Increasing the Production of Volatile Fatty Acids from Corn Stover Using Bioaugmentation of a Mixed Rumen Culture with Homoacetogenic Bacteria
Abstract
:1. Introduction
2. Materials and Methods
2.1. Inoculum
2.2. Substrate
2.3. Bacterial Strains
2.4. Fermentation
2.4.1. Control Fermenter
2.4.2. Bioaugmented Fermenters
2.5. Analyses
2.5.1. Measurement of VFA Using High Performance Liquid Chromatography
2.5.2. Gas Analyses Using Gas Analyzer
2.5.3. Calculations
2.6. Feedstock Characterization
3. Results and Discussion
3.1. Effect of BES on Rumen Fermentation and VFA Production
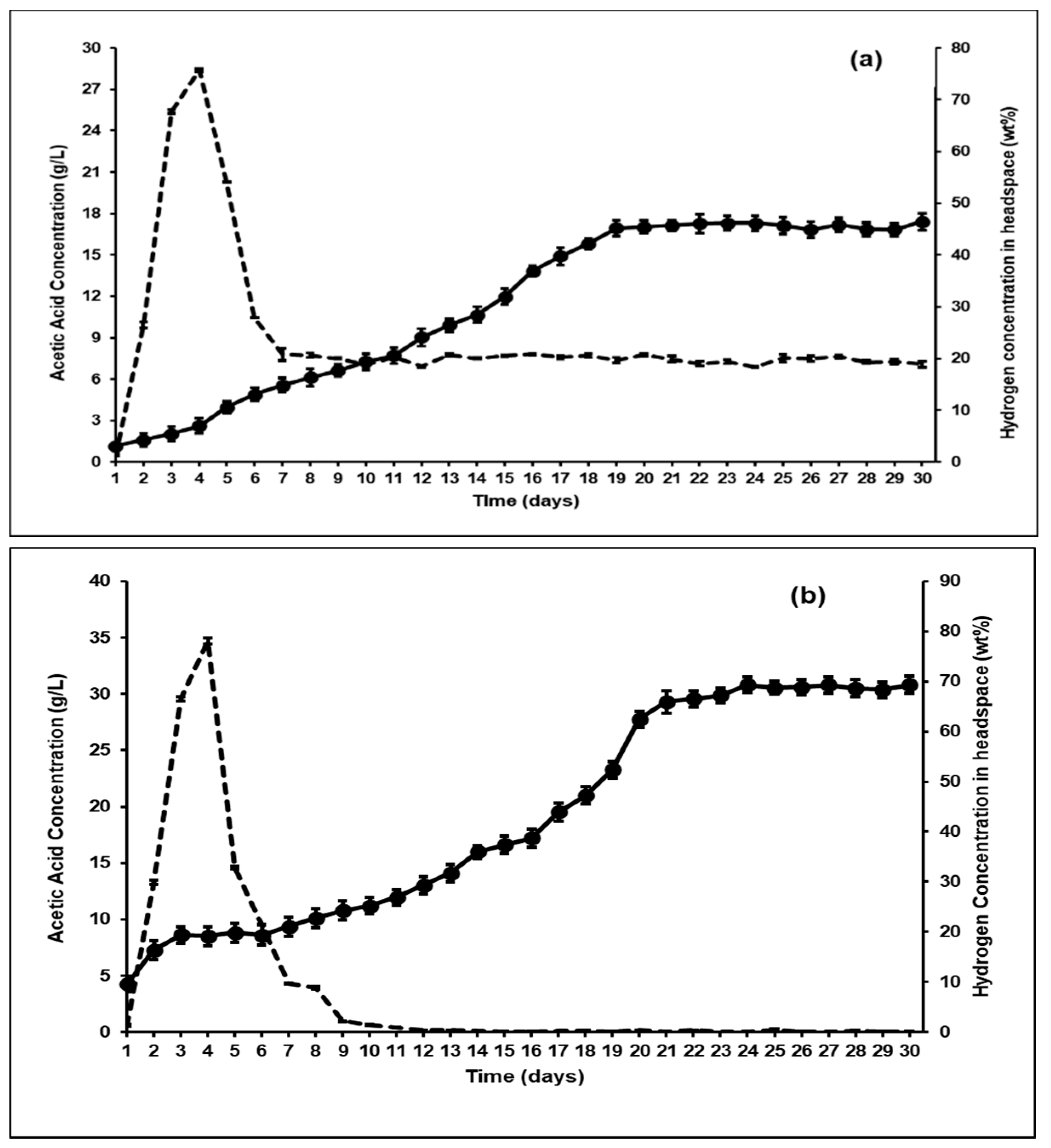
3.2. Effect of Bioaugmentation on Overall VFA Yield
3.3. Effect of Bioaugmentation on Individual VFA Yield
3.4. Mass Balance Analysis and Feedstock/Effluent Characterization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wan, C.X.; Li, Y.B. Microbial pretreatment of corn stover with Ceriporiopsis subvermispora for enzymatic hydrolysis and ethanol production. Bioresour. Technol. 2010, 101, 6398–6403. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.-Q.; Xie, H.; Chen, W.; Wang, E.-T.; Du, F.-G.; Song, A.-D. Biological pretreatment of corn stover with ligninolytic enzyme for high efficient enzymatic hydrolysis. Bioresour. Technol. 2013, 144, 572–578. [Google Scholar] [CrossRef] [PubMed]
- Himmel, M.E.; Ding, S.Y.; Johnson, D.K.; Adney, W.S.; Nimlos, M.R.; Brady, J.W.; Foust, T.D. Biomass recalcitrance: Engineering plants and enzymes for biofuels production. Science 2007, 315, 804–807. [Google Scholar] [CrossRef] [Green Version]
