Ten Years of Micro-CT in Dentistry and Maxillofacial Surgery: A Literature Overview
Abstract
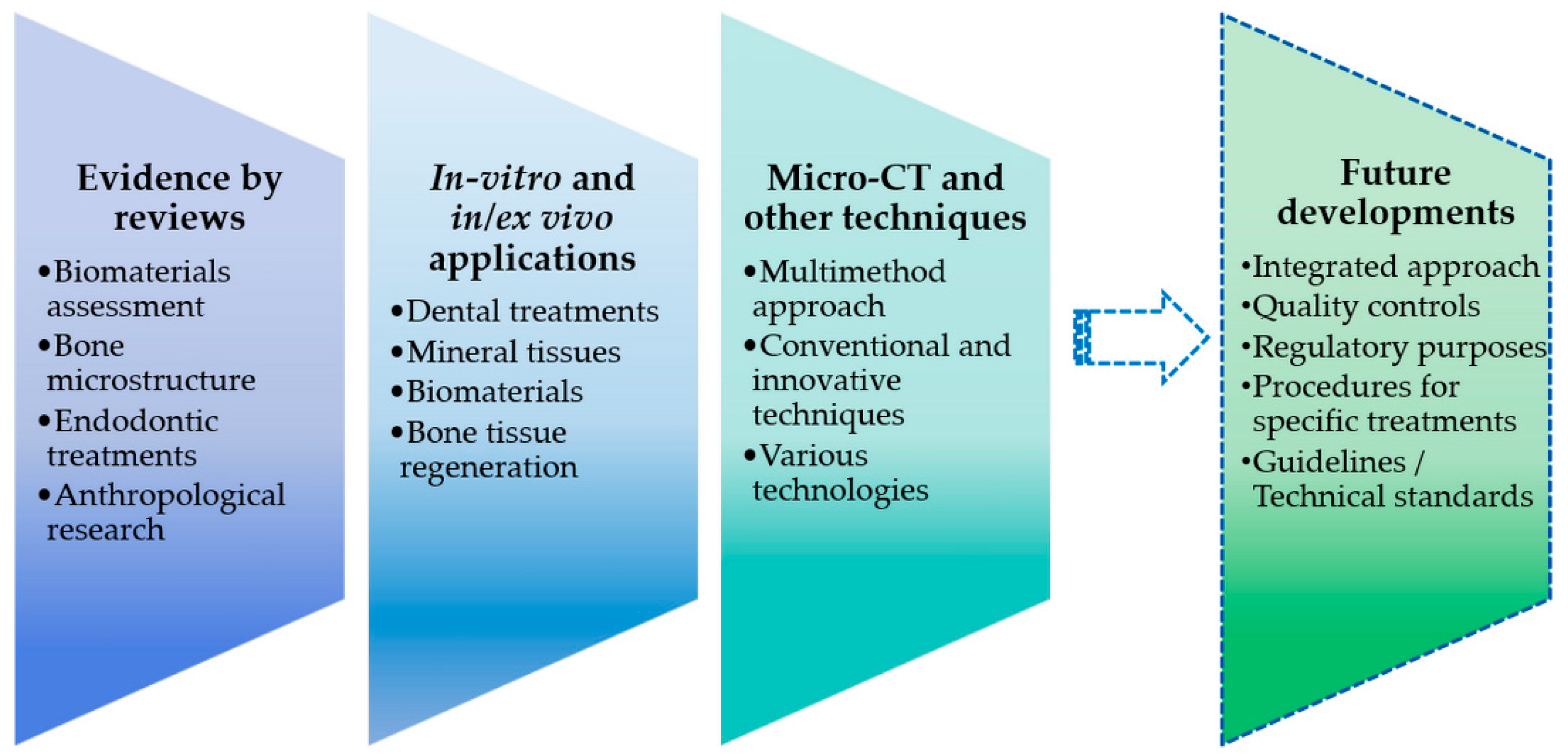
:1. Introduction
- Which have been the main dentistry and MFS applications of micro-CT during the last 10 years?
- What are the main literature reviews in 10 years?
- What will be the possible future scenario for the micro-CT development?
2. Evidence and Effectiveness by Literature Reviews
3. Micro-CT Studies: In Vitro and In/Ex Vivo Applications
3.1. Dental Treatments and Mineral Tissues
3.2. Biomaterials and Bone Tissue Regeneration
- Granular deproteinized bovine bone and b-TCP alone or with dental pulp stem cells [62].
- Polycaprolactone-tricalcium phosphate (PCL-TCP) [102].
- Nano-hydroxyapatite/collagen composite [106].
- Nanofibrous bone graft coupled with osteoinductive proteins/peptides—poly(D,L-lactide-co-glycolide)/collagen/gelatin [99].
- Silk fibroin scaffolds [108].
- Injectable scaffold based on oxidized alginate microbeads encapsulating periodontal ligament stem cells and gingival mesenchymal stem cells [109].
