Abstract
The Antarctic continent is widely considered to be one of the most hostile biological habitats on Earth. Despite extreme environmental conditions, the ice-free areas of the continent, which constitute some 0.44% of the total continental land area, harbour substantial and diverse communities of macro-organisms and especially microorganisms, particularly in the more “hospitable” maritime regions. In the more extreme non-maritime regions, exemplified by the McMurdo Dry Valleys of South Victoria Land, nutrient cycling and ecosystem servicing processes in soils are largely driven by microbial communities. Nitrogen turnover is a cornerstone of ecosystem servicing. In Antarctic continental soils, specifically those lacking macrophytes, cold-active free-living diazotrophic microorganisms, particularly Cyanobacteria, are keystone taxa. The diazotrophs are complemented by heterotrophic bacterial and archaeal taxa which show the genetic capacity to perform elements of the entire N cycle, including nitrification processes such as the anammox reaction. Here, we review the current literature on nitrogen cycling genes, taxa, processes and rates from studies of Antarctic soils. In particular, we highlight the current gaps in our knowledge of the scale and contribution of these processes in south polar soils as critical data to underpin viable predictions of how such processes may alter under the impacts of future climate change.
Keywords:
N-cycling; soils; Antarctic; diazotrophy; anammox; ecosystem services; bacteria; archaea; Cyanobacteria 1. Introduction
Continental Antarctica is largely ice-covered, with limited coastal, montane and maritime ice-free areas (approx. 54,000 km2, est. 0.44%) []. Following the advent of modern microbial phylogenetics over two decades ago, the microbiology of Antarctic terrestrial soils has become the subject of extensive research. This research has led to the discovery that these remote and extreme edaphic habitats harbour microbial populations that are much more phylogenetically and functionally diverse than ever previously recognised [].
Comprehensive phylogenetic surveys of Antarctic soils and soil-related habitats from most of the dominant ice-free areas of the continent, based on 16S (prokaryotic) and ITS (lower eukaryote) amplicon sequencing data or shotgun metagenome sequences, have now been published. Many of the analyses relate to the South Victoria Land McMurdo Dry Valleys, but more remote edaphic zones such as the Vestfold Hills, Mars Oasis and Robinson Ridge have also been covered [,,,]. Additionally, more “exotic” soil types, including heated alpine soils, ornithogenic soils and niche soil habitats such as biological soil crusts and hypolithons [], have been the focus of detailed phylogenetics surveys.
There is a growing worldwide emphasis on large-scale biogeographic studies of soil microbial diversity. While such published studies in Antarctica are currently restricted to sub-regions of the continent (such as the McMurdo Dry Valleys [] and the Antarctic Peninsula []), a number of continent-wide soil microbiology biogeographical surveys are currently underway, at least one of which includes samples recovered by the 20th December 2016–19th March 2017 Antarctic Circumnavigation Expedition (ACE) cruise [], which accessed soils from a series of remote and rarely visited sub-Antarctic islands.
The Antarctic continent is largely devoid of higher eukaryotes, with the lower latitude regions of the peninsula being the only regions to harbour angiosperms (two species: Deschampsia antarctica and Colobanthus quitensis) and only highly localized peri-coastal areas impacted by marine mammals and birds. Antarctic soils are dominated by prokaryotes, although some lower eukaryotes (fungi, chlorophytes and microfauna) are widespread and others, such as bryophytes and microalgae, occur locally []. In consequence, in much of the continental Antarctic edaphic habitat, ecosystem services, including nitrogen turnover, are thought to be largely performed by prokaryotes.
Most Antarctic soils are considered to be severely oligotrophic and are particularly low in organic nitrogen []. Nitrate accumulation, as commonly found in hot desert soils, is less prevalent in Antarctic cold desert soils. Highly localized coastal soils, sites of penguin rookeries and elephant seal colonies, are hugely enriched in uric acid and organic nitrogen [], while lacustrine cyanobacterial and algal mat biomass is a source of edaphic N input to soils in some of the McMurdo Dry Valleys []. Elsewhere, N inputs are probably restricted to trace amounts in snowfall [] and dinitrogen fixation by diazotrophic microorganisms, particularly Cyanobacteria [].
While an understanding of the distribution of N-fixing and N-processing organisms in Antarctic soils, and knowledge of the presence or absence of key N-cycling genes, is increasingly comprehensive, quantitative data remain remarkably scarce. The very limited data on N-processing rates in any of the different Antarctic soil habitats make it virtually impossible, at the current state of knowledge, to model the nitrogen turnover contributions to ecosystem services in any part of terrestrial Antarctica, with the consequence that an accurate baseline for future estimations of the effects of continental climate change is equally remote.
Here, we review the current state of knowledge of nitrogen processing in Antarctic soils (Figure 1), with an emphasis on the critical gaps in our knowledge base.
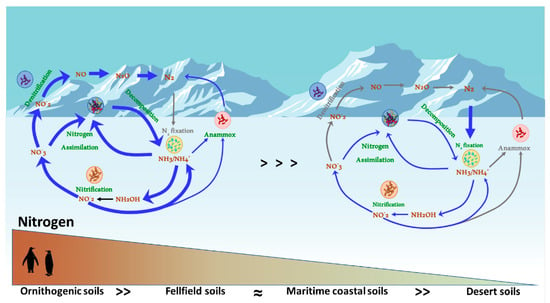
Figure 1.
N-cycling processes in Antarctic soils, across the nitrogen hetero-oligotrophy spectrum. Line colour and thickness represent the relative importance of the individual N biotransformation processes in the various soil types.
2. N Compound Speciation and Quantitation in Antarctic Soils
The continental and sub-Antarctic regions encompass a very large latitudinal scale (over 40 degrees of latitude). This geographical scale has led to the delineation of three biogeographic zones, comprising maritime, sub-Antarctic, and continental Antarctica. Maritime Antarctica includes the Scotia Arc archipelagos, South Orkney and South Shetland Islands and the majority of the Antarctic Peninsula southward to Alexander Island. The sub-Antarctic region is represented by various islands close to the Antarctic polar frontal zone. Continental Antarctica includes the eastern and southern regions of the Antarctic Peninsula and typically includes other Antarctic continental territories [,].
These biogeographic regions harbour a wide spectrum of varyingly constituted soils. The soils are largely oligotrophic, with widespread stoichiometric imbalances []. The availability of total N varies substantially across the different biogeographical zones (Table 1) [,,,,,,]. These variations in nutrient availability certainly contribute to the phylogenetic and functional differences in microbial communities in soils from different biogeographic regions (e.g., []).

Table 1.
Soil nitrogen levels in various Antarctic soils. (* Not determined)
Until the recent recognition that understanding the drivers of microbial communities in Antarctic soils requires a systematic quantification of physiochemical variables and biogeochemical cycling, few studies quantified soil chemistry. These studies have, however, demonstrated remarkably low levels of total organic nitrogen (TON) and NH4+-N in several non-coastal continental soils, although there is evidence of nitrate accumulation in some continental soils [,,]. This excess nitrate is likely to influence soil N stoichiometries and the biological capacity to sequester nitrogen and might ultimately influence the net accumulation of other key elements such as carbon in Antarctic soils. However, due to the sporadic and incomplete data across a range of Antarctic soils, we lack the quantitative estimates required to validate such predictions.
A systematic review of the previous studies shows evidence of low or very low N levels across a range of Antarctic soils. In some regions, N levels are at or below detection levels [,]. There have been suggestions that, in regions such as the McMurdo Dry Valleys, the soil N may be derived from nearby coastal and lacustrine algal mats, where katabatic wind episodes transport organic nitrogen from localized high productivity areas to nearby oligotrophic soils [,,]. These studies are supported, in part, by the detection of known cosmopolitan marine Cyanobacteria including Leptolyngbya, Phormidium, Oscillatoria and Nostoc in these soils [,].
At the opposite end of the scale, ornithogenic soils have high levels of NH4+-N. Guano deposits from penguins and pinnipeds in large breeding colonies appear to be the primary source of this NH4+-N, which is derived from the hydrolysis of uric acid []. The extent to which diazotrophs in these soils may sequester N is unclear, since the effects of high uric acid concentrations are likely to inhibit N sequestration []. Organic nitrogen in coastal soils may be supplemented by marine aerosols or marine algal deposits [], while nitrogen in sub-Antarctic fellfield soils is probably largely plant-derived [].
3. N-Cycling Taxa in Soils
With the complete absence of higher plants from much of ice-free continental Antarctica, much of the soil nutrient cycling is thought to be driven by microbial communities [,] (Table 2). This prediction is corroborated by evidence of the extensive genetic capacity for nitrogen cycling in continental soils and soil-associated niche habitats [,]. These functional nitrogen cycling pathways are similar to those previously observed in bacterial and archaeal phyla [,].

