The Combination of Diode Laser and Ozonated Water in the Treatment of Complicated Pulp Gangrene
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Participant
2.2. Materials, Devices, and Techiques
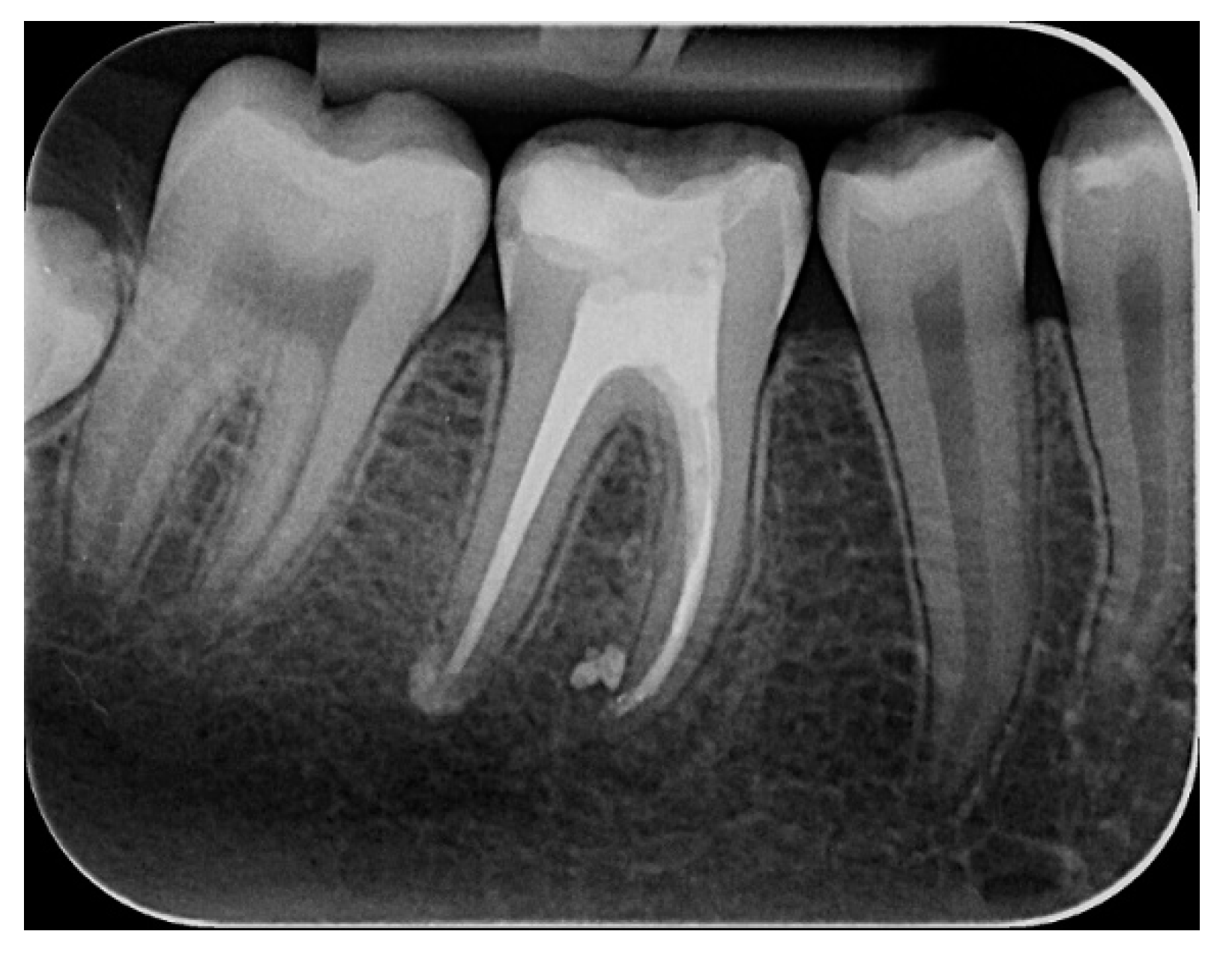
2.2.1. Chemomechanical Debridement of Endodontic Space and Root Canal Filling
2.2.2. Identification and Quantification of E. faecalis
Endodontic Sample Collection
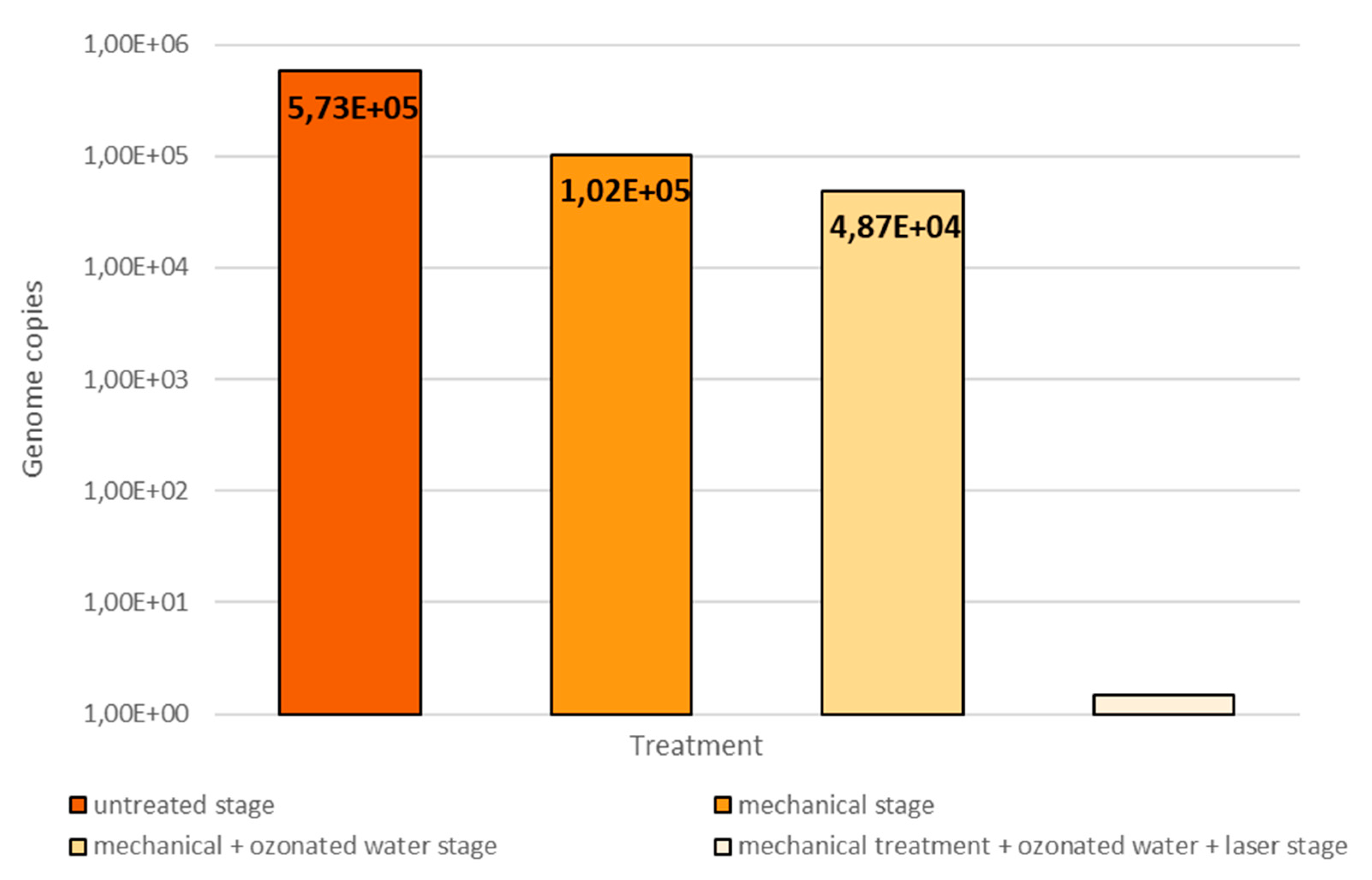
- after opening the pulp chamber, before starting the chemomechanical decontamination of the endodontic space (untreated stage)
- after mechanical debridement with physiological serum (mechanical stage)
- after mechanical debridement and ozone therapy, where ozone was the substitute for chemical decontaminants (mechanical + ozonated water stage)
- after mechanical debridement, ozone therapy and diode laser therapy, the equivalent of a complete chemomechanical preparation (mechanical + ozonated water + laser stage)
Quantification of Enterococcus faecalis
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Shah, D.; Lynd, T.; Ho, D.; Chen, J.; Vines, J.; Jung, H.D.; Kim, J.H.; Zhang, P.; Wu, H.; Jun, H.W.; et al. Pulp-Dentin Tissue Healing Response: A Discussion of Current Biomedical Approaches. J. Clin. Med. 2020, 9, 434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshizawa, J.M.; Schafer, C.A.; Schafer, J.J.; Farrell, J.J.; Paster, B.J.; Wong, D.T.W. Salivary Biomarkers: Toward Future Clinical and Diagnostic Utilities. Clin. Microbiol. Rev. 2013, 26, 781–791. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Isola, G.; Alibrandi, A.; Rapisarda, E.; Matarese, G.; Williams, R.C.; Leonardi, R. Association of vitamin D in patients with periodontitis: A cross-sectional study. J Periodontal Res. 2020. [Google Scholar] [CrossRef] [PubMed]
- Isola, G.; Alibrandi, A.; Currò, M.; Matarese, M.; Ricca, S.; Matarese, G.; Ientile, R.; Kocher, T. Evaluation of salivary and serum ADMA levels in patients with periodontal and cardiovascular disease as subclinical marker of cardiovascular risk. J Periodontol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Bordea, I.R.; Hanna, R.; Chinifoursh, N.; Gradinaru, E.; Campean, R.S.; Sîrbu, A.; Amaroli, A.; Benedicenti, S. Evaluation the Outcome of Various Laser Therapy Applications in Root Canal Disinfection: A Systematic Review. Photodiagnosis Photodyn. Ther. 2020, 29, 101611. [Google Scholar] [CrossRef] [PubMed]