4. Micro-CT Analysis Combined with Other Techniques or Technologies
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Rüegsegger, P.; Koller, B.; Müller, R. A microtomographic system for the nondestructive evaluation of bone architecture. Calcif. Tissue Int. 1996, 58, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Lin, A.S.P.; Stock, S.R.; Guldberg, R.E. Microcomputed tomography. In Springer Handbook of Microscopy; Hawkes, P.W., Spence, J.C.H., Eds.; Springer International Publishing: Cham, Germany, 2019; p. 2. ISBN 978-3-030-00069-1. [Google Scholar]
- Liao, C.-W.; Fuh, L.-J.; Shen, Y.-W.; Huang, H.-L.; Kuo, C.-W.; Tsai, M.-T.; Hsu, J.-T. Self-assembled micro-computed tomography for dental education. PLoS ONE 2018, 13, e0209698. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deyhle, H.; Schmidli, F.; Krastl, G.; Müller, B. Evaluating tooth restorations: Micro-computed tomography in practical training for students in dentistry. Int. Soc. Optical Eng. 2010, 7804, 780417. [Google Scholar] [CrossRef]
- Daly, S.M. Biophotonics for Blood Analysis; Elsevier: Amsterdam, The Netherlands, 2015; ISBN 9780857096746. [Google Scholar]
- Campioni, I.; Cacciotti, I.; Gupta, N. Additive manufacturing of reconstruction devices for maxillofacial surgery: Design and accuracy assessment of a mandibular plate prototype. Ann. Ist. Super. Sanità 2020, 56, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Mangione, F.; Meleo, D.; Talocco, M.; Pecci, R.; Pacifici, L.; Bedini, R. Comparative evaluation of the accuracy of linear measurements between cone beam computed tomography and 3D microtomography. Ann. Ist. Super. Sanità 2013, 49, 261–265. [Google Scholar] [CrossRef]
- Sinibaldi, R.; Pecci, R.; Somma, F.; Penna, S.D.; Bedini, R. A new software for dimensional measurements in 3D endodontic root canal instrumentation. Ann. Ist. Super. Sanità 2012, 48, 42–48. [Google Scholar] [CrossRef]
- Vögtlin, C.; Schulz, G.; Jäger, K.; Müller, B. Comparing the accuracy of master models based on digital intra-oral scanners with conventional plaster casts. Phys. Med. 2016, 1, 20–26. [Google Scholar] [CrossRef] [Green Version]
- Cengiz, I.F.; Oliveira, J.M.; Reis, R.L. Micro-computed tomography characterization of tissue engineering scaffolds: Effects of pixel size and rotation step. J. Mater. Sci. Mater. Med. 2017, 28. [Google Scholar] [CrossRef]
- Boerckel, J.D.; Mason, D.E.; McDermott, A.M.; Alsberg, E. Microcomputed tomography: Approaches and applications in bioengineering. Stem Cell Res. Ther. 2014, 5, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Sandholzer, M.A.; Walmsley, A.D.; Lumley, P.J.; Landini, G. Radiologic evaluation of heat-induced shrinkage and shape preservation of human teeth using micro-CT. J. Forensic Radiol. Imaging 2013, 1, 107–111. [Google Scholar] [CrossRef]
- Rutty, G.N.; Brough, A.; Biggs, M.J.P.; Robinson, C.; Lawes, S.D.A.; Hainsworth, S.V. The role of micro-computed tomography in forensic investigations. Forensic Sci. Int. 2013, 225, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Bibb, R.; Thompson, D.; Winder, J. Computed tomography characterisation of additive manufacturing materials. Med. Eng. Phys. 2011, 33, 590–596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pecci, R.; Baiguera, S.; Ioppolo, P.; Bedini, R.; Del Gaudio, C. 3D printed scaffolds with random microarchitecture for bone tissue engineering applications: Manufacturing and characterization. J. Mech. Behav. Biomed. Mater. 2020, 103, 103583. [Google Scholar] [CrossRef] [PubMed]
- Center for Devices and Radiological Health. Technical Considerations for Additive Manufactured Medical Devices-Guidance for Industry and Food and Drug Administration Staff; Center for Devices and Radiological Health: Silver Spring, MD, USA, 2017; ISBN ISBN 3014271934. [Google Scholar]
- Deyhle, H.; Dziadowiec, I.; Kind, L.; Thalmann, P.; Schulz, G.; Müller, B. Mineralization of early stage carious lesions in vitro—A quantitative approach. Dent. J. 2015, 3, 111–122. [Google Scholar] [CrossRef] [Green Version]
- Davis, G.R.; Mills, D.; Anderson, P. Real-time observations of tooth demineralization in 3 dimensions using X-ray microtomography. J. Dent. 2018, 69, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Davis, G.; Mills, D. High-contrast x-ray microtomography in dental research. In Proceedings of the Developments in X-Ray Tomography XI, San Diego, CA, USA, 26 September 2017; Volume 10391, pp. 187–194. [Google Scholar]
- Leeson, D. The digital factory in both the modern dental lab and clinic. Dent. Mater. 2019, 1, 43–52. [Google Scholar] [CrossRef]
- Chalas, R.; Szlazak, K.; Wojcik-Checinska, I.; Jaroszewicz, J.; Molak, R.; Czechowicz, K.; Paris, S.; Swieszkowski, W.; Kurzydlowski, K.J. Observations of mineralised tissues of teeth in X-ray micro-computed tomography. Folia Morphol. 2017, 76, 143–148. [Google Scholar] [CrossRef] [Green Version]
- Davis, G.R.; Evershed, A.N.Z.; Mills, D. Quantitative high contrast X-ray microtomography for dental research. J. Dent. 2013, 41, 475–482. [Google Scholar] [CrossRef]
- Moinzadeh, A.T.; Zerbst, W.; Boutsioukis, C.; Shemesh, H.; Zaslansky, P. Porosity distribution in root canals filled with gutta percha and calcium silicate cement. Dent. Mater. 2015, 31, 1100–1108. [Google Scholar] [CrossRef]
- Grande, N.M.; Plotino, G.; Gambarini, G.; Testarelli, L.; D’Ambrosio, F.; Pecci, R.; Bedini, R. Present and future in the use of micro-CT scanner 3D analysis for the study of dental and root canal morphology. Ann. Ist. Super. Sanità 2012, 48, 26–34. [Google Scholar] [CrossRef]
