Phosphine-Free-Synthesized ZnSe/ZnS Core/Shell Quantum Dots for White Light-Emitting Diodes
Abstract
:1. Introduction
2. Materials and Methods
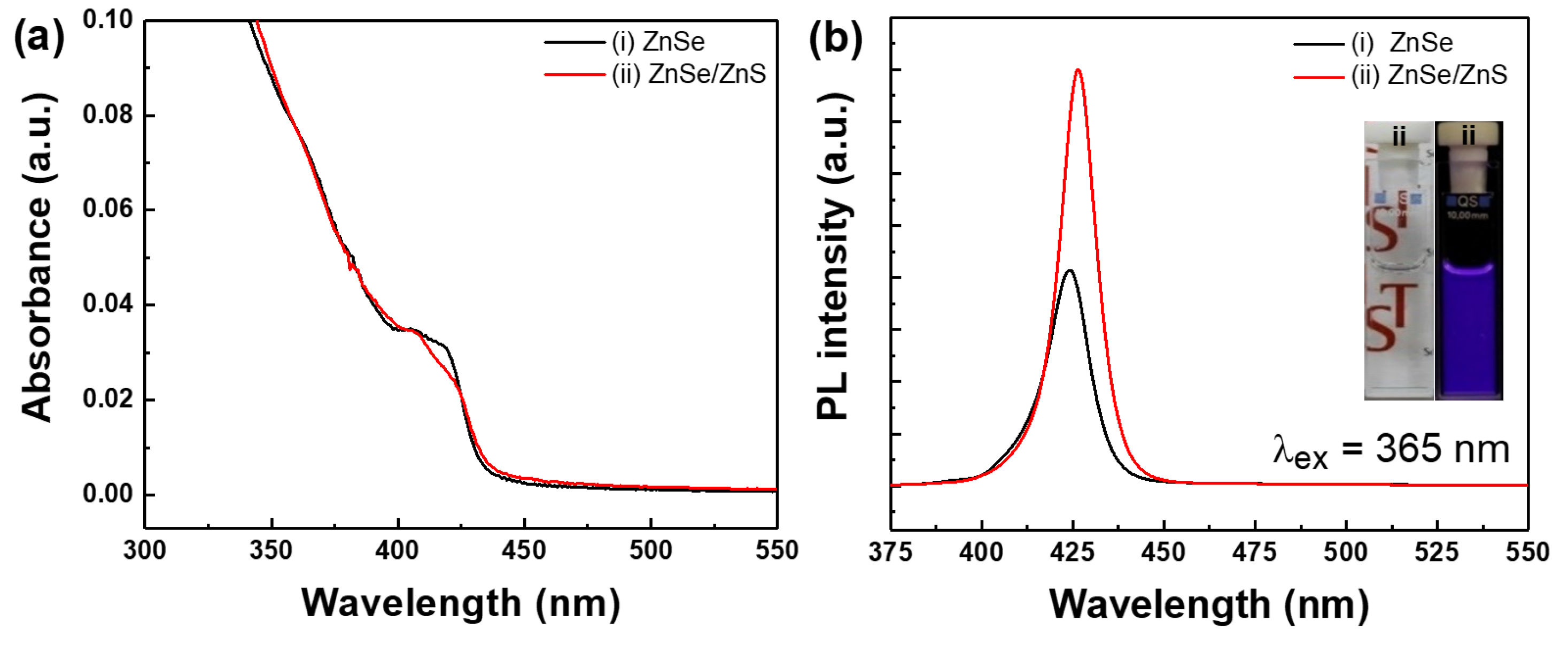
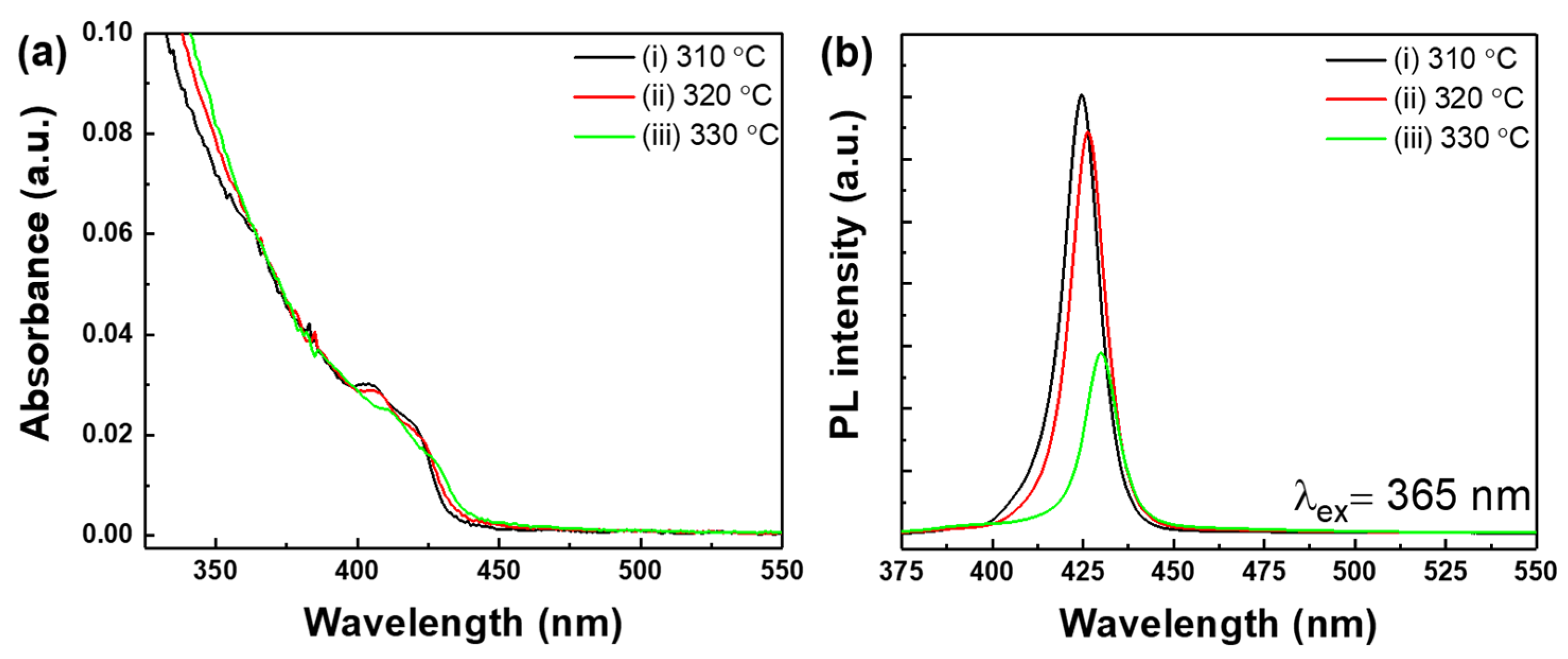
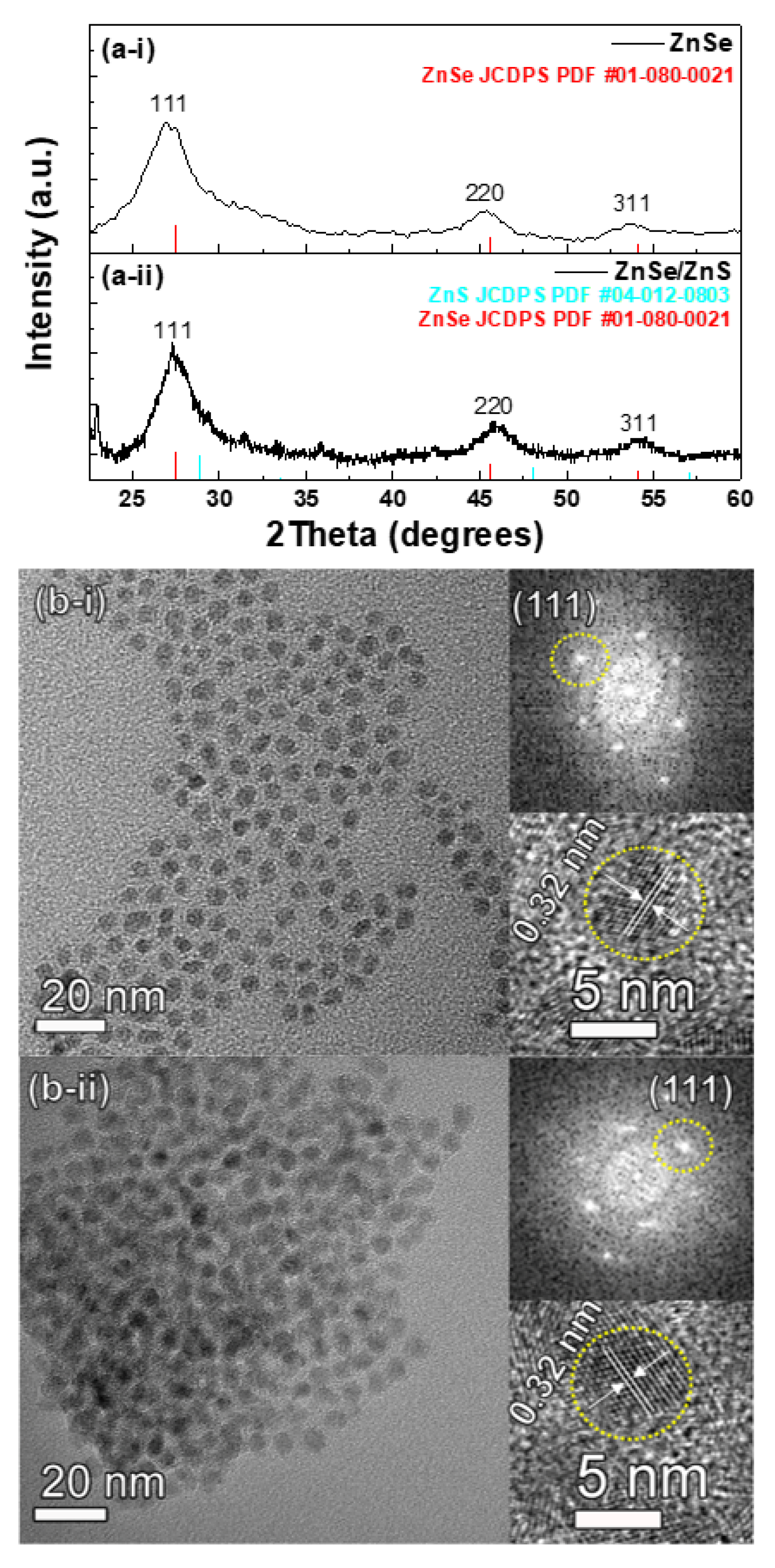
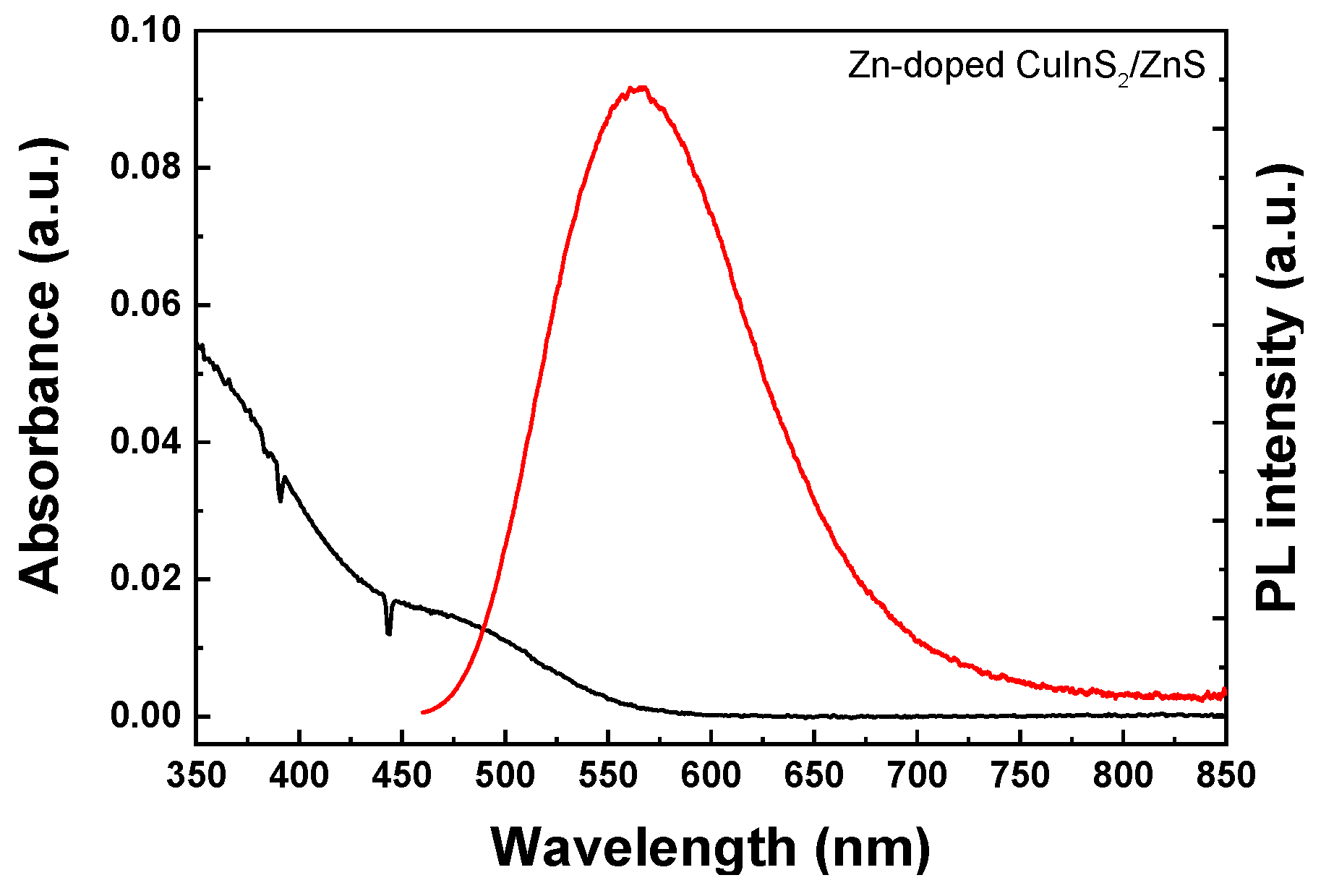
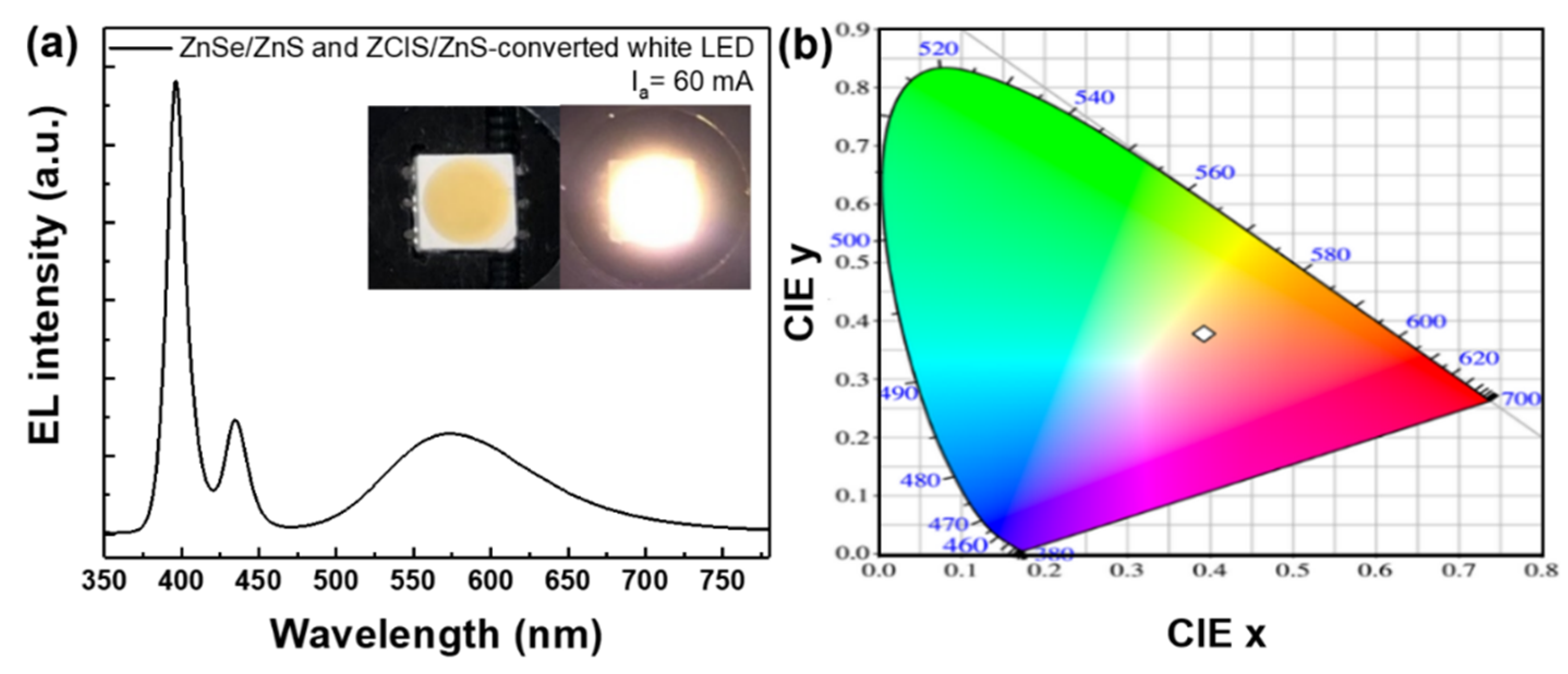
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Alivisatos, A.P. Perspectives on the Physical Chemistry of Semiconductor Nanocrystals. J. Phys. Chem. 1996, 100, 13226–13239. [Google Scholar] [CrossRef]
- Smith, A.M.; Nie, S. Semiconductor Nanocrystals: Structure, Properties, and Band Gap Engineering. Acc. Chem. Res. 2010, 43, 190–200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Todescato, F.; Fortunati, I.; Minotto, A.; Signorini, R.; Jasieniak, J.J.; Bozio, R. Engineering of Semiconductor Nanocrystals for Light Emitting Applications. Materials 2016, 9, 672. [Google Scholar] [CrossRef] [Green Version]
- Fuke, N.; Hoch, L.B.; Koposov, A.Y.; Manner, V.W.; Werder, D.J.; Fukui, A.; Koide, N.; Katayama, H.; Sykora, M. CdSe Quantum-Dot-Sensitized Solar Cell with ∼100% Internal Quantum Efficiency. ACS Nano 2010, 4, 6377–6386. [Google Scholar] [CrossRef]
