The Effect of Adsorbed Volatile Organic Compounds on an Ultrathin Water Film Measurement
Abstract
:Featured Application
Abstract
1. Introduction
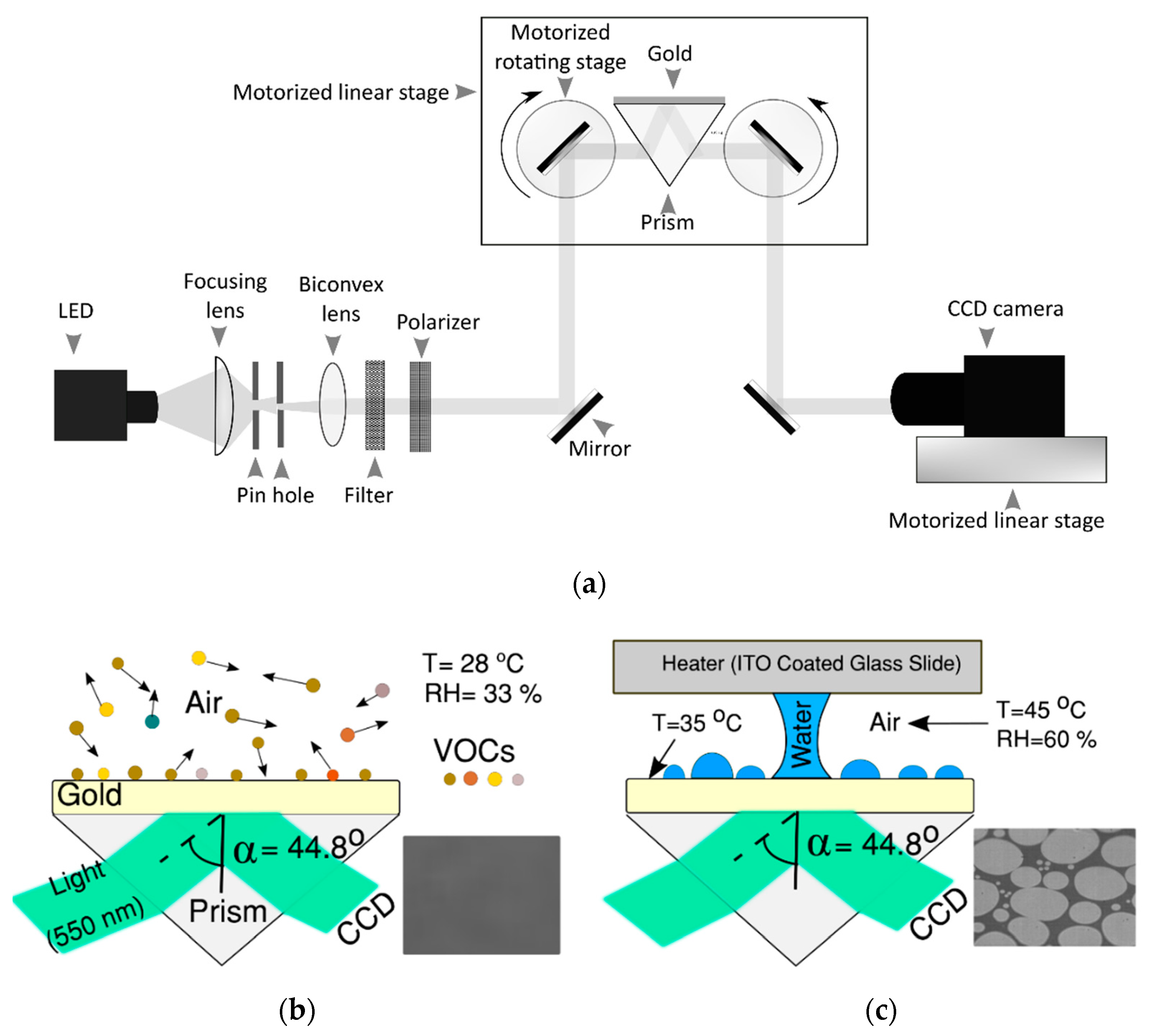
2. Materials and Methods
2.1. Sample Fabrication
2.2. Surface Characterization
2.3. Adsorbed VOC Characterization Using SPRi
3. Results and Discussion
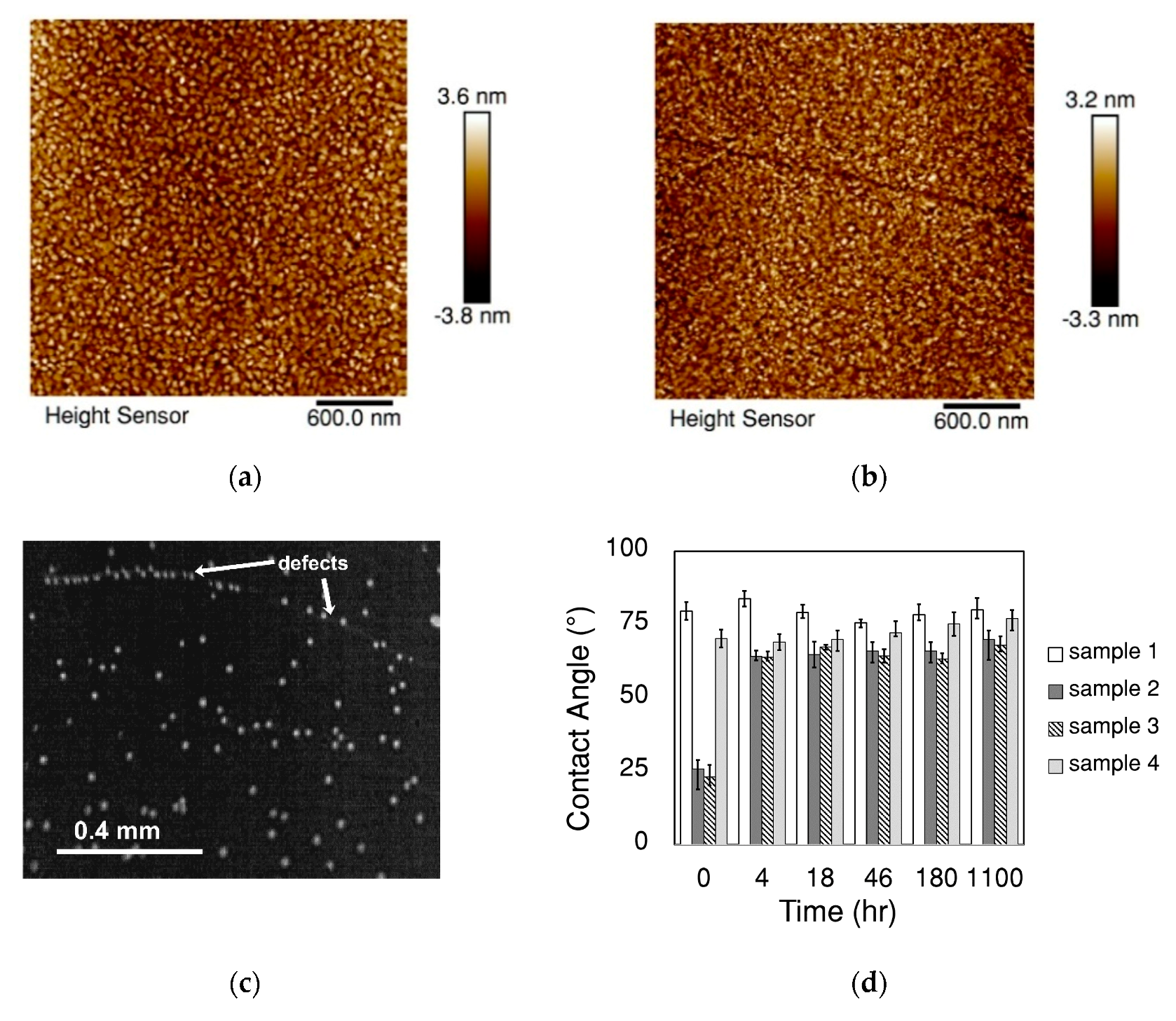
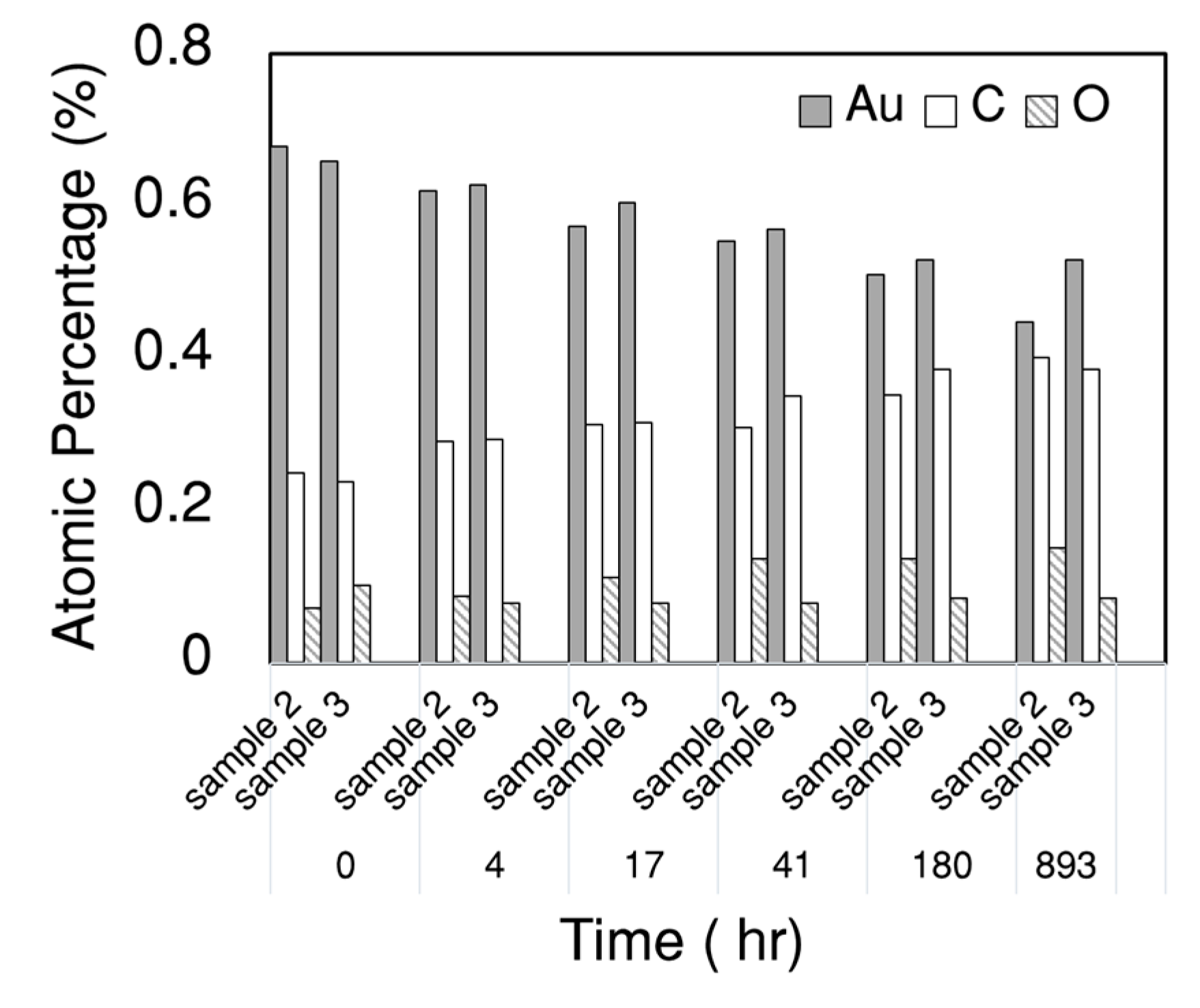
3.1. Proper Cleaning Procedure Prior to the Dropwise Condensation Experiment
3.2. Proper Storing Methods of Gold-Coated Samples
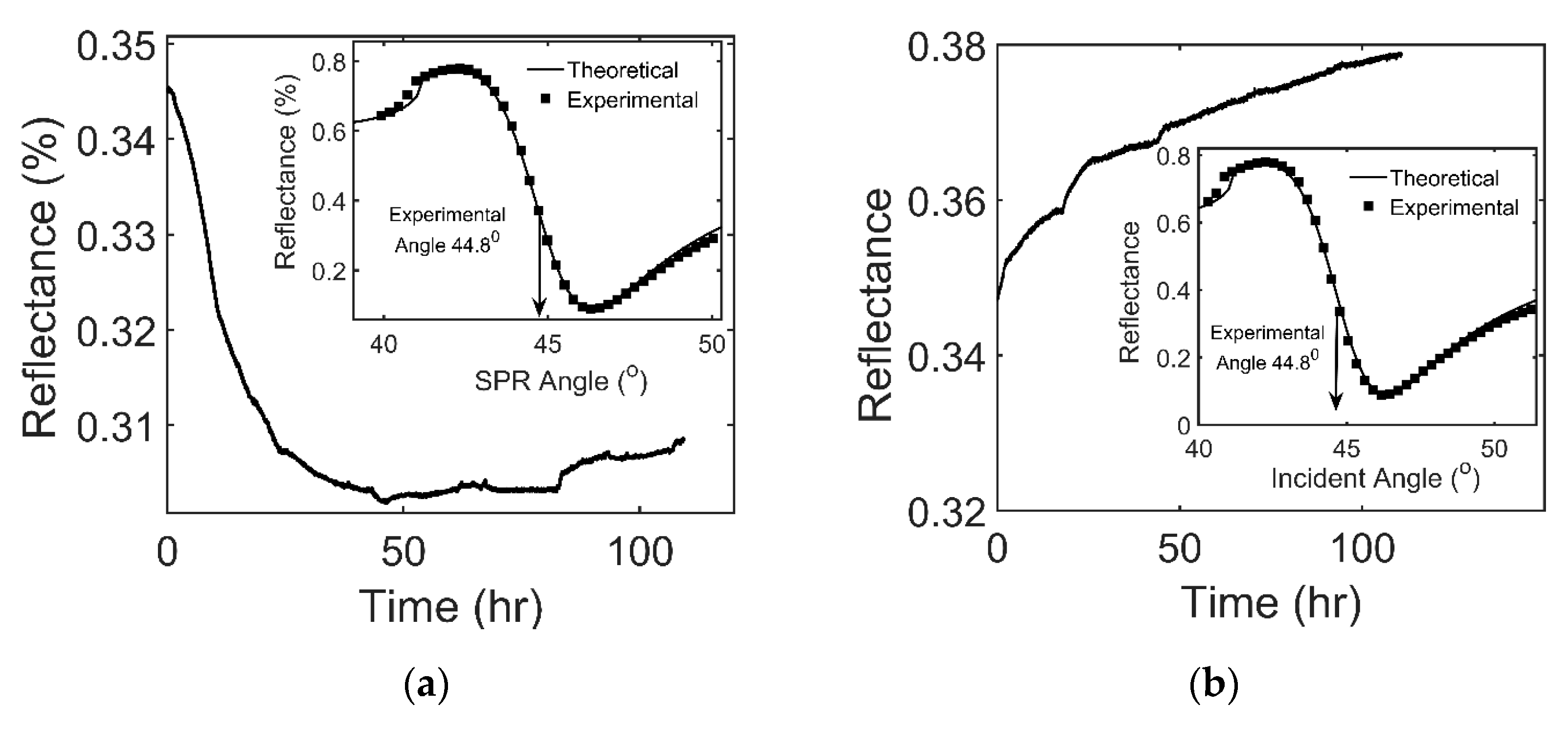
3.3. The Effect of Adsorption of VOCs on SPR Reflectance
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
List of Symbols
| C | Offset | min | Minimum (at the resonance angle) |
| d | Thickness of a layer (nm) | over | Overlayer |
| I | Intensity value | SPR | Associated with SPRi |
| K | Wave vector (1/nm) | sub | Sublayer |
| n | Refractive index | t | Theoretical |
| r | Coefficient of reflection between layers | XPS | Associated with XPS microscopy |
| R | Reflectance | 0 | First measurement |
| S | Scaling factor | 1 | Layer 1 |
| α | Incident angle (°) in SPRi | 2 | Lauer 2 |