- Meleo, D.; Bedini, R.; Pecci, R.; Mangione, F.; Pacifici, L. Microtomographic and morphometric characterization of a bioceramic bone substitute in dental implantology. Ann. Ist. Super. Sanità 2012, 48, 59–64. [Google Scholar] [CrossRef]
- Barboni, B.; Mangano, C.; Valbonetti, L.; Marruchella, G.; Berardinelli, P.; Martelli, A.; Muttini, A.; Mauro, A.; Bedini, R.; Turriani, M.; et al. Synthetic bone substitute engineered with amniotic epithelial cells enhances bone regeneration after maxillary sinus augmentation. PLoS ONE 2013, 8, e63256. [Google Scholar] [CrossRef] [Green Version]
- Bassi, M.A.; Bedini, R.; Pecci, R.; Ioppolo, P.; Lauritano, D.; Carinci, F. Mechanical properties of resin glass fiber-reinforced abutment in comparison to titanium abutment. J. Indian Soc. Periodontol. 2015, 19, 273–278. [Google Scholar]
- Bedini, R.; Pecci, R.; Marinozzi, F.; Bini, F.; Rizzo, G.; Campioni, I. Valutazione Morfometrica e Strutturale della Architettura del Tessuto Osseo Trabecolare del Collo del Femore: Analisi Microtomografica; Rapp. ISTISAN; Istituto Superiore di Sanità: Rome, Italy, 2018; pp. 1–36. [Google Scholar]
- Campioni, I.; Cacciotti, I.; Gupta, N. Additive manufacturing in ambito medicale: Valutazione di prototipi di dispositivi di fissaggio per chirurgia maxillo-facciale.In: Bedini R, Pecci R, Meleo D, Meli P, Scarano A. 6° Convegno Nazionale FORM. Forum On Regenerative Methods. Istisan Congr. 2019, 19/C2, 12. [Google Scholar]
- Campioni, I.; Pecci, R.; Pepe, E.; Bedini, R. Metodiche computazionali: Studio di fattibilità per l’analisi di strutture osso-biomateriale. In: Bedini R, Pecci R, Meli P, Meleo D. 2° Convegno Nazionale FORM. Forum On Regenerative Methods. Le metodiche rigenerative nel Servizio Sanitario Nazionale. Istisan Congr. 2015, 15/C1, 7. [Google Scholar]
- Bedini, R.; Pecci, R.; Meleo, D.; Campioni, I. Bone substitutes scaffold in human bone: Comparative Evaluation by 3D Micro-CT Technique. Appl. Sci. 2020, 10, 3451. [Google Scholar] [CrossRef]
- Barbetta, A.; Bedini, R.; Pecci, R.; Dentini, M. Role of X-ray microtomography in tissue engineering. Ann. Ist. Super. Sanità 2012, 48, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Brunke, O.; Brockdorf, K.; Drews, S.; Müller, B.; Donath, T.; Herzen, J.; Beckmann, F. Comparison between x-ray tube-based and synchrotron radiation-based μCT. In Proceedings of the Developments in X-Ray Tomography VI, San Diego, CA, USA, 16 September 2008; Volume 7078, p. 70780U. [Google Scholar]
- Botta, L.M.; White, S.N.; Deyhle, H.; Dziadowiec, I.; Schulz, G.; Thalmann, P.; Müller, B. Comparing natural and artificial carious lesions in human crowns by means of conventional hard x-ray micro-tomography and two-dimensional x-ray scattering with synchrotron radiation. In Proceedings of the Developments in X-Ray Tomography X, San Diego, CA, USA, 4 October 2016; Volume 9967, pp. 53–63. [Google Scholar]
- Dziadowiec, I.; Beckmann, F.; Schulz, G.; Deyhle, H.; Müller, B. Characterization of a human tooth with carious lesions using conventional and synchrotron radiation-based micro computed tomography. In Proceedings of the Developments in X-Ray Tomography IX, San Diego, CA, USA, 12 September 2014; Volume 9212, pp. 227–233. [Google Scholar]
- Müller, B.; Deyhle, H.; Lang, S.; Schulz, G.; Bormann, T.; Fierz, F.C.; Hieber, S.E. Three-dimensional registration of tomography data for quantification in biomaterials science. Int. J. Mater. Res. 2012, 103, 242–249. [Google Scholar] [CrossRef]
- Ogodescu, A.; Manescu, A.; Ogodescu, A.E.; Giuliani, A.; Todea, C. Micro-CT application for infiltration technology in paedodontics and orthodontics. In Proceedings of the Fifth International Conference on Lasers in Medicine: Biotechnologies Integrated in Daily Medicine, Imisoara, Romania, 19–21 September 2013; Todea, C., Podoleanu, A.G., Duma, V.-F., Eds.; SPIE: Bellingham, WA, USA, 2014; Volume 8925, pp. 51–56. [Google Scholar]
- Brogle-Kim, Y.-C.; Deyhle, H.; Müller, B.; Schulz, G.; Bormann, T.; Beckmann, F.; Jäger, K. Evaluation of oral scanning in comparison to impression using three-dimensional registration. In Proceedings of the Developments in X-Ray Tomography VIII, San Diego, CA, USA, 13–15 August 2012; Stock, S.R., Ed.; SPIE: Bellingham, WA, USA, 2012; Volume 8506, pp. 437–445. [Google Scholar]
- Luckow, M.; Deyhle, H.; Beckmann, F.; Dagassan-Berndt, D.; Müller, B. Tilting the jaw to improve the image quality or to reduce the dose in cone-beam computed tomography. Eur. J. Radiol. 2011, 80, e389–e393. [Google Scholar] [CrossRef]
- Gugger, J.; Krastl, G.; Huser, M.; Deyhle, H.; Müller, B. The morphology of amputated human teeth and its relation to mechanical properties after restoration treatment. In Proceedings of the Developments in X-Ray Tomography VII, San Diego, CA, USA, 2–5 August 2010; Stock, S.R., Ed.; SPIE: Bellingham, WA, USA, 2010; Volume 7804, pp. 397–406. [Google Scholar]
- Kofmehl, L.; Schulz, G.; Deyhle, H.; Filippi, A.; Hotz, G.; Berndt-Dagassan, D.; Kramis, S.; Beckmann, F.; Müller, B. Computed tomography to quantify tooth abrasion. In Proceedings of the Developments in X-Ray Tomography VII, San Diego, CA, USA, 2–5 August 2010; Stock, S.R., Ed.; SPIE: Bellingham, WA, USA, 2010; Volume 7804, pp. 375–384. [Google Scholar]
