Effects of Impactor Size on Biomechanical Characteristics of Spinal Cord in Hemicontusion Injury Model Using Finite Element Analysis
Abstract
:1. Introduction
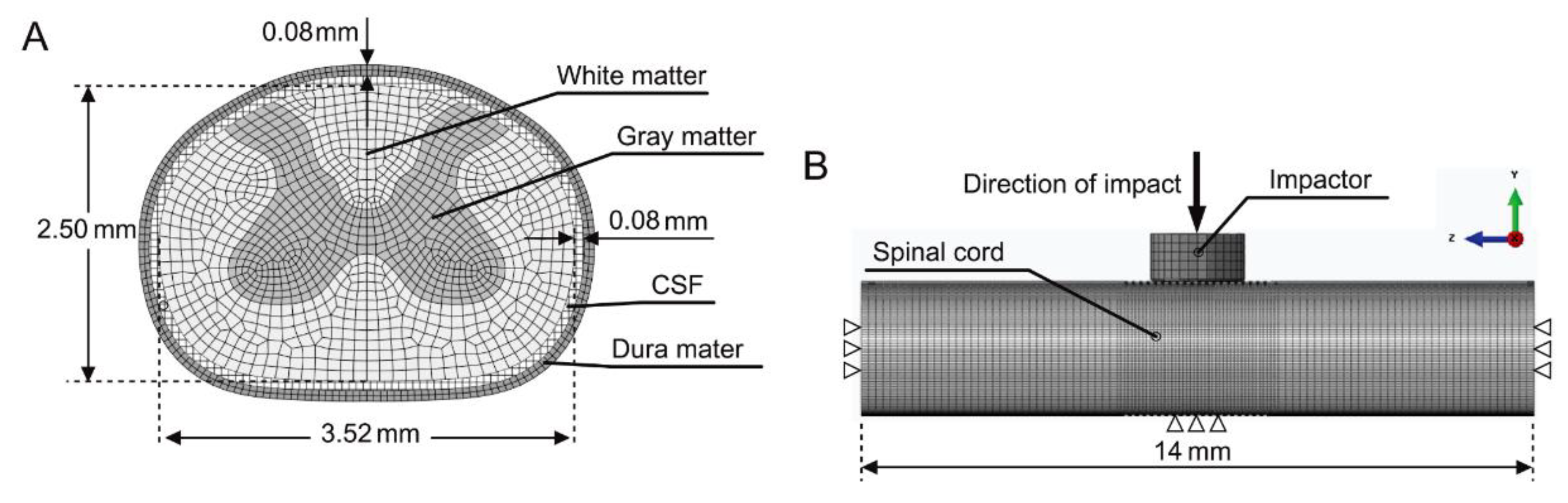
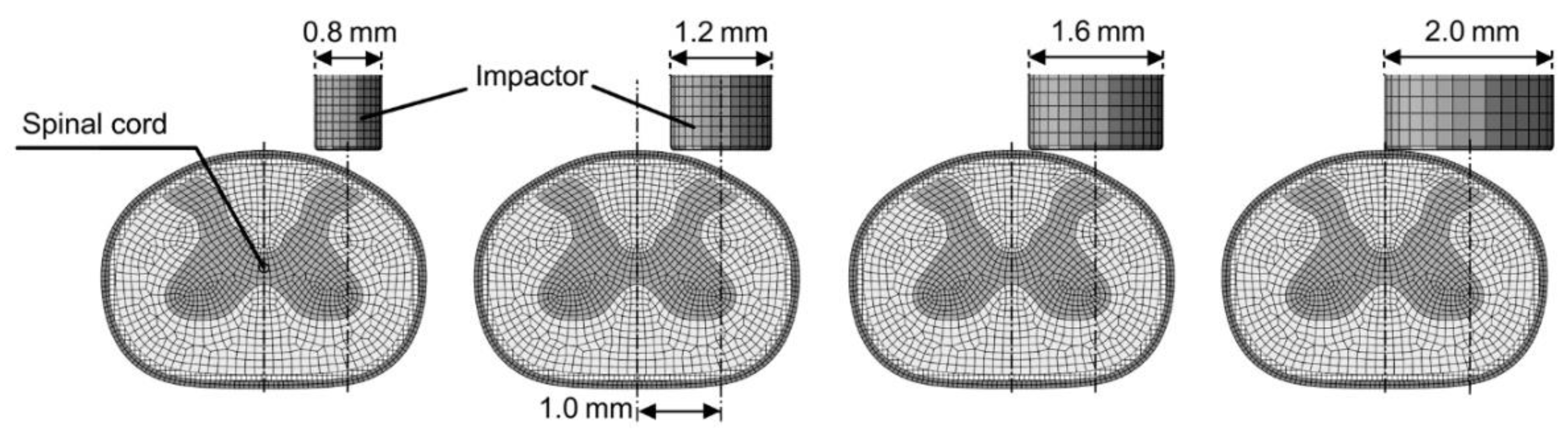
2. Materials and Methods
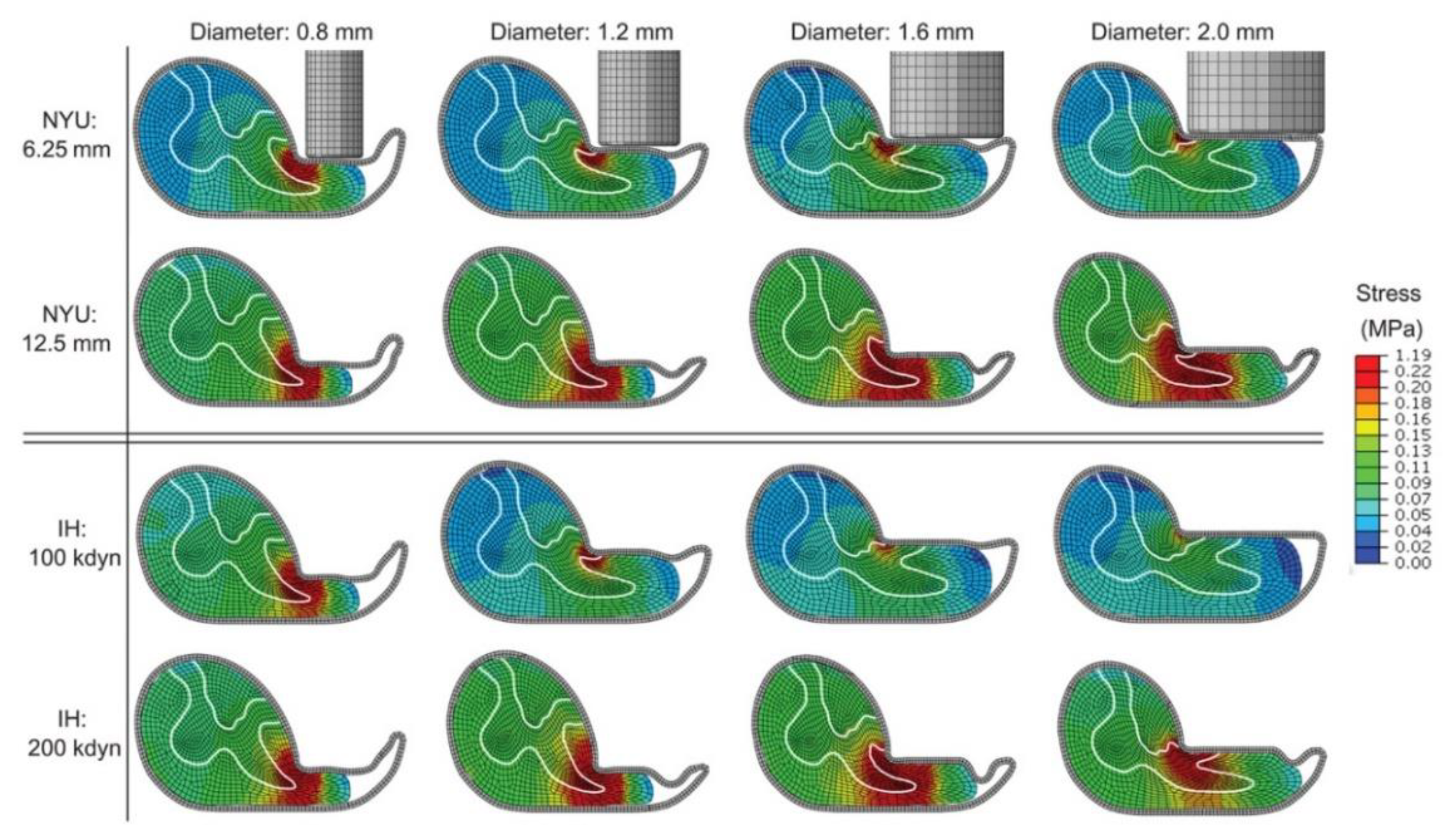
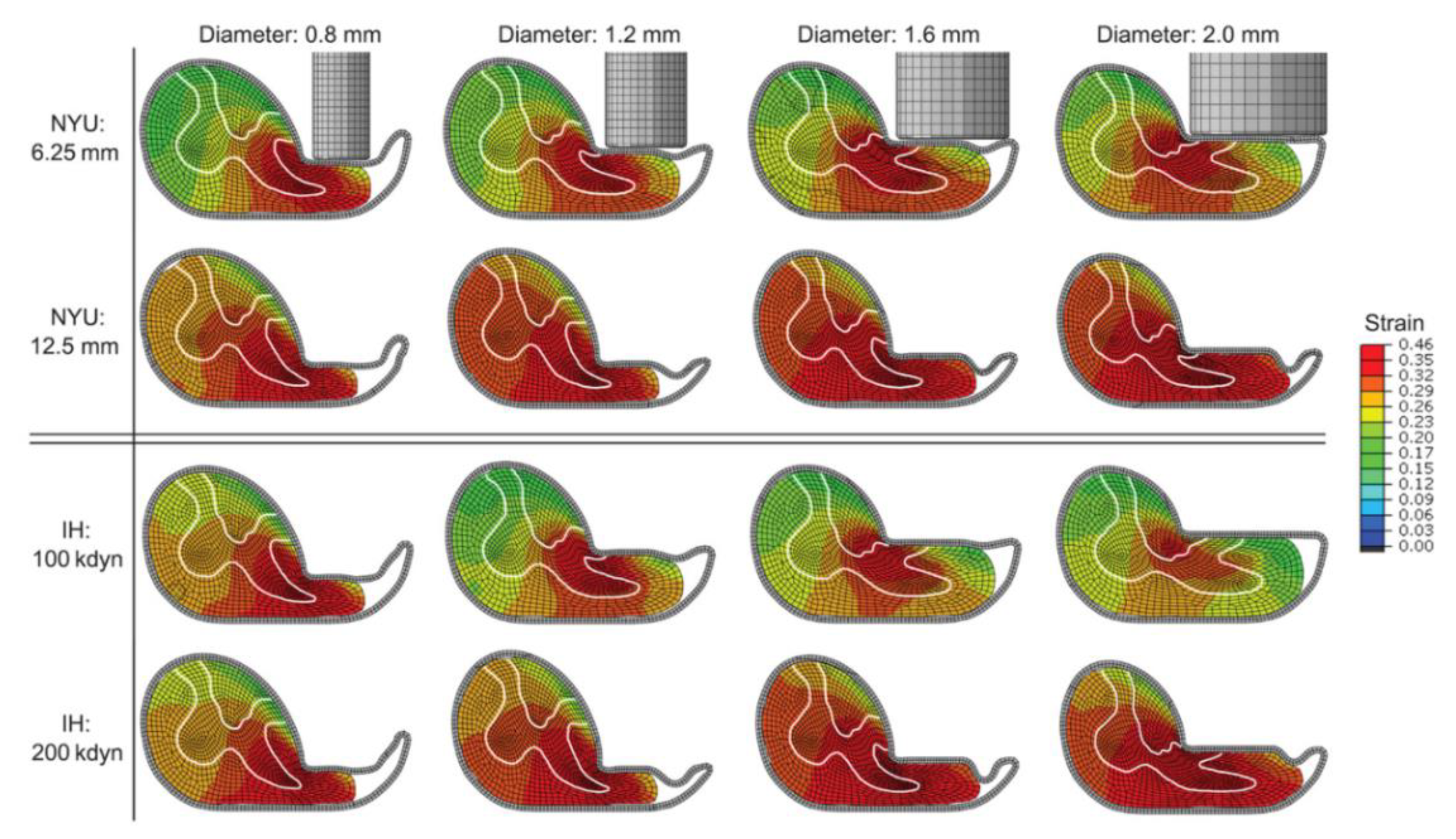
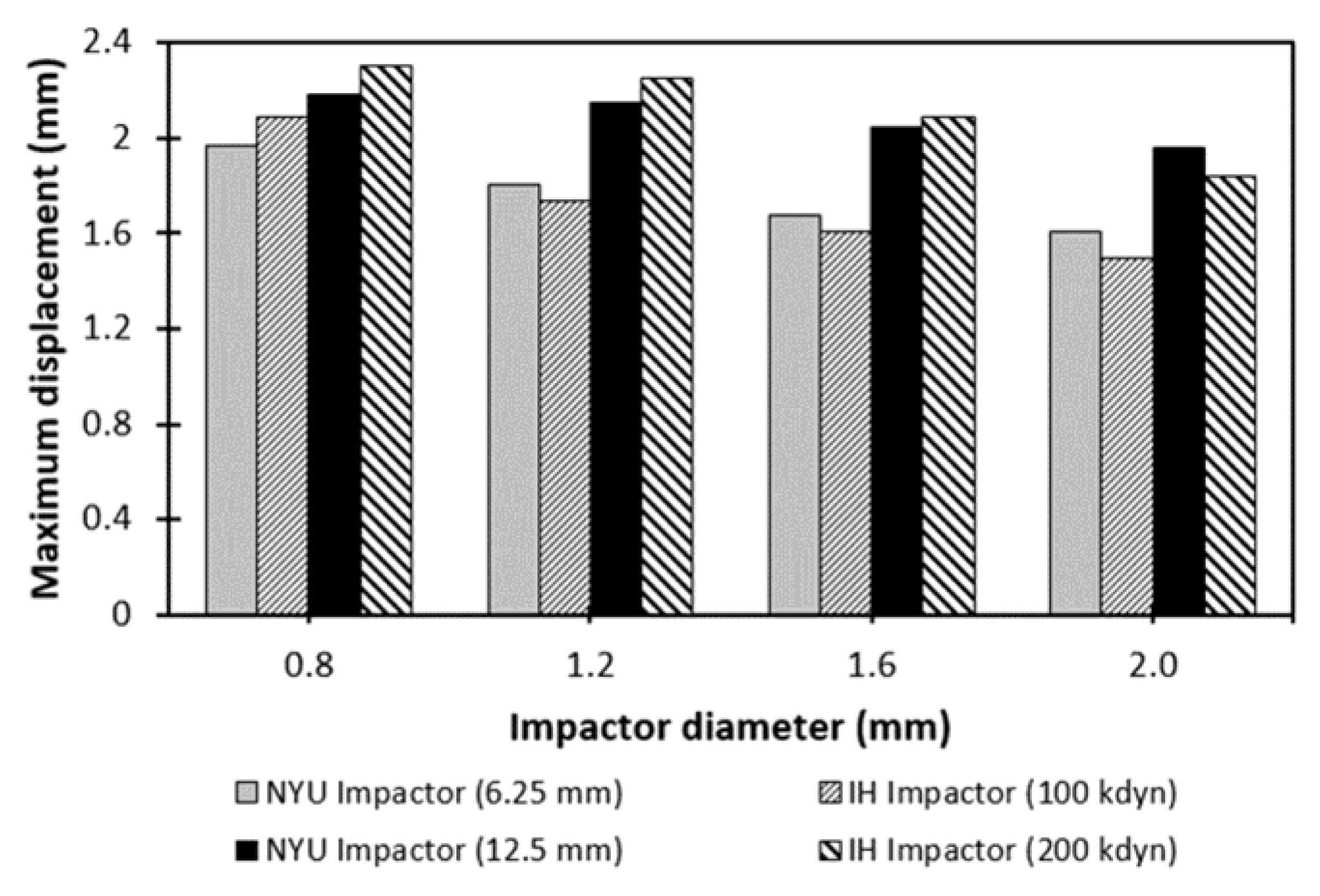
3. Results
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Sekhonm, L.H.; Fehlings, M.G. Epidemiology, demographics, and pathophysiology of acute spinal cord injury. Spine 2001, 26, S2–S12. [Google Scholar] [CrossRef] [PubMed]
- Noyes, D.H. Electromechanical impactor for producing experimental spinal cord injury in animals. Med. Biol. Eng. Comput. 1987, 25, 335–340. [Google Scholar] [CrossRef] [PubMed]
- Constantini, S.; Young, W. The effects of methylprednisolone and the ganglioside GM1 on acute spinal cord injury in rats. J. Neurosurg. 1994, 80, 97–111. [Google Scholar] [CrossRef] [PubMed]
- Basso, D.M.; Beattie, M.S.; Bresnahan, J.C. Graded histological and locomotor outcomes after spinal cord contusion using the NYU weight-drop device versus transection. Exp. Neurol. 1996, 139, 244–256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scheff, S.W.; Rabchevsky, A.G.; Fugaccia, I.; Main, J.A.; Lumpp, J.E., Jr. Experimental modeling of spinal cord injury: Characterization of a force-defined injury device. J. Neurotrauma 2003, 20, 179–193. [Google Scholar] [CrossRef]
- Rabchevsky, A.G.; Sullivan, P.G.; Fugaccia, I.; Scheff, S.W. Creatine diet supplement for spinal cord injury: Influences on functional recovery and tissue sparing in rats. J. Neurotrauma 2003, 20, 659–669. [Google Scholar] [CrossRef]
