 3075–3128. [Google Scholar]
- Delgado, V.; Ajmone Marsan, N.; De Waha, S.; Bonaros, N.; Brida, M.; Burri, H.; Caselli, S.; Doenst, T.; Ederhy, S.; Erba, P.A.; et al. 2023 ESC Guidelines for the management of endocarditis. Eur. Heart J. 2023, 44, 3948–4042. [Google Scholar]
- Tinica, G.; Tarus, A.; Enache, M.; Artene, B.; Rotaru, I.; Bacusca, A.; Burlacu, A. Infective endocarditis after TAVI: A meta-analysis and systematic review of epidemiology, risk factors and clinical consequences. Rev. Cardiovasc. Med. 2020, 21, 263. [Google Scholar] [PubMed]
- Panagides, V.; Cuervo, G.; Llopis, J.; Abdel-Wahab, M.; Mangner, N.; Habib, G.; Regueiro, A.; Mestres, C.A.; Tornos, P.; Durand, E.; et al. Infective Endocarditis after Transcatheter versus Surgical Aortic Valve Replacement. Clin. Infect. Dis. 2024, 78, 179–187. [Google Scholar] [CrossRef] [PubMed]
- del Val, D.; Panagides, V.; Mestres, C.A.; Miró, J.M.; Rodés-Cabau, J. Infective Endocarditis after Transcatheter Aortic Valve Replacement. J. Am. Coll. Cardiol. 2023, 81, 394–412. [Google Scholar] [CrossRef] [PubMed]
- Sanghavi, R.; Ravikumar, N.; Sarodaya, V.; Haq, M.; Sherif, M.; Harky, A. Outcomes in Cardiac Implantable Electronic device-related Infective Endocarditis: A Systematic Review of Current Literature. Future Cardiol. 2022, 18, 891–899. [Google Scholar] [CrossRef]
- Jędrzejczyk-Patej, E.; Mazurek, M.; Kowalski, O.; Sokal, A.; Kozieł, M.; Adamczyk, K.; Przybylska-Siedlecka, K.; Morawski, S.; Liberska, A.; Szulik, M.; et al. Device-related infective endocarditis in cardiac resynchronization therapy recipients—Single center registry with over 2500 person-years follow up. Int. J. Cardiol. 2017, 227, 18–24. [Google Scholar] [CrossRef]
- Fernández-Hidalgo, N.; Almirante, B.; Tornos, P.; Pigrau, C.; Sambola, A.; Igual, A.; Pahissa, A. Contemporary Epidemiology and Prognosis of Health Care—Associated Infective Endocarditis. Clin. Infect. Dis. 2008, 47, 1287–1297. [Google Scholar] [CrossRef]
- Lomas, J.M.; Martínez-Marcos, F.J.; Plata, A.; Ivanova, R.; Gálvez, J.; Ruiz, J.; Reguera, J.M.; Noureddine, M.; De La Torre, J.; De Alarcón, A. Healthcare—Associated Infective Endocarditis. An Undesirable Effect of Health Care Universalization. Clin. Microbiol. Infect. 2009, 26, 1683–1690. [Google Scholar]
- Park, L.P.; Chu, V.H.; Peterson, G.; Skoutelis, A.; Lejko-Zupa, T.; Bouza, E.; Tattevin, P.; Habib, G.; Tan, R.; Gonzalez, J.; et al. Validated Risk Score for Predicting 6-Month Mortality in Infective Endocarditis. J. Am. Heart Assoc. 2016, 5, e003016. [Google Scholar] [CrossRef]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic. Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef]
- Durack, D.T.; Lukes, A.S.; Bright, D.K. New criteria for diagnosis of infective endocarditis: Utilization of specific echocardiographic findings. Duke Endocarditis Service. Am. J. Med. 1994, 96, 200–209. [Google Scholar] [CrossRef]
- Sunder, S.; Grammatico-Guillon, L.; Lemaignen, A.; Lacasse, M.; Gaborit, C.; Boutoille, D.; Tattevin, P.; Denes, E.; Guimard, T.; Dupont, M.; et al. Incidence, characteristics, and mortality of infective endocarditis in France in 2011. PLoS ONE 2019, 14, e0223857. [Google Scholar] [CrossRef] [PubMed]