- Rana, D.; Rana, V.; Ahring, B.K. Producing high sugar concentrations from loblolly pine using wet explosion pretreatment. Bioresour. Technol. 2012, 121, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Biswas, R.; Uellendahl, H.; Ahring, B.K. Wet explosion pretreatment of sugarcane bagasse for enhanced enzymatic hydrolysis. Biomass Bioenergy 2014, 61, 104–113. [Google Scholar] [CrossRef]
- Biswas, R.; Uellendahl, H.; Ahring, B.K. Wet explosion: A universal and efficient pretreatment process for lignocellulosic biorefineries. BioEnergy Res. 2015, 8, 1101–1106. [Google Scholar] [CrossRef]
- Njoku, S.I.; Uellendahl, H.; Ahring, B.K. Comparing oxidation and dilute acid wet explosion pretreatment of Cocksfoot grass at high dry matter concentration for cellulosic ethanol production. Energy Sci. Eng. 2013, 1, 89–98. [Google Scholar] [CrossRef]
- Sawatdeenarunat, C.; Sung, S.; Khanal, S.K. Enhanced volatile fatty acids production during anaerobic digestion of lignocellulosic biomass via micro-oxygenation. Bioresour. Technol. 2017, 237, 139–145. [Google Scholar] [CrossRef]
- Murali, N.; Srinivas, K.; Ahring, B.K. Biochemical production and separation of carboxylic acids for biochemical applications. Fermentation 2017, 3, 22. [Google Scholar] [CrossRef] [Green Version]
- Weimer, P.J.; Nerdahl, M.; Brandl, D.J. Production of medium-chain volatile fatty acids by mixed ruminal microorganisms is enhanced to ethanol in co-culture with Clostridum Kluyveri. Bioresour. Technol. 2015, 175, 97–101. [Google Scholar] [CrossRef]