- Ghasemlou, N.; Kerr, B.J.; David, S. Tissue displacement and impact force are important contributors to outcome after spinal cord contusion injury. Exp. Neurol. 2005, 196, 9–17. [Google Scholar] [CrossRef]
- Nishi, R.A.; Liu, H.; Chu, Y.; Hamamura, M.; Su, M.Y.; Nalcioglu, O.; Anderson, A.J. Behavioral, histological, and ex vivo magnetic resonance imaging assessment of graded contusion spinal cord injury in mice. J. Neurotrauma 2007, 24, 674–689. [Google Scholar] [CrossRef]
- Soblosky, J.S.; Song, J.H.; Dinh, D.H. Graded unilateral cervical spinal cord injury in the rat: Evaluation of forelimb recovery and histological effects. Behav. Brain Res. 2001, 119, 1–13. [Google Scholar] [CrossRef]
- Pearse, D.D.; Lo, T.P., Jr.; Cho, K.S.; Lynch, M.P.; Garg, M.S.; Marcillo, A.E.; Sanchez, A.R.; Cruz, Y.; Dietrich, W.D. Histopathological and behavioral characterization of a novel cervical spinal cord displacement contusion injury in the rat. J. Neurotrauma 2005, 22, 680–702. [Google Scholar] [CrossRef]
- Dunham, K.A.; Siriphorn, A.; Chompoopong, S.; Floyd, C.L. Characterization of a graded cervical hemicontusion spinal cord injury model in adult male rats. J. Neurotrauma 2010, 27, 2091–2106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gensel, J.C.; Tovar, C.A.; Hamers, F.P.; Deibert, R.J.; Beattie, M.S.; Bresnahan, J.C. Behavioral and histological characterization of unilateral cervical spinal cord contusion injury in rats. J. Neurotrauma 2006, 23, 36–54. [Google Scholar] [CrossRef] [PubMed]
