Evaluation of the In Vitro and In Vivo Antitumor Efficacy of Peanut Sprout Extracts Cultivated with Fermented Sawdust Medium Against Bladder Cancer
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.3. Cell Culture
2.4. PSEFS Treatment, MTT Assay, and Cell Counting
2.5. Cell Cycle Analysis
2.6. Immunoblots and IP
2.7. Apoptosis Assay
2.8. Wound-Healing Migration Assay
2.9. Boyden Chamber Invasion Assay
2.10. Zymography
2.11. Nuclear Extracts and EMSA
2.12. Mouse Xenograft Inoculation
2.13. Immunohistochemistry
2.14. Plasma Preparation and Biochemical Analysis
2.15. Statistical Analysis
3. Results
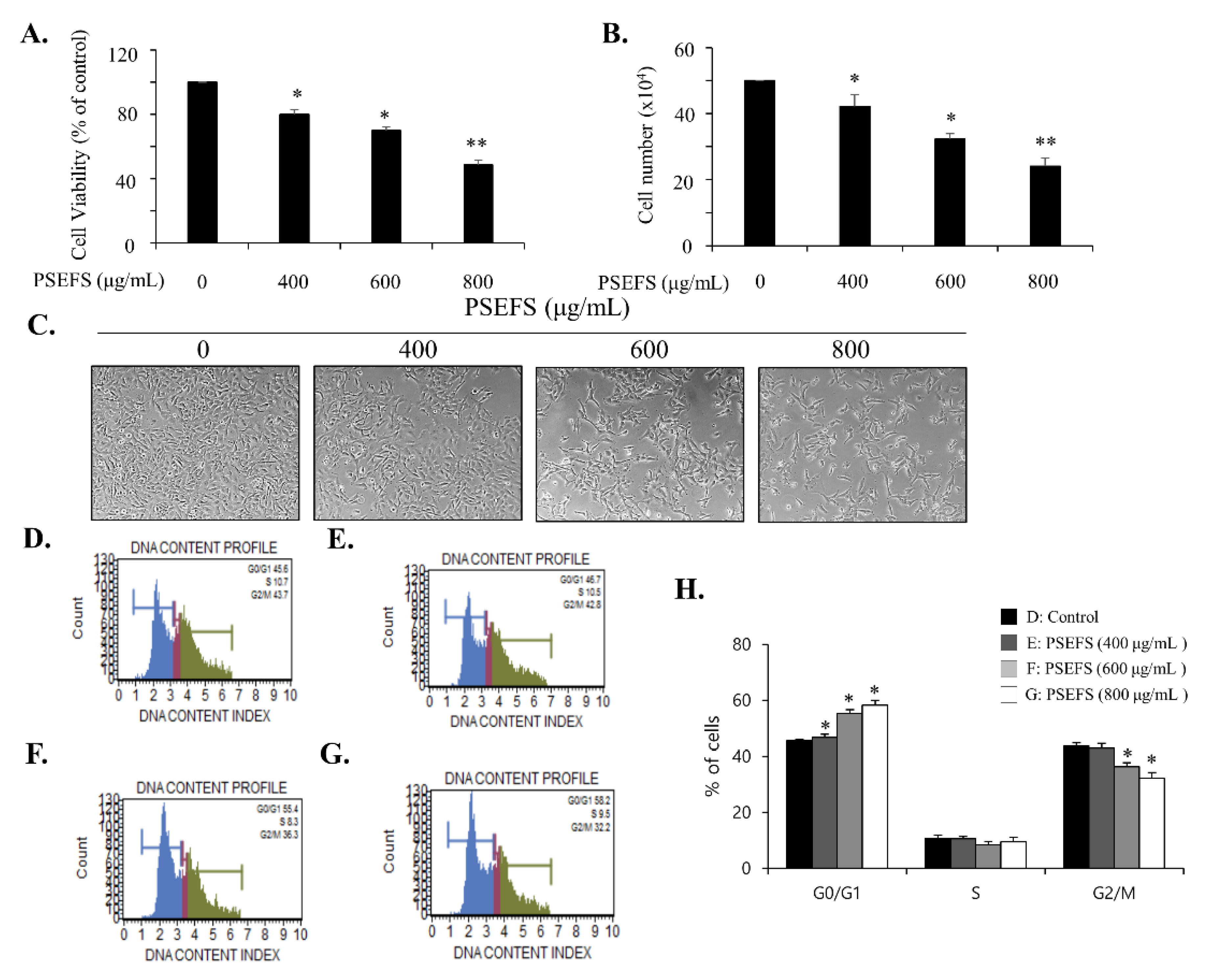
3.1. PSEFS Prohibits the Proliferation of Bladder Cancer T24 Cells by Stimulating Cell Cycle Arrest at the G1 Phase
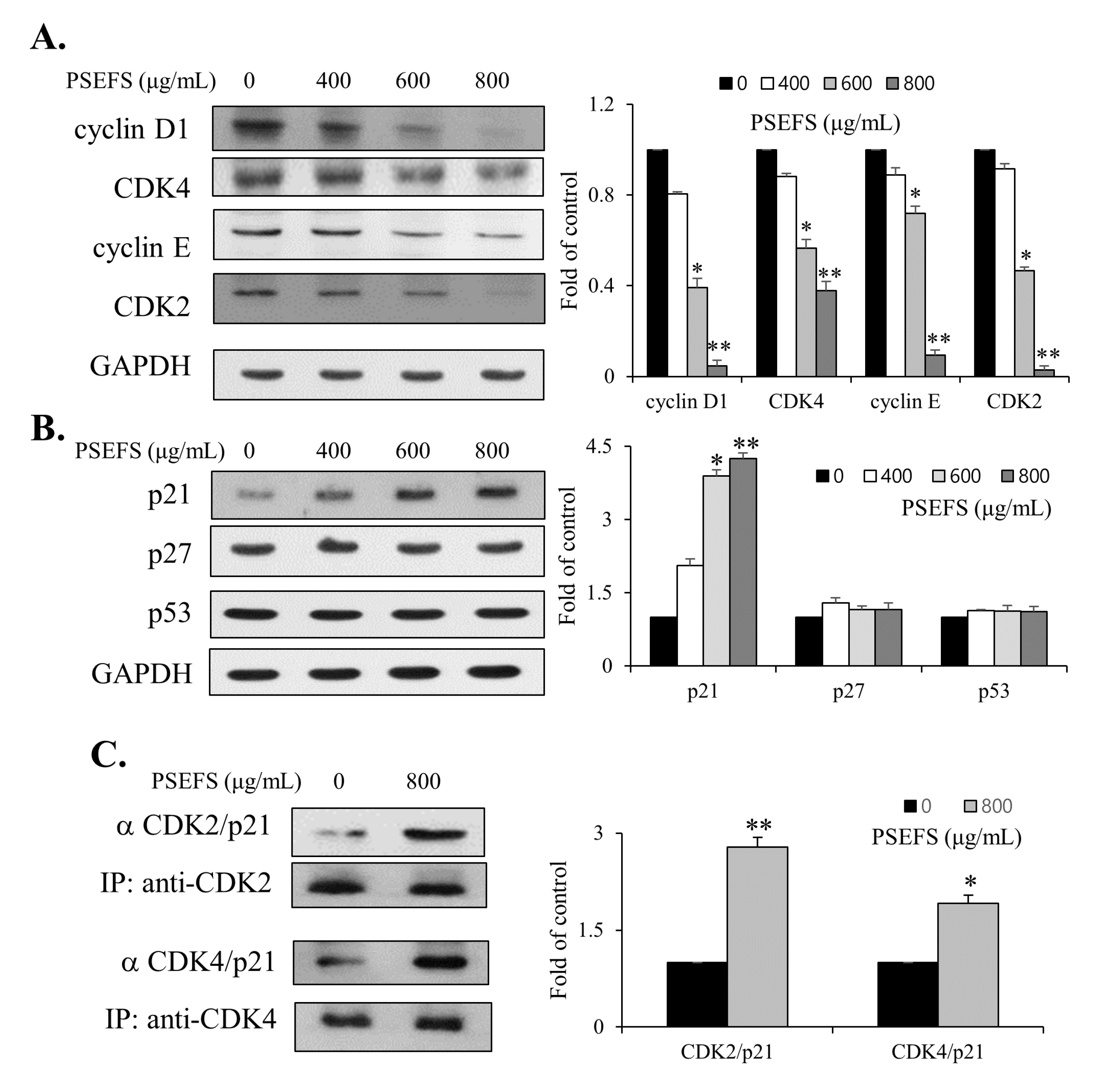
3.2. PSEFS Stimulates G1-Phase Arrest of the Cell Cycle Via Reduced Expression of CDKs and Cyclins by Increasing p21WAF1 Protein Level
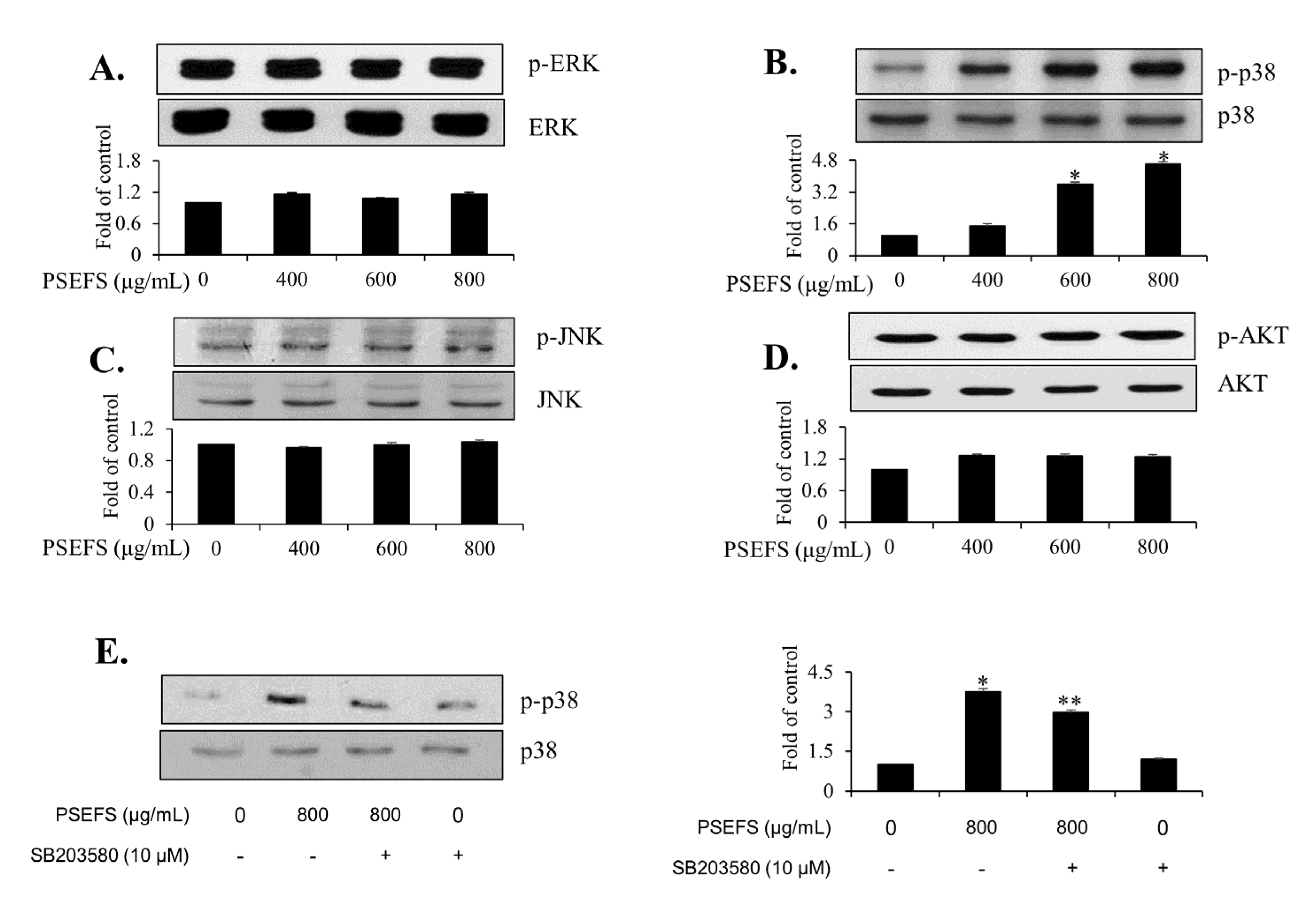
3.3. PSEFS Suppresses the Phosphorylation of p38 MAPK in T24 Cells
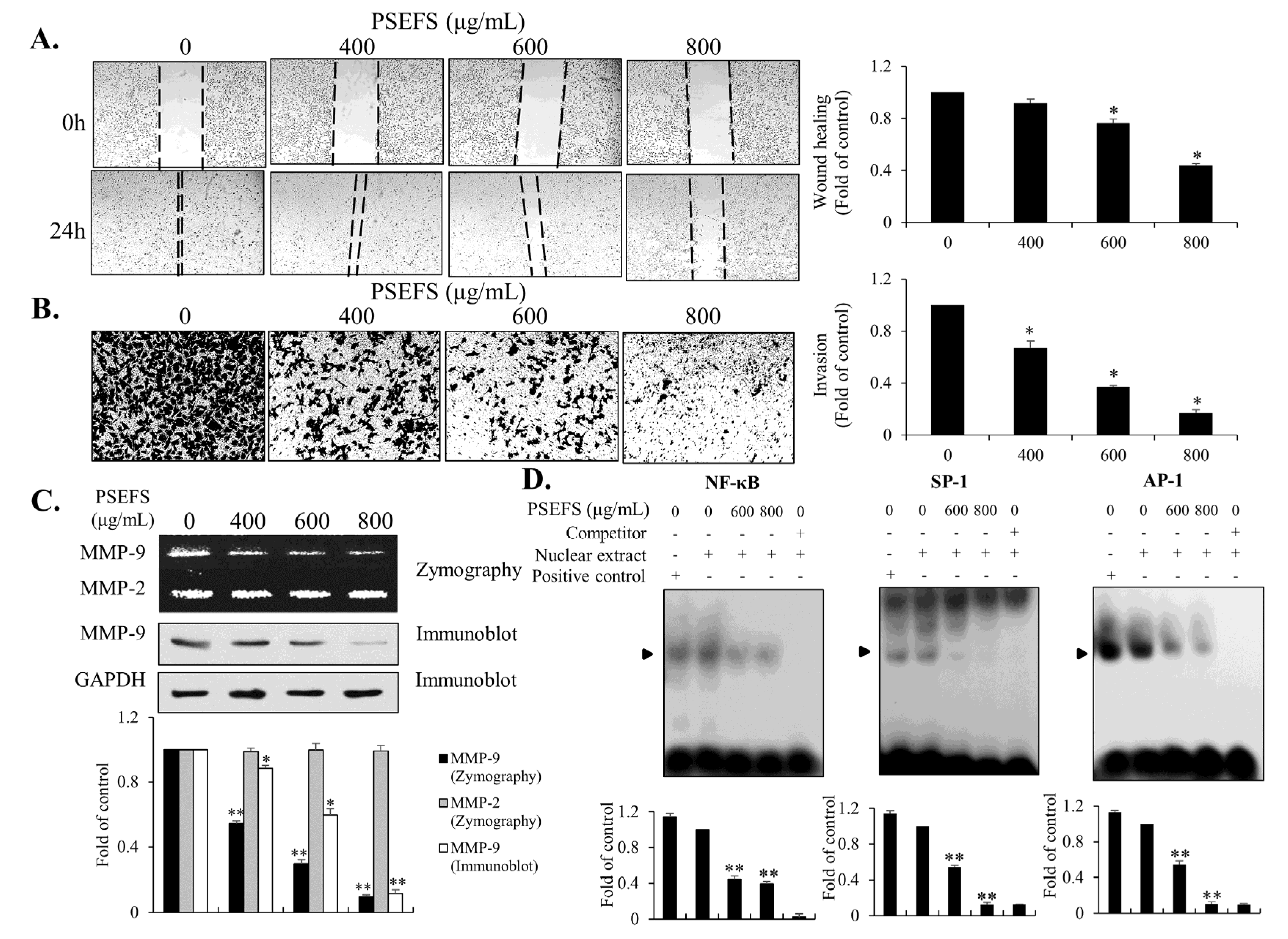
3.4. The MMP-9-Related Transcription Factors SP1, AP-1, and NF-κB are Associated with PSEFS-Induced Mitigation of T24 Cell Migration and Invasion
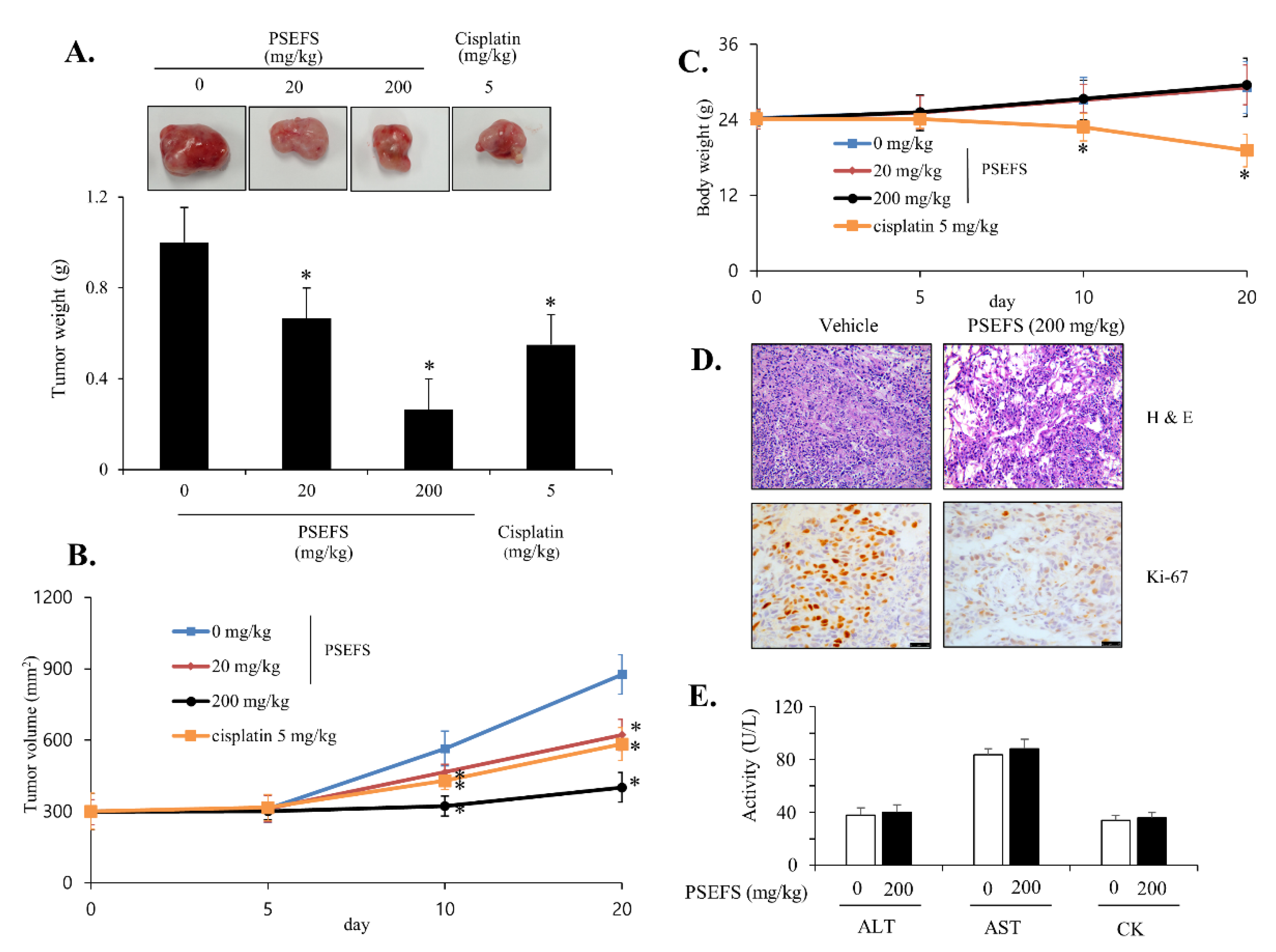
3.5. PSEFS Abolishes Tumor Growth in Xenograft Model Bearing Bladder Cancer T24 Cells