- Weimer, P.J.; Russell, J.B.; Muck, R.E. Lessons from the cow: What the ruminant animal can teach us about consolidated bioprocessing of cellulosic biomass. Bioresour. Technol. 2009, 100, 5323–5331. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.-H.; Yu, H.-Q. Application of rumen microorganisms for enhanced anaerobic fermentation of corn stover. Process Biochem. 2005, 40, 2371–2377. [Google Scholar] [CrossRef]
- Graeme, A.; Christopher, M. Methanogen genomics to discover targets for methane mitigation technologies and options for alternative H2 utilization in the rumen. Aust. J. Exp. Agric. 2008, 48, 28–37. [Google Scholar]
- Angelidaki, I.; Ellengaard, L.; Ahring, B.K. Applications of the anaerobic digestion process. In Biomethanation II; Ahring, B.K., Angelidaki, I., Dolfing, J., Ellengaard, L., Gavala, H.N., Haagensen, F., Mogensen, A.S., Eds.; Springer: New York, NY, USA, 2003; pp. 1–34. [Google Scholar]
- Ahring, B.K.; Biomethanation, I. Biomethanation I; Ahring, B.K., Ed.; Springer: New York, NY, USA, 2003; pp. 1–31. [Google Scholar]
- Ungerfeld, E.M. Inhibition of rumen methanogenesis and ruminant productivity: A meta analysis. Front. Vet. Sci. 2018, 5, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Van Kessel, J.A.S.; Russell, J. The effect of pH on ruminal methanogenesis. FEMS Microbiol. Ecol. 1996, 20, 205–210. [Google Scholar] [CrossRef]
- Ahring, B.K.; Murali, N.; Srinivas, K. Fermentation of cellulose with a mixed microbial rumen culture with and without methanogenesis. Ferment. Technol. 2018, 7, 152. [Google Scholar] [CrossRef]