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P.; et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef]
- Falcone, M.; Tiseo, G.; Durante-Mangoni, E.; Ravasio, V.; Barbaro, F.; Ursi, M.P.; Pasticci, M.B.; Bassetti, M.; Grossi, P.; Venditti, M.; et al. Risk Factors and Outcomes of Endocarditis Due to Non-HACEK Gram-Negative Bacilli: Data from the Prospective Multicenter Italian Endocarditis Study Cohort. Antimicrob. Agents Chemother. 2018, 62, 02208-17. [Google Scholar] [CrossRef] [PubMed]
- Calderón Parra, J.; De Castro-Campos, D.; Muñoz García, P.; Olmedo Samperio, M.; Marín Arriaza, M.; De Alarcón, A.; Gutierrez-Carretero, E.; Alvarez, M.C.F.; Meda, J.M.M.; Sanchez, M.Á.G.; et al. Non-HACEK gram negative bacilli endocarditis: Analysis of a national prospective cohort. Eur. J. Intern. Med. 2021, 92, 71–78. [Google Scholar] [CrossRef]
- Sebillotte, M.; Boutoille, D.; Declerck, C.; Talarmin, J.P.; Lemaignen, A.; Piau, C.; Revest, M.; Tattevin, P.; Gousseff, M. Non-HACEK gram-negative bacilli endocarditis: A multicentre retrospective case-control study. Infect. Dis. 2023, 55, 599–606. [Google Scholar] [CrossRef] [PubMed]
- Pericàs, J.M.; Hernández-Meneses, M.; Muñoz, P.; Martínez-Sellés, M.; Álvarez-Uria, A.; de Alarcón, A.; Gutiérrez-Carretero, E.; Goenaga, M.A.; Zarauza, M.J.; Falces, C.; et al. Characteristics and Outcome of Acute Heart Failure in Infective Endocarditis: Focus on Cardiogenic Shock. Clin. Infect. Dis. 2021, 73, 765–774. [Google Scholar] [CrossRef] [PubMed]
- Mir, T.; Uddin, M.; Qureshi, W.T.; Regmi, N.; Tleyjeh, I.M.; Saydain, G. Predictors of Complications Secondary to Infective Endocarditis and Their Associated Outcomes: A Large Cohort Study from the National Emergency Database (2016–2018). Infect. Dis. Ther. 2022, 11, 305–321. [Google Scholar] [CrossRef]
- Bohbot, Y.; Habib, G.; Laroche, C.; Stöhr, E.; Chirouze, C.; Hernandez-Meneses, M.; Melissopoulou, M.; Mutlu, B.; Scheggi, V.; Branco, L.; et al. Characteristics, management, and outcomes of patients with left-sided infective endocarditis complicated by heart failure: A substudy of the ESC-EORP EURO-ENDO (European infective endocarditis) registry. Eur. J. Heart Fail. 2022, 24, 1253–1265. [Google Scholar] [CrossRef]
- Pericàs, J.M.; Hernández-Meneses, M.; Muñoz, P.; Álvarez-Uría, A.; Pinilla-Llorente, B.; de Alarcón, A.; Reviejo, K.; Fariñas, M.C.; Falces, C.; Goikoetxea-Agirre, J.; et al. Outcomes and Risk Factors of Septic Shock in Patients With Infective Endocarditis: A Prospective Cohort Study. Open Forum Infect. Dis. 2021, 8, ofab119. [Google Scholar] [CrossRef]
- Sousa, C.; Nogueira, P.; Pinto, F.J. Insight into the epidemiology of infective endocarditis in Portugal: A contemporary nationwide study from 2010 to 2018. BMC Cardiovasc. Disord. 2021, 21, 138. [Google Scholar] [CrossRef]
