Expediting Finite Element Analyses for Subject-Specific Studies of Knee Osteoarthritis: A Literature Review
Abstract
:1. Introduction
2. Development of Knee FEA
3. Knee FEA Workflow
3.1. Pre-Processing
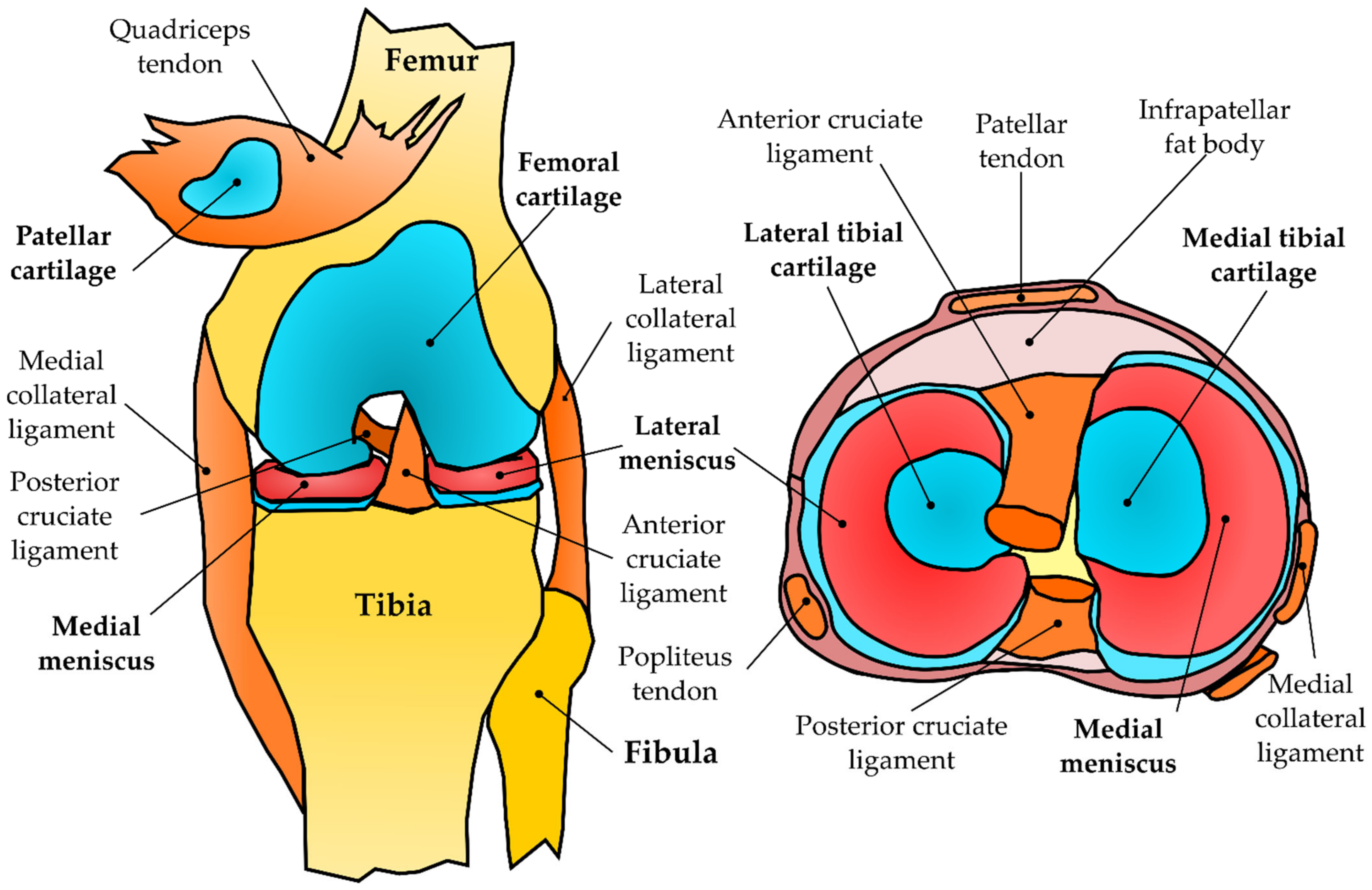
3.1.1. Geometry
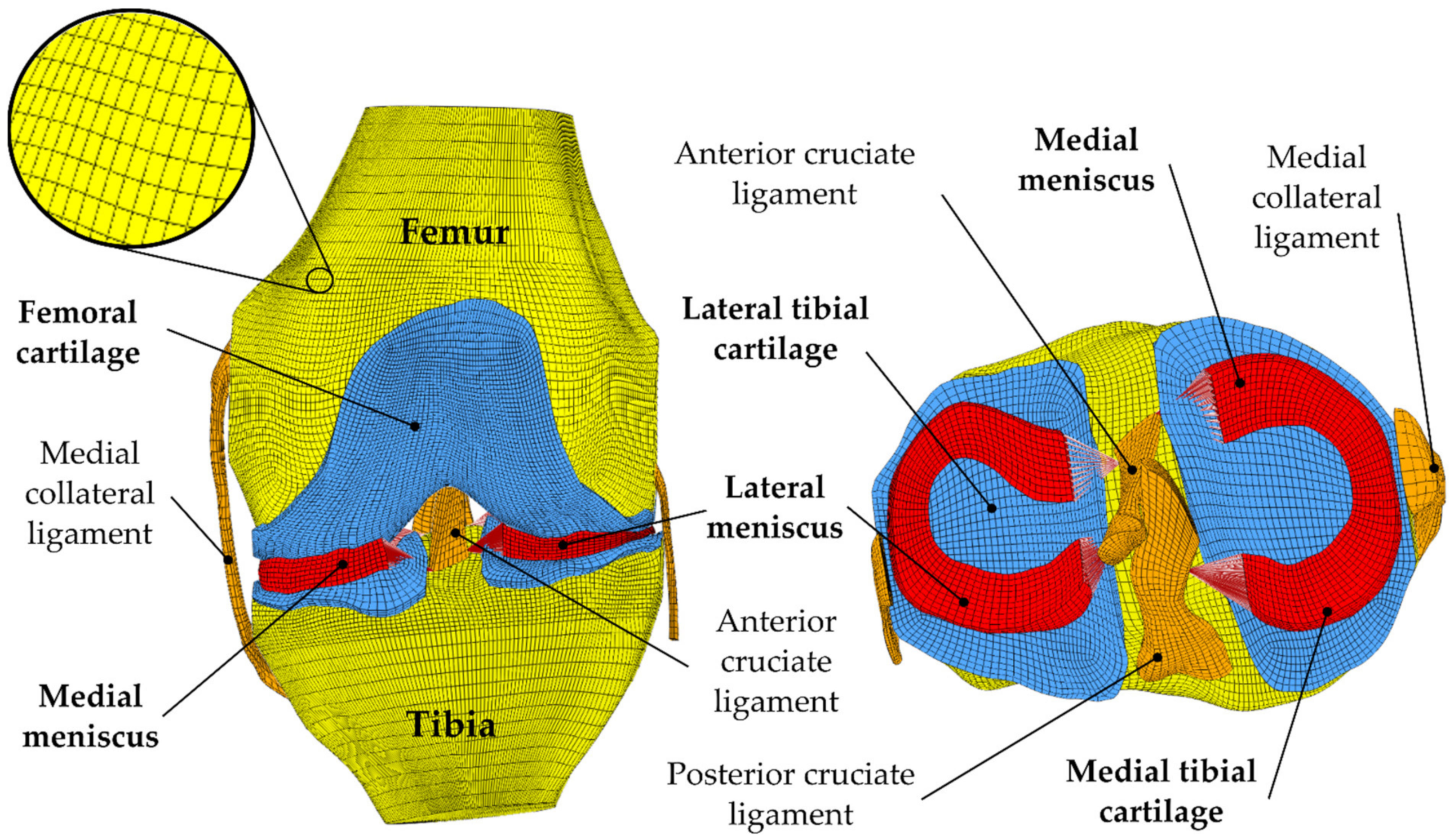
3.1.2. Mesh
3.1.3. Material Constitutive Models
3.1.4. Subject-Specific Motion and Loading
3.1.5. Model Configuration
3.2. Processing
3.3. Post-Processing
3.4. Onset and Progression of OA
3.5. Verification and Validation
4. Discussion
- Automate pre-processing tasks using a minimum amount of information (geometry generation and loading specification);
- Incorporate site-specific tissue composition and mechanical properties;
- Develop accurate algorithms for OA prognosis (theory and validation);
- Develop efficient multiscale modeling methods (from joint to tissue level);
- Combine advances in FEA, musculoskeletal modeling, and AI.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Harris, M.D.; Cyr, A.J.; Ali, A.A.; Fitzpatrick, C.K.; Rullkoetter, P.J.; Maletsky, L.P.; Shelburne, K.B. A Combined Experimental and Computational Approach to Subject-Specific Analysis of Knee Joint Laxity. J. Biomech. Eng. 2016, 138, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Dhaher, Y.Y.; Kwon, T.; Barry, M. The effect of connective tissue material uncertainties on knee joint mechanics under isolated loading conditions. J. Biomech. 2010, 43, 3118–3125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fregly, B.J.; Besier, T.F.; Lloyd, D.G.; Delp, S.L.; Banks, S.A.; Pandy, M.G.; D’Lima, D.D. Grand Challenge Competition to Predict In Vivo Knee Loads. J. Ortopaedic Res. 2014, 30, 503–513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ali, A.A.; Harris, M.D.; Shalhoub, S.; Maletsky, L.P.; Rullkoetter, P.J.; Shelburne, K.B. Combined measurement and modeling of specimen-specific knee mechanics for healthy and ACL-deficient conditions. J. Biomech. 2017, 57, 117–124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Litwic, A.; Edwards, M.H.; Dennison, E.M.; Cooper, C. Epidemiology and burden of osteoarthritis. Br. Med. Bull. 2013, 105, 185–199. [Google Scholar] [CrossRef] [Green Version]
- Gersing, A.S.; Schwaiger, B.J.; Nevitt, M.C.; Joseph, G.B.; Chanchek, N.; Guimaraes, J.B.; Wamba, J.M.; Facchetti, L.; McCulloch, C.E.; Link, T.M. Is weight loss associated with less progression of changes in knee articular cartilage among obese and overweight patients as assessed with MR imaging over 48 months? Data from the osteoarthritis initiative. Radiology 2017, 284, 508–520. [Google Scholar] [CrossRef]
- Neogi, T.; Zhang, Y. Epidemiology of Osteoarthritis. Rheum. Dis. Clin. N. Am. 2013, 39, 1–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vos, T.; Flaxman, A.D.; Naghavi, M.; Lozano, R.; Michaud, C.; Ezzati, M.; Shibuya, K.; Salomon, J.A.; Abdalla, S.; Aboyans, V.; et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2163–2196. [Google Scholar] [CrossRef]
- March, L.M.; Bachmeier, C.J.M. Economics of osteoarthritis: A global perspective. Baillieres. Clin. Rheumatol. 1997, 11, 817–834. [Google Scholar] [CrossRef]
- O’Neill, T.W.; McCabe, P.S.; McBeth, J. Update on the epidemiology, risk factors and disease outcomes of osteoarthritis. Best Pract. Res. Clin. Rheumatol. 2018, 32, 312–326. [Google Scholar] [CrossRef] [PubMed]
- United States Bone and Joint Initiative. The Burden of Musculoskeletal Diseases in the United States (BMUS), 3rd ed.; United States Bone and Joint Initiative: Rosemont, IL, USA, 2014; Available online: http://www.boneandjointburden.org (accessed on 5 February 2019).
- Cram, P.; Lu, X.; Kates, S.L.; Singh, J.; Li, Y.; Wolf, B.R. Total knee arthroplasty volume, utilization, and outcomes among Medicare beneficiaries, 1991–2010. JAMA 2012, 308, 1227–1236. [Google Scholar] [CrossRef]
- Englund, M. The role of biomechanics in the initiation and progression of OA of the knee. Best Pract. Res. Clin. Rheumatol. 2010, 24, 39–46. [Google Scholar] [CrossRef] [PubMed]
- DeFrate, L.E.E.; Kim-Wang, S.Y.Y.; Englander, Z.A.A.; McNulty, A.L.L. Osteoarthritis year in review 2018: Mechanics. Osteoarthr. Cartil. 2019, 27, 392–400. [Google Scholar] [CrossRef] [Green Version]
- Nieminen, M.T.; Casula, V.; Nevalainen, M.T.; Saarakkala, S. Osteoarthritis year in review 2018: Imaging. Osteoarthr. Cartil. 2019, 27, 401–411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allen, K.D.; Golightly, Y.M. Epidemiology of osteoarthritis: State of the evidence. Curr. Opin. Rheumatol. 2015, 27, 276–283. [Google Scholar] [CrossRef]
- Murphy, L.; Schwartz, T.A.; Helmick, C.G.; Renner, J.B.; Tudor, G.; Koch, G.; Dragomir, A.; Kalsbeek, W.D.; Luta, G.; Jordan, J.M. Lifetime risk of symptomatic knee osteoarthritis. Arthritis Care Res. 2008, 59, 1207–1213. [Google Scholar] [CrossRef]
- Segal, N.A.; Anderson, D.D.; Iyer, K.S.; Baker, J.; Torner, J.C.; Lynch, J.A.; Felson, D.T.; Lewis, C.E.; Brown, T.D. Baseline articular contact stress levels predict incident symptomatic knee osteoarthritis development in the MOST cohort. J. Orthop. Res. 2009, 27, 1562–1568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chan, L.C.; Li, H.H.T.; Chan, P.K.; Wen, C. A machine learning-based approach to decipher multi-etiology of knee osteoarthritis onset and deterioration. Osteoarthr. Cartil. Open 2021, 3, 100135. [Google Scholar] [CrossRef]
- Halilaj, E.; Le, Y.; Hicks, J.L.; Hastie, T.J.; Delp, S.L. Modeling and predicting osteoarthritis progression: Data from the osteoarthritis initiative. Osteoarthr. Cartil. 2018, 26, 1643–1650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Orozco, G.A.; Tanska, P.; Florea, C.; Grodzinsky, A.J.; Korhonen, R.K. A novel mechanobiological model can predict how physiologically relevant dynamic loading causes proteoglycan loss in mechanically injured articular cartilage. Sci. Rep. 2018, 8, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Klets, O.; Mononen, M.E.; Liukkonen, M.K.; Nevalainen, M.T.; Nieminen, M.T.; Saarakkala, S.; Korhonen, R.K. Estimation of the Effect of Body Weight on the Development of Osteoarthritis Based on Cumulative Stresses in Cartilage: Data from the Osteoarthritis Initiative. Ann. Biomed. Eng. 2018, 46, 334–344. [Google Scholar] [CrossRef] [Green Version]