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matulay, J.T.; Kamat, A.M. Advances in Risk Stratification of Bladder Cancer to Guide Personalized Medicine. F1000Research 2018, 7, 1137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chromecki, T.F.; Bensalah, K.; Remzi, M.; Verhoest, G.; Cha, E.K.; Scherr, D.S.; Novara, G.; Karakiewicz, P.I.; Shariat, S.F. Prognostic Factors for Upper Urinary Tract Urothelial Carcinoma. Nat. Rev. Urol. 2011, 8, 440–447. [Google Scholar] [CrossRef] [PubMed]
- Berdik, C. Unlocking Bladder Cancer. Nature 2017, 551, S34–S35. [Google Scholar] [CrossRef] [PubMed]
- Ghagane, S.C.; Puranik, S.I.; Kumbar, V.M.; Nerli, R.B.; Jalalpure, S.S.; Hiremath, M.B.; Neelagund, S.; Aladakatti, R. In Vitro Antioxidant and Anticancer Activity of Leea Indica Leaf Extracts on Human Prostate Cancer Cell Lines. Integr. Med. Res. 2017, 6, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Knowles, M.A.; Hurst, C.D. Molecular Biology of Bladder Cancer: New Insights into Pathogenesis and Clinical Diversity. Nat. Rev. Cancer 2015, 15, 25–41. [Google Scholar] [CrossRef] [PubMed]
- Gerhardt, D.; Bertola, G.; Dietrich, F.; Figueiró, F.; Zanotto-Filho, A.; Moreira Fonseca, J.C.; Morrone, F.B.; Barrios, C.H.; Battastini, A.M.O.; Salbego, C.G. Boldine Induces Cell Cycle Arrest and Apoptosis in T24 Human Bladder Cancer Cell Line Via Regulation of ERK, AKT, and GSK-3β. Urol. Oncol. 2014, 32, 36.e1–36.e9. [Google Scholar] [CrossRef] [PubMed]
- Mayer, I.A.; Arteaga, C.L. The PI3K/AKT Pathway as a Target for Cancer Treatment. Annu. Rev. Med. 2016, 67, 11–28. [Google Scholar] [CrossRef]