- Dalstra, M.; Schulz, G.; Dagassan-Berndt, D.; Verna, C.; Müller-Gerbl, M.; Müller, B. Hard x-ray micro-tomography of a human head post-mortem as a gold standard to compare x-ray modalities. In Proceedings of the Developments in X-Ray Tomography X, San Diego, CA, USA, 29–31 August 2016; Stock, S.R., Müller, B., Wang, G., Eds.; SPIE: Bellingham, WA, USA, 2016; Volume 9967, pp. 37–42. [Google Scholar]
- Latief, F.D.E.; Sari, D.S.; Fitri, L.A. Applications of Micro-CT scanning in medicine and dentistry: Microstructural analyses of a Wistar Rat mandible and a urinary tract stone. J. Phys. Conf. Ser. 2017, 884. [Google Scholar] [CrossRef] [Green Version]
- Davis, G.R.; Mills, D. Brute force absorption contrast microtomography. In Proceedings of the Developments in X-Ray Tomography IX, San Diego, CA, USA, 12 September 2014; Volume 9212, p. 92120I. [Google Scholar]
- Kaisarly, D.; Gezawi, M. El Polymerization shrinkage assessment of dental resin composites: A literature review. Odontology 2016, 104, 257–270. [Google Scholar] [CrossRef]
- Contrepois, M.; Soenen, A.; Bartala, M.; Laviole, O. Marginal adaptation of ceramic crowns: A systematic review. J. Prosthet. Dent. 2013, 110, 447–454. [Google Scholar] [CrossRef]
- Ortiz, F.G.; Jimeno, E.B. Analysis of the porosity of endodontic sealers through micro-computed tomography: A systematic review. J. Conserv. Dent. 2018, 21, 238–242. [Google Scholar] [CrossRef]
- Kalatzis-Sousa, N.G.; Spin-Neto, R.; Wenzel, A.; Tanomaru-Filho, M.; Faria, G. Use of micro-computed tomography for the assessment of periapical lesions in small rodents: A systematic review. Int. Endod. J. 2017, 50, 352–366. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, H.M.A.; Versiani, M.A.; De-Deus, G.; Dummer, P.M.H. A new system for classifying root and root canal morphology. Int. Endod. J. 2017, 50, 761–770. [Google Scholar] [CrossRef] [Green Version]
- de Sousa-Neto, M.D.; Silva-Sousa, Y.C.; Mazzi-Chaves, J.F.; Carvalho, K.K.T.; Barbosa, A.F.S.; Versiani, M.A.; Jacobs, R.; Leoni, G.B. Root canal preparation using micro-computed tomography analysis: A literature review. Braz. Oral Res. 2018, 32 (Suppl. 1), e66. [Google Scholar] [CrossRef] [Green Version]
- Velozo, C.; Albuquerque, D. Microcomputed Tomography studies of the effectiveness of XP-endo shaper in root canal preparation: A review of the literature. Sci. World J. 2019, 2019, 3570870. [Google Scholar] [CrossRef]
- Junior, J.F.S.; das Rocas, I.N.; Marceliano-Alves, M.F.; Perez, A.R.; Ricucci, D. Unprepared root canal surface areas: Causes, clinical implications, and therapeutic strategies. Braz. Oral Res. 2018, 32 (Suppl. 1), e65. [Google Scholar] [CrossRef]
- Rossi-Fedele, G.; Ahmed, H.M.A. Assessment of root canal filling removal effectiveness using micro-computed tomography: A systematic review. J. Endod. 2017, 43, 520–526. [Google Scholar] [CrossRef]
- Zou, W.; Hunter, N.; Swain, M.V. Application of polychromatic microCT for mineral density determination. J. Dent. Res. 2011, 90, 18–30. [Google Scholar] [CrossRef] [Green Version]
- Yeler, D.Y.; Koraltan, M.; Hocaoglu, T.P.; Arslan, C.; Erselcan, T.; Yeler, H. Bone quality and quantity measurement techniques in dentistry. Cumhur. Dent. J. 2016, 19, 73–86. [Google Scholar]
- Ibrahim, N.; Parsa, A.; Hassan, B.; van der Stelt, P.; Wismeijer, D. Diagnostic imaging of trabecular bone microstructure for oral implants: A literature review. Dentomaxillofac. Radiol. 2013, 42, 20120075. [Google Scholar] [CrossRef]
- Anderson, P.J.; Yong, R.; Surman, T.L.; Rajion, Z.A.; Ranjitkar, S. Application of three-dimensional computed tomography in craniofacial clinical practice and research. Aust. Dent. J. 2014, 59, 174–185. [Google Scholar] [CrossRef]
- Irie, M.S.; Rabelo, G.D.; Spin-Neto, R.; Dechichi, P.; Borges, J.S.; Soares, P.B.F. Use of micro-computed tomography for bone evaluation in dentistry. Braz. Dent. J. 2018, 29, 227–238. [Google Scholar] [CrossRef] [Green Version]
- Denry, I.; Kuhn, L.T. Design and characterization of calcium phosphate ceramic scaffolds for bone tissue engineering. Dent. Mater. 2016, 32, 43–53. [Google Scholar] [CrossRef] [Green Version]
- Batool, F.; Strub, M.; Petit, C.; Bugueno, I.M.; Bornert, F.; Clauss, F.; Huck, O.; Kuchler-Bopp, S.; Benkirane-Jessel, N. Periodontal tissues, maxillary jaw bone, and tooth regeneration approaches: From animal models analyses to clinical applications. Nanomaterials 2018, 8, 337. [Google Scholar] [CrossRef] [Green Version]
- Faot, F.; Chatterjee, M.; de Camargos, G.V.; Duyck, J.; Vandamme, K. Micro-CT analysis of the rodent jaw bone micro-architecture: A systematic review. Bone Rep. 2015, 2, 14–24. [Google Scholar] [CrossRef] [Green Version]
- Annibali, S.; Bellavia, D.; Ottolenghi, L.; Cicconetti, A.; Cristalli, M.P.; Quaranta, R.; Pilloni, A. Micro-CT and PET analysis of bone regeneration induced by biodegradable scaffolds as carriers for dental pulp stem cells in a rat model of calvarial “critical size” defect: Preliminary data. J. Biomed. Mater. Res. B Appl. Biomater. 2014, 102, 815–825. [Google Scholar] [CrossRef]
- Ide, Y.; Nakahara, T.; Nasu, M.; Matsunaga, S.; Iwanaga, T.; Tominaga, N.; Tamaki, Y. Postnatal mandibular cheek tooth development in the miniature pig based on two-dimensional and three-dimensional X-ray analyses. Anat. Rec. 2013, 296, 1247–1254. [Google Scholar] [CrossRef]
- Baltacioglu, I.H.; Demirel, G.; Kolsuz, M.E.; Orhan, K. In-vitro analysis of maxillary first molars morphology using three dimensional Micro-CT imaging: Considerations for restorative dentistry. Eur. Oral Res. 2019, 52, 75–81. [Google Scholar] [CrossRef]