- Nollet, L.; Demeyer, D.; Verstraete, W. Effect of 2-Bromoethanesulfonic acid and Peptostreptococcus productus ATCC 35244 addition of stimulation of reductive acetogenesis in the ruminal ecosystem by selective inhibition of methanogenesis. Appl. Environ. Microbiol. 1997, 63, 194–200. [Google Scholar] [CrossRef] [Green Version]
- Xu, K.; Liu, H.; Chen, J. Effect of classic methanogenic inhibitors on the quantity and diversity of archae community and the reductive homoacetogenic activity during the process of anaerobic sludge digestion. Bioresour. Technol. 2010, 101, 2600–2607. [Google Scholar] [CrossRef]
- Gagen, E.J.; Denman, S.E.; McSweeney, C.S. Acetogenesis as an alternative to methanogenesis in the rumen. In Livestock Production and Climate; Mallik, P.K., Bhatta, R., Takahashi, J., Kohn, R., Prasad, C.S., Eds.; CAB International: Oxfordshire, UK, 2015; pp. 292–303. [Google Scholar]
- Schiel-Bengelsdorf, B.; Durre, P. Pathway engineering and synthetic biology using acetogens. FEBS Lett. 2012, 586, 2191–2198. [Google Scholar] [CrossRef] [Green Version]
- Herrero, M.; Stuckey, D.C. Bioaugmentation and its application in wastewater treatment: A review. Chemosphere 2015, 140, 119–128. [Google Scholar] [CrossRef]
- Cycon, M.A.; Mrozik, A.; Piotrowska-Seget, Z. Bioaugmentation as a strategy for the remediation of pesticide-polluted soil: A review. Chemosphere 2017, 172, 52–71. [Google Scholar] [CrossRef]
- Ren, N.Q.; Chua, H.; Chan, S.Y.; Tsang, Y.F.L.; Wang, Y.J.; Sin, N. Assessing optimal fermentation type for bio-hydrogen production in continuous-flow acidogenic reactors. Bioresour. Technol. 2007, 98, 1774–1780. [Google Scholar] [CrossRef]