Table 2.
Summary of microorganisms participating in nitrogen cycling.
3.1. Bacterial Nitrogen Cycling in Soils and Cryptic Niches
As for other terrestrial environments, bacteria dominate Antarctic terrestrial niches [,]. Evidence from phylogenetic gene surveys suggests that bacterial taxa mediate the bulk of primary productivity []. Cyanobacteria are widely considered to be the central regulators of nitrogen cycling in soils []. As direct and indirect mediators of nutrient recycling in Antarctic soils, Cyanobacteria play important functional roles as “ecosystem engineers” []. Heterocystous Cyanobacteria, predominantly Nostoc commune, appear to drive nitrogen fixation in Antarctic soils. Other heterocystous Cyanobacteria including Calothrix, Dichothrix, Nodularia and Hydrocoryne may play a role in sequestering nitrogen in soils and rock-associated niches such as hypoliths and endoliths [,,]. Several studies have reported that nitrogen sequestration genes, linked to rate limiting steps in the cycle including ammonia oxidation, are homologous to those previously found in Cyanobacteria including Nitrosospira and Nitrosomonas [,].
In the McMurdo Dry Valleys, hypoliths and endoliths are important sources of nitrogen in hyperoligotrophic soils [,]. In these systems, nitrification is driven by several Cyanobacteria including Nostoc and Anabaena [,]. Denitrification, the reduction of nitrate to N2 gas, is mediated by Deltaproteobacteria, Bacteroidetes [,,] and Actinobacteria [,]. Betaproteobacteria and Planctomycetes are also key taxa implicated in soil nitrate removal via the denitrification and anaerobic ammonium oxidation (annamox) pathways []. It has been suggested [] that Burkholderiales, diazotropic Betaproteobacteria, may also play an important role in N input in newly colonized soils. Other taxa, including Actinobacteria (genus Streptomyces and family Frankeniaceae) and Chloroflexi, are involved in nitrogen fixation in Antarctic soils [,], although their quantitative contributions are unknown.
These studies support the conclusion that the capacity for diazotrophy is widespread in nutrient-poor Antarctic soils, other than in certain high altitude edaphic areas [,,]. However, the extent and importance of interactions among Antarctic taxa, and their importance in diazotrophy, is not well understood, although there is some evidence that cooperativity between Cyanobacteria and other taxa (e.g., Actinobacteria, Bacteroidetes and Proteobacteria) is essential for completion of the nitrogen cycle [,,].
3.2. Archaea Are Drivers of Nitrification in Antarctic Soils and Niche Habitats
Although the occurrence of Archaea in coastal soils has been well documented [], they are the least understood members of the microbial community in Antarctic soils. Archaea have been implicated in nitrogen fixation in soils collected from several sites in the Miers Valley [], while Thaumarchaeota (formerly known as Crenarchaeota Marine Group 1.1b) represents more than 80% of all archaeal sequences in McMurdo Dry Valleys soils []. It was suggested that these taxa (collectively known as ammonia oxidizing archaea: AOA) may be the dominant ammonia oxidizers in this edaphic habitat []. Thaumarchaeota are known to be key players within the global nitrogen cycle due to their involvement in nitrification across a wide range of habitats [,,] by oxidizing ammonia to nitrite, mediated by ammonia monooxygenase (amoA).
Other studies have also demonstrated the presence of AOA, with high abundances of Nitrososphaerales lineages, in Antarctic coastal soils [,,,,]. Quantification of the relative abundances of AOA and AOB (ammonia oxidizing bacteria) in Antarctic Peninsula soils indicated a general dominance of AOA over AOB [], but it was noted that the functional importance of AOB vs. AOA could vary in natural ecosystems [].
Sequences linked to AOA are sporadic in data repositories, with the majority related to Thaumarchaeota previously reported from Antarctic waters and soils [,,]. Overall, a synthesis of the current literature appears to confirm that AOA are ecologically rare in non-maritime Antarctic soils [,,].
3.3. The Role of Fungi in Nitrogen Cycling
Eukaryotes in Antarctic soils are largely fungal and dominated by relatively few ascomycete taxa [,]. Free-living fungi and yeasts are generally of limited abundance [,] and are primarily restricted to lithobiont niches []. However, as for Archaea, low apparent abundance does not necessarily imply that these organisms are not important in functional processes related to nitrogen cycling. For example, fungi in Miers Valley soils have been shown to contain nitrification pathway genes []. Many fungi, including yeasts commonly found in Antarctic habitats such as Rhodotorula muscorum, Rhodotorula mucilaginosa, Cryptococcus aerius and Cryptococcus albidus [], produce enzymes such urease [] and may play an essential role in nitrogen mineralization or ammonification. These species can also assimilate many inorganic and organic nitrogenous compounds and are considered to play an important role in nitrogen turnover in soils, including in nitrogen storage [,,]. In addition, although denitrification is generally considered as a prokaryotic process, fungal denitrifiers, identified as Candida sp and Trichosporon cutaneum, have been found in Antarctic soils []. However, to date, the role of fungi and yeasts in nitrogen cycling in Antarctic soils is still poorly understood.
3.4. Viruses as Drivers of Nitrogen Cycling
While the composition of some Antarctic soil microbial communities is now generally well understood, the role of associated phages and viruses, and their potential influences on microbial dynamics and nitrogen cycling via host cell lysis, remains poorly understood. It has been speculated that viruses may play a very significant role in biogeochemical cycling of Antarctic soils, particularly by inducing species diversification and consequent functionality []. Recent investigations suggest that viruses in Antarctic soils and hypoliths are highly diverse, mainly dominated by Mycobacterium phages [,]. Studies in other systems have hinted at the importance of viruses in metabolic control [,]. It is tempting to speculate that the extreme environmental conditions may promote a lysogenic rather than lytic phage lifestyle, and there is circumstantial evidence of this from Antarctic “metaviromic” studies []. While processes such as phage infection-driven niche differentiation may play important roles in modulating nitrogen cycling in Antarctic soils, we lack the corroborating evidence from viral–host interaction studies to confirm this speculation.
4. N-Cycling Genes in Soils
Most of the surveys of N-cycling functional markers in Antarctic soils (summarised in Figure 1) have focused on the core genes involved in N-fixation (nifH), nitrification (amoA) and denitrification processes (narG for nitrate reduction, nirK and nirS for nitrite reduction, norB for nitric oxide reduction and nosZ for nitrous oxide reduction). Shotgun metagenomic and amplicon sequencing approaches have been used to explore the presence/absence and diversity of key nitrogen cycling genes [,], while gene abundances have been monitored using qPCR and its variations [,,,,] and Geochip microarray technologies [,,].
N-fixation: Diazotrophic members of microbial communities encoding the nitrogenase enzymatic complex are responsible for the biological fixation of atmospheric N2 []. This key, high energy-cost and irreversible reaction involves the reduction of N2 into NH4+ by the canonical Mo-nitrogenase [,]. A functional and mature nitrogenase enzymatic complex can involve multiple genes encoded on different operons [,], with a minimal conserved core of six structural and cofactor biosynthetic genes []. The canonical nitrogenase contains the electron transfer Fe subunit, referred to as nitrogenase reductase and encoded by the nifH gene, and the MoFe protein, a heterodimer containing the active site encoded by the nifDK gene [,].
Nif genes, particularly nifH, are highly conserved and present in a considerable number of phylogenetically divergent bacteria and archaea [,]. Most studies take advantage of the robustness of the nifH gene as a functional marker to identify diazotroph diversity in natural environments []. Across the Antarctic region, nifH gene analysis has been used to determine the abundance of autotrophic Cyanobacteria, particularly in cryptic soil habitats such as hypoliths and endoliths [,,,]. However, the presence of Cyanobacteria is not an absolute indicator of N-fixation as there are reported examples of loss of the nifH gene from their genomes []. However, strong NifH signatures assigned to heterotrophic N-fixers are now considered as evidence for large non-phototroph related inputs of nitrogen into oligotrophic Antarctic soils [,,,]. Diversity analysis of nifH markers in McMurdo Dry Valley hypolithic communities indicated that all potential diazotrophs were associated with Proteobacteria taxa []. Similar results, at the functional level, showed the presence of heterotrophic diazotrophs in association with Cyanobacteria, where over 50% of total nitrogen fixation was assigned to non-autotrophic taxa [].
Nitrification: Nitrification represents the oxidative portion of the nitrogen (N) cycle, the two-step process whereby ammonia is oxidized to nitrite and subsequently to nitrate []. Although most of the surveys of microbial N processes in Antarctic soils have focused on N-fixation, several studies of the diversity and abundance of nitrifiers have been conducted in different regions of Antarctica, including lakes in the Ross Sea region [,,], on the Antarctic Peninsula [,] and on McMurdo Dry Valley soils [,,].