| θ | XPS angle relative to the surface (°) | 3 | Layer 3 |
| λ | Inelastic mean free path in gold (nm) | 4 | Layer 4 |
| subscript | Thick Au 4f layer with no overlayer | ||
| max | Maximum (at the total internal reflection angle) | ||
References
- Hwang, K.W.; Kim, D.; Jo, H.; Park, H.S.; Moriyama, K.; Kim, M.H. Effects of heat flux on dropwise condensation on a superhydrophobic surface. J. Mech. Sci. Technol. 2016, 30, 2141–2149. [Google Scholar] [CrossRef]
- Chung, B.-J.; Kim, M.C.; Ahmadinejad, M. Film-wise and drop-wise condensation of steam on short inclined plates. J. Mech. Sci. Technol. 2008, 22, 127–133. [Google Scholar] [CrossRef]
- Leipertz, A. J3 Dropwise Condensation. In VDI Heat Atlas; Springer: Berlin, Germany, 2010. [Google Scholar]
- Khandekar, S.; Muralidhar, K. Dropwise Condensation on Inclined Textured Surfaces; Springer: Berlin, Germany, 2014. [Google Scholar]
- Ahangar, S.B.; Konduru, V.; Allen, J.S.; Miljkovic, N.; Lee, S.H.; Choi, C.K. Development of automated angle-scanning, high-speed surface plasmon resonance imaging and SPRi visualization for the study of dropwise condensation. Exp. Fluids 2019, 61, 12. [Google Scholar] [CrossRef] [Green Version]