- Acs, N.; Bagi, Z.; Rakhely, G.; Minarovics, J.; Nagy, K.; Kovacs, K.L. Bioaugmentation of biogas production by a hydrogen producing bacterium. Bioresour. Technol. 2015, 186, 286–293. [Google Scholar] [CrossRef] [Green Version]
- Nielsen, H.B.; Mlandenovska, Z.; Ahring, B.K. Bioaugmentation of a two stage thermophilic (68C/55C) anaerobic digestion concept for improvement of the methane yield from cattle manure. Biotechnol. Bioeng. 2007, 97, 1638–1643. [Google Scholar] [CrossRef] [PubMed]
- Costa, J.C.; Barbosa, S.G.; Alves, M.M.; Sousa, D.Z. Thermo-chemical pre- and biological co-treatments to improve hydrolysis and methane production from poultry litter. Bioresour. Technol. 2013, 111, 141–147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strang, O.; Acs, N.; Wirth, R.; Maroti, G.; Bagi, Z.; Rakhely, G.; Kovacs, K.L. Bioaugmentation of the thermophilic anaerobic biodegradation of cellulose and corn stover. Anaerobe 2017, 46, 104–113. [Google Scholar] [CrossRef]
- Murali, S.; Fernandez, S.; Ahring, B.K. Fermentation of wet-exploded corn stover for the production of volatile fatty acids. Bioresour. Technol. 2017, 227, 197–204. [Google Scholar] [CrossRef]
- Chidthaisong, A.; Conrad, R. Specificity of chloroform, 2-bromoethanesulfonate and fluoroacetate to inhibit methanogenesis and other anaerobic processes in anoxic rice field soil. Soil Biol. Biochem. 2000, 32, 977–988. [Google Scholar] [CrossRef]
- Ungerfeld, E.M. Shifts in metabolic hydrogen sinks in the methanogenesis-inhibited ruminal fermentation: A meta-analysis. Bioresour. Technol. 2010, 101, 6398–6403. [Google Scholar] [CrossRef]