- Sclafani, R.A.; Holzen, T.M. Cell Cycle Regulation of DNA Replication. Annu. Rev. Genet 2007, 41, 237–280. [Google Scholar] [CrossRef] [Green Version]
- Li, A.; Blow, J.J. The Origin of CDK Regulation. Nat. Cell Biol. 2001, 3, 182. [Google Scholar] [CrossRef] [Green Version]
- Otto, T.; Sicinski, P. Cell Cycle Proteins as Promising Targets in Cancer Therapy. Nat. Rev. Cancer 2017, 17, 93–115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeggo, P.A.; Pearl, L.H.; Carr, A.M. DNA Repair, Genome Stability and Cancer: A Historical Perspective. Nat. Rev. Cancer 2016, 16, 35–42. [Google Scholar] [CrossRef] [Green Version]
- Robertson, A.G.; Kim, J.; Al-Ahmadie, H.; Bellmunt, J.; Guo, G.; Cherniack, A.D.; Hinoue, T.; Laird, P.W.; Hoadley, K.A.; Akbani, R.; et al. Comprehensive Molecular Characterization of Muscle-Invasive Bladder Cancer. Cell 2017, 171, 540–556.e25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bianco, F.J.; Gervasi, D.C.; Tiguert, R.; Grignon, D.J.; Pontes, J.E.; Crissman, J.D.; Fridman, R.; Wood, D.P. Matrix Metalloproteinase-9 Expression in Bladder Washes from Bladder Cancer Patients Predicts Pathological Stage and Grade. Clin. Cancer Res. 1998, 4, 3011–3016. [Google Scholar] [PubMed]
- Davies, B.; Waxman, J.; Wasan, H.; Abel, P.; Williams, G.; Krausz, T.; Neal, D.; Thomas, D.; Hanby, A.; Balkwill, F. Levels of Matrix Metalloproteases in Bladder Cancer Correlate with Tumor Grade and Invasion. Cancer Res. 1993, 53, 5365–5369. [Google Scholar] [PubMed]
