Comparison of PCR Primers for Analyzing Denitrifying Microorganisms in the Hyporheic Zone
Abstract
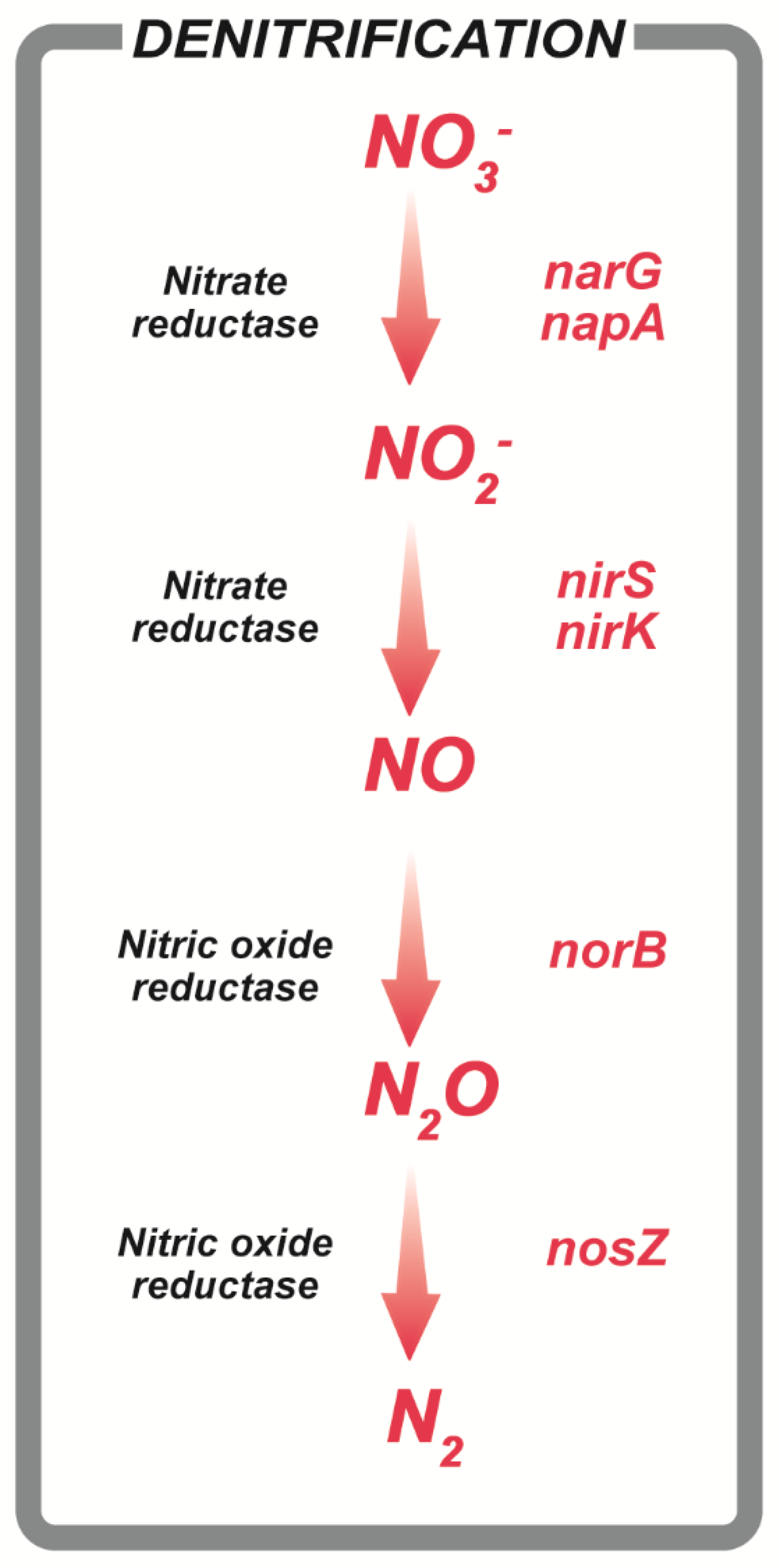
1. Introduction
2. Materials and Methods
2.1. Study Area and Sampling
2.2. Type Strain Collection and Nucleic Acid Extraction
2.3. Comparison of the PCR Primers for Denitrification-Associated Genes
2.4. PCR Amplification Testing of Denitrification-Associated Genes
3. Results
3.1. Type Strain Collection and Nucleic Acid Extraction
3.2. Comparison of PCR Primers for Denitrification Associated Genes
3.3. PCR Amplification Testing of Denitrification-Associated Genes
4. Discussion
5. Conclusions
Supplementary Materials
Funding
Conflicts of Interest
References
- Hayatsu, M.; Tago, K.; Saito, M. Various players in the nitrogen cycle: Diversity and functions of the microorganisms involved in nitrification and denitrification. Soil Sci. Plant Nutr. 2008, 54, 33–45. [Google Scholar] [CrossRef]
- Stein, L.Y.; Kiotz, M.G. The nitrogen cycle. Curr. Biol. 2016, 26, 94–98. [Google Scholar] [CrossRef] [PubMed]
- Hauck, R.D. Nitrogen fertilizer effects in nitrogen cycle processes. In Terrestrial Nitrogen Cycles; Clark, F.E., Rosswall, T., Eds.; Swedish Natural Science Research Council: Stockholm, Sweden, 1981; pp. 551–562. [Google Scholar]
- Pichinoty, F.; Garcia, J.L.; Job, C.; Durand, M. Denitrification by Bacillus licheniformis. Can. J. Microbiol. 1978, 24, 45–49. [Google Scholar] [CrossRef]
- Shoun, H.; Kano, M.; Baba, I.; Takaya, N. Denitrification by actinomycetes and purification of dissimilatory nitrite reductase and azurin from Streptomyces thioluteus. J. Bacteriol. 1998, 180, 4413–4415. [Google Scholar] [CrossRef] [PubMed]
- Gamble, T.N.; Betlach, H.R.; Tiedje, J.M. Numerically dominant denitrifying bacteria from world soils. Appl. Environ. Microbiol. 1977, 33, 926–939. [Google Scholar] [CrossRef] [PubMed]
- Mancinelli, R.L.; Hochstein, L.I. The occurrence of denitrification in extremely halophilic bacteria. FEMS Microbiol. Lett. 1986, 35, 55–58. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Matsuo, Y.; Takimoto, A.; Suzuki, S.; Maruo, F.; Shoun, H. Denitrification, a novel type of respiratory metabolism in fungal denitrification. J. Biol. Chem. 1996, 5, 16263–16267. [Google Scholar] [CrossRef] [PubMed]
- Philippot, L.; Hallin, S. Molecular analyses of soil denitrifying bacteria. In Molecular Techniqeus for Soil, Rhizosphere; Cooper, J.E., Rao, J.R., Eds.; CAB International: Willingford, UK, 2006; pp. 146–165. [Google Scholar]