- Zhao, J.-Y.; Chen, G.; Gu, Y.-P.; Cui, R.; Zhang, Z.-L.; Yu, Z.-L.; Tang, B.; Zhao, Y.-F.; Pang, D.-W. Ultrasmall Magnetically Engineered Ag2Se Quantum Dots for Instant Efficient Labeling and Whole-Body High-Resolution Multimodal Real-Time Tracking of Cell-Derived Microvesicles. J. Am. Chem. Soc. 2016, 138, 1893–1903. [Google Scholar] [CrossRef]
- Li, J.; Liang, Z.; Su, Q.; Jin, H.; Wang, K.; Xu, G.; Xu, X. Small Molecule-Modified Hole Transport Layer Targeting Low Turn-On-Voltage, Bright, and Efficient Full-Color Quantum Dot Light Emitting Diodes. ACS Appl. Mater. Interfaces 2018, 10, 3865–3873. [Google Scholar] [CrossRef] [PubMed]
- Kuehnel, M.F.; Sahm, C.D.; Neri, G.; Lee, J.R.; Orchard, K.L.; Cowan, A.J.; Reisner, E. ZnSe quantum dots modified with a Ni(cyclam) catalyst for efficient visible-light driven CO2 reduction in water. Chem. Sci. 2018, 9, 2501–2509. [Google Scholar] [CrossRef] [Green Version]
- Xu, Z.; Tang, X.; Liu, Y.; Zhang, Z.; Chen, W.; Liu, K.; Yuan, Z. CsPbBr3 Quantum Dot Films with High Luminescence Efficiency and Irradiation Stability for Radioluminescent Nuclear Battery Application. ACS Appl. Mater. Interfaces 2019, 11, 14191–14199. [Google Scholar] [CrossRef]