Nitrification processes, carried out by AOA and AOB, are restricted to a limited range of taxa [,]. Magalhães et al. identified only four AOA and three AOB amoA OTUs in four different and highly heterogeneous Dry Valley soils []. These were derived from Nitrosospira-like taxa, typically associated with pristine environments and low soil NH4-N levels [,,,,]. While several studies have shown a predominance of AOA over AOB [], the ratio of the two clades varies for different Antarctic soils. In the Antarctic Peninsula, archaeal amoA genes were dominant compared to their bacterial counterparts [], but large variations in AOA and AOB amoA gene abundance were detected in four Dry Valley soils []. It was concluded that soil geochemical properties (i.e., pH, C/N, Mg, Cr, Mn, Co, Ni and Cu) and other environmental variables such as water availability have a significant impact on the relative abundances of AOA and AOB amoA genes [,,].
Denitrification: To complete the cycle and return the N2 to the atmosphere, nitrate reductase, encoded by the narGHJI operon, is responsible for the reduction of nitrate to nitrite []. In the second step, two types of nitrite reductase catalyse the reduction of nitrite to nitric oxide: a cytochrome cd1 encoded by nirS or a Cu-containing enzyme encoded by nirK [,]. Subsequently, nitric oxide is reduced by the nitrite oxide reductase encoded by norB, which produces nitrous oxide, a powerful greenhouse product with significant implications for global warming. Finally, nitrous oxide is reduced to N2 by nitrous oxide reductase encoded by nosZ [].
In general, Antarctic soils harbour the genetic capacity to complete the denitrification process []. The genes responsible for denitrification have been detected in widely different Antarctic soil habitats, including the sub-Antarctic, maritime Antarctica and desert soils and lithic niches in the McMurdo Dry Valleys [,,,]. However, the abundance and diversity of denitrification functional markers can differ significantly between sampling locations and can be affected by temperature, vegetation type and macrofauna [,,].
5. N-Cycling in Antarctic Soils: Rates, Processes and Ecosystem Services
Microbial communities in Antarctic terrestrial habitats are thought to be primary ecosystem service providers through the input of nitrogen gas into the biosphere via nitrogen fixation into ammonia and subsequent nitrification []. This assumption is partly informed by the abundance of N2-fixing nitrifying microorganisms in both open soils and cryptic niches, such as diazotrophic Cyanobacteria and Proteobacteria [,,], as well as ammonia oxidizing mosses that dominate several Antarctic landscapes []. However, only a limited number of studies have focused on the kinetics of the nitrogen cycle within these terrestrial systems (Table 3).

Table 3.
Quantification of N2-fixation and nitrification processes in terrestrial Antarctic soil habitats.
Studies based on the acetylene reduction assay, which measures nifH activity of N2-fixing microbes [], have shown that moss-associated Cyanobacteria Nostoc commune in the slopes of Vestfold Hills, Eastern Antarctica, contributed between 52 and 119 mg N m−2 yr−1 to the local ecosystem [], while cyanobacteria-dominated hypoliths nearby the McMurdo Station are estimated to contribute a combined 0.38 kg N yr−1 []. Similarly, studies measuring the rates of ammonia nitrification provided evidence for the nitrification potential of soils from geothermally distinct Antarctic Dry Valley [,]. While the inconsistency in rate measurement units between studies make them difficult to compare, the consensus is that these rates are biologically relevant for the ecosystem services of Antarctic terrestrial systems [,]. In addition to the innate ability of soil microbial communities to fix N2, the N-cycle kinetics of Antarctic terrestrial systems is also affected by external inputs such as water incursions from lakes and ornithogenic sources [,]. For instance, the wet deposition of guano in the Penguin Colony, Ardley Island, was shown to increase the nitrate input into the soils, which in turn had a negative effect on N2-fixation rates while driving N loss through the increase in denitrification rates []. Glacial melt streams in the McMurdo Valley have been also shown to contribute to the N input in downstream soil microbial mats through the transport of Nostoc-derived mineralized ammonia and nitrate []. Another study focusing on ephemerally wetted soils showed that hyporheic sites close to the margins of streams in the Miers Valley exhibited much higher N2 fixation rates (0.04 to 5.8 nmol N cm−3 h−1) than arid sites in the same region, thus demonstrating that the availability of water plays an important role in N-cycling rates of Antarctic soil communities []. As transcriptomics and metagenomics data on Antarctic terrestrial microbial communities become increasingly available, more studies need to be conducted to link the stoichiometry of N-cycle gene presence and expression to actual kinetics of ecosystem services in these habitats.
6. N Supplementation Experiments
Given the extreme environmental conditions prevailing in terrestrial Antarctic habitats, the indigenous microbial communities may be particularly vulnerable to rapid changes in microenvironment, including nutrient status [,,]. Various in situ and ex situ microcosm studies have addressed the effects of N supplementation on microbial community composition; the results are frequently contradictory, their interpretation is often made more complex by the inclusion of co-variables (temperature, C supplementation) and there is little consistency between studies in the analytical methods.
Nitrogen addition to McMurdo Dry Valley soils, in the form of glycine and NH4Cl, induced increases in microbial enzymatic activities, respiration rates and ELFA (ester linked fatty acid) titres [,,,]. Garwood Valley soils, supplemented with NH4Cl, showed a rapid increase in respiration rates (cf. glucose supplemented soils), suggesting that the soil microbial community was N limited []. A study of the combined effect of warming and tryptic soy broth supplementation in maritime Antarctica soils on microbial community composition concluded that nitrogen addition, but not temperature, could induce short-term changes in community structure []. More specifically, the addition of NH4Cl, along with warming in open top chambers, resulted in a reduction in Gram-positive bacterial markers in EFLA analyses [].
Warmer temperatures increase the availability of meltwater from permafrost and glaciers, affecting N-cycling by soil microbial communities [,]. For instance, warm and wet conditions stimulated microbial activity when glycine and tryptic soy broth were added to a maritime Antarctic soil in the Signy Island [], resulting in the increase in both organic and inorganic N. In particular, the input of carbon in Antarctic soils (either through meltwater or other sources) has been shown to significantly change the kinetics of N-cycling, resulting in net N immobilization [,] and changes in the N mineralization process [,,,,]. Meltwater pulses with C and N addition induced a positive nutrient cycling response in McMurdo Dry Valley soils [], but increased water and organic substrate availability led to a loss of prokaryotic taxonomic diversity [,,].
Despite the observed changes in the microbial community and functional responses to N supplementation, there is still no clear consensus on the impact of N addition, at any level. There is a clear need for further investigations of microbial community dynamics, coupled with gene expression studies using metatranscriptomics and metabolomics, in order to better understand the resilience and responsiveness of N-cycling taxa in these Antarctic soils.
7. Gaps in Current Knowledge
One of the gaps that has become apparent during this review of the current literature is that the data on nitrogen compound speciation and quantification, while well distributed over the different Antarctic soil environments, are old, having been mostly acquired several decades ago. There are questions as to how relevant these data still are as nitrogen availability may have changed in the intervening years [,].
Data on both the nitrogen cycling taxa and genes, largely derived from the application of modern next generation sequencing approaches, are abundant and well distributed over the ice-free soils of Antarctica. However, there are still important questions which remain unanswered. Studies of Antarctic soil microbial composition, outside of manipulation experiments such as the addition of water [], have been performed at single time points. Such experiments do not permit valid conclusions on how the microbial communities change over time. Previous research has shown that Antarctic microbial communities are sensitive to changes in climatic conditions [,], so repeated observations will be important to understand how Antarctic soil microbial communities respond to a changing climate and how this affects nitrogen cycling.
Transcriptomic data from Antarctic soils are, generally, sorely lacking, with a few notable exceptions (e.g., []). Database searches of EMBL-EBI MGnify, and transcriptome BioProjects in NCBI, using “Antarctica soil” as a search term do not identify any current Antarctic metatranscriptome datasets. This is, in part, a result of the well-known (but generally not reported) difficulties associated with RNA extractions from extremely oligotrophic continental Antarctic soils. Knowledge of which genes are present is important but it does not tell us whether the bacteria that possess these genes are active or not. It is clear that more studies need to make use of metatranscriptomics to identify which genes (and pathways and processes) are expressed and at what levels.
In addition, approaches which rely on PCR primers targeted to conserved gene regions may struggle to cope with the diversity of sequences present in Antarctic habitats (unpublished data). This may be exacerbated if Antarctic microorganisms make use of pathways that are rare or absent elsewhere. For example, alternative nitrogenases may be expressed and have equal or better function than the canonical molybdenum nitrogenases. There is evidence that the contribution of alternative nitrogenases has been underestimated [].