- Kaizer, M.R.; Bano, S.; Borba, M.; Garg, V.; Dos Santos, M.B.F.; Zhang, Y. Wear Behavior of graded glass/zirconia crowns and their antagonists. J. Dent. Res. 2019, 98, 437–442. [Google Scholar] [CrossRef]
- Lin, P.-Y.; Lyu, H.-C.; Hsu, C.-Y.S.; Chang, C.-S.; Kao, F.-J. Imaging carious dental tissues with multiphoton fluorescence lifetime imaging microscopy. Biomed. Opt. Express 2010, 2, 149–158. [Google Scholar] [CrossRef] [Green Version]
- Al-Rimawi, A.; EzEldeen, M.; Schneider, D.; Politis, C.; Jacobs, R. 3D Printed temporary veneer restoring autotransplanted teeth in children: Design and concept validation ex vivo. Int. J. Environ. Res. Public Health 2019, 16, 496. [Google Scholar] [CrossRef] [Green Version]
- Kind, L.; Stevanovic, S.; Wuttig, S.; Wimberger, S.; Hofer, J.; Muller, B.; Pieles, U. Biomimetic remineralization of carious lesions by self-assembling peptide. J. Dent. Res. 2017, 96, 790–797. [Google Scholar] [CrossRef]
- Maia, A.C.; Mangabeira, A.; Vieira, R.; de Neves, A.A.; Lopes, R.T.; Pires, T.M.; Viana, G.M.; Cabral, L.M.; Cavalcante, L.M.; Portela, M.B. Experimental composites containing quaternary ammonium methacrylates reduce demineralization at enamel-restoration margins after cariogenic challenge. Dent. Mater. 2019, 35, e175–e183. [Google Scholar] [CrossRef]
- Ostapiuk, M.; Tarczydlo, B.; Surowska, B.; Orlowski, M.; Tymczyna, B.; Bachanek, T.; Rzepecka, A.; Mroz, A. Qualitative analysis of the margins of restorations made with different filling resins. Microsc. Res. Tech. 2018, 81, 823–831. [Google Scholar] [CrossRef]
- Iwashita, T.; Mine, A.; Matsumoto, M.; Nakatani, H.; Higashi, M.; Kawaguchi-Uemura, A.; Kabetani, T.; Tajiri, Y.; Imai, D.; Hagino, R.; et al. Effects of three drying methods of post space dentin bonding used in a direct resin composite core build-up method. J. Prosthodont. Res. 2018, 62, 449–455. [Google Scholar] [CrossRef]
- Matsumoto, M.; Mine, A.; Miura, J.; Minamino, T.; Iwashita, T.; Nakatani, H.; Nishida, T.; Takeshige, F.; Yatani, H. Bonding effectiveness and multi-interfacial characterization of two direct buildup resin core systems bonded to post-space dentin. Clin. Oral Investig. 2017, 21, 309–317. [Google Scholar] [CrossRef]
- Belgın, C.A.; Serindere, G.; Orhan, K. Accuracy and reliability of enamel and dentin thickness measurements on micro-computed tomography and digital periapical radiographs#. J. Forensic Radiol. Imaging 2019, 18, 32–36. [Google Scholar]
- Saito, K.; Takahashi, K.; Asahara, M.; Kiso, H.; Togo, Y.; Tsukamoto, H.; Huang, B.; Sugai, M.; Shimizu, A.; Motokawa, M.; et al. Effects of Usag-1 and Bmp7 deficiencies on murine tooth morphogenesis. BMC Dev. Biol. 2016, 16, 14. [Google Scholar] [CrossRef] [Green Version]
- Minamino, T.; Mine, A.; Shintani, A.; Higashi, M.; Kawaguchi-Uemura, A.; Kabetani, T.; Hagino, R.; Imai, D.; Tajiri, Y.; Matsumoto, M.; et al. Advanced statistical analyses to reduce inconsistency of bond strength data. J. Dent. Res. 2017, 96, 1400–1405. [Google Scholar] [CrossRef]
- Paredes, S.E.Y.; Segato, R.A.B.; Moreira, L.D.; Moreira, A.; Serrano, K.V.D.; Rodrigues, C.T.; Almeida, L.Y.; León, J.E. Dentoalveolar abscesses not associated with caries or trauma: A diagnostic hallmark of hypophosphatemic rickets initially misdiagnosed as hypochondroplasia. Head Neck Pathol. 2018, 12, 604–609. [Google Scholar] [CrossRef]
- Khominsky, A.; Yong, R.; Ranjitkar, S.; Townsend, G.; Anderson, P.J. Extensive phenotyping of the orofacial and dental complex in Crouzon syndrome. Arch. Oral Biol. 2018, 86, 123–130. [Google Scholar] [CrossRef]
- Yüksel, B.N.; Orhan, K.; Tulga Öz, F. Micro-CT evaluation of taurodontism in a deciduous molar and a permanent molar: Case report. Cumhur. Dent. J. 2019, 22, 486–490. [Google Scholar] [CrossRef]
- Kaya, S.; Orhan, K.; Tulga Öz, F. Williams-beuren syndrome—A case report. Cumhur. Dent. J. 2019, 22, 481–485. [Google Scholar] [CrossRef]
- Dannemann, M.; Kucher, M.; Kirsch, J.; Binkowski, A.; Modler, N.; Hannig, C.; Weber, M.-T. An approach for a mathematical description of human root canals by means of elementary parameters. J. Endod. 2017, 43, 536–543. [Google Scholar] [CrossRef]
- Ziya, M.; Yüksel, B.N.; Şari, S. Root canal morphology of mandibular primary molars: A micro-ct study. Cumhur. Dent. J. 2019, 22, 382–389. [Google Scholar] [CrossRef] [Green Version]
- Marceliano-Alves, M.F.; De Lima, C.O.; Augusto, C.M.; Barbosa, A.F.A.; Bruno, A.M.V.; Rosa, A.M.; Lopes, R.T. The internal root canal morphology of single-rooted mandibular canines revealed by micro-computed tomography. J. Conserv. Dent. 2018, 21, 588–591. [Google Scholar] [CrossRef]
- Espir, C.; Nascimento, C.; Guerreiro-Tanomaru, J.; Bonetti-Filho, I.; Tanomaru-Filho, M. Radiographic and micro-computed tomography classification of root canal morphology and dentin thickness of mandibular incisors. J. Conserv. Dent. 2018, 21, 57–62. [Google Scholar]
- Sachdeva, N.; Nikhil, V.; Jha, P. Effect of ultrasonic root-end cavity preparation on dentinal microcrack formation: A micro-computed tomography study. J. Conserv. Dent. 2019, 22, 362–366. [Google Scholar] [CrossRef]
- Singh, V.; Nikhil, V.; Bansal, P. Induction of dentinal microcracks during postspace preparation: A comparative microcomputed tomography study. J. Conserv. Dent. 2018, 21, 646–650. [Google Scholar] [PubMed]
- Oishi, A.; Terashima, T.; Miyashin, M.; Takagi, Y. Repair processes of experimental root fractures in rat molars examined by histopathological techniques and 3D micro-CT imaging. Pediatr. Dent. J. 2013, 23, 8–15. [Google Scholar] [CrossRef]