- van Spanning, R.J.M.; Richardson, D.J.; Ferguson, S.J. Introduction to the biochemistry and molecular biology of denitrification. In Biology of the Nitrogen Cycle; Bothe, H., Ferguson, S.J., Newton, W.E., Eds.; Elsevier: Amsterdam, The Netherlands, 2007; pp. 3–20. [Google Scholar]
- Braker, G.; Tiedje, J.M. Nitric oxide reductase (norB) genes from pure cultures and environmental samples. Appl. Environ. Microbiol. 2003, 69, 3476–3483. [Google Scholar] [CrossRef]
- Bremer, C.; Braker, G.; Matthies, D.; Reuter, A.; Engels, C.; Conrad, R. Impact of plant functional group, plant species, and sampling time on the composition of nirK-type denitrifier communities in soil. Appl. Environ. Microbiol. 2007, 73, 6876–6884. [Google Scholar] [CrossRef]
- Hannig, M.; Braker, G.; Dippner, J.; Jürgens, K. Linking denitrifier community structure and prevalent biogeochemical parameters in the pelagial of the central Baltic Proper (Baltic Sea). FEMS Microbiol. Ecol. 2006, 57, 260–271. [Google Scholar] [CrossRef][Green Version]
- Kandeler, E.; Deiglmayr, K.; Tscherko, D.; Bru, D.; Philippor, L. Abundance of narG, nirS, nirK, and nosZ genes of denitrifying bacteria during primary successions of a glacier foreland. Appl. Environ. Microbiol. 2006, 72, 5957–5962. [Google Scholar] [CrossRef] [PubMed]
- Bothe, H.; Jost, G.; Schloter, M.; Ward, B.B.; Witzel, K.P. Molecular analysis of ammonia oxidation and denitrification in natural environments. FEMS Microbiol. Rev. 2000, 24, 673–690. [Google Scholar] [CrossRef] [PubMed]
- Enwall, K.; Hallin, S. Comparison of T-RFLP and DGGE techniques to assess denitrifier community composition in soil. Lett. Appl. Microbiol. 2009, 48, 145–148. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, Y.; Zhou, H.; Li, L.; Zheng, J.; Zhang, X.; Zheng, J.; Pan, G. Abundance, composition and activity of denitrifier communities in metal polluted paddy soils. Sci. Rep. 2016, 6, 19086. [Google Scholar] [CrossRef]
- Zhang, W.; Gao, Y.; Yi, N.; Wang, C.; Di, P.; Yan, S. Variations in abundance and community composition of denitrifying bacteria during a cyanobacterial bloom in a eutrophic shallow lake in China. J. Freshw. Ecol. 2017, 32, 467–476. [Google Scholar] [CrossRef]
- Bowen, J.L.; Byrnes, J.E.; Weisman, D.; Colaneri, C. Functional gene pyrosequencing and network analysis: An approach to examine the response of denitrifying bacteria to increased nitrogen supply in salt marsh sediments. Front. Microbiol. 2013, 4, 342. [Google Scholar] [CrossRef]