- Bond, M.; Fabunmi, R.P.; Baker, A.H.; Newby, A.C. Synergistic Upregulation of Metalloproteinase-9 by Growth Factors and Inflammatory Cytokines: An Absolute Requirement for Transcription Factor NF-Kappa B. FEBS Lett. 1998, 435, 29–34. [Google Scholar] [CrossRef] [Green Version]
- Sato, H.; Seiki, M. Regulatory Mechanism of 92 kDa Type IV Collagenase Gene Expression which is Associated with Invasiveness of Tumor Cells. Oncogene 1993, 8, 395–405. [Google Scholar]
- Lee, S.; Cho, S.; Lee, E.; Kim, S.; Lee, S.; Lim, J.; Choi, Y.H.; Kim, W.; Moon, S. Interleukin-20 Promotes Migration of Bladder Cancer Cells through Extracellular Signal-Regulated Kinase (ERK)-Mediated MMP-9 Protein Expression Leading to Nuclear Factor (NF-κB) Activation by Inducing the Up-Regulation of p21(WAF1) Protein Expression. J. Biol. Chem. 2013, 288, 5539–5552. [Google Scholar] [CrossRef] [Green Version]
- De Vries, K.; Strydom, M.; Steenkamp, V. Journal of Herbal Medicine. J. Herb. Med. 2011, 11, 71–77. [Google Scholar] [CrossRef] [Green Version]
- Howitz, K.T.; Bitterman, K.J.; Cohen, H.Y.; Lamming, D.W.; Lavu, S.; Wood, J.G.; Zipkin, R.E.; Chung, P.; Kisielewski, A.; Zhang, L.; et al. Small Molecule Activators of Sirtuins Extend Saccharomyces Cerevisiae Lifespan. Nature 2003, 425, 191–196. [Google Scholar] [CrossRef]
- Choi, J.; Choi, D.; Lee, J.; Yun, S.; Lee, D.; Eun, J.; Lee, S. Ethanol Extract of Peanut Sprout Induces Nrf2 Activation and Expression of Antioxidant and Detoxifying Enzymes in Human Dermal Fibroblasts: Implication for its Protection Against UVB-Irradiated Oxidative Stress. Photochem. Photobiol. 