Studies on the rates of nitrogen cycling in Antarctic soils are rare [] and tend to be limited in scope, focusing on very specific situations and only a few geographical locations. Part of this may be due to suggestions that there is little to no biological nitrogen fixation in Antarctic soils [], making the question seem unimportant. There is a clear need for a broader view of nitrogen pathways, rates and stoichiometry, and future investigations will benefit from addressing the previously identified limitations.
Published nitrogen supplementation experiments have investigated how community compositions change but there is still a need to identify changes in gene expression. Such experiments are also good opportunities for metabolomic investigations in order to gain a more in-depth understanding of how nitrogen influences microbial community functionality [,]. To the authors’ knowledge, there has only been a single reported metabolomic investigation of Antarctic soil microbial communities [].
Given that there are only limited data on nitrogen cycling processes in Antarctic soils, and given the importance of N-cycling as a core component of ecosystem servicing, we offer the following recommendations:
- (1)
- In agreement with Guerra et al. [], there is a need to establish a wider international effort for regular, long-term monitoring of different Antarctic environments in order to acquire data which can show temporal shifts in both soil nutrient content and in microbial community composition.
- (2)
- Given that much of the basic data on soil chemistry from terrestrial Antarctica are decades out of date, new datasets are desperately needed if we are to understand the effects of a changing Antarctic climate.
- (3)
- While there are now numerous published metagenomic studies available for various Antarctic regions and habitats, there is a distinct lack of transcriptomic, proteomic and metabolomic data, all of which are required for a deeper understanding of community function and nutrient cycling dynamics.
- (4)
- Investigations into Antarctic microbial community composition and function should be performed with an awareness of the uniqueness of the continent and its biota. A reliance on homology-based molecular screening methods may skew community structure and function data, given the possibility that Antarctic microorganisms may make use of pathways and genes which may be absent or considered inconsequential in other less “extreme” environments.
Author Contributions
All authors contributed equally to the preparation of this manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
No external funding was required to support this work.
Acknowledgments
The authors wish to thank the National Research Foundation SANAP program for project support (grant number 110717) and for postdoctoral bursary funding for M.O. We also wish to thank the University of Pretoria for postdoctoral bursary funding for C.C., J.B., S.V. and J.J. and for Senior Postdoctoral Fellowship funding for P.L.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Brooks, S.T.; Jabour, J.; van den Hoff, J.; Bergstrom, D.M. Our footprint on Antarctica competes with nature for rare ice-free land. Nat. Sustain. 2019, 2, 185–190. [Google Scholar] [CrossRef]
- Lee, C.K.; Laughlin, D.C.; Bottos, E.M.; Caruso, T.; Joy, K.; Barrett, J.E.; Hopkins, D.W.; Pointing, S.B.; McDonal, I.R.; Cowan, D.A.; et al. Biotic interactions are an unexpected yet critical control on the complexity of an abiotically driven polar ecosystem. Commun. Biol. 2019, 2, 62. [Google Scholar] [CrossRef]
- Bottos, E.M.; Laughlin, D.C.; Herbold, C.W.; Lee, C.K.; McDonald, I.R.; Cary, S.C. Abiotic factors influence patterns of bacterial diversity and community composition in the Dry Valleys of Antarctica. FEMS Microbiol. Ecol. 2020, 96, fiaa042. [Google Scholar] [CrossRef]
- Lee, C.K.; Barbier, B.A.; Bottos, E.M.; McDonald, I.R.; Cary, S.C. The inter-valley soil comparative survey: The ecology of dry valley edaphic microbial communities. ISME J. 2012, 6, 1046–1057. [Google Scholar] [CrossRef]
- Lepane, V.; Künnis-Beres, K.; Kaup, E.; Sharma, B. Dissolved organic matter, nutrients, and bacteria in Antarctic soil core from Schirmacher Oasis. J. Soils Sediments 2018, 18, 2715–2726. [Google Scholar] [CrossRef]
- Cowan, D.A. Microbiology of Antarctic Soils; Springer: Berlin/Heidelberg, Germany, 2014; 328p, ISBN 978-3-642-45212-3. [Google Scholar]
- Dennis, P.G.; Newsham, K.K.; Rushton, S.P.; O’Donnell, A.G.; Hopkins, D.W. Soil bacterial diversity is positively associated with air temperature in the maritime Antarctic. Sci. Rep. 2019, 9, 2686. [Google Scholar] [CrossRef]
- Walton, D.W.H.; Thomas, J. Cruise Report—Antarctic Circumnavigation Expedition (ACE) 20th December 2016—19th March 2017; OpenAIRE: Birmingham, UK, 2018; pp. 1–380. [Google Scholar] [CrossRef]
- Terauds, A.; Lee, J.R. Antarctic biogeography revisited: Updating the Antarctic Conservation Biogeographic Regions. Divers. Distribut. 2016, 22, 836–840. [Google Scholar] [CrossRef]
- Bokhorst, S.; Convey, P.; Aerts, R. Nitrogen inputs by marine vertebrates drive abundance and richness in Antarctic terrestrial ecosystems. Curr. Biol. 2019, 29, 1721–1729. [Google Scholar] [CrossRef]
- Winton, V.H.L.; Ming, A.; Caillon, N.; Hauge, L.; Jones, A.E.; Savarino, J.; Yang, X.; Frey, M.M. Deposition, recycling and archival of nitrate stable isotopes between the air-snow interface: Comparison beteen Dronning Maud Land and Dome C, Antarctica. Atmos. Chem. Phys. 2020, 20, 5861–5885. [Google Scholar] [CrossRef]
- Makhalanyane, T.P.; Valverde, A.; Velázquez, D.; Gunnigle, E.; Van Goethem, M.W.; Quesada, A.; Cowan, D.A. Ecology and biogeochemistry of cyanobacteria in soils, permafrost, aquatic and cryptic polar habitats. Biodivers. Conserv. 2015, 24, 819–840. [Google Scholar] [CrossRef]
- Selkirk, P.M. The nature and importance of the sub-Antarctic. In Papers and Proceedings of the Royal Society of Tasmania; The Royal Society of Tasmania: Hobart, Australia, 2007; Volume 1, pp. 1–6. [Google Scholar] [CrossRef]
- Convey, P. Antarctic ecosystems. In Encyclopedia of Biodiversity, 2nd ed.; Levin, S.A., Ed.; Elsevier: San Diego, CA, USA, 2013; pp. 179–188. [Google Scholar]
- Cameron, R.E. Cold desert characteristic and problems relevant to other arid land. In Arid Lands in Perspective; McGinnies, W.G., Goldman, B.G., Eds.; University of Arizona Press: Tucson, AZ, USA, 1969; pp. 167–205. [Google Scholar]
- Cameron, R.E.; Devaney, J.R. Antarctic soil algal crusts: A scanning electron and optical microscope study. Trans. Am. Microsc. Soc. 1970, 80, 264–273. [Google Scholar] [CrossRef]
- Cameron, R.E. Antarctic soil microbial and ecological investigations. In Research in the Antarctic; Quam, L.O., Porter, H.D., Eds.; Springer: Washington, DC, USA, 1971; pp. 137–189. [Google Scholar]