- Park, S.; Lee, S.; Yoon, J.; Chae, S.W. Finite element analysis of knee and ankle joint during gait based on motion analysis. Med. Eng. Phys. 2019, 63, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Shu, L.; Yamamoto, K.; Yao, J.; Saraswat, P.; Liu, Y.; Mitsuishi, M.; Sugita, N. A subject-specific finite element musculoskeletal framework for mechanics analysis of a total knee replacement. J. Biomech. 2018, 77, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Mononen, M.E.; Tanska, P.; Isaksson, H.; Korhonen, R.K. New algorithm for simulation of proteoglycan loss and collagen degeneration in the knee joint: Data from the osteoarthritis initiative. J. Orthop. Res. 2018, 36, 1673–1683. [Google Scholar] [CrossRef] [PubMed]
- Mononen, M.E.; Liukkonen, M.K.; Korhonen, R.K. Utilizing Atlas-Based Modeling to Predict Knee Joint Cartilage Degeneration: Data from the Osteoarthritis Initiative. Ann. Biomed. Eng. 2019, 47, 813–825. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hosseini, S.M.; Wilson, W.; Ito, K.; Van Donkelaar, C.C. A numerical model to study mechanically induced initiation and progression of damage in articular cartilage. Osteoarthr. Cartil. 2014, 22, 95–103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stender, M.E.; Carpenter, R.D.; Regueiro, R.A.; Ferguson, V.L. An evolutionary model of osteoarthritis including articular cartilage damage, and bone remodeling in a computational study. J. Biomech. 2016, 49, 3502–3508. [Google Scholar] [CrossRef]
- Párraga Quiroga, J.M.; Wilson, W.; Ito, K.; van Donkelaar, C.C. The effect of loading rate on the development of early damage in articular cartilage. Biomech. Model. Mechanobiol. 2017, 16, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Cooper, R.J.; Wilcox, R.K.; Jones, A.C. Finite element models of the tibiofemoral joint: A review of validation approaches and modelling challenges. Med. Eng. Phys. 2019, 74, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Chandrupatla, T.R.; Belegundu, A.D. Introduction to Finite Elements in Engineering, 3rd ed.; Prentice Hall: Hoboken, NJ, USA, 2002; ISBN 0130615919. [Google Scholar]
- Bolcos, P.O.; Mononen, M.E.; Mohammadi, A.; Ebrahimi, M.; Tanaka, M.S.; Samaan, M.A.; Souza, R.B.; Li, X.; Suomalainen, J.S.; Jurvelin, J.S.; et al. Comparison between kinetic and kinetic-kinematic driven knee joint finite element models. Sci. Rep. 2018, 8, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodriguez-Vila, B.; Sánchez-González, P.; Oropesa, I.; Gomez, E.J.; Pierce, D.M. Automated hexahedral meshing of knee cartilage structures—Application to data from the osteoarthritis initiative. Comput. Methods Biomech. Biomed. Engin. 2017, 20, 1543–1553. [Google Scholar] [CrossRef] [PubMed]
- Roberts, G.L.; Pallister, I. Finite element analysis in trauma & orthopaedics—An introduction to clinically relevant simulation & its limitations. Orthop. Trauma 2012, 26, 410–416. [Google Scholar] [CrossRef]
- Henak, C.R.; Anderson, A.E.; Weiss, J.A. Subject-specific analysis of joint contact mechanics: Application to the study of osteoarthritis and surgical planning. J. Biomech. Eng. 2013, 135, 1–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wismans, J.; Veldpaus, F.; Jannsen, J. A three-dimensional mathematical model of the knee-joint. J. Biomech. 1980, 13, 677–685. [Google Scholar] [CrossRef] [Green Version]
- Andriacchi, T.P.; Mikosz, R.P.; Hampton, S.J.; Galante, J.O. Model studies of the stiffness characteristics of the human knee joint. J. Biomech. 1983, 16, 23–29. [Google Scholar] [CrossRef]
- Bendjaballah, M.Z.; Shirazi-Adl, A.; Zukor, D.J. Biomechanics of the human knee joint in compression: Reconstruction, mesh generation and finite element analysis. Knee 1995, 2, 69–79. [Google Scholar] [CrossRef]
- Mukherjee, S.; Nazemi, M.; Jonkers, I.; Geris, L. Use of Computational Modeling to Study Joint Degeneration: A Review. Front. Bioeng. Biotechnol. 2020, 8, 1–20. [Google Scholar] [CrossRef]
- Yang, N.H.; Canavan, P.K.; Nayeb-Hashemi, H.; Najafi, B.; Vaziri, A. Protocol for constructing subject-specific biomechanical models of knee joint. Comput. Methods Biomech. Biomed. Engin. 2010, 13, 589–603. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, D.; Roemer, F.W.; Guermazi, A. Imaging for osteoarthritis. Ann. Phys. Rehabil. Med. 2016, 59, 161–169. [Google Scholar] [CrossRef]
- Boswell, M.A.; Uhlrich, S.D.; Kidziński, T.K.; Kolesar, J.A.; Gold, G.E.; Beaupre, G.S.; Delp, S.L. A neural network to predict the knee adduction moment in patients with osteoarthritis using anatomical landmarks obtainable from 2D video analysis. Osteoarthr. Cartil. 2021, 29, 346–356. [Google Scholar] [CrossRef] [PubMed]
- Neubert, A.; Wilson, K.J.; Engstrom, C.; Surowiec, R.K.; Paproki, A.; Johnson, N.; Crozier, S.; Fripp, J.; Ho, C.P. Comparison of 3D bone models of the knee joint derived from CT and 3T MR imaging. Eur. J. Radiol. 2017, 93, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Ambellan, F.; Tack, A.; Ehlke, M.; Zachow, S. Automated Segmentation of Knee Bone and Cartilage combining Statistical Shape Knowledge and Convolutional Neural Networks: Data from the Osteoarthritis Initiative. Med. Image Anal. 2018, 52, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, M.A.; Langenderfer, J.E.; Rullkoetter, P.J.; Laz, P.J. Development of subject-specific and statistical shape models of the knee using an efficient segmentation and mesh-morphing approach. Comput. Methods Programs Biomed. 2010, 97, 232–240. [Google Scholar] [CrossRef]
- Tack, A.; Mukhopadhyay, A.; Zachow, S. Knee menisci segmentation using convolutional neural networks: Data from the Osteoarthritis Initiative. Osteoarthr. Cartil. 2018, 26, 680–688. [Google Scholar] [CrossRef] [PubMed]
- Anderson, D.D.; Segal, N.A.; Kern, A.M.; Nevitt, M.C.; Torner, J.C.; Lynch, J.A. Reliability of Semiautomated Computational Methods for Estimating Tibiofemoral Contact Stress in the Multicenter Osteoarthritis Study. Comput. Math. Methods Med. 2012, 2012, 8. [Google Scholar] [CrossRef] [PubMed]
- Das Neves Borges, P.; Forte, A.E.; Vincent, T.L.; Dini, D.; Marenzana, M. Rapid, automated imaging of mouse articular cartilage by microCT for early detection of osteoarthritis and finite element modelling of joint mechanics. Osteoarthr. Cartil. 2014, 22, 1419–1428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rao, C.; Fitzpatrick, C.K.; Rullkoetter, P.J.; Maletsky, L.P.; Kim, R.H.; Laz, P.J. A statistical finite element model of the knee accounting for shape and alignment variability. Med. Eng. Phys. 2013, 35, 1450–1456. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, A.; Myller, K.A.H.; Tanska, P.; Hirvasniemi, J.; Saarakkala, S.; Töyräs, J.; Korhonen, R.K.; Mononen, M.E. Rapid CT-based Estimation of Articular Cartilage Biomechanics in the Knee Joint Without Cartilage Segmentation. Ann. Biomed. Eng. 2020, 48, 2965–2975. [Google Scholar] [CrossRef]