- Celikten, B.; Uzuntas, C.F.; Orhan, A.I.; Orhan, K.; Tufenkci, P.; Kursun, S.; Demiralp, K.O. Evaluation of root canal sealer filling quality using a single-cone technique in oval shaped canals: An in vitro Micro-CT study. Scanning 2016, 38, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Helvacioglu-Yigit, D.; Yilmaz, A.; Kiziltas-Sendur, G.; Aslan, O.S.; Abbott, P.V. Efficacy of reciprocating and rotary systems for removing root filling material: A micro-computed tomography study. Scanning 2014, 36, 576–581. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Tsoi, J.K.H.; Cheung, G.S.P.; Matinlinna, J. An in vitro evaluation on a novel root canal cleansing method by using nylon fibers. Fibers 2015, 3, 197–205. [Google Scholar] [CrossRef] [Green Version]
- Alovisi, M.; Pasqualini, D.; Musso, E.; Bobbio, E.; Giuliano, C.; Mancino, D.; Scotti, N.; Berutti, E. Influence of contracted endodontic access on root canal geometry: An in vitro study. J. Endod. 2018, 44, 614–620. [Google Scholar] [CrossRef] [Green Version]
- Krastl, G.; Gugger, J.; Deyhle, H.; Zitzmann, N.U.; Weiger, R.; Müller, B. Impact of adhesive surface and volume of luting resin on fracture resistance of root filled teeth. Int. Endod. J. 2011, 44, 432–439. [Google Scholar] [CrossRef]
- Alshehri, M.; Alamri, H.M.; Alshwaimi, E.; Kujan, O. Micro-computed tomographic assessment of quality of obturation in the apical third with continuous wave vertical compaction and single match taper sized cone obturation techniques. Scanning 2016, 38, 352–356. [Google Scholar] [CrossRef]
- Goymen, M.; Gulec, A. Effect of photobiomodulation therapies on the root resorption associated with orthodontic forces: A pilot study using micro computed tomography. Clin. Oral Investig. 2019, 24, 1431–1438. [Google Scholar] [CrossRef]
- Minozzi, S.; Panetta, D.; De Sanctis, M.; Giuffra, V. A dental prosthesis from the early modern age in Tuscany (Italy). Clin. Implant Dent. Relat. Res. 2017, 19, 365–371. [Google Scholar] [CrossRef] [Green Version]
- Nicklisch, N.; Maier, F.; Schulz, G.; Rossbach, A.; Pichler, S.L.; Zeilhofer, H.F.; Gutwald, R.; Dresely, V.; Meller, H.; Baumhoer, D.; et al. An osseous lesion in the maxillary sinus—Tumour or tumour-like? Int. J. Osteoarchaeol. 2019, 29, 183–190. [Google Scholar] [CrossRef]
- Ozaki, M.; Takayama, T.; Yamamoto, T.; Ozawa, Y.; Nagao, M.; Tanabe, N.; Nakajima, A.; Suzuki, N.; Maeno, M.; Yamano, S.; et al. A collagen membrane containing osteogenic protein-1 facilitates bone regeneration in a rat mandibular bone defect. Arch. Oral Biol. 2017, 84, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, N.; Smith, E.E.; Angstadt, S.; Zhang, W.; Khademhosseini, A.; Yelick, P.C. Dental cell sheet biomimetic tooth bud model. Biomaterials 2016, 106, 167–179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Charwat-Pessler, J.; Musso, M.; Petutschnigg, A.; Entacher, K.; Plank, B.; Wernersson, E.; Tangl, S.; Schuller-Gotzburg, P. A bone sample containing a bone graft substitute analyzed by correlating density information obtained by X-ray micro tomography with compositional information obtained by raman microscopy. Materials 2015, 8, 3831–3853. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boda, S.K.; Almoshari, Y.; Wang, H.; Wang, X.; Reinhardt, R.A.; Duan, B.; Wang, D.; Xie, J. Mineralized nanofiber segments coupled with calcium-binding BMP-2 peptides for alveolar bone regeneration. Acta. Biomater. 2019, 85, 282–293. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, S.; Zhou, W.; Fu, H.; Qian, L.; Miron, R.J. Addition of a synthetically fabricated osteoinductive biphasic calcium phosphate bone graft to BMP2 improves new bone formation. Clin. Implant Dent. Relat. Res. 2016, 18, 1238–1247. [Google Scholar] [CrossRef]
- Zhang, W.; Vazquez, B.; Oreadi, D.; Yelick, P.C. Decellularized tooth bud scaffolds for tooth regeneration. J. Dent. Res. 2017, 96, 516–523. [Google Scholar] [CrossRef] [Green Version]
- Yeo, A.; Cheok, C.; Teoh, S.H.; Zhang, Z.Y.; Buser, D.; Bosshardt, D.D. Lateral ridge augmentation using a PCL-TCP scaffold in a clinically relevant but challenging micropig model. Clin. Oral Implants Res. 2012, 23, 1322–1332. [Google Scholar] [CrossRef]
- Sha, J.; Kanno, T.; Miyamoto, K.; Bai, Y.; Hideshima, K.; Matsuzaki, Y. Application of a bioactive/bioresorbable three-dimensional porous uncalcined and unsintered Hydroxyapatite/Poly-D/L-lactide composite with human mesenchymal stem cells for bone regeneration in maxillofacial surgery: A pilot animal study. Materials 2019, 12, 705. [Google Scholar] [CrossRef] [Green Version]
- Bai, Y.; Sha, J.; Kanno, T.; Miyamoto, K.; Hideshima, K.; Matsuzaki, Y. Comparison of the bone regenerative capacity of three-dimensional uncalcined and unsintered Hydroxyapatite/Poly-d/l-lactide and beta-tricalcium phosphate used as bone graft substitutes. J. Invest. Surg. 2019, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.T.S.; Hashimi, S.; Saifzadeh, S.; Ivanovski, S.; Vaquette, C. Additively manufactured biphasic construct loaded with BMP-2 for vertical bone regeneration: A pilot study in rabbit. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 92, 554–564. [Google Scholar] [CrossRef] [PubMed]
- Hatakeyama, W.; Taira, M.; Takafuji, K.; Kihara, H.; Kondo, H. Bone-regeneration trial of rat critical-size calvarial defects using nano-apatite/collagen composites. Nano Biomed. 2013, 5, 98–103. [Google Scholar]