- Pourhajibagher, M.; Chinifoursh, N.; Bahador, A. Antimicrobial action photoactivated C-Phycocyanin against Enterococcus faecalis biofilms: Attenuation of quorum-sensing system. Photodiagnosis Photodyn. Ther. 2019, 28, 286–291. [Google Scholar] [CrossRef]
- Zan, R.; Kutlu, G.; Hubbezoglu, I.; Sumer, Z.; Tunc, T.; Mutlu, Z. Bactericidal effects of various irrigation solutions against staphylococcus aureus in human root canal. J. Istanb. Univ. Fac. Dent. 2015, 49, 19–26. [Google Scholar] [CrossRef]
- Mohammadi, Z.; Shalavi, S.; Soltani, M.K.; Asgary, S. A review of the properties and applications of ozone in endodontics: An update. Iran Endod. J. 2013, 8, 40–43. [Google Scholar]
- Burke, F.J. Ozone and caries: A review of the literature. Dent. Update 2012, 39, 271–278. [Google Scholar] [CrossRef]
- Azarpazhooh, A.; Limeback, H. The application of ozone in dentistry: A systematic review of literature. J. Dent. 2008, 36, 104–116. [Google Scholar] [CrossRef]
- Ricucci, D.; Siqueira, J.F., Jr. Biofilms and apical periodontitis: Study of prevalence and association with clinical and histopathologic findings. J. Endod. 2010, 36, 1277–1288. [Google Scholar] [CrossRef] [PubMed]
- Love, R.M. Enterococcus faecalis—A mechanism for its role in endodontic failure. Int. Endod. J. 2001, 34, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Antunes, H.S.; Rocas, I.N.; Alves, F.R.; Siqueira, J.F., Jr. Total and Specific Bacterial Levels in the Apical Root Canal System of Teeth with Post-treatment Apical Periodontitis. J. Endod. 2015, 41, 1037–1042. [Google Scholar] [CrossRef]
- Nair, P.N. On the causes of persistent apical periodontitis: A review. Int. Endod. J. 2006, 39, 249–281. [Google Scholar] [CrossRef]
- Cobzeanu, B.M.; Costan, V.D.; Danciu, M.; Pasca, S.A.; Sulea, D.; Ungureanu, L.B.; Moscalu, M.; Cobzeanu, M.D.; Popescu, E. Environmental factors involved in genesis of retromolar oropharynx junction cancer. Environ. Eng. Manag. J. 2017, 16, 1101–1106. [Google Scholar] [CrossRef]