- Ahangar, S.B.; Allen, J.S.; Lee, S.H.; Choi, C.K. Surface Plasmon Resonance Imaging: A Technique to Reveal the Dropwise Condensation Mechanism. J. Heat Transf. 2020, 142. [Google Scholar] [CrossRef] [Green Version]
- Xu, K.; Cao, P.; Heath, J.R. Graphene Visualizes the First Water Adlayers on Mica at Ambient Conditions. Science 2010, 329, 1188–1191. [Google Scholar] [CrossRef] [Green Version]
- Verdaguer, A.; Cardellach, M.; Fraxedas, J. Thin water films grown at ambient conditions on BaF2(111) studied by scanning polarization force microscopy. J. Chem. Phys. 2008, 129, 174705. [Google Scholar] [CrossRef]
- Cao, P.; Xu, K.; Varghese, J.O.; Heath, J.R. The Microscopic Structure of Adsorbed Water on Hydrophobic Surfaces under Ambient Conditions. Nano Lett. 2011, 11, 5581–5586. [Google Scholar] [CrossRef] [Green Version]
- Cha, H.; Wu, A.; Kim, M.-K.; Saigusa, K.; Liu, A.; Miljkovic, N. Nanoscale-agglomerate-mediated heterogeneous nucleation. Nano Lett. 2017, 17, 7544–7551. [Google Scholar] [CrossRef]
- Yan, X.; Huang, Z.; Sett, S.; Oh, J.; Cha, H.; Li, L.; Feng, L.; Wu, Y.; Zhao, C.; Orejon, D.; et al. Atmosphere-Mediated Superhydrophobicity of Rationally Designed Micro/Nanostructured Surfaces. ACS Nano 2019, 13, 4160–4173. [Google Scholar] [CrossRef] [Green Version]