- Dabbousi, B.O.; Rodriguez-Viejo, J.; Mikulec, F.V.; Heine, J.R.; Mattoussi, H.; Ober, R.; Jensen, K.F.; Bawendi, M.G. (CdSe)ZnS Core−Shell Quantum Dots: Synthesis and Characterization of a Size Series of Highly Luminescent Nanocrystallites. J. Phys. Chem. B 1997, 101, 9463–9475. [Google Scholar] [CrossRef]
- Yang, Y.; Zheng, Y.; Cao, W.; Titov, A.; Hyvonen, J.; Manders, J.R.; Xue, J.; Holloway, P.H.; Qian, L. High-efficiency light-emitting devices based on quantum dots with tailored nanostructures. Nat. Photon. 2015, 9, 259–266. [Google Scholar] [CrossRef]
- Peng, X. An essay on synthetic chemistry of colloidal nanocrystals. Nano Res. 2009, 2, 425–447. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.Y.; Naik Mude, N.; Lampande, R.; Eun, K.J.; Yeom, J.E.; Choi, H.S.; Sohn, S.H.; Yoo, J.M.; Kwon, J.H. Efficient Cadmium-Free Inverted Red Quantum Dot Light-Emitting Diodes. ACS Appl. Mater. Interfaces 2019, 11, 36917–36924. [Google Scholar] [CrossRef]
- Zhang, J.; Sun, W.; Yin, L.; Miao, X.; Zhang, D. One-pot synthesis of hydrophilic CuInS2 and CuInS2–ZnS colloidal quantum dots. J. Mater. Chem. C 2014, 2, 4812–4817. [Google Scholar] [CrossRef]