- Ebrahimkhani, S.; Jaward, M.H.; Cicuttini, F.M.; Dharmaratne, A.; Wang, Y.; de Herrera, A.G.S. A review on segmentation of knee articular cartilage: From conventional methods towards deep learning. Artif. Intell. Med. 2020, 106, 101851. [Google Scholar] [CrossRef]
- Paproki, A.; Engstrom, C.; Chandra, S.S.; Neubert, A.; Fripp, J.; Crozier, S. Automated segmentation and analysis of normal and osteoarthritic knee menisci from magnetic resonance images—Data from the Osteoarthritis Initiative. Osteoarthr. Cartil. 2014, 22, 1259–1270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Viceconti, M.; Bellingeri, L.; Cristofolini, L.; Toni, A. A comparative study on different methods of automatic mesh generation of human femurs. Med. Eng. Phys. 1998, 20, 1–10. [Google Scholar] [CrossRef]
- Ramos, A.; Simões, J.A. Tetrahedral versus hexahedral finite elements in numerical modelling of the proximal femur. Med. Eng. Phys. 2006, 28, 916–924. [Google Scholar] [CrossRef]
- McErlain, D.D.; Milner, J.S.; Ivanov, T.G.; Jencikova-Celerin, L.; Pollmann, S.I.; Holdsworth, D.W. Subchondral cysts create increased intra-osseous stress in early knee OA: A finite element analysis using simulated lesions. Bone 2011, 48, 639–646. [Google Scholar] [CrossRef] [PubMed]
- Tadepalli, S.C.; Erdemir, A.; Cavanagh, P.R. Comparison of hexahedral and tetrahedral elements in finite element analysis of the foot and footwear. J. Biomech. 2011, 44, 2337–2343. [Google Scholar] [CrossRef] [PubMed]
- Maas, S.A.; Ellis, B.J.; Rawlins, D.S.; Weiss, J.A. Finite element simulation of articular contact mechanics with quadratic tetrahedral elements. J. Biomech. 2016, 49, 659–667. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Erdemir, A. Open Knee: Open Source Modeling & Simulation to Enable Scientific Discovery and Clinical Care in Knee Biomechanics. J. Knee Surg. 2016, 29, 107–116. [Google Scholar] [CrossRef] [Green Version]
- Wilson, W.; Van Donkelaar, C.C.; Van Rietbergen, B.; Ito, K.; Huiskes, R. Stresses in the local collagen network of articular cartilage: A poroviscoelastic fibril-reinforced finite element study. J. Biomech. 2004, 37, 357–366. [Google Scholar] [CrossRef]
- Klets, O.; Mononen, M.E.; Tanska, P.; Nieminen, M.T.; Korhonen, R.K.; Saarakkala, S. Comparison of different material models of articular cartilage in 3D computational modeling of the knee: Data from the Osteoarthritis Initiative (OAI). J. Biomech. 2016, 49, 3891–3900. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- García, J.J.; Cortés, D.H. A biphasic viscohyperelastic fibril-reinforced model for articular cartilage: Formulation and comparison with experimental data. J. Biomech. 2007, 40, 1737–1744. [Google Scholar] [CrossRef]
- Ateshian, G.A.; Rajan, V.; Chahine, N.O.; Canal, C.E.; Hung, C.T. Modeling the Matrix of Articular Cartilage Using a Continuous Fiber Angular Distribution Predicts Many Observed Phenomena. J. Biomech. Eng. 2009, 131, 612–615. [Google Scholar] [CrossRef]
- Peters, A.E.; Akhtar, R.; Comerford, E.J.; Bates, K.T. Tissue material properties and computational modelling of the human tibiofemoral joint: A critical review. PeerJ 2018, 6, 1–48. [Google Scholar] [CrossRef] [Green Version]
- Shirazi, R.; Shirazi-Adl, A.; Hurtig, M. Role of cartilage collagen fibrils networks in knee joint biomechanics under compression. J. Biomech. 2008, 41, 3340–3348. [Google Scholar] [CrossRef] [PubMed]
- Keenan, K.E.; Pal, S.; Lindsey, D.P.; Besier, T.F.; Beaupre, G.S. A viscoelastic constitutive model can accurately represent entire creep indentation tests of human patella cartilage. J. Appl. Biomech. 2013, 29, 292–302. [Google Scholar] [CrossRef] [Green Version]
- Thomas, G.C.; Asanbaeva, A.; Vena, P.; Sah, R.L.; Klisch, S.M. A nonlinear constituent based viscoelastic model for articular cartilage and analysis of tissue remodeling due to altered glycosaminoglycan-collagen interactions. J. Biomech. Eng. 2009, 131, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Soltz, M.A.; Ateshian, G.A. A conewise linear elasticity mixture model for the analysis of tension-compression nonlinearity in articular cartilage. J. Biomech. Eng. 2000, 122, 576–586. [Google Scholar] [CrossRef] [Green Version]
- Soltz, M.A.; Ateshian, G.A. Experimental verification and theoretical prediction of cartilage interstitial fluid pressurization at an impermeable contact interface in confined compression. J. Biomech. 1998, 31, 927–934. [Google Scholar] [CrossRef]
- Biot, M.A. General theory of three-dimensional consolidation. J. Appl. Phys. 1941, 12, 155–164. [Google Scholar] [CrossRef]
- Mow, V.C.; Kuei, S.C.; Lai, W.M.; Armstrong, C.G. Biphasic creep and stress relaxation of articular cartilage in compression: Theory and experiments. J. Biomech. Eng. 1980, 102, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Holmes, M.H.; Mow, V.C. The nonlinear characteristics of soft gels and hydrated connective tissues in ultrafiltration. J. Biomech. 1990, 23, 1145–1156. [Google Scholar] [CrossRef]
- Lai, W.M.; Mow, V.C. Drag-induced compression of articular cartilage during a permeation experiment. Biorheology 1980, 17, 111–123. [Google Scholar] [CrossRef] [PubMed]
- Simon, B.R. Multiphase poroelastic finite element models for soft tissue structures. Appl. Mech. Rev. 1992, 45, 191–218. [Google Scholar] [CrossRef]
- DiSilvestro, M.R.; Suh, J.K.F. A cross-validation of the biphasic poroviscoelastic model of articular cartilage in unconfined compression, indentation, and confined compression. J. Biomech. 2001, 34, 519–525. [Google Scholar] [CrossRef]
- García, J.J.; Cortés, D.H. A nonlinear biphasic viscohyperelastic model for articular cartilage. J. Biomech. 2006, 39, 2991–2998. [Google Scholar] [CrossRef] [PubMed]
- Suh, J.K.; Bai, S. Finite element formulation of biphasic poroviscoelastic model for articular cartilage. J. Biomech. Eng. 1998, 120, 195–201. [Google Scholar] [CrossRef]
- Mak, A.F. The apparent viscoelastic behavior of articular cartilage–the contributions from the intrinsic matrix viscoelasticity and interstitial fluid flows. J. Biomech. Eng. 1986, 108, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Pierce, D.M.; Unterberger, M.J.; Trobin, W.; Ricken, T.; Holzapfel, G.A. A microstructurally based continuum model of cartilage viscoelasticity and permeability incorporating measured statistical fiber orientations. Biomech. Model. Mechanobiol. 2016, 15, 229–244. [Google Scholar] [CrossRef]