- Anderson, K.D.; Sharp, K.G.; Steward, O. Bilateral cervical contusion spinal cord injury in rats. Exp. Neurol. 2009, 220, 9–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aguilar, R.M.; Steward, O. A bilateral cervical contusion injury model in mice: Assessment of gripping strength as a measure of forelimb motor function. Exp. Neurol. 2010, 221, 38–53. [Google Scholar] [CrossRef] [Green Version]
- Sandrow-Feinberg, H.R.; Izzi, J.; Shumsky, J.S.; Zhukareva, V.; Houle, J.D. Forced exercise as a rehabilitation strategy after unilateral cervical spinal cord contusion injury. J. Neurotrauma 2009, 26, 721–731. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.H.; Streijger, F.; Tigchelaar, S.; Maloon, M.; Liu, J.; Tetzlaff, W.; Kwon, B.K. A contusive model of unilateral cervical spinal cord injury using the infinite horizon impactor. J. Vis. Exp. 2012, 65, e3313. [Google Scholar] [CrossRef] [Green Version]
- Streijger, F.; Beernink, T.M.; Lee, J.H.; Bhatnagar, T.; Park, S.; Kwon, B.K.; Tetzlaff, W. Characterization of a cervical spinal cord hemicontusion injury in mice using the infinite horizon impactor. J. Neurotrauma 2013, 30, 869–883. [Google Scholar] [CrossRef] [PubMed]
- Mondello, S.E.; Sunshine, M.D.; Fischedick, A.E.; Moritzm, C.T.; Horner, P.J. A cervical hemi-contusion spinal cord injury model for the investigation of novel therapeutics targeting proximal and distal forelimb functional recovery. J. Neurotrauma 2015, 32, 1994–2007. [Google Scholar] [CrossRef] [Green Version]
- Ungerer, G.; Cui, J.; Ndam, T.; Bekemeier, M.; Song, H.; Li, R.; Siedhoff, H.R.; Yang, B.; Appenteng, M.K.; Greenlief, C.M.; et al. Harpagophytum procumbens extract ameliorates allodynia and modulates oxidative and antioxidant stress pathways in a rat model of spinal cord injury. Neuromol. Med. 2020, 22, 278–292. [Google Scholar] [CrossRef]