- Terada, S.; Xin, Y.; Saitow, K.-i. Cost-Effective Synthesis of Silicon Quantum Dots. Chem. Mater. 2020, 32, 8382–8392. [Google Scholar] [CrossRef]
- Zhang, Y.; Xiao, J.; Zhuo, P.; Yin, H.; Fan, Y.; Liu, X.; Chen, Z. Carbon Dots Exhibiting Concentration-Dependent Full-Visible-Spectrum Emission for Light-Emitting Diode Applications. ACS Appl. Mater. Interfaces 2019, 11, 46054–46061. [Google Scholar] [CrossRef] [PubMed]
- Reiss, P. ZnSe based colloidal nanocrystals: Synthesis, shape control, core/shell, alloy and doped systems. New J. Chem. 2007, 31, 1843–1852. [Google Scholar] [CrossRef]
- Wang, A.; Shen, H.; Zang, S.; Lin, Q.; Wang, H.; Qian, L.; Niu, J.; Song Li, L. Bright, efficient, and color-stable violet ZnSe-based quantum dot light-emitting diodes. Nanoscale 2015, 7, 2951–2959. [Google Scholar] [CrossRef]
- Murray, C.B.; Norris, D.J.; Bawendi, M.G. Synthesis and characterization of nearly monodisperse CdE (E = S, Se, Te) semiconductor nanocrystallites. J. Am. Chem. Soc. 1993, 115, 8706–8715. [Google Scholar] [CrossRef]
- Chen, H.S.; Wang, S.J.J.; Lo, C.J.; Chi, J.Y. White-light emission from organics-capped ZnSe quantum dots and application in white-light-emitting diodes. Appl. Phys. Lett. 2005, 86, 131905. [Google Scholar] [CrossRef] [Green Version]