- Pierce, D.M.; Ricken, T.; Holzapfel, G.A. A hyperelastic biphasic fibre-reinforced model of articular cartilage considering distributed collagen fibre orientations: Continuum basis, computational aspects and applications. Comput. Methods Biomech. Biomed. Engin. 2013, 16, 1344–1361. [Google Scholar] [CrossRef] [PubMed]
- Julkunen, P.; Kiviranta, P.; Wilson, W.; Jurvelin, J.S.; Korhonen, R.K. Characterization of articular cartilage by combining microscopic analysis with a fibril-reinforced finite-element model. J. Biomech. 2007, 40, 1862–1870. [Google Scholar] [CrossRef] [PubMed]
- Wilson, W.; Van Donkelaar, C.C.; Huyghe, J.M. A comparison between mechano-electrochemical and biphasic swelling theories for soft hydrated tissues. J. Biomech. Eng. 2005, 127, 158–165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilson, W.; Van Donkelaar, C.C.; Van Rietbergen, B.; Huiskes, R. A fibril-reinforced poroviscoelastic swelling model for articular cartilage. J. Biomech. 2005, 38, 1195–1204. [Google Scholar] [CrossRef] [PubMed]
- Huyghe, J.M.; Jannsen, J. Quadriphasic mechanics of swelling incompressible porous media. Int. J. Eng. Sci. 1997, 35, 793–802. [Google Scholar] [CrossRef] [Green Version]
- Lai, W.M.; Hou, J.S.; Mow, V.C. A triphasic theory for the swelling and deformation behaviors of articular cartilage. J. Biomech. Eng. 1991, 113, 245–258. [Google Scholar] [CrossRef]
- Linka, K.; Schäfer, A.; Hillgärtner, M.; Itskov, M.; Knobe, M.; Kuhl, C.; Hitpass, L.; Truhn, D.; Thuering, J.; Nebelung, S. Towards Patient-Specific Computational Modelling of Articular Cartilage on the Basis of Advanced Multiparametric MRI Techniques. Sci. Rep. 2019, 9, 1–13. [Google Scholar] [CrossRef]
- Räsänen, L.P.; Tanska, P.; Mononen, M.E.; Lammentausta, E.; Zbýň, Š.; Venäläinen, M.S.; Szomolanyi, P.; van Donkelaar, C.C.; Jurvelin, J.S.; Trattnig, S.; et al. Spatial variation of fixed charge density in knee joint cartilage from sodium MRI—Implication on knee joint mechanics under static loading. J. Biomech. 2016, 49, 3387–3396. [Google Scholar] [CrossRef] [PubMed]
- Räsänen, L.P.; Tanska, P.; Zbýň, Š.; van Donkelaar, C.C.; Trattnig, S.; Nieminen, M.T.; Korhonen, R.K. The effect of fixed charge density and cartilage swelling on mechanics of knee joint cartilage during simulated gait. J. Biomech. 2017, 61, 34–44. [Google Scholar] [CrossRef] [Green Version]
- Park, J.Y.; Kim, J.K.; Cheon, J.E.; Lee, M.C.; Han, H.S. Meniscus Stiffness Measured with Shear Wave Elastography is Correlated with Meniscus Degeneration. Ultrasound Med. Biol. 2020, 46, 297–304. [Google Scholar] [CrossRef]
- Adam, C.; Eckstein, F.; Milz, S.; Schulte, E.; Becker, C.; Putz, R. The distribution of cartilage thickness in the knee-joints of old-aged individuals measurement by A-mode ultrasound. Clin. Biomech. 1998, 13, 1–10. [Google Scholar] [CrossRef]
- Liukkonen, J.; Lehenkari, P.; Hirvasniemi, J.; Joukainen, A.; Virén, T.; Saarakkala, S.; Nieminen, M.T.; Jurvelin, J.S.; Töyräs, J. Ultrasound Arthroscopy of Human Knee Cartilage and Subchondral Bone in vivo. Ultrasound Med. Biol. 2014, 40, 2039–2047. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, R.J.; Wang, H.M.; Polprasert, D.R.; Kraft, R.A.; Pietrosimone, B.G. Evaluation of knee cartilage thickness: A comparison between ultrasound and magnetic resonance imaging methods. Knee 2017, 24, 217–223. [Google Scholar] [CrossRef]
- Hatami-Marbini, H.; Maulik, R. A Biphasic Transversely Isotropic Poroviscoelastic Model for the Unconfined Compression of Hydrated Soft Tissue. J. Biomech. Eng. 2016, 138, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Cohen, B.; Lai, W.M.; Mow, V.C. A transversely isotropic biphasic model for unconfined compression of growth plate and chondroepiphysis. J. Biomech. Eng. 1998, 120, 491–496. [Google Scholar] [CrossRef] [PubMed]
- Garcia, J.J.; Altiero, N.J.; Haut, R.C. An approach for the stress analysis of transversely isotropic biphasic cartilage under impact load. J. Biomech. Eng. 1998, 120, 608–613. [Google Scholar] [CrossRef] [PubMed]
- Bolcos, P.O.; Mononen, M.E.; Tanaka, M.S.; Yang, M.; Suomalainen, J.-S.; Nissi, M.J.; Töyräs, J.; Ma, B.; Li, X.; Korhonen, R.K. Identification of locations susceptible to osteoarthritis in patients with anterior cruciate ligament reconstruction: Combining knee joint computational modelling with follow-up T1ρ and T2 imaging. Clin. Biomech. 2020, 79, 104844. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vaziri, A.; Nayeb-Hashemi, H.; Singh, A.; Tafti, B.A. Influence of meniscectomy and meniscus replacement on the stress distribution in human knee joint. Ann. Biomed. Eng. 2008, 36, 1335–1344. [Google Scholar] [CrossRef]
- Danso, E.K.; Honkanen, J.T.J.; Saarakkala, S.; Korhonen, R.K. Comparison of nonlinear mechanical properties of bovine articular cartilage and meniscus. J. Biomech. 2014, 47, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Zielinska, B.; Haut Donahue, T.L. 3D finite element model of meniscectomy: Changes in joint contact behavior. J. Biomech. Eng. 2006, 128, 115–123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Párraga-Quiroga, J.M.; Emans, P.; Wilson, W.; Ito, K.; Donkelaar, C.C. Van Should a native depth-dependent distribution of human meniscus constitutive components be considered in FEA-models of the knee joint? J. Mech. Behav. Biomed. Mater. 2014, 38, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Guess, T.M.; Razu, S.; Jahandar, H.; Stylianou, A. Predicted loading on the menisci during gait: The effect of horn laxity. J. Biomech. 2015, 48, 1490–1498. [Google Scholar] [CrossRef] [Green Version]
- Guess, T.M.; Thiagarajan, G.; Kia, M.; Mishra, M. A subject specific multibody model of the knee with menisci. Med. Eng. Phys. 2010, 32, 505–515. [Google Scholar] [CrossRef] [PubMed]
- Peña, E.; Peña, J.A.; Doblaré, M. On modelling nonlinear viscoelastic effects in ligaments. J. Biomech. 2008, 41, 2659–2666. [Google Scholar] [CrossRef]
- Peña, E.; Calvo, B.; Martínez, M.A.; Doblaré, M. An anisotropic visco-hyperelastic model for ligaments at finite strains. Formulation and computational aspects. Int. J. Solids Struct. 2007, 44, 760–778. [Google Scholar] [CrossRef] [Green Version]
- Freutel, M.; Schmidt, H.; Dürselen, L.; Ignatius, A.; Galbusera, F. Finite element modeling of soft tissues: Material models, tissue interaction and challenges. Clin. Biomech. 2014, 29, 363–372. [Google Scholar] [CrossRef] [PubMed]
- Peña, E.; Calvo, B.; Martínez, M.A.; Doblaré, M. A three-dimensional finite element analysis of the combined behavior of ligaments and menisci in the healthy human knee joint. J. Biomech. 2006, 39, 1686–1701. [Google Scholar] [CrossRef] [PubMed]