- Spider, T.W.; Cowling, J.C. Ornithogenic soils of the Cape Bird Adelie Penguin rookeries, Antarctica. Polar Biol. 1984, 2, 199–205. [Google Scholar] [CrossRef]
- Vishniac, H. The microbiology of Antarctic soils. In Antarctic Microbiology; Friedmann, E.I., Ed.; Wiley-Liss: New York, NY, USA, 1993; pp. 297–341. [Google Scholar]
- Cowan, D.A.; Ah Tow, L. Endangered Antarctic Environments. Annu. Rev. Microbiol. 2004, 58, 649–690. [Google Scholar] [CrossRef]
- Lachacz, A.; Kalisz, B.; Gielwanoska, I.; Olech, M.; Chwedorzewka, K.; Kellmann-Sopyla, W. Nutrient abundance and variability from soils in the coast of King George Island. Soil Sci. Plant Nutr. 2018, 2, 294–311. [Google Scholar] [CrossRef]
- Michalski, G.; Bockheim, J.G.; Kendall, C.; Thiemens, M. Isotopic composition of Antarctic Dry Valley nitrate: Implications for NOy sources and cycling in Antarctica. Geophys. Res. Lett. 2005, 32. [Google Scholar] [CrossRef]
- Barrett, J.E.; Virginia, R.A.; Wall, D.H.; Cary, S.C.; Adams, B.J.; Hacker, A.L.; Aislabie, J.M. Co-variation in soil biodiversity and biogeochemistry in northern and southern Victoria Land, Antarctica. Antarct. Sci. 2006, 18, 535–548. [Google Scholar] [CrossRef]
- Zhu, R.; Liu, Y.; Ma, E.; Sun, J.; Xu, H.; Sun, L. Nutrient compositions and potential greenhouse gas production in penguin guano, ornithogenic soils and seal colony soils in coastal Antarctica. Antarct. Sci. 2009, 21, 427–428. [Google Scholar] [CrossRef]
- Jung, J.; Yeom, J.; Kim, J.; Han, J.; Lim, H.S.; Park, H.; Hyun, S.; Park, W. Change in gene abundance in the nitrogen biogeochemical cycle with temperature and nitrogen addition in Antarctic soils. Res. Microbiol. 2011, 162, 1018–1026. [Google Scholar] [CrossRef]
- Cowan, D.A.; Makhalanyane, T.P.; Dennis, P.G.; Hopkins, D.W. Microbial ecology and biogeochemistry of continental antarctic soils. Front. Microbiol. 2014, 5, 154. [Google Scholar] [CrossRef]
- Parish, T.R. A numerical study of strong katabatic winds over Antarctica. Mon. Weather Rev. 1984, 112, 545–554. [Google Scholar] [CrossRef]
- Nylen, T.H.; Fountain, A.G. Climatolotogy of katabatic winds in the McMurdo Dry Valleys, Antarctica. J. Geophs. Res. 2004, 109, D03114. [Google Scholar] [CrossRef]
- Wood, S.; Rueckert, A.; Cowan, D.; Cary, S.C. Sources of edaphic cyanobacterial diversity in the Dry Valleys of Eastern Antarctica. ISME J. 2008, 2, 308–320. [Google Scholar] [CrossRef] [PubMed]
- Jungblut, A.D.; Hawes, I.; Mountfort, D.; Hitzfeld, B.; Dietrich, D.R.; Burns, B.P.; Neilan, B.A. Diversity within Cyanobacterial mat communities in variable salinity meltwater ponds of McMurdo Ice Shelf, Antarctica. Environ. Microbiol. 2005, 7, 519–529. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.M.; Elster, J. Cyanobacteria in Antarctic lake Environments. In Algae and Cyanobacteria in Extreme Environments: Cellular Origin, Life in Extreme Habitats and Astrobiology; Seckbach, J., Ed.; Springer: Dordrecht, The Netherlands, 2007; Volume 11. [Google Scholar] [CrossRef]
- Hutchinson, G.E. Survey of existing knowledge of biogeochemistry. 3. of vertebrate excretion. Bull. Am. Mus. Natl. Hist. 1950, 96, 71–94. [Google Scholar]
- Tatur, A.; Myrcha, A. Ornithogenic soils on King George Island, South Shetland Island (Maritime Antarctic Zone). Pol. Polar Res. 1984, 5, 31–60. [Google Scholar]
- Chan, Y.; Van Nostrand, J.D.; Zhou, J.; Pointing, S.B.; Farrell, R.L. Functional ecology of an Antarctic Dry Valley. Proc. Natl. Acad. Sci. USA 2013, 110, 8990–8995. [Google Scholar] [CrossRef] [PubMed]
- Pointing, S.B.; Chan, Y.; Lacap, D.C.; Lau, M.C.Y.; Jurgens, J.A.; Farrell, R.L. Highly specialized microbial diversity in hyper-arid polar desert. Proc. Natl. Acad. Sci. USA 2009, 106, 19964–19969. [Google Scholar] [CrossRef] [PubMed]
- Cowan, D.A.; Sohm, J.A.; Makhalanyane, T.P.; Capone, D.G.; Green, T.G.A.; Cary, S.C.; Tuffin, I.M. Hypolithic communities: Important nitrogen sources in Antarctic desert soils. Environ. Microbiol. Rep. 2011, 3, 581–586. [Google Scholar] [CrossRef]
- Wei, S.T.S.; Lacap-Bugler, D.C.; Lau, M.C.Y.; Caruso, T.; Rao, S.; de los Rios, A.; Archer, S.K.; Chiu, J.M.Y.; Higgins, C.; Van Nostrand, J.D.; et al. Taxonomic and functional diversity of soil and hypolithic microbial communities in Miers Valley, McMurdo Dry Valleys, Antarctica. Front. Microbiol. 2016, 7, 1642. [Google Scholar] [CrossRef]
- Cary, S.C.; McDonald, I.R.; Barrett, J.E.; Cowan, D.A. On the rocks: The microbiology of Antarctic Dry Valley soils. Nat. Rev. Microbiol. 2010, 8, 129–138. [Google Scholar] [CrossRef]
- Komárek, J.; Genuário, D.B.; Fiore, M.F.; Elster, J. Heterocytous cyanobacteria of the Ulu Peninsula, James Ross Island, Antarctica. Polar Biol. 2015, 38, 475–492. [Google Scholar] [CrossRef]
- Van Goethem, M.W.; Cowan, D.A. Role of Cyanobacteria in the Ecology of Polar Environments. In The Ecological Role of Micro-Organisms in the Antarctic Environment; Springer Polar Sciences: Cham, Switzerland, 2019; pp. 3–23. ISBN 9783030027865. [Google Scholar]
- Hayashi, K.; Tanabe, Y.; Fujitake, N.; Kida, M.; Wang, Y.; Hayatsu, M.; Kudoh, S. Ammonia oxidation potentials and ammonia oxidizers of lichen–moss vegetated soils at two ice-free areas in east antarctica. Microbes Environ. 2020, 35, 2–6. [Google Scholar] [CrossRef]
- Magalhães, C.; Machado, A.; Frank-Fahle, B.; Lee, C.K.; Cary, C.S. The ecological dichotomy of ammonia-oxidizing archaea and bacteria in the hyper-arid soils of the Antarctic Dry Valleys. Front. Microbiol. 2014, 5, 515. [Google Scholar] [CrossRef]
- Fernández-Valiente, E.; Quesada, A.; Howard-Williams, C.; Hawes, I. N2-fixation in cyanobacterial mats from ponds on the McMurdo Ice Shelf, Antarctica. Microb. Ecol. 2001, 42, 338–349. [Google Scholar] [CrossRef]
- de Scally, S.Z.; Makhalanyane, T.P.; Frossard, A.; Hogg, I.D.; Cowan, D.A. Antarctic microbial communities are functionally redundant, adapted and resistant to short term temperature perturbations. Soil Biol. Biochem. 2016, 103, 160–170. [Google Scholar] [CrossRef]
- Papale, M.; Conte, A.; Mikkonen, A.; Michaud, L.; La Ferla, R.; Azzaro, M.; Caruso, G.; Paranhos, R.; Cabral Anderson, S.; Maimone, G.; et al. Prokaryotic assemblages within permafrost active layer at Edmonson Point (Northern Victoria Land, Antarctica). Soil Biol. Biochem. 2018, 123, 165–179. [Google Scholar] [CrossRef]
- Garrido-Benavent, I.; Pérez-Ortega, S.; Durán, J.; Ascaso, C.; Pointing, S.B.; Rodríguez-Cielos, R.; Navarro, F.; de los Ríos, A. Differential Colonization and Succession of Microbial Communities in Rock and Soil Substrates on a Maritime Antarctic Glacier Forefield. Front. Microbiol. 2020, 11, 126. [Google Scholar] [CrossRef]
- Richter, I.; Herbold, C.W.; Lee, C.K.; McDonald, I.R.; Barrett, J.E.; Cary, S.C. Influence of soil properties on archaeal diversity and distribution in the McMurdo Dry Valleys, Antarctica. FEMS Microbiol. Ecol. 2014, 89, 347–359. [Google Scholar] [CrossRef]