- McGuinness, L.M.; Salganik, M.; Vega, L.; Pickering, K.D.; Kerkhof, L.J. Replicability of bacterial communities in denitrifying bioreactors as measured by PCR/T-RFLP analysis. Environ. Sci. Technol. 2006, 40, 509–515. [Google Scholar] [CrossRef]
- Yi, N.; Gao, Y.; Zhang, Z.; Wang, Y.; Liu, X.; Zhang, L.; Yan, S. Response of spatial patterns of denitrifying bacteria communities to water properties in the stream inlets at Dianchi Lake, China. Int. J. Genom. 2015, 2015, 572121. [Google Scholar] [CrossRef]
- Kim, H.; Kaown, D.; Mayer, B.; Lee, J.Y.; Lee, K.K. Combining pyrosequencing and isotopic approaches to assess denitrification in a hyporheic zone. Sci. Total Environ. 2018, 631, 755–764. [Google Scholar] [CrossRef]
- Kim, H.; Lee, K.K.; Lee, J.Y. Numerical verification of hyporheic zone depth estimation using streambed temperature. J. Hydrol. 2014, 511, 861–869. [Google Scholar] [CrossRef]
- Kim, H.; Kaown, D.; Mayer, B.; Lee, J.Y.; Hyun, Y.; Lee, K.K. Identifying the sources of nitrate contamination of groundwater in an agricultural area (Haean basin, Korea) using isotope and microbial community analyses. Sci. Total Environ. 2015, 533, 566–575. [Google Scholar] [CrossRef] [PubMed]
- Suárez, S.P.; Peiffer, S.; Gebauer, G. Origin and fate of nitrate runoff in an agricultural catchment: Haean, South Korea–Comparison of two extremely different monsoon seasons. Sci. Total Environ. 2019, 648, 66–79. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Uchida, Y.; Shimomura, Y.; Akiyama, H.; Hayatsu, M. Responses of denitrifying bacterial communities to short-term waterlogging of soils. Sci. Rep. 2017, 7, 803. [Google Scholar] [CrossRef] [PubMed]
- Findlay, S.E.G.; Gregory, S.V.; Grimm, N.B.; Johnson, S.L.; McDowell, W.H.; Meyer, J.L.; Valett, H.M.; Webster, J.R.; Arango, C.P.; Beaulieu, J.J.; et al. Stream denitrification across biomes and its response to anthropogenic nitrate loading. Nature 2008, 452, 202–205. [Google Scholar]
- Joo, H.S.; Hirai, M.; Shoda, M. Characteristics of ammonium removal by heterotrophic nitrification-aerobic denitrification by Alcaligenes faecalis No. 4. J. Biosci. Bioeng. 2005, 100, 184–191. [Google Scholar] [CrossRef]