2013, 89, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.; Kim, J.; Kwon, S.; Park, K.; Kang, J.; Seo, K.I. Antioxidative Effects of Peanut Sprout Extracts. J. Korean Soc. Food Sci. Nutr. 2010, 39, 941–946. [Google Scholar] [CrossRef]
- Kang, N.E.; Ha, A.W.; Woo, H.W.; Kim, W.K. Peanut Sprouts Extract (Arachis Hypogaea L.) has Anti-Obesity Effects by Controlling the Protein Expressions of PPARγ and Adiponectin of Adipose Tissue in Rats Fed High-Fat Diet. Nutr. Res. Pract. 2014, 8, 158–164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Limmongkon, A.; Nopprang, P.; Chaikeandee, P.; Somboon, T.; Wongshaya, P.; Pilaisangsuree, V. LC-MS/MS Profiles and Interrelationships between the Anti-Inflammatory Activity, Total Phenolic Content and Antioxidant Potential of Kalasin 2 Cultivar Peanut Sprout Crude Extract. Food Chem. 2018, 239, 569–578. [Google Scholar] [CrossRef]
- Li, T.; Luo, L.; Kim, S.; Moon, S.; Moon, B. Trans-Resveratrol Extraction from Peanut Sprouts Cultivated using Fermented Sawdust Medium and its Antioxidant Activity. J. Food Sci. 2020, 85, 639–646. [Google Scholar] [CrossRef] [PubMed]
- Song, J.H.; Hwang, B.; Chung, H.J.; Moon, B.; Kim, J.W.; Ko, K.; Kim, B.W.; Kim, W.R.; Kim, W.J.; Myung, S.C.; et al. Peanut Sprout Extracts Cultivated with Fermented Sawdust Medium Inhibits Benign Prostatic Hyperplasia in Vitro and in Vivo. World J. Mens Health 2020, 38, 385–396. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, L.G.; Wu, X.; Guan, J. Wound-Healing Assay. Methods Mol. Biol. 2005, 294, 23–29. [Google Scholar]