- Popovich, P.G.; Lemeshow, S.; Gensel, J.C.; Tovar, C.A. Independent evaluation of the effects of glibenclamide on reducing progressive hemorrhagic necrosis after cervical spinal cord injury. Exp. Neurol. 2012, 233, 615–622. [Google Scholar] [CrossRef] [Green Version]
- Simard, J.M.; Popovich, P.G.; Tsymbalyuk, O.; Gerzanich, V. Spinal cord injury with unilateral versus bilateral primary hemorrhage—Effects of glibenclamide. Exp. Neurol. 2012, 233, 829–835. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nicaise, C.; Hala, T.J.; Frank, D.M.; Parker, J.L.; Authelet, M.; Leroy, K.; Brion, J.P.; Wright, M.C.; Lepore, A.C. Phrenic motor neuron degeneration compromises phrenic axonal circuitry and diaphragm activity in a unilateral cervical contusion model of spinal cord injury. Exp. Neurol. 2012, 235, 539–552. [Google Scholar] [CrossRef] [PubMed]
- Maikos, J.T.; Qian, Z.; Metaxas, D.; Shreiber, D.I. Finite element analysis of spinal cord injury in the rat. J. Neurotrauma 2008, 25, 795–816. [Google Scholar] [CrossRef] [PubMed]
- Khuyagbaatar, B.; Kim, K.; Kim, Y.H. Effect of bone fragment impact velocity on biomechanical parameters related to spinal cord injury: A finite element study. J. Biomech. 2014, 44, 2820–2825. [Google Scholar] [CrossRef] [PubMed]
- Khuyagbaatar, B.; Kim, K.; Park, W.M.; Lee, S.; Kim, Y.H. Increased stress and strain on the spinal cord due to ossification of the posterior longitudinal ligament in the cervical spine under flexion after laminectomy. Proc. Inst. Mech. Eng. Part H 2017, 231, 898–906. [Google Scholar] [CrossRef]