- Clark, I.M.; Buchkina, N.; Jhurreea, D.; Goulding, K.W.T.; Hirsch, P.R. Impacts of nitrogen application rates on the activity and diversity of denitrifying bacteria in the Broadbalk Wheat Experiment. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2012, 367, 1235–1244. [Google Scholar] [CrossRef]
- Cuhel, J.; Simek, M.; Laughlin, R.J.; Bru, D.; Cheneby, D.; Watson, C.J.; Philippot, L. Insights into the effect of soil pH on N2O and N2 emissions and denitrifier community size and activity. Appl. Environ. Microbiol. 2010, 76, 1870–1878. [Google Scholar] [CrossRef]
- Milenkovski, S. Structure and Function of Microbial Communities in Constructed Wetlands—Influence of Environmental Parameters and Pesticides on Denitrifying Bacteria. Ph.D. Thesis, Lund University, Lund, Sweden, 2009; p. 119. [Google Scholar]
- Coyne, M.S.; Lal, R.; Stewart, B.A. Soil Nitrogen Uses and Environmental Impacts; CRC Press: Boca Raton, FL, USA, 2018; pp. 95–139. [Google Scholar]
- Tomasek, A.; Kozarek, J.L.; Hondzo, M.; Lurndahl, N.; Sadowsky, M.J.; Wang, P.; Staley, C. Environmental drivers of denitrification rates and denitrifying gene abundances in channels and riparian areas. Water Res. Res. 2017, 53, 6523–6538. [Google Scholar] [CrossRef]
- Green, J.L.; Bohannan, B.J.M.; Whitaker, R.J. Microbial biogeography: From taxonomy to traits. Science 2008, 320, 1039–1043. [Google Scholar] [CrossRef]

| No. | Bacteria | Culture Collection # | Condition | ||
|---|---|---|---|---|---|
| Medium | Temp (°C) | Time (days) | |||
| 01 | Achromobacter denitrificans | KACC 12986 | NA | 24 | 1 |
| 02 | Aquitalea denitrificans | KACC 12729 | R2A | 30 | 4 |
| 03 | Blastobacter denitrificans | KACC 14837 | BYTGA | 28 | 3–5 |
| 04 | Burkholderia denitrificans | KACC 12733 | R2A | 30 | 2 |
| 05 | Castellaniella denitrificans | KACC 13903 | R2A | 30 | 1 |
| 06 | Comamonas denitrificans | KACC 13430 | TSA | 28 | 1 |
| 07 | Flavobacterium denitrificans | KACC 12500 | R2A | 30 | 2 |
| 08 | Leucobacter denitrificans | KACC 14055 | R2A | 30 | 3 |
| 09 | Rhodanobacter denitrificans | KACC 12251 | NA | 30 | 2 |
| 10 | Roseobacter denitrificans | KACC 17256 | R2A | 30 | 2 |
| 11 | Paracoccus denitrificans | KACC 13175 | MA | 30 | 1–2 |
| Gene | Primer Set No. | Denitrifying Bacteria (Type Strain) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | Remark | |||
| narG | 1 | Non-specific band #2 | ||||||||||||
| 2 | ● | ● | ● | ● | ● | ● | ||||||||
| 3 | ● | |||||||||||||
| 4 | Non-specific band | |||||||||||||
| 5 | ● | ● | ● | |||||||||||
| napA | 1 | ● | ND | ● | ● | Multi-band | ||||||||