- Nagaraja, T.G. Ionophores and antibiotics in ruminants. In Biotechnology in Animal Feeds and Animal Feeding; Wallace, R.J., Chesson, A., Eds.; VCH: Weinheim, Germany, 1995; pp. 171–204. [Google Scholar]
- Ungerfeld, E.M. Metabolic hydrogen flows in rumen fermentation: Principles and possibilities of interventions. Front. Microbiol. 2020, 11, 589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, M.; Ungerfeld, E.M.; Wang, R.; Zhou, C.S.; Basang, Z.Z.; Ao, S.M.; Tan, Z.L. Supersaturation of dissolved hydrogen and methane in rumen of Tibetian sheep. Front. Microbiol. 2016, 7, 850. [Google Scholar] [CrossRef]
- Zhou, Z.; Meng, Q.; Yu, Z. Effect of methanogenic inhibitors on methane production and abundances of methanogens and cellulolytic bacteria in In Vitro ruminal cultures. Appl. Environ. Microbiol. 2011, 77, 2634–2639. [Google Scholar] [CrossRef] [Green Version]
- Yu, F.B.; Ali, S.W.; Guan, L.B.; Li, S.P.; Zhou, S. Bioaugmentation of a sequencing batch reactor with Pesudomonas putida ONBA-17 and its impact on reactor bacterial communities. J. Hazard. Mater. 2010, 176, 20–26. [Google Scholar] [CrossRef]
- Yan, B.H.; Selvam, A.; Xu, S.Y.; Wong, J.W.C. A novel way to utilize hydrogen and carbon dioxide in acidogenic reactor through homoacetogenesis. Bioresour. Technol. 2014, 159, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Lopez, S.; Mcintosh, F.M.; Wallace, R.J.; Newbold, C.J. Effect of adding acetogenic bacteria on methane production by mixed rumen microorganisms. Anim. Feed Sci. Technol. 1999, 78, 1–9. [Google Scholar] [CrossRef]
- Demirel, B.; Scherer, P. The roles of acetotrophicc and hydrogenotrophic methanogens during anaerobic conversion of biomass to methane: A review. Rev. Environ. Sci. Biotechnol. 2008, 7, 173–190. [Google Scholar] [CrossRef]