- Greaves, C.Y.; Gadala, M.S.; Oxland, T.R. A threedimensional finite element model of the cervical spine with spinal cord: An investigation of three injury mechanisms. Ann. Biomed. Eng. 2008, 36, 396–405. [Google Scholar] [CrossRef]
- Khuyagbaatar, B.; Kim, K.; Park, W.M.; Kim, Y.H. Effect of posterior decompression extent on biomechanical parameters of the spinal cord in cervical ossification of the posterior longitudinal ligament. Proc. Inst. Mech. Eng. Part H 2016, 230, 545–552. [Google Scholar] [CrossRef]
- Russell, C.M.; Choo, A.M.; Tetzlaff, W.; Chung, T.E.; Oxland, T.R. Maximum principal strain correlates with spinal cord tissue damage in contusion and dislocation injuries in the rat cervical spine. J. Neurotrauma 2012, 29, 1574–1585. [Google Scholar] [CrossRef]
- Khuyagbaatar, B.; Kim, K.; Purevsuren, T.; Lee, S.H.; Kim, Y.H. Biomechanical effects on cervical spinal cord and nerve root following laminoplasty for ossification of the posterior longitudinal ligament in the cervical spine: A comparison between open-door and double-door laminoplasty using finite element analysis. J. Biomech. Eng. 2018, 140, 071006. [Google Scholar] [CrossRef]
- Lam, C.J.; Assinck, P.; Liu, J.; Tetzlaff, W.; Oxland, T.R. Impact depth and the interaction with impact speed affect the severity of contusion spinal cord injury in rats. J. Neurotrauma 2014, 31, 1985–1997. [Google Scholar] [CrossRef] [Green Version]
- Khuyagbaatar, B.; Kim, K.; Kim, Y.H. Conversion Equation between the Drop Height in the New York University Impactor and the Impact Force in the Infinite Horizon Impactor in the Contusion Spinal Cord Injury Model. J. Neurotrauma 2015, 32, 1987–1993. [Google Scholar] [CrossRef] [PubMed]