- Li, L.S.; Pradhan, N.; Wang, Y.; Peng, X. High Quality ZnSe and ZnS Nanocrystals Formed by Activating Zinc Carboxylate Precursors. Nano Lett. 2004, 4, 2261–2264. [Google Scholar] [CrossRef]
- Kim, T.; Kim, K.-H.; Kim, S.; Choi, S.-M.; Jang, H.; Seo, H.-K.; Lee, H.; Chung, D.-Y.; Jang, E. Efficient and stable blue quantum dot light-emitting diode. Nature 2020, 586, 385–389. [Google Scholar] [CrossRef]
- Han, C.-Y.; Lee, S.-H.; Song, S.-W.; Yoon, S.-Y.; Jo, J.-H.; Jo, D.-Y.; Kim, H.-M.; Lee, B.-J.; Kim, H.-S.; Yang, H. More Than 9% Efficient ZnSeTe Quantum Dot-Based Blue Electroluminescent Devices. ACS Energy Lett. 2020, 5, 1568–1576. [Google Scholar] [CrossRef]
- Shen, H.; Wang, H.; Li, X.; Niu, J.Z.; Wang, H.; Chen, X.; Li, L.S. Phosphine-free synthesis of high quality ZnSe, ZnSe/ZnS, and Cu-, Mn-doped ZnSe nanocrystals. Dalton Trans. 2009, 10534–10540. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.-J.; Shen, X.-C.; Liang, H.; Chen, F.-Y.; Huang, H.-J. Phosphine-free synthesis of ZnSe:Mn and ZnSe:Mn/ZnS doped quantum dots using new Se and S precursors. New J. Chem. 2014, 38, 448–454. [Google Scholar] [CrossRef]