- Orejon, D.; Askounis, A.; Takata, Y.; Attinger, D. Dropwise Condensation on Multiscale Bioinspired Metallic Surfaces with Nanofeatures. ACS Appl. Mater. Interfaces 2019, 11, 24735–24750. [Google Scholar] [CrossRef]
- Kurokawa, A.; Odaka, K.; Azuma, Y.; Fujimoto, T.; Kojima, I. Diagnosis and cleaning of carbon contamination on SiO2 thin film. J. Surf. Anal. 2009, 15, 337–340. [Google Scholar] [CrossRef] [Green Version]
- Robinson, R.; Sandberg, R.; Allred, D.; Jackson, A.; Johnson, J.; Evans, W.; Doughty, T.; Baker, A.; Adamson, K.; Jacquier, A. Removing surface contaminants from silicon wafers to facilitate EUV optical characterization. In Proceedings of the Annual Technical Conference-Society of Vacuum Coaters, Santa Clara, CA, USA, 9–14 May 2009; p. 368. [Google Scholar]
- Pires, J.; Carvalho, A.; de Carvalho, M.B. Adsorption of volatile organic compounds in Y zeolites and pillared clays. Microporous Mesoporous Mater. 2001, 43, 277–287. [Google Scholar] [CrossRef]
- Chapter 3—Surface Preparation for Film and Coating Deposition Processes. In Handbook of Deposition Technologies for Films and Coatings, 3rd ed.; Martin, P.M. (Ed.) William Andrew Publishing: Boston, MA, USA, 2010; pp. 93–134. [Google Scholar] [CrossRef]
- Hubadillah, S.K.; Harun, Z.; Aminudin, N.N.; Rosman, N. Ceramic membrane surface roughness induced by modified phase inversion: The effect of thermodynamic properties. Aust. J. Basic Appl. Sci. 2014, 8, 233–240. [Google Scholar]
- Nouri, N.M.; Sekhavat, S.; Bayani Ahangar, S.; Faal Nazari, N. Effect of curing condition on superhydrophobic surface for 7075Al. J. Dispers. Sci. Technol. 2012, 33, 771–774. [Google Scholar] [CrossRef]
- Raja, R.S.S.; Selvakumar, P.; Babu, P.D. A novel fabrication of superhydrophobic surfaces on aluminium substrate by picosecond pulsed laser. J. Mech. Sci. Technol. 2020, 34, 1667–1674. [Google Scholar] [CrossRef]
- Huang, T.-H.; Bhalothia, D.; Lin, S.; Huang, Y.-R.; Wang, K.-W. The Ethanol Oxidation Reaction Performance of Carbon-Supported PtRuRh Nanorods. Appl. Sci. 2020, 10, 3923. [Google Scholar] [CrossRef]
- Kim, D.Y.; Jeong, C.H.; Lee, H.J.; Choi, C.K.; Lee, S.H. Modeling of the finite boundary limit of evaporation flux in the contact line region using the surface plasmon resonance imaging. Int. Commun. Heat Mass Transf. 2020, 116, 104598. [Google Scholar] [CrossRef]