- Han, J.; Jung, J.; Park, M.; Hyun, S.; Park, W. Short-term effect of elevated temperature on the abundance and diversity of bacterial and archaeal amoA genes in antarctic soils. J. Microbiol. Biotechnol. 2013, 23, 1187–1196. [Google Scholar] [CrossRef]
- Karaevskaya, E.S.; Demchenko, L.S.; Demidov, N.E.; Rivkina, E.M.; Bulat, S.A.; Gilichinsky, D.A. Archaeal diversity in permafrost deposits of Bunger Hills Oasis and King George Island (Antarctica) according to the 16S rRNA gene sequencing. Microbiology 2014, 83, 398–406. [Google Scholar] [CrossRef]
- Buzzini, P.; Branda, E.; Goretti, M.; Turchetti, B. Psychrophilic yeasts from worldwide glacial habitats: Diversity, adaptation strategies and biotechnological potential. FEMS Microbiol. Ecol. 2012, 82, 217–241. [Google Scholar] [CrossRef]
- Buzzini, P.; Margesin, R. Cold-adapted yeasts: A lesson from the cold and a challenge for the XXI century. In Cold-Adapted Yeasts; Springer: Berlin/Heidelberg, Germany, 2014; pp. 3–22. [Google Scholar]
- Vishniac, H.S. Yeast Biodiversity in the Antarctic. In Biodiversity and Ecophysiology of Yeasts; Springer: Berlin/Heidelberg, Germany, 2006; pp. 419–440. [Google Scholar] [CrossRef]
- Amarelle, V.; Carrasco, V.; Fabiano, E. The hidden life of Antarctic rocks. In The Ecological Role of Micro-Organisms in the Antarctic Environment; Springer: Cham, Switzerland, 2019; pp. 221–237. [Google Scholar] [CrossRef]
- Makhalanyane, T.P.; Valverde, A.; Lacap, D.C.; Pointing, S.B.; Tuffin, M.I.; Cowan, D.A. Evidence of species recruitment and development of hot desert hypolithic communities. Environ. Microbiol. Rep. 2013, 5, 219–224. [Google Scholar] [CrossRef]
- Van Dorst, J.; Benaud, N.; Ferrari, B. New Insights into the Microbial Diversity of Polar Desert Soils: A Biotechnological Perspective. In Microbial Ecology of Extreme Environments; Springer: Cham, Switzerland, 2017; pp. 169–183. ISBN 9783319516868. [Google Scholar]
- Ferrari, B.C.; Bissett, A.; Snape, I.; van Dorst, J.; Palmer, A.S.; Ji, M.; Siciliano, S.D.; Stark, J.S.; Winsley, T.; Brown, M.V. Geological connectivity drives microbial community structure and connectivity in polar, terrestrial ecosystems. Environ. Microbiol. 2016, 18, 1834–1849. [Google Scholar] [CrossRef]
- Ji, M.; Greening, C.; Vanwonterghem, I.; Carere, C.R.; Bay, S.K.; Steen, J.A.; Montgomery, K.; Lines, T.; Beardall, J.; Van Dorst, J.; et al. Atmospheric trace gases support primary production in Antarctic desert surface soil. Nature 2017, 552, 400–403. [Google Scholar] [CrossRef]
- Yergeau, E.; Hogues, H.; Whyte, L.G.; Greer, C.W. The functional potential of high Arctic permafrost revealed by metagenomic sequencing, qPCR and microarray analyses. ISME J. 2010, 4, 1206–1214. [Google Scholar] [CrossRef]
- Treusch, A.H.; Leininger, S.; Kietzin, A.; Schuster, S.C.; Klenk, H.P.; Schleper, C. Novel genes for nitrite reductase and Amo-related proteins indicate a role of uncultivated mesophilic crenarchaeota in nitrogen cycling. Environ. Microbiol. 2005, 7, 1985–1995. [Google Scholar] [CrossRef]
- Hatzenpichler, R.; Lebedeva, E.V.; Spieck, E.; Stoecker, K.; Richter, A.; Daims, H.; Wagner, M. A moderately thermophilic ammonia-oxidizing crenarchaeote from a hot spring. Proc. Natl. Acad. Sci. USA 2008, 105, 2134–2139. [Google Scholar] [CrossRef]
- Leininger, S.; Urich, T.; Schloter, M.; Schwark, L.; Qi, J.; Nicol, G.W.; Prosser, J.I.; Schuster, S.C.; Schleper, C. Archaea predominate among ammonia-oxidizing prokaryotes in soils. Nature 2006, 442, 806–809. [Google Scholar] [CrossRef]
- Magalhães, C.; Stevens, M.I.; Cary, S.C.; Ball, B.A.; Storey, B.C.; Wall, D.H.; Türk, R.; Ruprecht, U. At Limits of Life: Multidisciplinary Insights Reveal Environmental Constraints on Biotic Diversity in Continental Antarctica. PLoS ONE 2012, 7, e44578. [Google Scholar] [CrossRef]
- Barnard, S.; Van Goethem, M.W.; de Scally, S.Z.; Cowan, D.A.; van Rensburg, P.J.; Claassens, S.; Makhalanyane, T.P. Increased temperatures alter viable microbial biomass, ammonia oxidizing bacteria and extracellular enzymatic activities in Antarctic soils. FEMS Microbiol. Ecol. 2020, 96, fiaa065. [Google Scholar] [CrossRef]
- Rao, S.; Chan, Y.; Lacap, D.C.; Hyde, K.D.; Pointing, S.B.; Farrell, R.L. Low-diversity fungal assemblage in an Antarctic Dry Valleys soil. Polar Biol. 2012, 35, 567–574. [Google Scholar] [CrossRef]
- Vero, S.; Garmendia, G.; Martínez-Silveira, A.; Cavello, I.; Wisniewski, M. Yeast Activities Involved in Carbon and Nitrogen Cycles in Antarctica. In The Ecological Role of Micro-organisms in the Antarctic Environment; Springer: Cham, Switzerland, 2019; pp. 45–64. [Google Scholar]
- Kurtzman, C.; Fell, J.W.; Boekhout, T. The Yeasts: A Taxonomic Study; Elsevier: Amsterdam, The Netherlands, 2011. [Google Scholar]
- Anesio, A.M.; Bellas, C.M. Are low temperature habitats hot spots of microbial evolution driven by viruses? Trends Microbiol. 2011, 19, 52–57. [Google Scholar] [CrossRef]
- Wei, S.T.S.; Higgins, C.M.; Adriaenssens, E.M.; Cowan, D.A.; Pointing, S.B. Genetic signatures indicate widespread antibiotic resistance and phage infection in microbial communities of the McMurdo Dry Valleys, East Antarctica. Polar Biol. 2015, 38, 919–925. [Google Scholar] [CrossRef]
- Zablocki, O.; van Zyl, L.; Adriaenssens, E.M.; Rubagotti, E.; Tuffin, M.; Cary, S.C.; Cowan, D. High-level diversity of tailed phages, eukaryote-associated viruses, and virophage-like elements in the metaviromes of antarctic soils. Appl. Environ. Microbiol. 2014, 80, 6879–6887. [Google Scholar] [CrossRef]
- Adriaenssens, E.M.; Kramer, R.; Van Goethem, M.W.; Makhalanyane, T.P.; Hogg, I.; Cowan, D.A. Environmental drivers of viral community composition in Antarctic soils identified by viromics. Microbiome 2017, 5, 83. [Google Scholar] [CrossRef]
- Dávila-Ramos, S.; Castelán-Sánchez, H.G.; Martínez-ávila, L.; Sánchez-Carbente, M.D.R.; Peralta, R.; Hernández-Mendoza, A.; Dobson, A.D.W.; Gonzalez, R.A.; Pastor, N.; Batista-García, R.A. A review on viral metagenomics in extreme environments. Front. Microbiol. 2019, 10, 2403. [Google Scholar] [CrossRef]
- Hutchins, P.R.; Miller, S.R. Genomics of variation in nitrogen fixation activity in a population of the thermophilic cyanobacterium Mastigocladus laminosus. ISME J. 2017, 11, 78–86. [Google Scholar] [CrossRef]
- Coyne, K.J.; Parker, A.E.; Lee, C.K.; Sohm, J.A.; Kalmbach, A.; Gunderson, T.; León-Zayas, R.; Capone, D.G.; Carpenter, E.J.; Cary, S.C. The distribution and relative ecological roles of autotrophic and heterotrophic diazotrophs in the McMurdo Dry Valleys, Antarctica. FEMS Microbiol. Ecol. 2020, 96, fiaa010. [Google Scholar] [CrossRef]
- Yergeau, E.; Kang, S.; He, Z.; Zhou, J.; Kowalchuk, G.A. Functional microarray analysis of nitrogen and carbon cycling genes across an Antarctic latitudinal transect. ISME J. 2007, 1, 163–179. [Google Scholar] [CrossRef]
- Niederberger, T.; Sohm, J.; Tirindelli, J.; Gunderson, T.; Capone, D.; Carpenter, E.; Cary, S. Diverse and highly active diazotrophic assemblages inhabit ephermally wetted soils of the Antarctic Dry Valleys. FEMS Microbiol. Ecol. 2012, 82, 376–390. [Google Scholar] [CrossRef]