- Banski, M.; Afzaal, M.; Malik, M.A.; Podhorodecki, A.; Misiewicz, J.; O’Brien, P. Special Role for Zinc Stearate and Octadecene in the Synthesis of Luminescent ZnSe Nanocrystals. Chem. Mater. 2015, 27, 3797–3800. [Google Scholar] [CrossRef]
- Guidelli, E.J.; Lignos, I.; Yoo, J.J.; Lusardi, M.; Bawendi, M.G.; Baffa, O.; Jensen, K.F. Mechanistic Insights and Controlled Synthesis of Radioluminescent ZnSe Quantum Dots Using a Microfluidic Reactor. Chem. Mater. 2018, 30, 8562–8570. [Google Scholar] [CrossRef]
- Chen, H.-S.; Lo, B.; Hwang, J.-Y.; Chang, G.-Y.; Chen, C.-M.; Tasi, S.-J.; Wang, S.-J.J. Colloidal ZnSe, ZnSe/ZnS, and ZnSe/ZnSeS Quantum Dots Synthesized from ZnO. J. Phys. Chem. B 2004, 108, 17119–17123. [Google Scholar] [CrossRef]
- Jang, H.S.; Won, Y.H.; Jeon, D.Y. Improvement of electroluminescent property of blue LED coated with highly luminescent yellow-emitting phosphors. Appl. Phys. B 2009, 95, 715–720. [Google Scholar] [CrossRef]
- Li, F.; You, L.; Nie, C.; Zhang, Q.; Jin, X.; Li, H.; Gu, X.; Huang, Y.; Li, Q. Quantum dot white light emitting diodes with high scotopic/photopic ratios. Opt. Express 2017, 25, 21901–21913. [Google Scholar] [CrossRef]