- Prada, I.; Mico-Munoz, P.; Giner-Lluesma, T.; Mico-Martinez, P.; Muwaquet-Rodiquez, S.; Albero-Monteagudo, A. Update of therapeutic planning of irrigation and intracanal medication in root canal treatment. A literature review. J. Clin. Exp. Dent. 2019, 11, 185–193. [Google Scholar] [CrossRef]
- Restaino, L.; Frampton, E.W.; Hemphill, J.B.; Palnikar, P. Efficacy of ozonated water against various food-related microorganisms. Appl. Environ. Microbiol. 1995, 61, 3471–3475. [Google Scholar] [CrossRef] [Green Version]
- Estrela, C.; Estrela, C.R.; Decurcio, D.A.; Hollanda, A.C.; Silva, J.A. Antimicrobial efficacy of ozonated water, gaseous ozone, sodium hypochlorite and chlorhexidine in infected human root canals. Int. Endod. J. 2007, 40, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, F.; Kitamura, C.; Nagayoshi, M.; Chen, K.K.; Terashita, M.; Nishihara, T. Ozonated water improves lipopolysaccharide-induced responses of an odontoblast-like cell line. J. Endod. 2009, 35, 668–672. [Google Scholar] [CrossRef] [PubMed]
- Huth, K.C.; Jakob, F.M.; Saugel, B.; Cappello, C.; Paschos, E.; Hollweck, R.; Hickel, R.; Brand, K. Effect of ozone on oral cells compared with established antimicrobials. Eur. J. Oral. Sci. 2006, 114, 435–440. [Google Scholar] [CrossRef] [PubMed]
- Filippi, A. The effects of ozonized water on epithelial wound healing. Dtsch. Zahnarztl. Z. 2001, 56, 104–108. [Google Scholar]
- Nagayoshi, M.; Kitamura, C.; Fukuizumi, T.; Nishihara, T.; Terashita, M. Antimicrobial effect of ozonated water on bacteria invading dentinal tubules. J. Endod. 2004, 30, 778–781. [Google Scholar] [CrossRef]
- Dai, T.; Huang, Y.Y.; Hamblin, M.R. Photodynamic therapy for localized infections—State of the art. Photodiagnosis Photodyn. Ther. 2009, 6, 170–188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oliveira, B.P.; Aguiar, C.M.; Camara, A.C. Photodynamic therapy in combating the causative microorganisms from endodontic infections. Eur. J. Dent. 2014, 8, 424–430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ricardo, A.L.; Britto, M.L.; Genovese, W.J. In vivo study of the Nd:YAP laser in persistent periapical lesion. Photomed. Laser Surg. 2005, 23, 582–585. [Google Scholar] [CrossRef] [PubMed]