- Urina-Jassir, M.; Jaimes-Reyes, M.A.; Martinez-Vernaza, S.; Quiroga-Vergara, C.; Urina-Triana, M. Clinical, Microbiological, and Imaging Characteristics of Infective Endocarditis in Latin America: A Systematic Review. Int. J. Infect. Dis. 2022, 117, 312–321. [Google Scholar] [CrossRef] [PubMed]
- Vahabi, A.; Gül, F.; Garakhanova, S.; Sipahi, H.; Sipahi, O.R. Pooled analysis of 1270 infective endocarditis cases in Turkey. J. Infect. Dev. Ctries. 2019, 13, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Alves, S.G.; Pivatto, F.; Filippini, F.B.; Dannenhauer, G.P.; Seroiska, G.; Bischoff, H.M.; Birk, L.F.S.; Terra, D.H.; Sganzerla, D.; Miglioranza, M.H. Desempenho do Escore SHARPEN e do Índice de Comorbidade de Charlson para Predição de Mortalidade durante a Internação Hospitalar e após a Alta na Endocardite Infecciosa. Arq. Bras. Cardiol. 2023, 120, e20230441. [Google Scholar] [CrossRef]
- Lu, K.J.; Kearney, L.G.; Ord, M.; Jones, E.; Burrell, L.M.; Srivastava, P.M. Age adjusted Charlson Co-morbidity Index is an independent predictor of mortality over long-term follow-up in infective endocarditis. Int. J. Cardiol. 2013, 168, 5243–5248. [Google Scholar] [CrossRef] [PubMed]
- Wei, D.; Sun, Y.; Chen, R.; Meng, Y.; Wu, W. Age-adjusted Charlson comorbidity index and in-hospital mortality in critically ill patients with cardiogenic shock: A retrospective cohort study. Exp. Ther. Med. 2023, 25, 299. [Google Scholar] [CrossRef] [PubMed]
- Noubiap, J.J.; Nkeck, J.R.; Kwondom, B.S.; Nyaga, U.F. Epidemiology of infective endocarditis in Africa: A systematic review and meta-analysis. Lancet Glob. Health 2022, 10, e77–e86. [Google Scholar] [CrossRef]
- Hase, R.; Otsuka, Y.; Yoshida, K.; Hosokawa, N. Profile of infective endocarditis at a tertiary-care hospital in Japan over a 14-year period: Characteristics, outcome and predictors for in-hospital mortality. Int. J. Infect. Dis. 2015, 33, 62–66. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Zaballos, S.; González-Ramallo, V.; Quintana, E.; Muñoz, P.; de la Villa-Martínez, S.; Fariñas, M.C.; Arnáiz-de las Revillas, F.; de Alarcón, A.; Rodríguez-Esteban, M.Á.; Miró, J.M.; et al. Multivalvular Endocarditis: A Rare Condition with Poor Prognosis. J. Clin. Med. 2022, 11, 4736. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, H.J.; Ku, N.S.; Lee, S.H.; Lee, S.; Choi, J.Y.; Yeom, J.S. Infective endocarditis at a tertiary care hospital in South Korea. Heart 2021, 107, 135–141. [Google Scholar] [CrossRef]
- Maguire, D.J.; Arora, R.C.; Hiebert, B.M.; Dufault, B.; Thorleifson, M.D. The Epidemiology of Endocarditis in Manitoba: A Retrospective Study. CJC Open 2021, 3, 1471–1481. [Google Scholar] [CrossRef]
- Prendergast, B.; Tornos, P. Surgery for Infective Endocarditis. Circulation 2010, 121, 1141–1152. [Google Scholar] [CrossRef] [PubMed]
- Jensen, A.; Bundgaard, H.; Fosbøl, E.; Butt, J.H.; Bruun, N.E.; Voldstedlund, M.; Torp-Pedersen, C.; Gislason, G.; Iversen, K.; Chamat, S.; et al. Temporal changes in the incidence of infective endocarditis in Denmark 1997–2017: A nationwide study. Int. J. Cardiol. 2021, 326, 145–152. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).