- Boonsin, R.; Barros, A.; Donat, F.; Boyer, D.; Chadeyron, G.; Schneider, R.; Boutinaud, P.; Mahiou, R. Optical Properties and Reliability Studies of Gradient Alloyed Green Emitting (CdSe)x(ZnS)1–x and Red Emitting (CuInS2)x(ZnS)1–x Quantum Dots for White Light-Emitting Diodes. ACS Photon. 2018, 5, 462–470. [Google Scholar] [CrossRef]
- Jiang, T.; Shen, M.; Dai, P.; Wu, M.; Yu, X.; Li, G.; Xu, X.; Zeng, H. Cd-free Cu–Zn–In–S/ZnS quantum dots@SiO2 multiple cores nanostructure: Preparation and application for white LEDs. Nanotechnology 2017, 28, 435702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, S.H.; Hong, A.; Kim, J.-H.; Yang, H.; Lee, K.; Jang, H.S. Highly Bright Yellow-Green-Emitting CuInS2 Colloidal Quantum Dots with Core/Shell/Shell Architecture for White Light-Emitting Diodes. ACS Appl. Mater. Interfaces 2015, 7, 6764–6771. [Google Scholar] [CrossRef] [PubMed]
- Toufanian, R.; Zhong, X.; Kays, J.C.; Saeboe, A.M.; Dennis, A.M. Correlating ZnSe Quantum Dot Absorption with Particle Size and Concentration. Chem. Mater. 2021, 33, 7527–7536. [Google Scholar] [CrossRef]
- Reiss, P.; Protière, M.; Li, L. Core/Shell Semiconductor Nanocrystals. Small 2009, 5, 154–168. [Google Scholar] [CrossRef] [PubMed]
- Qu, L.; Peng, X. Control of Photoluminescence Properties of CdSe Nanocrystals in Growth. J. Am. Chem. Soc. 2002, 124, 2049–2055. [Google Scholar] [CrossRef]