- Orozco, G.A.; Tanska, P.; Mononen, M.E.; Halonen, K.S.; Korhonen, R.K. The effect of constitutive representations and structural constituents of ligaments on knee joint mechanics. Sci. Rep. 2018, 8, 1–15. [Google Scholar] [CrossRef]
- Venäläinen, M.S.; Mononen, M.E.; Väänänen, S.P.; Jurvelin, J.S.; Töyräs, J.; Virén, T.; Korhonen, R.K. Effect of bone inhomogeneity on tibiofemoral contact mechanics during physiological loading. J. Biomech. 2016, 49, 1111–1120. [Google Scholar] [CrossRef] [PubMed]
- Stender, M.E.; Regueiro, R.A.; Ferguson, V.L. A poroelastic finite element model of the bone–cartilage unit to determine the effects of changes in permeability with osteoarthritis. Comput. Methods Biomech. Biomed. Engin. 2017, 20, 319–331. [Google Scholar] [CrossRef]
- Donahue, T.L.H.; Hull, M.L.; Rashid, M.M.; Jacobs, C.R. A finite element model of the human knee joint for the study of tibio-femoral contact. J. Biomech. Eng. 2002, 124, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Anwar, A.; Hu, Z.; Zhang, Y.; Gao, Y.; Tian, C.; Wang, X. Multiple Subchondral Bone Cysts Cause Deterioration of Articular Cartilage in Medial OA of Knee: A 3D Simulation Study. Front. Bioeng. Biotechnol. 2020, 8, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.A.; Shalhoub, S.S.; Cyr, A.J.; Fitzpatrick, C.K.; Maletsky, L.P.; Rullkoetter, P.J.; Shelburne, K.B. Validation of predicted patellofemoral mechanics in a finite element model of the healthy and cruciate-deficient knee. J. Biomech. 2016, 49, 302–309. [Google Scholar] [CrossRef] [Green Version]
- Richards, R.E.; Andersen, M.S.; Harlaar, J.; Noort, J.C. Van Den Relationship between knee joint contact forces and external knee joint moments in patients with medial knee osteoarthritis: Effects of gait modi fi cations. Osteoarthr. Cartil. 2018, 26, 1203–1214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marouane, H.; Shirazi-Adl, A.; Adouni, M. Alterations in knee contact forces and centers in stance phase of gait: A detailed lower extremity musculoskeletal model. J. Biomech. 2016, 49, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Adouni, M.; Shirazi-Adl, A. Evaluation of knee joint muscle forces and tissue stresses-strains during gait in severe OA versus normal subjects. J. Orthop. Res. 2014, 32, 69–78. [Google Scholar] [CrossRef]
- Besier, T.F.; Gold, G.E.; Delp, S.L.; Fredericson, M.; Beaupré, G.S. The influence of femoral internal and external rotation on cartilage stresses within the patellofemoral joint. J. Orthop. Res. 2008, 26, 1627–1635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Delp, S.L.; Anderson, F.C.; Arnold, A.S.; Loan, P.; Habib, A.; John, C.T.; Guendelman, E.; Thelen, D.G. OpenSim: Open-source software to create and analyze dynamic simulations of movement. IEEE Trans. Biomed. Eng. 2007, 54, 1940–1950. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burton, W.S.; Myers, C.A.; Rullkoetter, P.J. Machine learning for rapid estimation of lower extremity muscle and joint loading during activities of daily living. J. Biomech. 2021, 123, 110439. [Google Scholar] [CrossRef] [PubMed]
- Halilaj, E.; Rajagopal, A.; Fiterau, M.; Hicks, J.L.; Hastie, T.J.; Delp, S.L. Machine learning in human movement biomechanics: Best practices, common pitfalls, and new opportunities. J. Biomech. 2018, 81, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Ardestani, M.M.; Chen, Z.; Wang, L.; Lian, Q.; Liu, Y.; He, J.; Li, D.; Jin, Z. A neural network approach for determining gait modifications to reduce the contact force in knee joint implant. Med. Eng. Phys. 2014, 36, 1253–1265. [Google Scholar] [CrossRef]
- Ardestani, M.M.; Chen, Z.; Wang, L.; Lian, Q.; Liu, Y.; He, J.; Li, D.; Jin, Z. Feed forward artificial neural network to predict contact force at medial knee joint: Application to gait modification. Neurocomputing 2014, 139, 114–129. [Google Scholar] [CrossRef]
- Kokkotis, C.; Moustakidis, S.; Papageorgiou, E.; Giakas, G.; Tsaopoulos, D.E. Machine learning in knee osteoarthritis: A review. Osteoarthr. Cartil. Open 2020, 2, 100069. [Google Scholar] [CrossRef]
- Mononen, M.E.; Tanska, P.; Isaksson, H.; Korhonen, R.K. A novel method to simulate the progression of collagen degeneration of cartilage in the knee: Data from the osteoarthritis initiative. Sci. Rep. 2016, 6, 1–14. [Google Scholar] [CrossRef] [Green Version]
- De Brabandere, A.; Emmerzaal, J.; Timmermans, A.; Jonkers, I.; Vanwanseele, B.; Davis, J. A Machine Learning Approach to Estimate Hip and Knee Joint Loading Using a Mobile Phone-Embedded IMU. Front. Bioeng. Biotechnol. 2020, 8, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fitzpatrick, C.K.; Rullkoetter, P.J. Estimating total knee replacement joint load ratios from kinematics. J. Biomech. 2014, 47, 3003–3011. [Google Scholar] [CrossRef] [PubMed]
- Hume, D.R.; Navacchia, A.; Ali, A.A.; Shelburne, K.B. The interaction of muscle moment arm, knee laxity, and torque in a multi-scale musculoskeletal model of the lower limb. J. Biomech. 2018, 76, 173–180. [Google Scholar] [CrossRef]
- Rooks, N.B.; Schneider, M.T.Y.; Erdemir, A.; Halloran, J.P.; Laz, P.J.; Shelburne, K.B.; Hume, D.R.; Imhauser, C.W.; Zaylor, W.; Elmasry, S.; et al. Deciphering the “art” in Modeling and Simulation of the Knee Joint: Variations in Model Development. J. Biomech. Eng. 2021, 143, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Navacchia, A.; Hume, D.R.; Rullkoetter, P.J.; Shelburne, K.B. A computationally efficient strategy to estimate muscle forces in a finite element musculoskeletal model of the lower limb. J. Biomech. 2019, 84, 94–102. [Google Scholar] [CrossRef]
- Shelburne, K.B.; Torry, M.R.; Pandy, M.G. Muscle, ligament, and joint-contact forces at the knee during walking. Med. Sci. Sports Exerc. 2005, 37, 1948–1956. [Google Scholar] [CrossRef] [Green Version]
- Tanska, P.; Mononen, M.E.; Korhonen, R.K. A multi-scale finite element model for investigation of chondrocyte mechanics in normal and medial meniscectomy human knee joint during walking. J. Biomech. 2015, 48, 1397–1406. [Google Scholar] [CrossRef] [PubMed]