- Sparrey, C.J.; Salegio, E.A.; Camisa, W.; Tam, H.; Beattie, M.S.; Bresnahan, J.C. Mechanical design and analysis of a unilateral cervical spinal cord contusion injury model in non-human primates. J. Neurotrauma 2016, 33, 1136–1149. [Google Scholar] [CrossRef] [PubMed]
- Fournely, M.; Petit, Y.; Wagnac, E.; Evin, M.; Arnoux, P.J. Effect of experimental, morphological and mechanical factors on the murine spinal cord subjected to transverse contusion: A finite element study. PLoS ONE 2020, 15, e0232975. [Google Scholar] [CrossRef] [PubMed]
- Laing, A.C.; Brenneman, E.C.; Yung, A.; Liu, J.; Kozlowski, P.; Oxland, T. The effects of age on the morphometry of the cervical spinal cord and spinal column in adult rats: An MRI-based study. Anat. Rec. 2014, 297, 1885–1895. [Google Scholar] [CrossRef] [PubMed]
- Jaumard, N.V.; Leung, J.; Gokhale, A.J.; Guarino, B.B.; Welch, W.C.; Winkelstein, B.A. Relevant anatomic and morphological measurements of the rat spine: Considerations for rodent models of human spine trauma. Spine 2015, 40, E1084–E1092. [Google Scholar] [CrossRef] [Green Version]
- Khuyagbaatar, B.; Kim, K.; Park, W.M.; Kim, Y.H. Biomechanical investigation of post-operative C5 palsy due to ossification of the posterior longitudinal ligament in different types of cervical spinal alignment. J. Biomech. 2017, 57, 54–61. [Google Scholar] [CrossRef]
- Walker, C.L.; Zhang, Y.P.; Liu, Y.; Li, Y.; Walker, M.J.; Liu, N.K.; Shields, C.B.; Xu, X.M. Anatomical and functional effects of lateral cervical hemicontusion in adult rats. Restor. Neurol. Neurosci. 2016, 34, 389–400. [Google Scholar] [CrossRef]