- Chen, H. Boyden Chamber Assay. Methods Mol. Biol. 2005, 294, 15–22. [Google Scholar]
- Hellman, L.M.; Fried, M.G. Electrophoretic Mobility Shift Assay (EMSA) for Detecting Protein-Nucleic Acid Interactions. Nat. Protoc. 2007, 2, 1849–1861. [Google Scholar] [CrossRef]
- Qi, Z.; Tang, T.; Sheng, L.; Ma, Y.; Liu, Y.; Yan, L.; Qi, S.; Ling, L.; Zhang, Y. Salidroside Inhibits the Proliferation and Migration of Gastric Cancer Cells Via Suppression of Src associated Signaling Pathway Activation and Heat Shock Protein 70 Expression. Mol. Med. Rep. 2018, 18, 147–156. [Google Scholar] [CrossRef]
- Rajesh, Y.; Banerjee, A.; Pal, I.; Biswas, A.; Das, S.; Dey, K.K.; Kapoor, N.; Ghosh, A.K.; Mitra, P.; Mandal, M. Delineation of Crosstalk between HSP27 and MMP-2/MMP-9: A Synergistic Therapeutic Avenue for Glioblastoma Management. Biochim. Et Biophys. Acta (BBA)-Gen. Subj. 2019, 1863, 1196–1209. [Google Scholar] [CrossRef] [PubMed]
- Sulistyowati, E.; Lee, M.Y.; Wu, L.C.; Hsu, J.H.; Dai, Z.K.; Wu, B.N.; Lin, M.C.; Yeh, J.L. Exogenous Heat Shock Cognate Protein 70 Suppresses LPS-Induced Inflammation by Down-Regulating NF-kappaB through MAPK and MMP-2/-9 Pathways in Macrophages. Molecules 2018, 23, 2124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Osborne, C.K.; Boldt, D.H.; Clark, G.M.; Trent, J.M. Effects of Tamoxifen on Human Breast Cancer Cell Cycle Kinetics: Accumulation of Cells in Early G1 Phase. Cancer Res. 1983, 43, 3583–3585. [Google Scholar]