- Yue, W.; Wang, Z.; Yang, Y.; Chen, L.; Syed, A.; Wong, K.; Wang, X. Electron-beam lithography of gold nanostructures for surface-enhanced Raman scattering. J. Micromech. Microeng. 2012, 22, 125007. [Google Scholar] [CrossRef]
- Bayani, S.; Tabe, Y.; Kang, Y.T.; Lee, S.H.; Choi, C.K. Surface plasmon resonance imaging of drop coalescence at high-temporal resolution. J. Flow Vis. Image Process. 2018, 25, 191–205. [Google Scholar] [CrossRef]
- Ahangar, S.B.; Bellur, K.; Medici, E.; Tajiri, K.; Allen, J.S.; Choi, C.K. Optical properties and swelling of thin film perfluorinated sulfonic-acid ionomer. ECS Trans. 2019, 92, 197. [Google Scholar] [CrossRef]
- Baer, D.R.; Wang, Y.-C.; Castner, D.G. Use of XPS to Quantify Thickness of Coatings on Nanoparticles. Microsc. Today 2016, 24, 40–45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Passiu, C.; Rossi, A.; Weinert, M.; Tysoe, W.; Spencer, N.D. Probing the outermost layer of thin gold films by XPS and density functional theory. Appl. Surf. Sci. 2020, 507, 145084. [Google Scholar] [CrossRef]