- Ozel, E.; Yucekal-Tuncer, B.; Firatli, E. Short-term treatment of periapical lesion of anterior tooth affected by microleakage using Nd:YAG laser. Case report. N. Y. State Dent. J. 2010, 76, 48–51. [Google Scholar] [PubMed]
- Bourgeois, D.; David, A.; Inquimbert, C.; Tramini, P.; Molinari, N.; Carrouel, F. Quantification of carious pathogens in the interdental microbiota of young caries-free adults. PLoS ONE 2017, 12, e0185804. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jelihovschi, I.; Drochioi, C.; Badescu, A.C.; Lupusoru, R.V.; Munteanu, A.E.; Baranov, N.; Manuc, D.; Serban, R.I.; Cobzaru, R.G.; Ripa, C.V.; et al. Comparison of Sampling Techniques for qPCR Quantification of Periodontal Pathogens. Rev. Chim. 2017, 68, 2853–2856. [Google Scholar] [CrossRef]
- Figini, L.; Lodi, G.; Gorni, F.; Gagliani, M. Single versus multiple visits for endodontic treatment of permanent teeth: A Cochrane systematic review. J. Endod. 2008, 34, 1041–1047. [Google Scholar] [CrossRef]
- Cardoso, M.G.; Oliveira, L.D.; Koga-Ito, C.Y.; Jorge, A.O. Effectiveness of ozonated water on Candida albicans, Enterococcus faecalis, and endotoxins in root canals. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2008, 105, 85–91. [Google Scholar] [CrossRef]
- Tuncay, O.; Dincer, A.N.; Kustarci, A.; Er, O.; Dinc, G.; Demirbuga, S. Effects of ozone and photo-activated disinfection against E. Faecalis biofilms In Vitro. Niger J. Clin. Pract. 2015, 18, 814–818. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bocci, V. Biological and clinical effects of ozone. Has ozone therapy a future in medicine? Br. J. Biomed. Sci. 1999, 56, 270–279. [Google Scholar] [PubMed]
- Huth, K.C.; Saugel, B.; Jakob, F.M.; Cappelo, C.; Quirling, M.; Paschos, E.; Ern, K.; Hickel, R.; Brand, K. Effect of aqueous ozone on the NF-kappa B system. J. Dent. Res. 2007, 86, 451–456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baysan, A.; Lynch, E. Effect of ozone on the oral microbiota and clinical severity of primary root caries. Am. J. Dent. 2004, 17, 56–60. [Google Scholar]