- Crane, S.L.; van Dorst, J.; Hose, G.C.; King, C.K.; Ferrari, B.C. Microfluidic qPCR enables high throughput quantification of microbial functional genes but requires strict curation of primers. Front. Environ. Sci. 2018, 6, 145. [Google Scholar] [CrossRef]
- Asuming-Brempong, S. Microarray Technology and Its Applicability in Soil Science—A Short Review. Open J. Soil Sci. 2012, 2, 333–340. [Google Scholar] [CrossRef]
- Levy-Booth, D.J.; Prescott, C.E.; Grayston, S.J. Microbial functional genes involved in nitrogen fixation, nitrification and denitrification in forest ecosystems. Soil Biol. Biochem. 2014, 75, 11–25. [Google Scholar] [CrossRef]
- Bellenger, J.P.; Darnajoux, R.; Zhang, X.; Kraepiel, A.M.L. Biological nitrogen fixation by alternative nitrogenases in terrestrial ecosystems: A review. Biogeochemistry 2020, 149, 53–73. [Google Scholar] [CrossRef]
- Rubio, L.M.; Ludden, P.W. Maturation of nitrogenase: A biochemical puzzle. J. Bacteriol. 2005, 187, 405–414. [Google Scholar] [CrossRef]
- Dos Santos, P.C.; Fang, Z.; Mason, S.W.; Setubal, J.C.; Dixon, R. Distribution of nitrogen fixation and nitrogenase-like sequences amongst microbial genomes. BMC Genom. 2012, 13, 162. [Google Scholar] [CrossRef]
- Seefeldt, L.C.; Hoffman, B.M.; Dean, D.R. Mechanism of Mo-dependent nitrogenase. Annu. Rev. Biochem. 2009, 78, 701–722. [Google Scholar] [CrossRef]
- Hoffman, B.M.; Lukoyanov, D.; Dean, D.R.; Seefeldt, L.C. Nitrogenase: A draft mechanism. Acc. Chem. Res. 2013, 46, 587–595. [Google Scholar] [CrossRef]
- Falkowski, P.G.; Godfrey, L.V. Electrons, life and the evolution of Earth’s oxygen cycle. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2008, 363, 2705–2716. [Google Scholar] [CrossRef]
- Godfrey, L.V.; Falkowski, P.G. The cycling and redox state of nitrogen in the Archaean ocean. Nat. Geosci. 2009, 2, 725–729. [Google Scholar] [CrossRef]
- Zehr, J.P.; Jenkins, B.D.; Short, S.M.; Steward, G.F. Nitrogenase gene diversity and microbial community structure: A cross-system comparison. Environ. Microbiol. 2003, 5, 539–554. [Google Scholar] [CrossRef] [PubMed]
- Latysheva, N.; Junker, V.L.; Palmer, W.J.; Codd, G.A.; Barker, D. The evolution of nitrogen fixation in cyanobacteria. Bioinformatics 2012, 28, 603–606. [Google Scholar] [CrossRef]
- Lacap-Bugler, D.C.; Lee, K.K.; Archer, S.; Gillman, L.N.; Lau, M.; Leuzinger, S.; Lee, C.K.; Maki, T.; McKay, C.P.; Perrott, J.K.; et al. Global Diversity of Desert Hypolithic Cyanobacteria. Front. Microbiol. 2017, 8, 867. [Google Scholar] [CrossRef] [PubMed]
- Voytek, M.A.; Priscu, J.C.; Ward, B.B. The distribution and relative abundance of ammonia-oxidizing bacteria in lakes of the McMurdo Dry Valley, Antarctica. Hydrobiologia 1999, 401, 113–130. [Google Scholar] [CrossRef]
- Ayton, J.; Aislabie, J.; Barker, G.M.; Saul, D.; Turner, S. Crenarchaeota affiliated with group 1.1 b are prevalent in coastal mineral soils of the Ross Sea region of Antarctica. Environ. Microbiol. 2010, 12, 689–703. [Google Scholar] [CrossRef] [PubMed]
- Howard-Williams, C.; Hawes, I. Ecological processes in Antarctic inland waters: Interactions between physical processes and the nitrogen cycle. Antarct. Sci. 2007, 19, 205–217. [Google Scholar] [CrossRef]
- Tolar, B.B.; Ross, M.J.; Wallsgrove, N.J.; Liu, Q.; Aluwihare, L.I.; Popp, B.N. Contribution of ammonia oxidation to chemoautotrophy in Antarctic coastal waters. ISME J. 2016, 10, 2605–2619. [Google Scholar] [CrossRef]
- Monteiro, M.; Baptista, M.S.; Séneca, J.; Torgo, L.; Lee, C.K.; Cary, S.C.; Magalhães, C. Understanding the Response of Nitrifying Communities to Disturbance in the McMurdo Dry Valleys, Antarctica. Microorganisms 2020, 8, 404. [Google Scholar] [CrossRef]
- Stephen, J.R.; McCaig, A.E.; Smith, Z.; Prosser, J.I.; Embley, T.M. Molecular diversity of soil and marine 16S rRNA gene sequences related to b-subgroup ammonia-oxidizing bacteria. Appl. Environ. Microbiol. 1996, 62, 4147–4154. [Google Scholar] [CrossRef]
- Stephen, J.R.; Kowalchuk, G.A.; Bruns, M.A.V.; McCaig, A.E.; Phillips, C.J.; Embley, T.M.; Prosser, J.I. Analysis of β-subgroup Proteobacterial ammonia oxidizer populations in soil by denaturating gradient gel electrophoresis analysis and hierarchical phylogenetic probing. Appl. Environ. Microbiol. 1998, 64, 2958–2965. [Google Scholar] [CrossRef]
- McCaig, A.E.; Phillips, C.J.; Stephen, J.R.; Kowalchuk, G.A.; Harvey, S.M.; Herbert, R.A.; Embley, T.M.; Prosser, J.I. Nitrogen cycling and community structure of proteobacterial beta-subgroup ammonia-oxidizing bacteria within polluted marine fish farm sediments. Appl. Environ. Microbiol. 1999, 65, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Magalhães, C.; Machado, A.; Bordalo, A.A. Temporal variability of relative abundance of ammonia oxidizing bacteria vs. archaea in the sandy at of the Douro River estuary, Portugal. Aquat. Microb. Ecol. 2009, 56, 13–23. [Google Scholar] [CrossRef]
- Philippot, L.; Hojberg, O. Dissimilatory nitrate reductases in bacteria. Biochim. Biophys. Acta. 1999, 1577, 1–23. [Google Scholar] [CrossRef]
- Braker, G.; Zhou, J.; Wu, L.; Devol, A.H.; Tiedje, J.M. Nitrite reductase genes (nirK and nirS) as functional markers to investigate diversityof denitrifying bacteria in Pacific Northwest marine sediment communities. Appl. Environ. Microbiol. 2000, 66, 2096–2104. [Google Scholar] [CrossRef]
- Prieme, A.; Braker, G.; Tiedje, J.M. Diversity of nitrite reductase (nirK and nirS) gene fragments in forested upland and wetland soils. Appl. Environ. Microbiol. 2002, 68, 1893–1900. [Google Scholar] [CrossRef]
- Zumft, W.G. Cell biology and molecular basis of denitrification. Microbiol. Mol. Biol. 1997, 61, 533–616. [Google Scholar] [CrossRef]
- Pérez, C.A.; Aravena, J.C.; Ivanovich, C.; McCulloh, R. Effects of penguin guano and moisture on nitrogen biological fixation in maritime Antarctic soils. Polar Biol. 2017, 40, 437–448. [Google Scholar] [CrossRef]
- Chan, Y.; Lacap, D.C.; Lau, M.C.Y.; Ha, K.Y.; Warren-Rhodes, K.A.; Cockell, C.S.; Cowan, D.A.; McKay, C.P.; Pointing, S.B. Hypolithic microbial communities: Between a rock and a hard place. Environ. Microbiol. 2012, 14, 2272–2282. [Google Scholar] [CrossRef]
- Davey, A.; Marchant, H.J. Seasonal variation in nitrogen fixation by Nostoc commune Vaucher at the Vestfold Hills, Antarctica. Phycologia 1983, 22, 377–385. [Google Scholar] [CrossRef]
- Nakatsubo, T.; Ino, Y. Nitrogen cycling in an Antarctic ecosystem 2. Estimation of the amount of nitrogen fixation in a moss community on East Ongul Island. Ecol. Res. 1987, 2, 31–40. [Google Scholar] [CrossRef]
- Line, M.A. Nitrogen fixation in the sub-Antarctic Macquarie Island. Polar Biol. 1991, 11, 601–606. [Google Scholar] [CrossRef]