- Takayama, T.; Dai, J.; Tachi, K.; Shohara, R.; Kasai, H.; Imamura, K.; Yamano, S. The potential of stromal cell-derived factor-1 delivery using a collagen membrane for bone regeneration. J. Biomater. Appl. 2017, 31, 1049–1061. [Google Scholar] [CrossRef]
- Thai, T.H.; Nuntanaranont, T.; Kamolmatyakul, S.; Meesane, J. In vivo evaluation of modified silk fibroin scaffolds with a mimicked microenvironment of fibronectin/decellularized pulp tissue for maxillofacial surgery. Biomed. Mater. 2017, 13, 15009. [Google Scholar] [CrossRef]
- Moshaverinia, A.; Chen, C.; Akiyama, K.; Xu, X.; Chee, W.W.L.; Schricker, S.R.; Shi, S. Encapsulated dental-derived mesenchymal stem cells in an injectable and biodegradable scaffold for applications in bone tissue engineering. J. Biomed. Mater. Res. A 2013, 101, 3285–3294. [Google Scholar] [CrossRef] [PubMed]
- Tatullo, M.; Spagnuolo, G.; Codispoti, B.; Zamparini, F.; Zhang, A.; Esposti, M.D.; Aparicio, C.; Rengo, C.; Nuzzolese, M.; Manzoli, L.; et al. PLA-based mineral-doped scaffolds seeded with human periapical Cyst-Derived MSCs: A promising tool for regenerative healing in dentistry. Materials 2019, 12, 597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turco, G.; Porrelli, D.; Marsich, E.; Vecchies, F.; Lombardi, T.; Stacchi, C.; Lenarda, R. Di Three-dimensional bone substitutes for oral and maxillofacial surgery: Biological and structural characterization. J. Funct. Biomater. 2018, 9, 62. [Google Scholar] [CrossRef] [Green Version]
- Zacchetti, G.; Dayer, R.; Rizzoli, R.; Ammann, P. Systemic treatment with strontium ranelate accelerates the filling of a bone defect and improves the material level properties of the healing bone. Biomed Res. Int. 2014, 2014. [Google Scholar] [CrossRef]
- Schneider, D.A.; Smith, S.M.; Campbell, C.; Hayami, T.; Kapila, S.; Hatch, N.E. Locally limited inhibition of bone resorption and orthodontic relapse by recombinant osteoprotegerin protein. Orthod. Craniofac. Res. 2015, 18 (Suppl. 1), 187–195. [Google Scholar] [CrossRef] [Green Version]
- Alikhani, M.; Lopez, J.A.; Alabdullah, H.; Vongthongleur, T.; Sangsuwon, C.; Alikhani, M.; Alansari, S.; Oliveira, S.M.; Nervina, J.M.; Teixeira, C.C. High-frequency acceleration: Therapeutic tool to preserve bone following tooth extractions. J. Dent. Res. 2016, 95, 311–318. [Google Scholar] [CrossRef] [PubMed]
- da Fabris, A.L.S.; Mulinari-Santos, G.; Hassumi, J.S.; Freire, A.R.; Faverani, L.P.; Gruber, R.; Okamoto, R. Morphometric and histologic characterization of alveolar bone from hypertensive patients. Clin. Implant Dent. Relat. Res. 2017, 19, 1106–1113. [Google Scholar] [CrossRef] [PubMed]
- Nepal, M.; Li, L.; Bae, T.S.; Kim, B.I.; Soh, Y. Evaluation of osseointegration around tibial implants in rats by ibandronate-treated nanotubular Ti-32Nb-5Zr alloy. Biomol. Ther. 2014, 22, 563–569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qi, M.; Hu, J.; Li, J.; Li, J.; Dong, W.; Feng, X.; Yu, J. Effect of zoledronate acid treatment on osseointegration and fixation of implants in autologous iliac bone grafts in ovariectomized rabbits. Bone 2012, 50, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.; Lu, T.; Wang, X.; Xu, L.; Wu, Q.; Pan, H.; Wang, D.; Liu, X.; Jiang, X. In vitro and in vivo evaluation of silicate-coated polyetheretherketone fabricated by electron beam evaporation. ACS Appl. Mater. Interfaces 2016, 8, 13197–13206. [Google Scholar] [CrossRef]
- de Wild, M.; Schumacher, R.; Mayer, K.; Schkommodau, E.; Thoma, D.; Bredell, M.; Gujer, A.K.; Gratz, K.W.; Weber, F.E. Bone regeneration by the osteoconductivity of porous titanium implants manufactured by selective laser melting: A histological and micro computed tomography study in the rabbit. Tissue Eng. Part A 2013, 19, 2645–2654. [Google Scholar] [CrossRef] [Green Version]
- Grau, M.; Seiler, C.; Roland, L.; Matena, J.; Windhovel, C.; Teske, M.; Escobar, H.M.; Lupke, M.; Seifert, H.; Gellrich, N.-C.; et al. Osteointegration of porous poly-epsilon-caprolactone-coated and previtalised magnesium implants in critically sized calvarial bone defects in the mouse model. Materials 2017, 11, 6. [Google Scholar] [CrossRef] [Green Version]
- Kubasiewicz-Ross, P.; Hadzik, J.; Dominiak, M. Osseointegration of zirconia implants with 3 varying surface textures and a titanium implant: A histological and micro-CT study. Adv. Clin. Exp. Med. 2018, 27, 1173–1179. [Google Scholar] [CrossRef] [Green Version]
- Eger, M.; Sterer, N.; Liron, T.; Kohavi, D.; Gabet, Y. Scaling of titanium implants entrains inflammation-induced osteolysis. Sci. Rep. 2017, 7, 39612. [Google Scholar] [CrossRef]
- Zeitouni, J.; Clough, B.; Zeitouni, S.; Saleem, M.; Al Aisami, K.; Gregory, C. The effects of the Er: YAG laser on trabecular bone micro-architecture: Comparison with conventional dental drilling by micro-computed tomographic and histological techniques. F1000Research 2017, 6, 1133. [Google Scholar] [CrossRef] [Green Version]
- Umanjec-Korac, S.; Parsa, A.; Nikoozad, A.D.; Wismeijer, D.; Hassan, B. Accuracy of cone beam computed tomography in following simulated autogenous graft resorption in maxillary sinus augmentation procedure: An ex vivo study. Dentomaxillofac. Radiol. 2016, 45, 20160092. [Google Scholar] [CrossRef] [Green Version]