- Martínez-Hipatl, C.; Muñoz-Aguirre, S.; Beltrán-Pérez, G.; Castillo-Mixcóatl, J.; Rivera-De la Rosa, J. Detection of volatile organic compounds by an interferometric sensor. Sens. Actuators B Chem. 2010, 147, 37–42. [Google Scholar] [CrossRef]
- Tao, W.-H.; Yang, T.C.-K.; Chang, Y.-N.; Chang, L.-K.; Chung, T.-W. Effect of moisture on the adsorption of volatile organic compounds by zeolite 13×. J. Environ. Eng. 2004, 130, 1210–1216. [Google Scholar] [CrossRef]
- Huang, H.; Haghighat, F.; Blondeau, P. Volatile organic compound (VOC) adsorption on material: Influence of gas phase concentration, relative humidity and VOC type. Indoor Air 2006, 16, 236–247. [Google Scholar] [CrossRef]
- Huang, C.-Y.; Song, M.; Gu, Z.-Y.; Wang, H.-F.; Yan, X.-P. Probing the Adsorption Characteristic of Metal–Organic Framework MIL-101 for Volatile Organic Compounds by Quartz Crystal Microbalance. Environ. Sci. Technol. 2011, 45, 4490–4496. [Google Scholar] [CrossRef]

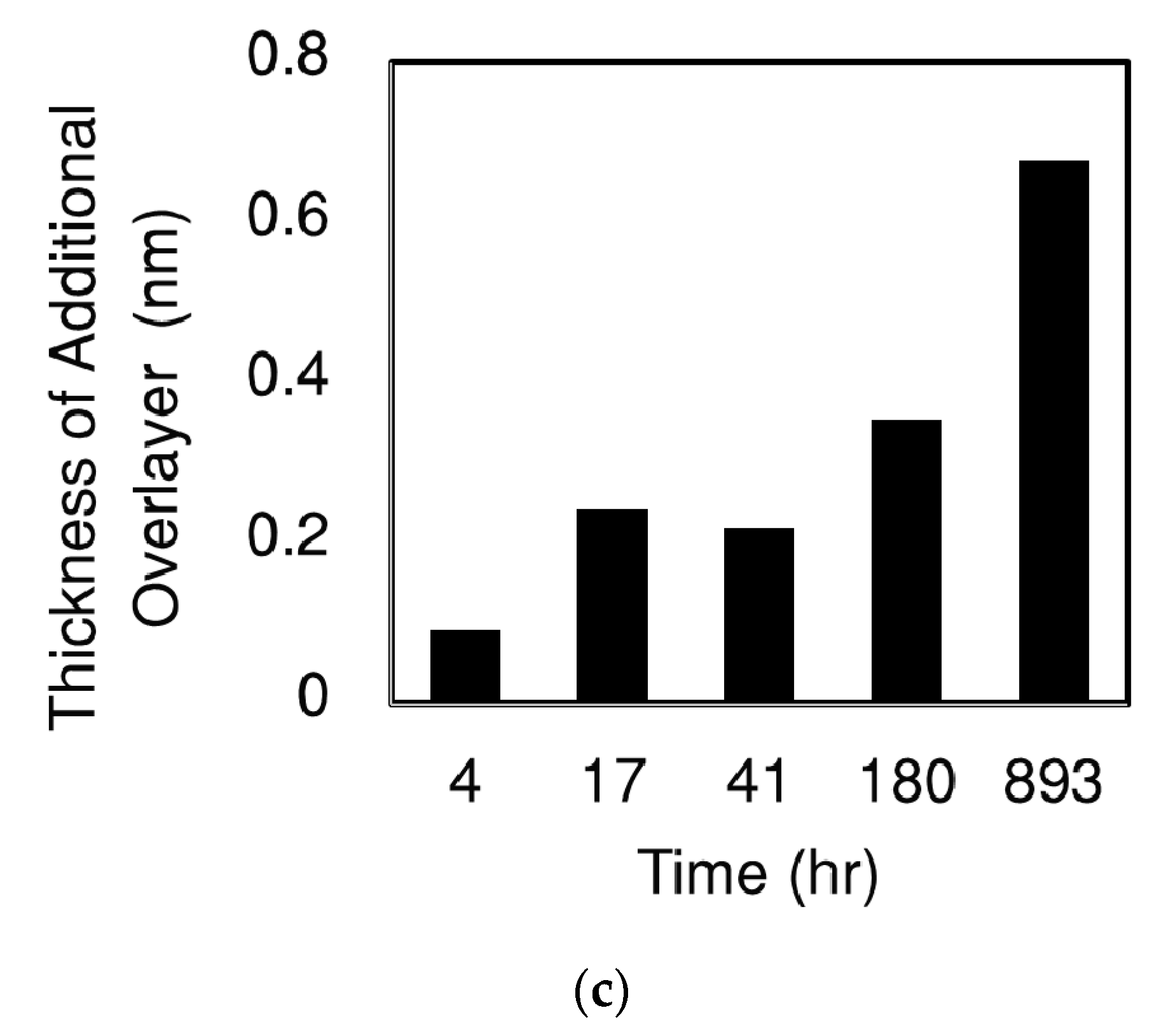
| Preparation | Sample 1 | Sample 2 | Sample 3 | Sample 4 |
|---|---|---|---|---|
| Cleaning procedure | - | SCP + Plasma | SCP + Plasma | SCP |
| Storing method | Open container | Open container | Sealed container | Open container |