- Vincent, W. Cyanobacterial Dominance in the Polar Regions. In The Ecology of Cyanobacteria; Whitton, B.A., Potts, M., Eds.; Kluwer: Dordrecht, The Netherlands, 2007; pp. 321–340. [Google Scholar]
- Hopkins, D.; Sparrow, A.; Elberling, B.; Gregorich, E.; Novis, P.; Greenfield, L.; Tilston, E. Carbon, nitrogen and temperature controls on microbial activity in soils from an Antarctic Dry Valley. Soil Biol. Biochem. 2006, 38, 3130–3140. [Google Scholar] [CrossRef]
- Strauss, S.L.; Garcia-Pichel, F.; Day, T.A. Soil microbial carbon and nitrogen transformations at a glacial foreland on Anvers Island, Antarctic Peninsula. Polar Biol. 2012, 35, 1459–1471. [Google Scholar] [CrossRef]
- Stewart, W.D.; Fitzgerald, G.P.; Burris, R.H. In situ studies on N2 fixation using the acetylene reduction technique. Proc. Nat. Acad. Sci. USA 1967, 58, 2071–2078. [Google Scholar] [CrossRef] [PubMed]
- Barrett, J.E.; Virginia, R.A.; Lyons, W.B.; McKnight, D.M.; Priscu, J.C.; Doran, P.T.; Fountain, A.G.; Wall, D.H.; Moorhead, D.L. Biogeochemical stoichiometry of Antarctic Dry Valley ecosystems. J. Geophys. Res. 2007, 112, G01010. [Google Scholar] [CrossRef]
- Barrett, J.; Virginia, R.; Hopkins, D.; Aislabie, J.; Bargagli, R.; Bockheim, J.; Campbell, I.; Lyons, W.; Moorhead, D.; Nkem, J.; et al. Terrestrial ecosystem processes of Victoria Land. Antarctica. Soil Biol. Biochem. 2006, 38, 3019–3034. [Google Scholar] [CrossRef]
- Kohler, T.J.; Stanish, L.F.; Liptzin, D.; Barrett, J.E.; McKnight, D.M. Catch and release: Hyporheic retention and mineralization of N-fixing Nostoc sustains downstream microbial mat biomass in two polar desert streams. Limnol. Oceanogr. 2018, 3, 357–364. [Google Scholar] [CrossRef]
- Dennis, P.G.; Newsham, K.K.; Rushton, S.P.; Ord, V.J.; O’Donnell, A.G.; Hopkins, D.W. Warming constrains bacterial community responses to nutrient inputs in a southern, but not northern, maritime Antarctic soil. Soil Biol. Biochem. 2013, 57, 248–255. [Google Scholar] [CrossRef]
- Van Horn, D.J.; Okie, J.G.; Buelow, H.N.; Gooseff, M.N.; Barrett, J.E.; Takacs-Vesbach, C.D. Soil microbial responses to increased moisture and organic resources along a salinity gradient in a polar desert. Appl. Environ. Microb. 2014, 80, 3034–3043. [Google Scholar] [CrossRef]
- Ball, B.A.; Adams, B.J.; Barrett, J.E.; Wall, D.H.; Virginia, R.A. Soil biological responses to C, N and P fertilization in a polar desert of Antarctica. Soil Biol. Biochem. 2018, 122, 7–18. [Google Scholar] [CrossRef]
- Hopkins, D.W.; Sparrow, A.D.; Shillam, L.L.; English, L.C.; Dennis, P.G.; Novis, P.; Elberling, B.; Gregorich, E.G.; Greenfield, L.G. Enzymatic activities and microbial communities in an Antarctic dry valley soil: Responses to C and N supplementation. Soil Biol. Biochem. 2008, 40, 2130–2136. [Google Scholar] [CrossRef]
- Sparrow, A.D.; Gregorich, E.G.; Hopkins, D.W.; Novis, P.; Elberling, B.; Greenfield, L.G. Resource limitations on soil microbial activity in an Antarctic dry valley. Soil Sci. Soc. Am. J. 2011, 75, 2188–2197. [Google Scholar] [CrossRef]
- Dennis, P.G.; Sparrow, A.D.; Gregorich, E.G.; Novis, P.M.; Elberling, B.; Greenfield, L.G.; Hopkins, D.W. Microbial responses to carbon and nitrogen supplementation in an Antarctic dry valley soil. Antarct. Sci. 2013, 25, 55–61. [Google Scholar] [CrossRef]
- Newsham, K.K.; Tripathi, B.M.; Dong, K.; Yamamoto, N.; Adams, J.M.; Hopkins, D.W. Bacterial community composition and diversity respond to nutrient amendment but not warming in a maritime Antarctic soil. Microb. Ecol. 2019, 78, 974–984. [Google Scholar] [CrossRef] [PubMed]
- Ball, B.A.; Virginia, R.A. Microbial biomass and respiration responses to nitrogen fertilization in a polar desert. Polar Biol. 2014, 3, 573–585. [Google Scholar] [CrossRef]
- Benhua, S.; Dennis, P.G.; Laudicina, V.A.; Ord, V.J.; Rushton, S.P.; O’Donnell, A.G.; Newsham, K.K.; Hopkins, D.W. Biogeochemical responses to nutrient, moisture and temperature manipulations of soil from Signy Island, South Orkney Islands in the Maritime Antarctic. Antarct. Sci. 2014, 26, 513–520. [Google Scholar] [CrossRef]
- Hopkins, D.W.; Dennis, P.G.; Rushton, S.P.; Newsham, K.K.; O’Donnell, T.G. Lean and keen: Microbial activity in soils from the Maritime Antarctic. Eur. J. Soil. Sci. 2020, 1–19. [Google Scholar] [CrossRef]
- Schwartz, E.; Van Horn, D.J.; Buelow, H.N.; Okie, J.G.; Gooseff, M.N.; Barrett, J.E.; Takacs-Vesbach, C.D. Characterization of growing bacterial populations in McMurdo Dry Valley soils through stable isotope probing with 18O-water. FEMS Microbiol. Ecol. 2014, 89, 415–425. [Google Scholar] [CrossRef]
- Buelow, H.N.; Winter, A.S.; Van Horn, D.J.; Barrett, J.E.; Gooseff, M.N.; Schwartz, E.; Takacs-Vesbach, C.D. Microbial community responses to increased water and organic matter in the arid soils of the mcmurdo dry valleys, Antarctica. Front. Microbiol. 2016, 7, 1040. [Google Scholar] [CrossRef]
- Cain, M.L.; Subler, S.; Evans, J.P.; Fortin, M.-J. Sampling spatial and temporal variation in soil nitrogen availability. Oecologia 1999, 118, 397–404. [Google Scholar] [CrossRef]
- Knops, J.M.H.; Tilman, D. Dynamics of soil nitrogen and carbon accumulation for 61 years after agricultural abandonment. Ecology 2000, 81, 88–98. [Google Scholar] [CrossRef]
- Niederberger, T.D.; Bottos, E.M.; Sohm, J.A.; Gunderson, T.; Parker, A.; Coyne, K.J.; Capone, D.G.; Carpenter, E.J.; Cary, S.C. Rapid Microbial Dynamics in Response to an Induced Wetting Event in Antarctic Dry Valley Soils. Front. Microbiol. 2019, 10, 621. [Google Scholar] [CrossRef] [PubMed]
- Doran, P.T.; Priscu, J.C.; Lyons, W.B.; Walsh, J.E.; Fountain, A.G.; McKnight, D.M.; Moorhead, D.L.; Virginia, R.A.; Wall, D.H.; Clow, G.D.; et al. Antarctic climate cooling and terrestrial ecosystem response. Nature 2002, 415, 517–520. [Google Scholar] [CrossRef] [PubMed]
- Barrett, J.E.; Virginia, R.A.; Wall, D.H.; Doran, P.T.; Fountain, A.G.; Welch, K.A.; Lyons, W.B. Persistent effects of a discrete warming event on a polar desert ecosystem. Glob. Chang. Biol. 2008, 14, 2249–2261. [Google Scholar] [CrossRef]
- Bundy, J.G.; Davey, M.P.; Viant, M.R. Environmental metabolomics: A critical review and future perspectives. Metabolomics 2009, 5, 3–21. [Google Scholar] [CrossRef]
- Sardans, J.; Peñuelas, J.; Rivas-Ubach, A. Ecological metabolomics: Overview of current developments and future challenges. Chemoecology 2011, 21, 191–225. [Google Scholar] [CrossRef]
- Coleine, C.; Gevi, F.; Fanelli, G.; Onofri, S.; Timperio, A.M.; Selbmann, L. Specific adaptations are selected in opposite sun exposed Antarctic cryptoendolithic communities as revealed by untargeted metabolomics. PLoS ONE 2020, 15, e0233805. [Google Scholar] [CrossRef]
- Guerra, C.A.; Heintz-Buschart, A.; Sikorski, J.; Chatzinotas, A.; Guerrero-Ramírez, N.; Cesarz, S.; Beaumelle, L.; Rillig, M.C.; Maestre, F.T.; Delgado-Baquerizo, M.; et al. Blind spots in global soil biodiversity and ecosystem function research. BioRxiv 2019, 20, 774356. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).