- Groher, A.; Weuster-Botz, D. Comparative reaction engineering analysis of different acetogenic bacteria for gas fermentation. J. Biotechnol. 2016, 228, 82–94. [Google Scholar] [CrossRef] [PubMed]
- Kantzow, C.; Mayer, A.L.; Weuster-Botz, D. Continuous gas fermentation by Acetobacterium woodii in a submerged membrane reactor with full cell retention. J. Biotechnol. 2015, 212, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Lamed, R.J.; Lobos, J.H.; Su, T.M. Effects of stirring and hydrogen on fermentation products of Clostridium thermocellum. Appl. Environ. Microbiol. 1988, 54, 1216–1221. [Google Scholar] [CrossRef] [Green Version]
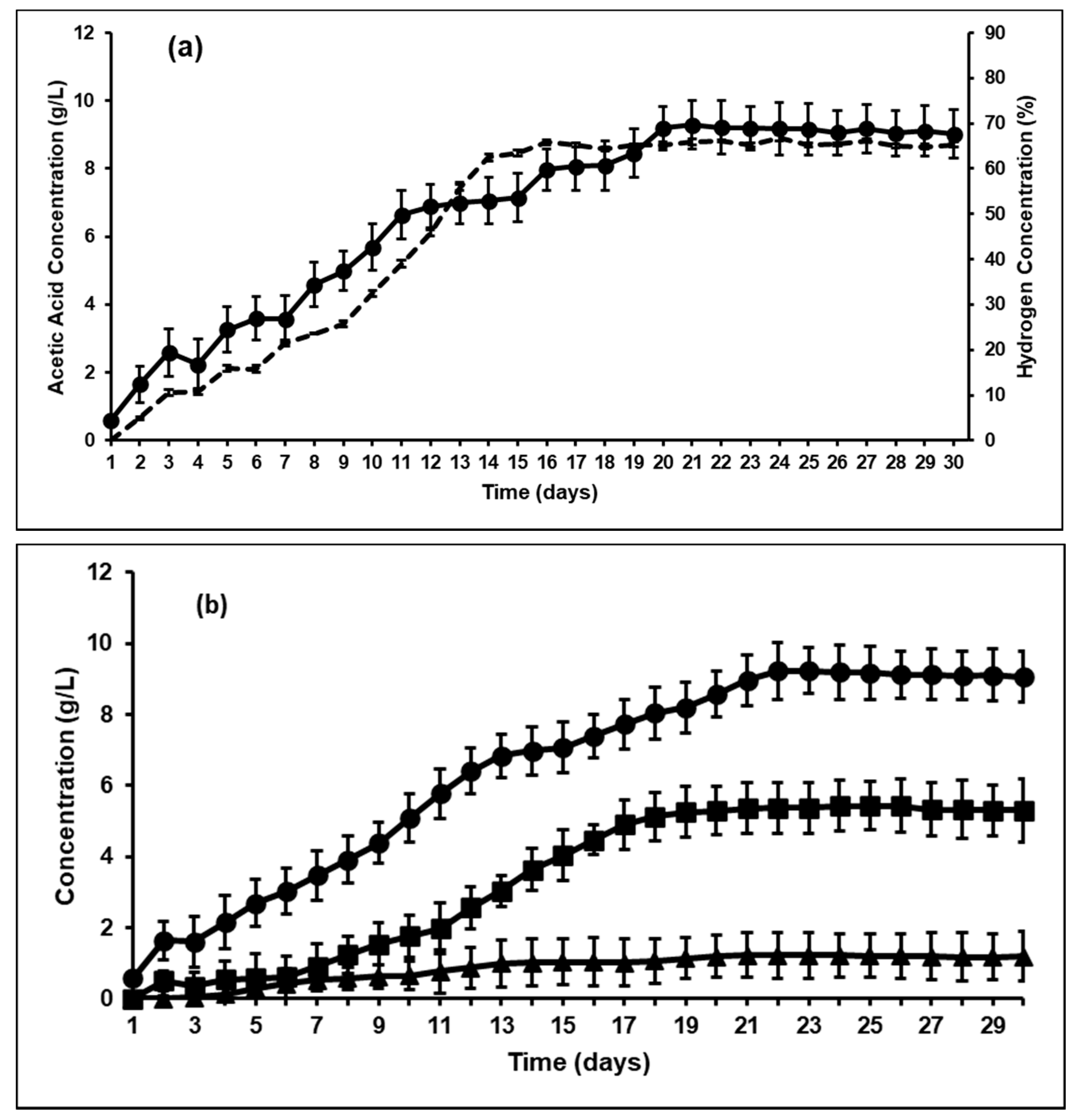

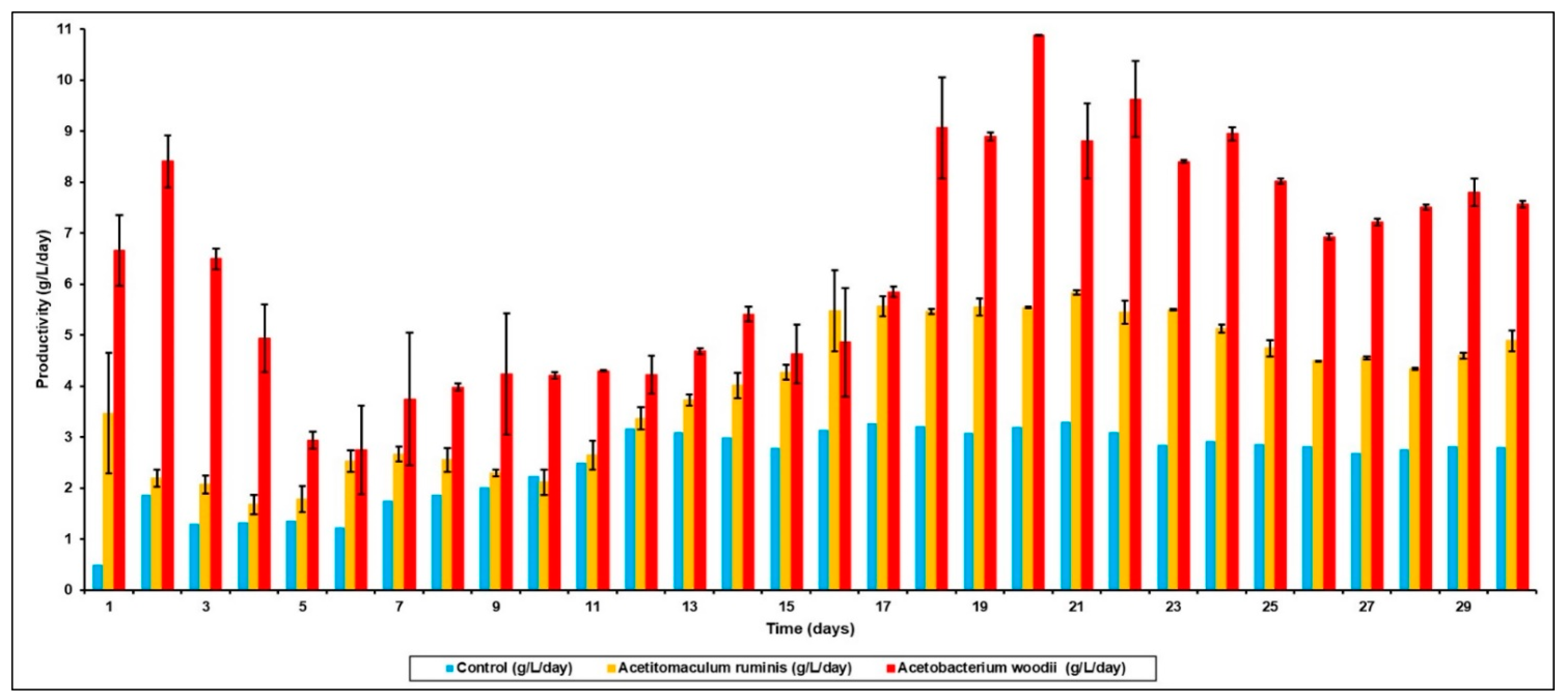
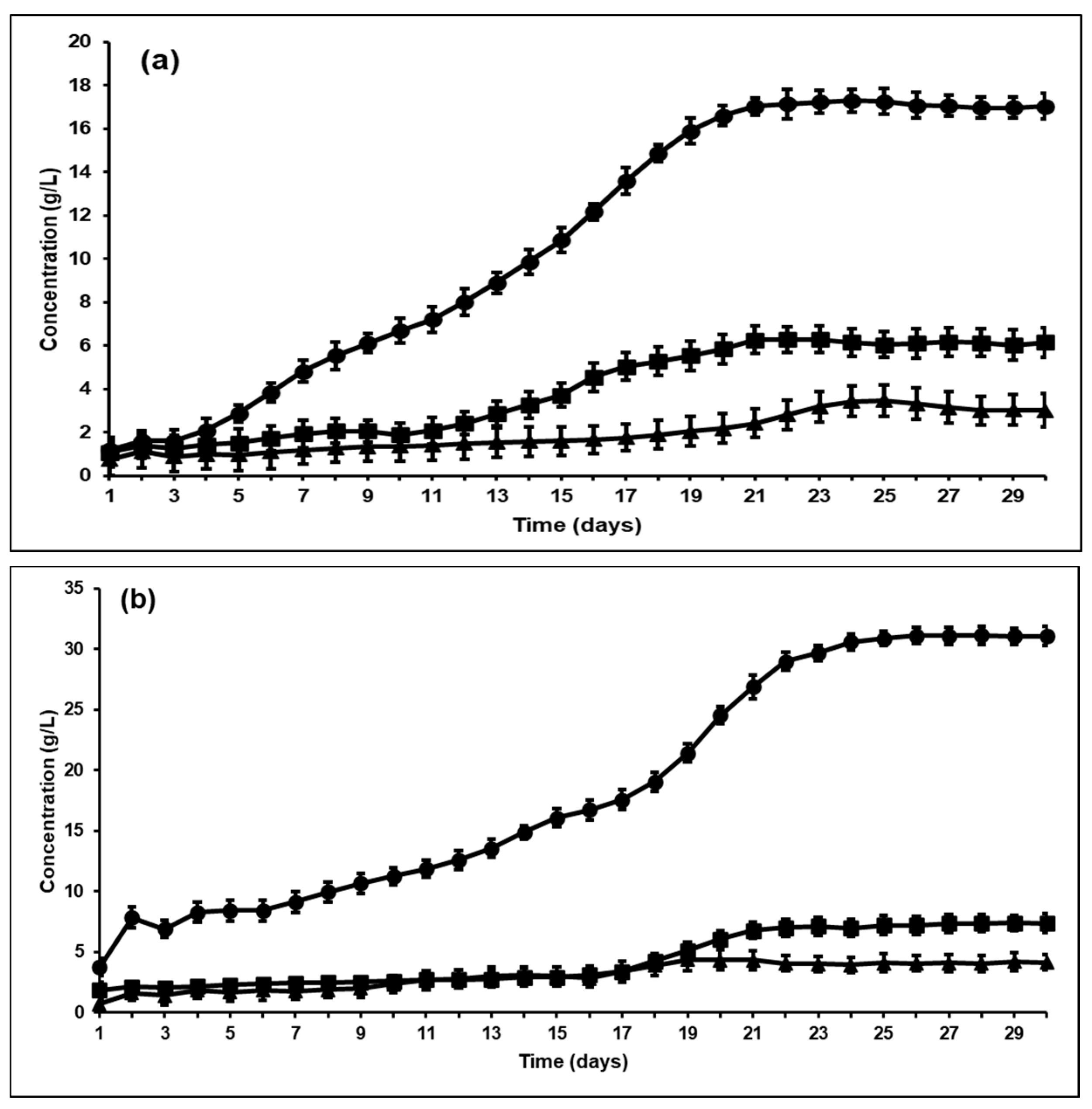
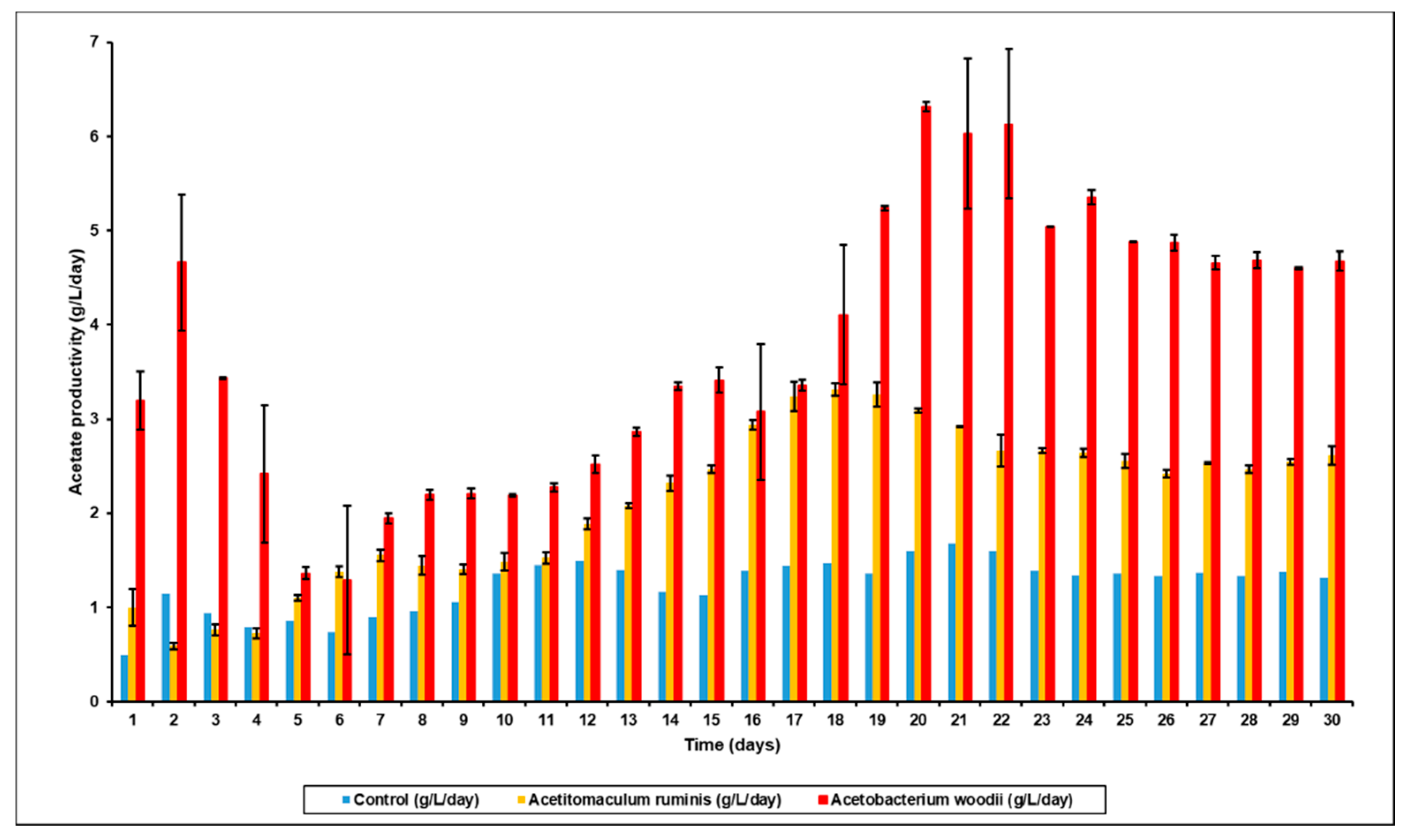
| Cellulose (wt%) | Hemicellulose (wt%) | Lignin (wt%) | Ash (wt%) | Total Solids (wt%) | Volatile Solids (wt%) | |
|---|---|---|---|---|---|---|
| Pretreated Corn Stover | 36.8 | 16.3 | 43.4 | 3.4 | 2.5 | 2.35 |
| Bioreactor | Acetic Acid (g/L) | Propionic Acid (g/L) | Butyric Acid (g/L) | Total VFA in Acetic Acid Equivalents (g/L) |
|---|---|---|---|---|
| Control; With Methanogenesis [30] | 12.26 | 10.08 | 2.42 | 31.09 (1.25 g/gVS) |
| Control; (BES-added) Without Methanogensis | 9.29 | 5.63 | 1.23 | 21.41 (0.95 g/gVS) |
| Bioaugmentation with A. ruminis after BES addition | 16.99 | 6.88 | 2.98 | 32.33 (1.34 g/gVS) |
| Bioaugmentation with A. woodii after BES addition | 30.8 | 7.91 | 3.89 | 49.31 (2.19 g/gVS) |