- Fang, Z.; Li, Y.; Zhang, H.; Zhong, X.; Zhu, L. Facile Synthesis of Highly Luminescent UV-Blue-Emitting ZnSe/ZnS Core/Shell Nanocrystals in Aqueous Media. J. Phys. Chem. C 2009, 113, 14145–14150. [Google Scholar] [CrossRef]
- Wysezecki, G.; Stiles, W.S. Color Science: Concepts and Methods, Quantitative Data and Formulae; John Wiley & Sons, Inc.: New York, NY, USA, 1967. [Google Scholar]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Byambasuren, N.; Jo, J.; Ko, H.; Ju, B.-K.; Byun, J.Y.; Jang, H.S. Phosphine-Free-Synthesized ZnSe/ZnS Core/Shell Quantum Dots for White Light-Emitting Diodes. Appl. Sci. 2021, 11, 10060. https://doi.org/10.3390/app112110060
Byambasuren N, Jo J, Ko H, Ju B-K, Byun JY, Jang HS. Phosphine-Free-Synthesized ZnSe/ZnS Core/Shell Quantum Dots for White Light-Emitting Diodes. Applied Sciences. 2021; 11(21):10060. https://doi.org/10.3390/app112110060
Chicago/Turabian StyleByambasuren, Nyamsuren, Jiyeon Jo, Hyungduk Ko, Byeong-Kwon Ju, Ji Young Byun, and Ho Seong Jang. 2021. "Phosphine-Free-Synthesized ZnSe/ZnS Core/Shell Quantum Dots for White Light-Emitting Diodes" Applied Sciences 11, no. 21: 10060. https://doi.org/10.3390/app112110060