- Lin, H.L.; Chang, Y.F.; Liu, T.Y.; Wu, C.W.; Chi, C.W. Submicromolar Paclitaxel Induces Apoptosis in Human Gastric Cancer Cells at Early G1 Phase. Anticancer. Res. 1998, 18, 3443–3449. [Google Scholar] [PubMed]
- Huang, K.; Kuo, C.; Chen, S.; Lin, C.; Lee, Y. Honokiol Inhibits in Vitro and in Vivo Growth of Oral Squamous Cell Carcinoma through Induction of Apoptosis, Cell Cycle Arrest and Autophagy. J. Cell. Mol. Med. 2018, 22, 1894–1908. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Cheng, X.; Chen, C.; Huijuan, W.; Zhao, H.; Liu, W.; Xiang, Z.; Wang, Q. Apigenin, a Flavonoid Constituent Derived from P. Villosa, Inhibits Hepatocellular Carcinoma Cell Growth by CyclinD1/CDK4 Regulation Via p38 MAPK-p21 Signaling. Pathol.-Res. Pract. 2020, 216, 152701. [Google Scholar] [CrossRef]
- Dhatwalia, S.K.; Kumar, M.; Dhawan, D.K. Role of EGCG in Containing the Progression of Lung Tumorigenesis–A Multistage Targeting Approach. Nutr. Cancer 2018, 70, 334–349. [Google Scholar] [CrossRef]
- Kamiyama, M.; Naguro, I.; Ichijo, H. In Vivo Gene Manipulation Reveals the Impact of Stress-Responsive MAPK Pathways on Tumor Progression. Cancer Sci. 2015, 106, 785–796. [Google Scholar] [CrossRef]
- Yu, X.; Zhong, J.; Yan, L.; Li, J.; Wang, H.; Wen, Y.; Zhao, Y. Curcumin Exerts Antitumor Effects in Retinoblastoma Cells by Regulating the JNK and p38 MAPK Pathways. Int. J. Mol. Med. 2016, 38, 861–868. [Google Scholar] [CrossRef] [Green Version]