- Esrafilian, A.; Stenroth, L.; Mononen, M.E.; Tanska, P.; Avela, J.; Korhonen, R.K. EMG-Assisted Muscle Force Driven Finite Element Model of the Knee Joint with Fibril-Reinforced Poroelastic Cartilages and Menisci. Sci. Rep. 2020, 10, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Halloran, J.P.; Sibole, S.; Van Donkelaar, C.C.; Van Turnhout, M.C.; Oomens, C.W.J.; Weiss, J.A.; Guilak, F.; Erdemir, A. Multiscale mechanics of articular cartilage: Potentials and challenges of coupling musculoskeletal, joint, and microscale computational models. Ann. Biomed. Eng. 2012, 40, 2456–2474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guilak, F.; Mow, V.C. The mechanical environment of the chondrocyte: A biphasic finite element model of cell-matrix interactions in articular cartilage. J. Biomech. 2000, 33, 1663–1673. [Google Scholar] [CrossRef]
- Fitzpatrick, C.K.; Baldwin, M.A.; Rullkoetter, P.J. Computationally efficient finite element evaluation of natural patellofemoral mechanics. J. Biomech. Eng. 2010, 132, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Maas, S.A.; Ateshian, G.A.; Weiss, J.A. FEBio: History and Advances. Annu. Rev. Biomed. Eng. 2017, 19, 279–299. [Google Scholar] [CrossRef]
- Maas, S.A.; Ellis, B.J.; Ateshian, G.A.; Weiss, J.A. FEBio: Finite elements for biomechanics. J. Biomech. Eng. 2012, 134, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Erdemir, A.; Guess, T.M.; Halloran, J.; Tadepalli, S.C.; Morrison, T.M. Considerations for reporting finite element analysis studies in biomechanics. J. Biomech. 2012, 45, 625–633. [Google Scholar] [CrossRef] [Green Version]
- Yao, J.; Salo, A.D.; Lee, J.; Lerner, A.L. Sensitivity of tibio-menisco-femoral joint contact behavior to variations in knee kinematics. J. Biomech. 2008, 41, 390–398. [Google Scholar] [CrossRef] [PubMed]
- Haut Donahue, T.L.; Hull, M.L.; Rashid, M.M.; Jacobs, C.R. How the stiffness of meniscal attachments and meniscal material properties affect tibio-femoral contact pressure computed using a validated finite element model of the human knee joint. J. Biomech. 2003, 36, 19–34. [Google Scholar] [CrossRef]
- Peña, E.; Calvo, B.; Martínez, M.A.; Doblaré, M. Effect of the size and location of osteochondral defects in degenerative arthritis. A finite element simulation. Comput. Biol. Med. 2007, 37, 376–387. [Google Scholar] [CrossRef]
- Kempson, G.E. Relationship between the tensile properties of articular cartilage from the human knee and age. Ann. Rheum. Dis. 1982, 41, 508–511. [Google Scholar] [CrossRef] [Green Version]
- Calce, S.E.; Kurki, H.K.; Weston, D.A.; Gould, L. The relationship of age, activity, and body size on osteoarthritis in weight-bearing skeletal regions. Int. J. Paleopathol. 2018, 22, 45–53. [Google Scholar] [CrossRef]
- Whittaker, J.L.; Woodhouse, L.J.; Nettel-Aguirre, A.; Emery, C.A. Outcomes associated with early post-traumatic osteoarthritis and other negative health consequences 3-10 years following knee joint injury in youth sport. Osteoarthr. Cartil. 2015, 23, 1122–1129. [Google Scholar] [CrossRef] [Green Version]
- Korhonen, R.K.; Laasanen, M.S.; Töyräs, J.; Lappalainen, R.; Helminen, H.J.; Jurvelin, J.S. Fibril reinforced poroelastic model predicts specifically mechanical behavior of normal, proteoglycan depleted and collagen degraded articular cartilage. J. Biomech. 2003, 36, 1373–1379. [Google Scholar] [CrossRef]
- DiSilvestro, M.R.; Francis Suh, J.K. Biphasic poroviscoelastic characteristics of proteoglycan-depleted articular cartilage: Simulation of degeneration. Ann. Biomed. Eng. 2002, 30, 792–800. [Google Scholar] [CrossRef] [PubMed]
- Korhonen, R.K.; Laasanen, M.S.; Töyräs, J.; Rieppob, J.; Hirvonen, J.; Helminen, H.J.; Jurvelin, J.S. Comparison of the equilibrium response of articular cartilage in unconfined compression, confined compression and indentation. J. Biomech. 2002, 35, 903–909. [Google Scholar] [CrossRef]
- Orozco, G.A.; Bolcos, P.; Mohammadi, A.; Tanaka, M.S.; Yang, M.; Link, T.M.; Ma, B.; Li, X.; Tanska, P.; Korhonen, R.K. Prediction of local fixed charge density loss in cartilage following ACL injury and reconstruction: A computational proof-of-concept study with MRI follow-up. J. Orthop. Res. 2021, 39, 1064–1081. [Google Scholar] [CrossRef]
- Eskelinen, A.S.A.; Tanska, P.; Florea, C.; Orozco, G.A.; Julkunen, P.; Grodzinsky, A.J.; Korhonen, R.K. Mechanobiological model for simulation of injured cartilage degradation via proinflammatory cytokines and mechanical. PLoS Comput. Biol. 2020, 16, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Shim, V.B.; Hunter, P.J.; Pivonka, P.; Fernandez, J.W. A multiscale framework based on the Physiome markup languages for exploring the initiation of osteoarthritis at the bone-cartilage interface. IEEE Trans. Biomed. Eng. 2011, 58, 3532–3536. [Google Scholar] [CrossRef]
- Andriacchi, T.P.; Briant, P.L.; Bevill, S.L.; Koo, S. Rotational Changes at the Knee after ACL Injury Cause Cartilage Thinning. Clin. Orthop. Relat. Res. 2006, 442, 39–44. [Google Scholar] [CrossRef]
- Mononen, M.E.; Julkunen, P.; Töyräs, J.; Jurvelin, J.S.; Kiviranta, I.; Korhonen, R.K. Alterations in structure and properties of collagen network of osteoarthritic and repaired cartilage modify knee joint stresses. Biomech. Model. Mechanobiol. 2011, 10, 357–369. [Google Scholar] [CrossRef] [PubMed]
- Mononen, M.E.; Mikkola, M.T.; Julkunen, P.; Ojala, R.; Nieminen, M.T.; Jurvelin, J.S.; Korhonen, R.K. Effect of superficial collagen patterns and fibrillation of femoral articular cartilage on knee joint mechanics—A 3D finite element analysis. J. Biomech. 2012, 45, 579–587. [Google Scholar] [CrossRef]
- Liukkonen, M.K.; Mononen, M.E.; Klets, O.; Arokoski, J.P.; Saarakkala, S.; Korhonen, R.K. Simulation of subject-specific progression of knee osteoarthritis and comparison to experimental follow-up data: Data from the osteoarthritis initiative. Sci. Rep. 2017, 7, 1–14. [Google Scholar] [CrossRef]
- Elahi, S.A.; Tanska, P.; Mukherjee, S.; Korhonen, R.K.; Geris, L.; Jonkers, I.; Famaey, N. Guide to mechanical characterization of articular cartilage and hydrogel constructs based on a systematic in silico parameter sensitivity analysis. J. Mech. Behav. Biomed. Mater. 2021, 124, 104795. [Google Scholar] [CrossRef] [PubMed]
- LeRoux, M.A.; Setton, L.A. Experimental and biphasic FEM determinations of the material properties and hydraulic permeability of the meniscus in tension. J. Biomech. Eng. 2002, 124, 315–321. [Google Scholar] [CrossRef]
- Wang, H.; Koff, M.F.; Potter, H.G.; Warren, R.F.; Rodeo, S.A.; Maher, S.A. An MRI-compatible loading device to assess knee joint cartilage deformation: Effect of preloading and inter-test repeatability. J. Biomech. 2015, 48, 2934–2940. [Google Scholar] [CrossRef] [PubMed]