| 2 | ||||||||||||||
| nirS | 1 | ● | ● | ● | ● | ● | Multi-band | |||||||
| 2 | ● | ● | ● | ● | Multi-band #10 | |||||||||
| 3 | Multi-band | |||||||||||||
| 4 | ● | ● | ● | Multi-band | ||||||||||
| 5 | ● | ● | ● | ● | Multi-band | |||||||||
| nirK | 1 | ● | ● | ● | ● | ND | Multi-band | |||||||
| 2 | ● | ● | ● | ● | Weak multi band | |||||||||
| 3 | ● | ● | ● | Multi-band | ||||||||||
| 4 | ● | ● | Multi-band | |||||||||||
| 5 | ● | ● | ● | ND | Multi-band | |||||||||
| norB | cnorB | 1 | ● | ● | ND | ● | Multi-band | |||||||
| 2 | ● | ● | ● | ● | Non-specific band | |||||||||
| 3 | ● | ND | ● | ● | ● | Non- specific band (cnor B) | ||||||||
| qnorB | 4 | ● | ● | ● | Multi-band | |||||||||
| 5 | ● | ● | Non-specific band (qnor B) | |||||||||||
| nosZ | 1 | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | Multi-band | ||
| 2 | ● | ● | ● | ● | ● | ● | ● | |||||||
| 3 | ● | ● | ● | |||||||||||
| 4 | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | Some non-specific band | ||
| 5 | ● | ● | ● | |||||||||||
| Aqueous Sample | narG | napA | nirS | nirK | cnorB | qnorB | nosZ |
|---|---|---|---|---|---|---|---|
| 174 | 152 | 425 | 514 | 389 | 637 | 760 | |
| SW * 1 | ● | ● | ● | ● | |||
| SW 2 | ● | ● | ● | ● | |||
| SW 3 | ● | ● | ● | ● | |||
| GW * 1 | ● | ● | |||||
| GW 2 | ● | ● | ● | ||||
| GW 3 | ● | ● | ▲ | ||||
| HW * 1 | ● | ● | ● | ▲ * | ● | ● | ● |
| HW 2 | ● | ● | ● | ● | ● | ● | |
| HW 3 | ● | ● | ▲ | ||||
| HW 4 | ● | ● | ● | ● | ● | ||
| HW 5 | ● | ● | ● | ● | ● | ||
| HW 6 | ● | ● | ● | ● | ● | ||
| HW7 | ● | ● | ● | ● | ● | ||
| HW 8 | ● | ● | ● | ● | ● | ||
| HW 9 | |||||||
| HW 10 | ● |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H. Comparison of PCR Primers for Analyzing Denitrifying Microorganisms in the Hyporheic Zone. Appl. Sci. 2020, 10, 4172. https://doi.org/10.3390/app10124172
Kim H. Comparison of PCR Primers for Analyzing Denitrifying Microorganisms in the Hyporheic Zone. Applied Sciences. 2020; 10(12):4172. https://doi.org/10.3390/app10124172
Chicago/Turabian StyleKim, Heejung. 2020. "Comparison of PCR Primers for Analyzing Denitrifying Microorganisms in the Hyporheic Zone" Applied Sciences 10, no. 12: 4172. https://doi.org/10.3390/app10124172
APA StyleKim, H. (2020). Comparison of PCR Primers for Analyzing Denitrifying Microorganisms in the Hyporheic Zone. Applied Sciences, 10(12), 4172. https://doi.org/10.3390/app10124172