| Analysis | Exposure Time (h) | |||
|---|---|---|---|---|
| Sample 1 | Sample 2 | Sample 3 | Sample 4 | |
| XPS | - | 0, 4, 17, 43, 180, 893 | 0, 4, 17, 43, 180, 893 | 0 |
| AFM | - | 2, 17, 41, 185 | 2, 17, 41, 185 | 2, 17, 41, 185 |
| Contact Angle | 0, 4, 18, 46, 180, 1100 | 0, 4, 18, 46, 180, 1100 | 0, 4, 18, 46, 180, 1100 | 0, 4, 18, 46, 180, 1100 |
| SPRi | - | 1~110 | 1~110 | 1~110 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahangar, S.B.; Jeong, C.H.; Long, F.; Allen, J.S.; Lee, S.H.; Choi, C.K. The Effect of Adsorbed Volatile Organic Compounds on an Ultrathin Water Film Measurement. Appl. Sci. 2020, 10, 5981. https://doi.org/10.3390/app10175981
Ahangar SB, Jeong CH, Long F, Allen JS, Lee SH, Choi CK. The Effect of Adsorbed Volatile Organic Compounds on an Ultrathin Water Film Measurement. Applied Sciences. 2020; 10(17):5981. https://doi.org/10.3390/app10175981
Chicago/Turabian StyleAhangar, Shahab Bayani, Chan Ho Jeong, Fei Long, Jeffrey S. Allen, Seong Hyuk Lee, and Chang Kyoung Choi. 2020. "The Effect of Adsorbed Volatile Organic Compounds on an Ultrathin Water Film Measurement" Applied Sciences 10, no. 17: 5981. https://doi.org/10.3390/app10175981