- Mootanah, R.; Imhauser, C.W.; Reisse, F.; Carpanen, D.; Walker, R.W.; Koff, M.F.; Lenhoff, M.W.; Rozbruch, S.R.; Fragomen, A.T.; Dewan, Z.; et al. Development and validation of a computational model of the knee joint for the evaluation of surgical treatments for osteoarthritis. Comput. Methods Biomech. Biomed. Engin. 2014, 17, 1502–1517. [Google Scholar] [CrossRef] [Green Version]
- Halonen, K.S.; Mononen, M.E.; Jurvelin, J.S.; To, J. Deformation of articular cartilage during static loading of a knee joint—Experimental and finite element analysis. J. Biomech. 2014, 47, 2467–2474. [Google Scholar] [CrossRef] [PubMed]
- Chan, D.D.; Cai, L.; Butz, K.D.; Trippel, S.B.; Nauman, E.A.; Neu, C.P. In vivo articular cartilage deformation: Noninvasive quantification of intratissue strain during joint contact in the human knee. Sci. Rep. 2016, 6, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adams, J.G.; McAlindon, T.; Dimasi, M.; Carey, J.; Eustace, S. Contribution of meniscal extrusion and cartilage loss to joint space narrowing in osteoarthritis. Clin. Radiol. 1999, 54, 502–506. [Google Scholar] [CrossRef]
- Gale, D.R.; Chaisson, C.E.; Totterman, S.M.S.; Schwartz, R.K.; Gale, M.E.; Felson, D. Meniscal subluxation: Association with osteoarthritis and joint space narrowing. Osteoarthr. Cartil. 1999, 7, 526–532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Besier, T.F.; Gold, G.E.; Beaupré, G.S.; Delp, S.L. A modeling framework to estimate patellofemoral joint cartilage stress in vivo. Med. Sci. Sports Exerc. 2005, 37, 1924–1930. [Google Scholar] [CrossRef] [Green Version]
- Scarvell, J.M.; Sc, B.A.; Smith, P.N.; Refshauge, K.M.; Galloway, H.R. Magnetic Resonance Imaging Analysis of Kinematics in Osteoarthritic Knees. J. Arthroplasty 2007, 22, 383–393. [Google Scholar] [CrossRef]
- Kawashima, K.; Tomita, T. In vivo three-dimensional motion analysis of osteoarthritic knees. Mod. Rheumatol. 2013, 23, 646–652. [Google Scholar] [CrossRef] [PubMed]
- Farrokhi, S.; Keyak, J.H.; Powers, C.M. Individuals with patellofemoral pain exhibit greater patellofemoral joint stress: A finite element analysis study. Osteoarthr. Cartil. 2011, 19, 287–294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, F.; Kozanek, M.; Hosseini, A.; Van de Velde, S.K.; Gill, T.J.; Rubash, H.E.; Li, G. In vivo tibiofemoral cartilage deformation during the stance phase of gait. J. Biomech. 2010, 43, 658–665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.S.; Tsai, T.Y.; Wang, S.; Li, P.; Kwon, Y.M.; Freiberg, A.; Rubash, H.E.; Li, G. Prediction of in Vivo Knee Joint Kinematics Using a Combined Dual Fluoroscopy Imaging and Statistical Shape Modeling Technique. J. Biomech. Eng. 2014, 136, 124503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Culvenor, A.G.; Wirth, W.; Boeth, H.; Duda, G.N.; Eckstein, F. Longitudinal changes in location-specific cartilage thickness and T2 relaxation-times after posterior cruciate ligament reconstruction for isolated and multiligament injury. Clin. Biomech. 2020, 79, 104935. [Google Scholar] [CrossRef]
- Pritzker, K.P.H.; Gay, S.; Jimenez, S.A.; Ostergaard, K.; Pelletier, J.P.; Revell, K.; Salter, D.; van den Berg, W.B. Osteoarthritis cartilage histopathology: Grading and staging. Osteoarthr. Cartil. 2006, 14, 13–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waldstein, W.; Perino, G.; Gilbert, S.L.; Maher, S.A.; Windhager, R.; Boettner, F. OARSI osteoarthritis cartilage histopathology assessment system: A biomechanical evaluation in the human knee. J. Orthop. Res. 2016, 34, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Caravaggi, P.; Assirelli, E.; Ensini, A.; Ortolani, M.; Mariani, E.; Leardini, A.; Neri, S.; Belvedere, C. Biomechanical-Based Protocol for in vitro Study of Cartilage Response to Cyclic Loading: A Proof-of-Concept in Knee Osteoarthritis. Front. Bioeng. Biotechnol. 2021, 9, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Almajalid, R.; Shan, J.; Zhang, M. A Novel Method to Predict Knee Osteoarthritis Progression on MRI Using Machine Learning Methods. IEEE Trans. Nanobiosci. 2018, 17, 228–236. [Google Scholar] [CrossRef]
- Kellgren, J.H.; Lawrence, J.S. Radiological Assessment of Osteo-Arthrosis. Ann. Rheum. Dis. 1957, 16, 494–502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peterfy, C.G.; Schneider, E.; Nevitt, M. The osteoarthritis initiative: Report on the design rationale for the magnetic resonance imaging protocol for the knee. Osteoarthr. Cartil. 2008, 16, 1433–1441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prendergast, P.J. Finite element models in tissue mechanics and orthopaedic implant design. Clin. Biomech. 1997, 12, 343–366. [Google Scholar] [CrossRef]
- Li, L.; Yang, L.; Zhang, K.; Zhu, L.; Wang, X.; Jiang, Q. Three-dimensional finite-element analysis of aggravating medial meniscus tears on knee osteoarthritis. J. Orthop. Transl. 2020, 20, 47–55. [Google Scholar] [CrossRef]
- Zhang, K.; Li, L.; Yang, L.; Shi, J.; Zhu, L.; Liang, H.; Wang, X.; Yang, X.; Jiang, Q. Effect of degenerative and radial tears of the meniscus and resultant meniscectomy on the knee joint: A finite element analysis. J. Orthop. Transl. 2019, 18, 20–31. [Google Scholar] [CrossRef]
- Ji, W.; Luo, C.; Zhan, S.; Zhan, Y.; Xie, X.; Zhang, B. Combined proximal tibial osteotomy for varus osteoarthritis of the knee: Biomechanical tests and finite-element analyses. Knee 2020, 27, 863–870. [Google Scholar] [CrossRef]
- Liukkonen, M.K.; Mononen, M.E.; Vartiainen, P.; Kaukinen, P.; Bragge, T.; Suomalainen, J.-S.; Malo, M.K.H.; Venesmaa, S.; Käkelä, P.; Pihlajamäki, J.; et al. Evaluation of the Effect of Bariatric Surgery-Induced Weight Loss on Knee Gait and Cartilage Degeneration. J. Biomech. Eng. 2018, 140, 041008. [Google Scholar] [CrossRef] [PubMed]
- Cheung, R.T.H.; Ho, K.K.W.; Au, I.P.H.; An, W.W.; Zhang, J.H.W.; Chan, Z.Y.S.; Deluzio, K.; Rainbow, M.J. Immediate and short-term effects of gait retraining on the knee joint moments and symptoms in patients with early tibiofemoral joint osteoarthritis: A randomized controlled trial. Osteoarthr. Cartil. 2018, 26, 1479–1486. [Google Scholar] [CrossRef] [Green Version]
- Pizzolato, C.; Reggiani, M.; Saxby, D.J.; Ceseracciu, E.; Modenese, L.; Lloyd, D.G. Biofeedback for Gait Retraining Based on Real-Time Estimation of Tibiofemoral Joint Contact Forces. IEEE Trans. Neural Syst. Rehabil. Eng. 2017, 25, 1612–1621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Richards, R.; Van Den Noort, J.C.; Van Der Esch, M.; Booij, M.J.; Harlaar, J. Gait retraining using real-time feedback in patients with medial knee osteoarthritis: Feasibility and effects of a six-week gait training program. Knee 2018, 25, 814–824. [Google Scholar] [CrossRef] [PubMed]