- Huang, G.; Tang, B.; Tang, K.; Dong, X.; Deng, J.; Liao, L.; Liao, Z.; Yang, H.; He, S. Isoquercitrin Inhibits the Progression of Liver Cancer in Vivo and in Vitro Via the MAPK Signalling Pathway. Oncol. Rep. 2014, 31, 2377–2384. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, H.; Song, J.-H.; Hwang, B.; Moon, B.; Yun, S.-J.; Kim, W.-J.; Moon, S.-K. Evaluation of the In Vitro and In Vivo Antitumor Efficacy of Peanut Sprout Extracts Cultivated with Fermented Sawdust Medium Against Bladder Cancer. Appl. Sci. 2020, 10, 8758. https://doi.org/10.3390/app10238758
Park H, Song J-H, Hwang B, Moon B, Yun S-J, Kim W-J, Moon S-K. Evaluation of the In Vitro and In Vivo Antitumor Efficacy of Peanut Sprout Extracts Cultivated with Fermented Sawdust Medium Against Bladder Cancer. Applied Sciences. 2020; 10(23):8758. https://doi.org/10.3390/app10238758
Chicago/Turabian StylePark, Hongbeom, Jun-Hui Song, Byungdoo Hwang, BoKyung Moon, Seok-Joong Yun, Wun-Jae Kim, and Sung-Kwon Moon. 2020. "Evaluation of the In Vitro and In Vivo Antitumor Efficacy of Peanut Sprout Extracts Cultivated with Fermented Sawdust Medium Against Bladder Cancer" Applied Sciences 10, no. 23: 8758. https://doi.org/10.3390/app10238758
APA StylePark, H., Song, J.-H., Hwang, B., Moon, B., Yun, S.-J., Kim, W.-J., & Moon, S.-K. (2020). Evaluation of the In Vitro and In Vivo Antitumor Efficacy of Peanut Sprout Extracts Cultivated with Fermented Sawdust Medium Against Bladder Cancer. Applied Sciences, 10(23), 8758. https://doi.org/10.3390/app10238758