- Lezcano, I.; Rey, R.P.; Gutiérrez, M.S.; Baluja, C.; Sánchez, E. Ozone inactivation of microorganisms in water. Gram-positive bacteria and yeast. Ozone Sci. Eng. 2001, 23, 183–187. [Google Scholar] [CrossRef]
- Giuroiu, C.; Dragomir, R.; Hamburda, T.; Salceanu, M.; Aminov, L.; Melian, A.; Andrian, S.; Vataman, M. The role of ozonated water in the success of therapy of chronic apical periodontitis. Case report. Rom. J. Oral Reabil. 2014, 6, 71–75. [Google Scholar]
- Silva, E.J.N.L.; Prado, M.C.; Soares, D.N.; Hecksher, F.; Martins, J.N.R.; Fidalgo, T.K.S. The effect of ozone therapy in root canal disinfection: A systematic review. Int. Endod. J. 2020, 53, 317–332. [Google Scholar] [CrossRef] [Green Version]
- De Groot, S.D.; Verhaagen, B.; Versluis, M.; Wu, M.K.; Wesselink, P.R.; van der Sluis, L.W. Laser-activated irrigation within root canals: Cleaning efficacy and flow visualization. Int. Endod. J. 2009, 42, 1077–1083. [Google Scholar] [CrossRef]
- Garcez, A.S.; Nuñez, S.C.; Hamblin, M.R.; Ribeiro, M.S. Antimicrobial effects of photodynamic therapy on patients with necrotic pulps and periapical lesion. J. Endod. 2008, 34, 138–142. [Google Scholar] [CrossRef] [Green Version]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giuroiu, C.L.; Andrian, S.; Stoleriu, S.; Scurtu, M.; Țănculescu, O.; Poroch, V.; Sălceanu, M. The Combination of Diode Laser and Ozonated Water in the Treatment of Complicated Pulp Gangrene. Appl. Sci. 2020, 10, 4203. https://doi.org/10.3390/app10124203
Giuroiu CL, Andrian S, Stoleriu S, Scurtu M, Țănculescu O, Poroch V, Sălceanu M. The Combination of Diode Laser and Ozonated Water in the Treatment of Complicated Pulp Gangrene. Applied Sciences. 2020; 10(12):4203. https://doi.org/10.3390/app10124203
Chicago/Turabian StyleGiuroiu, Cristian Levente, Sorin Andrian, Simona Stoleriu, Mihaela Scurtu, Oana Țănculescu, Vladimir Poroch, and Mihaela Sălceanu. 2020. "The Combination of Diode Laser and Ozonated Water in the Treatment of Complicated Pulp Gangrene" Applied Sciences 10, no. 12: 4203. https://doi.org/10.3390/app10124203