- Bowes, M.A.; Guillard, G.A.; Vincent, G.R.; Brett, A.D.; Brian, C.; Wolstenholme, H.; Conaghan, P.G. Precision, Reliability, and Responsiveness of a Novel Automated Quantification Tool for Cartilage Thickness: Data from the Osteoarthritis Initiative. J. Rheumatol. 2020, 47, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Litjens, G.; Kooi, T.; Bejnordi, B.E.; Setio, A.A.A.; Ciompi, F.; Ghafoorian, M.; van der Laak, J.A.W.M.; van Ginneken, B.; Sánchez, C.I. A survey on deep learning in medical image analysis. Med. Image Anal. 2017, 42, 60–88. [Google Scholar] [CrossRef] [Green Version]
- Teng, H.; Pedoia, V.; Link, T.M.; Majumdar, S.; Souza, R.B. Local associations between knee cartilage T1ρ and T2 relaxation times and patellofemoral joint stress during walking: A voxel-based relaxometry analysis. Knee 2018, 25, 406–416. [Google Scholar] [CrossRef] [PubMed]
- Teng, H.; Calixto, N.E.; Macleod, T.D.; Nardo, L.; Link, T.M.; Majumdar, S.; Souza, R.B. Associations between patellofemoral joint cartilage T1ρ and T2 and knee flexion moment and impulse during gait in individuals with and without patellofemoral joint osteoarthritis. Osteoarthr. Cartil. 2016, 24, 1554–1564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, S.Y.; Souza, R.B.; Ries, M.; Hansma, P.K.; Alliston, T.; Li, X. Local Tissue Properties of Human Osteoarthritic Cartilage Correlate with Magnetic Resonance T 1 rho Relaxation Times. J. Orthop. Res. 2011, 29, 1312–1319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaudhari, A.S.; Kogan, F.; Pedoia, V.; Majumdar, S.; Gold, G.E.; Hargreaves, B.A. Rapid Knee MRI Acquisition and Analysis Techniques for Imaging Osteoarthritis. J. Magn. Reson. Imaging 2020, 52, 1321–1339. [Google Scholar] [CrossRef]
- Zibetti, M.V.W.; Baboli, R.; Chang, G.; Otazo, R.; Regatte, R.R. Rapid Compositional Mapping of Knee Cartilage With Compressed Sensing MRI. J. Magn. Reson. Imaging 2018, 48, 1185–1198. [Google Scholar] [CrossRef]
- Marques, J.; Genant, H.K.; Lillholm, M.; Dam, E.B. Diagnosis of Osteoarthritis and Prognosis of Tibial Cartilage Loss by Quantification of Tibia Trabecular Bone from MRI. Magn. Reson. Med. 2013, 575, 568–575. [Google Scholar] [CrossRef] [PubMed]
- Ratzlaff, C.; Guermazi, A.; Collins, J.; Katz, J.N.; Losina, E.; Vanwyngaarden, C.; Russell, R.; Iranpour, T.; Duryea, J. A rapid, novel method of volumetric assessment of MRI-detected subchondral bone marrow lesions in knee osteoarthritis. Osteoarthr. Cartil. 2013, 21, 806–814. [Google Scholar] [CrossRef] [Green Version]
- Hirvasniemi, J.; Niinimäki, J.; Thevenot, J.; Saarakkala, S. Bone Density and Texture from Minimally Post-Processed Knee Radiographs in Subjects with Knee Osteoarthritis. Ann. Biomed. Eng. 2019, 47, 1181–1190. [Google Scholar] [CrossRef] [PubMed]
- Moerman, K.M.; Holt, C.A.; Evans, S.L.; Simms, C.K. Digital image correlation and finite element modelling as a method to determine mechanical properties of human soft tissue in vivo. J. Biomech. 2009, 42, 1150–1153. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.; Qian, Z.; Liang, W.; Liu, J.; Ren, L.; Ren, L. In vivo assessment of material properties of muscles and connective tissues around the knee joint based on shear wave elastography. J. Mech. Behav. Biomed. Mater. 2020, 109, 103829. [Google Scholar] [CrossRef] [PubMed]
- Teng, H.; MacLeod, T.D.; Kumar, D.; Link, T.M.; Majumdar, S.; Souza, R.B. Individuals with isolated patellofemoral joint osteoarthritis exhibit higher mechanical loading at the knee during the second half of the stance phase. Clin. Biomech. 2015, 30, 383–390. [Google Scholar] [CrossRef] [Green Version]
- Ho, K.; Blanchette, M.G.; Powers, C.M. The influence of heel height on patellofemoral joint kinetics during walking. Gait Posture 2012, 36, 271–275. [Google Scholar] [CrossRef] [PubMed]
- Brechter, J.H.; Powers, C.M. Patellofemoral stress during walking in persons with and without patellofemoral pain. Med. Sci. Sport. Exerc. 2002, 34, 1582–1593. [Google Scholar] [CrossRef] [PubMed]
- Van Eijden, T.M.G.J.; Kuowenhoven, E.; Verburg, J.; Weijs, W.A. A Mathematical Model of The Patellofemoral Joint. J. Biomech. 1986, 19, 219–229. [Google Scholar] [CrossRef]
- Gustafson, J.A.; Elias, J.J.; Debski, R.E.; Farrokhi, S. Development and validation of a kinematically-driven discrete element model of the patellofemoral joint. J. Biomech. 2019, 88, 164–172. [Google Scholar] [CrossRef]
- Li, M.; Venäläinen, M.S.; Chandra, S.S.; Patel, R.; Fripp, J.; Engstrom, C.; Korhonen, R.K.; Töyräs, J.; Crozier, S. Discrete element and finite element methods provide similar estimations for hip joint contact mechanics during walking gait. J. Biomech. 2021, 115, 110163. [Google Scholar] [CrossRef] [PubMed]
- Thomas-Aitken, H.D.; Willey, M.C.; Goetz, J.E. Joint contact stresses calculated for acetabular dysplasia patients using discrete element analysis are significantly influenced by the applied gait pattern. J. Biomech. 2018, 79, 45–53. [Google Scholar] [CrossRef]
- Chen, P.; Gao, L.; Shi, X.; Allen, K.; Yang, L. Fully automatic knee osteoarthritis severity grading using deep neural networks with a novel ordinal loss. Comput. Med. Imaging Graph. 2019, 75, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Kessler, D.A.; MacKay, J.W.; Crowe, V.A.; Henson, F.M.D.; Graves, M.J.; Gilbert, F.J.; Kaggie, J.D. The optimisation of deep neural networks for segmenting multiple knee joint tissues from MRIs. Comput. Med. Imaging Graph. 2020, 86, 101793. [Google Scholar] [CrossRef]
- Tiulpin, A.; Thevenot, J.; Rahtu, E.; Lehenkari, P.; Saarakkala, S. Automatic knee osteoarthritis diagnosis from plain radiographs: A deep learning-based approach. Sci. Rep. 2018, 8, 1–10. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paz, A.; Orozco, G.A.; Korhonen, R.K.; García, J.J.; Mononen, M.E. Expediting Finite Element Analyses for Subject-Specific Studies of Knee Osteoarthritis: A Literature Review. Appl. Sci. 2021, 11, 11440. https://doi.org/10.3390/app112311440
Paz A, Orozco GA, Korhonen RK, García JJ, Mononen ME. Expediting Finite Element Analyses for Subject-Specific Studies of Knee Osteoarthritis: A Literature Review. Applied Sciences. 2021; 11(23):11440. https://doi.org/10.3390/app112311440
Chicago/Turabian StylePaz, Alexander, Gustavo A. Orozco, Rami K. Korhonen, José J. García, and Mika E. Mononen. 2021. "Expediting Finite Element Analyses for Subject-Specific Studies of Knee Osteoarthritis: A Literature Review" Applied Sciences 11, no. 23: 11440. https://doi.org/10.3390/app112311440