| Feed 1 | Effluent A 2 | Effluent B 3 | Effluent C 4 | |
|---|---|---|---|---|
| Solid Fraction * | ||||
| Total Carbohydrates (%g/g biomass) | 53.1 | 44.4 | 34.8 | 30.4 |
| Cellulose (%g/g biomass) | 36.8 | 31.9 | 25.9 | 23.6 |
| Hemicellulose (%g/g biomass) | 16.3 | 12.5 | 8.9 | 6.8 |
| Soluble Lignin (%g/g biomass) | 2.64 | 3.7 | 3.6 | 4.2 |
| Insoluble Lignin (%g/g biomass) | 40.8 | 40.9 | 50.9 | 54.6 |
| Carbohydrate:Lignin Ratio | 1.22 | 0.99 | 0.64 | 0.52 |
| Liquid Fraction | ||||
| Acetic acid (g/L) | 0.34 | 9.29 | 16.99 | 30.8 |
| Propionic acid (g/L) | 0.17 | 5.63 | 6.88 | 7.91 |
| Butyric acid (g/L) | 0.11 | 1.23 | 2.98 | 3.89 |
| Hydrogen concentration (wt%) | N/A | 65% | 4% | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murali, N.; Srinivas, K.; Ahring, B.K. Increasing the Production of Volatile Fatty Acids from Corn Stover Using Bioaugmentation of a Mixed Rumen Culture with Homoacetogenic Bacteria. Microorganisms 2021, 9, 337. https://doi.org/10.3390/microorganisms9020337
Murali N, Srinivas K, Ahring BK. Increasing the Production of Volatile Fatty Acids from Corn Stover Using Bioaugmentation of a Mixed Rumen Culture with Homoacetogenic Bacteria. Microorganisms. 2021; 9(2):337. https://doi.org/10.3390/microorganisms9020337
Chicago/Turabian StyleMurali, Nanditha, Keerthi Srinivas, and Birgitte K. Ahring. 2021. "Increasing the Production of Volatile Fatty Acids from Corn Stover Using Bioaugmentation of a Mixed Rumen Culture with Homoacetogenic Bacteria" Microorganisms 9, no. 2: 337. https://doi.org/10.3390/microorganisms9020337