- Alvarez-Argote, S.; Gransee, H.M.; Mora, J.C.; Stowe, J.M.; Jorgenson, A.J.; Sieck, G.C.; Mantilla, C.B. The impact of midcervical contusion injury on diaphragm muscle function. J. Neurotrauma 2016, 33, 500–509. [Google Scholar] [CrossRef] [Green Version]
- Persson, C.; Summers, J.; Hall, R.M. The importance of fluid-structure interaction in spinal trauma models. J. Neurotrauma 2011, 28, 113–125. [Google Scholar] [CrossRef]
- Pleasant, J.M.; Carlson, S.W.; Mao, H.; Scheff, S.W.; Yang, K.H.; Saatman, K.E. Rate of neurodegeneration in the mouse controlled cortical impact model is influenced by impactor tip shape: Implications for mechanistic and therapeutic studies. J. Neurotrauma 2011, 28, 2245–2262. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khuyagbaatar, B.; Kim, K.; Batbayar, T.; Kim, Y.H. Effects of Impactor Size on Biomechanical Characteristics of Spinal Cord in Hemicontusion Injury Model Using Finite Element Analysis. Appl. Sci. 2020, 10, 4097. https://doi.org/10.3390/app10124097
Khuyagbaatar B, Kim K, Batbayar T, Kim YH. Effects of Impactor Size on Biomechanical Characteristics of Spinal Cord in Hemicontusion Injury Model Using Finite Element Analysis. Applied Sciences. 2020; 10(12):4097. https://doi.org/10.3390/app10124097
Chicago/Turabian StyleKhuyagbaatar, Batbayar, Kyungsoo Kim, Temuujin Batbayar, and Yoon Hyuk Kim. 2020. "Effects of Impactor Size on Biomechanical Characteristics of Spinal Cord in Hemicontusion Injury Model Using Finite Element Analysis" Applied Sciences 10, no. 12: 4097. https://doi.org/10.3390/app10124097