- Monje, A.; Wu, Y.; Huang, W.; Zhou, W.; Galindo-Moreno, P.; Montanero-Fernandez, J.; Sheridan, R.; Wang, H.-L.; Wang, F. Influence of posterior mandibular dimensions on alveolar bone microarchitecture. Int. J. Oral Maxillofac. Implants 2017, 32, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Son, K.; Lee, S.; Kang, S.H.; Park, J.; Lee, K.-B.; Jeon, M.; Yun, B.-J. A comparison study of marginal and internal fit assessment methods for fixed dental prostheses. J. Clin. Med. 2019, 8, 785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sen, S.; Erber, R.; Kunzmann, K.; Kirschner, S.; Weyer, V.; Schilling, L.; Brockmann, M.A.; Rues, S.; Orhan, G.; Lux, C.J.; et al. Assessing abrasion of orthodontic surface sealants using a modified ophthalmic optical coherence tomography device. Clin. Oral Investig. 2018, 22, 3143–3157. [Google Scholar] [CrossRef] [PubMed]
- Song, J.C.; Suwanprateeb, J.; Sae-Lee, D.; Sosakul, T.; Pitiphat, W.; Prajaneh, S.; Kositbowornchai, S.; Putraphan, B. 2D and 3D pore structure characterization of bi-layered porous polyethylene barrier membrane using SEM and micro-CT. ScienceAsia 2019, 45, 159–171. [Google Scholar] [CrossRef]
- Liu, S.; Broucek, J.; Virdi, A.S.; Sumner, D.R. Limitations of using micro-computed tomography to predict bone-implant contact and mechanical fixation. J. Microsc. 2012, 245, 34–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galibourg, A.; Dumoncel, J.; Telmon, N.; Calvet, A.; Michetti, J.; Maret, D. Assessment of automatic segmentation of teeth using a watershed-based method. Dentomaxillofac. Radiol. 2018, 47, 20170220. [Google Scholar] [CrossRef] [PubMed]
- Özcan, C.; Josset, Y.; Muraille, C.; Lestriez, P.; Taiar, R. A three dimensional (3D) finite element model of restored molar teeth and combination of restorative material. Ser. Biomech. 2017, 31, 12–18. [Google Scholar]
- Barbosa, J.M.; Tovar, N.; Tuesta, A.P.; Hirata, R.; Guimaraes, N.; Romanini, J.C.J.; Moghadam, M.; Coelho, P.G.; Jahangiri, L. Scan-layered reconstructions: A pilot study of a nondestructive dental histoanatomical analysis method and digital workflow to create restorations driven by natural dentin and enamel morphology. J. Esthet. Restor. Dent. 2017, 29, 256–263. [Google Scholar] [CrossRef]
- Hegazy, M.A.A.; Eldib, M.E.; Mun, Y.J.; Cho, M.H.; Cho, M.H.; Lee, S.Y. A bench-top micro-CT capable of simulating head motions. Biomed. Eng. Lett. 2017, 7, 237–244. [Google Scholar] [CrossRef]
- Lashgari, M.; Shahmoradi, M.; Rabbani, H.; Swain, M. Missing surface estimation based on modified tikhonov regularization: Application for Destructed dental tissue. IEEE Trans. Image Process. 2018, 27, 2433–2446. [Google Scholar] [CrossRef]
- Da Silva, L.H.; Ribeiro, S.; Borges, A.L.S.; Cesar, P.F.; Tango, R.N. FEA and microstructure characterization of a one-piece Y-TZP abutment. Dent. Mater. 2014, 30, e283–e288. [Google Scholar] [CrossRef] [PubMed]
- Magne, P. Virtual prototyping of adhesively restored, endodontically treated molars. J. Prosthet. Dent. 2010, 103, 343–351. [Google Scholar] [CrossRef]
- Kinoshita, H.; Nagahata, M.; Takano, N.; Takemoto, S.; Matsunaga, S.; Abe, S.; Yoshinari, M.; Kawada, E. Development of a drilling simulator for dental implant surgery. J. Dent. Educ. 2016, 80, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.H.; Lei, Y.N.; Ho, Y.H.; Sung, Y.H.; Lin, T.S. Predicting the holistic force-displacement relation of the periodontal ligament: In-vitro experiments and finite element analysis. Biomed. Eng. Online 2014, 13, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benazzi, S.; Grosse, I.R.; Gruppioni, G.; Weber, G.W.; Kullmer, O. Comparison of occlusal loading conditions in a lower second premolar using three-dimensional finite element analysis. Clin. Oral Investig. 2014, 18, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Ossareh, A.; Rosentritt, M.; Kishen, A. Biomechanical studies on the effect of iatrogenic dentin removal on vertical root fractures. J. Conserv. Dent. 2018, 21, 290–296. [Google Scholar] [PubMed]
- Kato, C.N.; Barra, S.G.; Tavares, N.P.; Amaral, T.M.; Brasileiro, C.B.; Mesquita, R.A.; Abreu, L.G. Use of fractal analysis in dental images: A systematic review. Dentomaxillofac. Radiol. 2019, 20180457. [Google Scholar] [CrossRef]


| Specifications | Micro-CT Model: µ40 * | CBCT Model: Accuitomo 170 ** | Micro-CT Model: SkyScan 1172 *** | Micro-CT Model: Phoenix Nanotom M **** |
|---|---|---|---|---|
| X-ray Source | 30–70 kV | 60–90 kV, 8 mA | 20–100 kV, 10 W | 180 kV, 20 W |
| X-ray Detector/Focal Spot Size | 2048 × 256 elements, 24 µm pitch | Spot Size 0.5 mm | 11 MP, 12-Bit Cooled CCD Fiber-Optically Coupled to Scintillator | GE DXR, 14 Bit, 3072 × 2400 Pixels |
| Max. Object Size/Field of View | 36.9 × 80 mm (Ø x L) | Min. Ø 40 × 40 mmMax. Ø 170 × 120 mm | Ø 27 mm Single Scan Ø 50 mm Offset Scan | 240 mm Ø × 250 mm in Height |
| Detail Detectability | 3–72 µm | 80–250 µm voxel size | 0.5 µm at Highest Magnification | Down to 0.2 µm |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Campioni, I.; Pecci, R.; Bedini, R. Ten Years of Micro-CT in Dentistry and Maxillofacial Surgery: A Literature Overview. Appl. Sci. 2020, 10, 4328. https://doi.org/10.3390/app10124328
Campioni I, Pecci R, Bedini R. Ten Years of Micro-CT in Dentistry and Maxillofacial Surgery: A Literature Overview. Applied Sciences. 2020; 10(12):4328. https://doi.org/10.3390/app10124328
Chicago/Turabian StyleCampioni, Ilaria, Raffaella Pecci, and Rossella Bedini. 2020. "Ten Years of Micro-CT in Dentistry and Maxillofacial Surgery: A Literature Overview" Applied Sciences 10, no. 12: 4328. https://doi.org/10.3390/app10124328
APA StyleCampioni, I., Pecci, R., & Bedini, R. (2020). Ten Years of Micro-CT in Dentistry and Maxillofacial Surgery: A Literature Overview. Applied Sciences, 10(12), 4328. https://doi.org/10.3390/app10124328







