Comparative Evaluation of Bond Strength and Microleakage of Three Ion-Releasing Restorative Materials at Various pH Levels
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
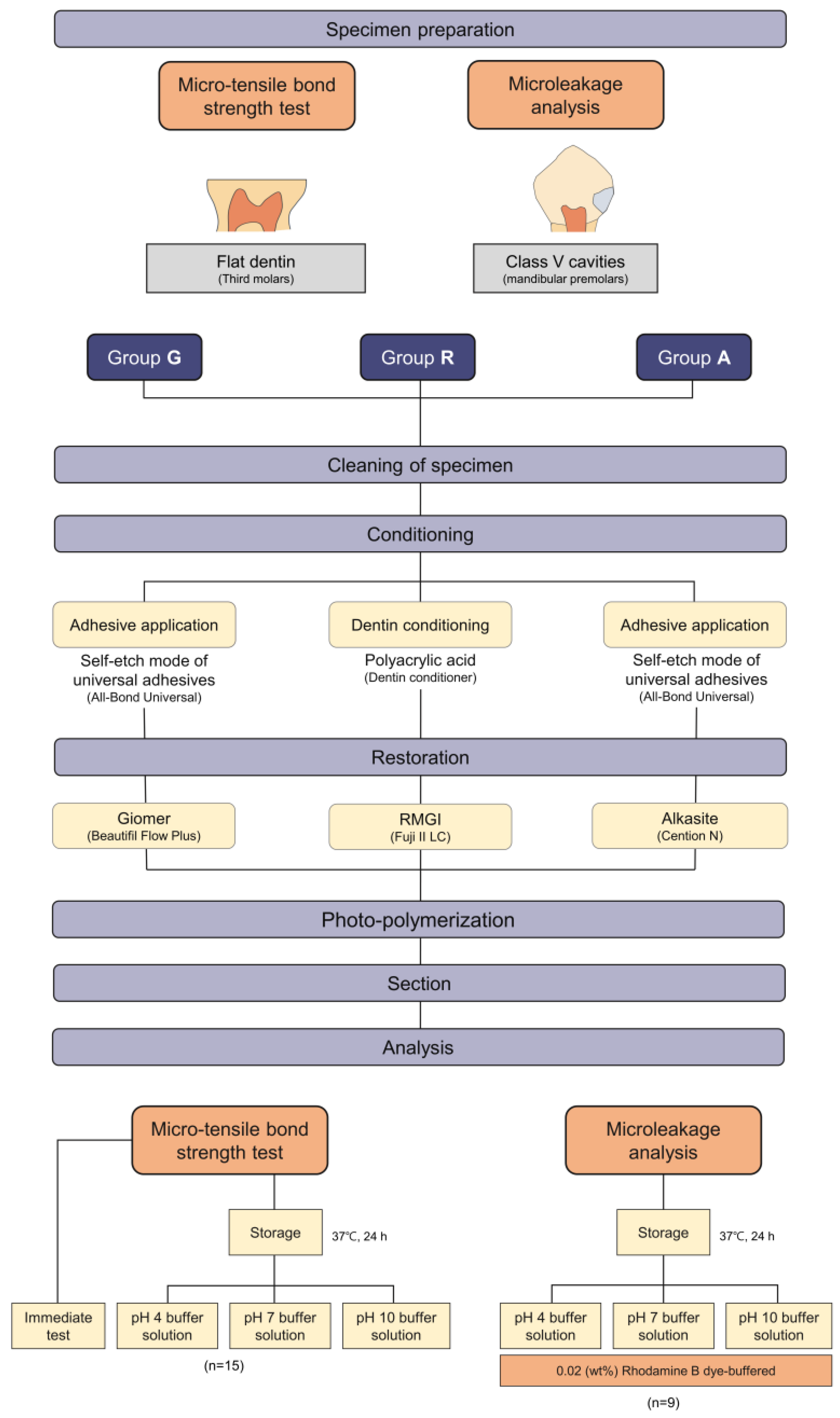
2.2. Micro-Tensile Bond Strength (μTBS) Test
2.2.1. Dentin Specimen Preparation
2.2.2. Group Assignment, Surface Treatment and Restoration Procedure
2.2.3. Micro-Tensile Bond Strength (μTBS) Test and Failure Mode Analysis
2.3. Microleakage Evaluation
2.3.1. Specimen Preparation for Microleakage Evaluation
2.3.2. Group Assignment and Confocal Laser Scanning Microscopic Analysis
2.3.3. Assessment of Microleakage
- No dye penetration.
- Dye penetration to one-half of the occlusal or gingival wall.
- Dye penetration extending to the end of the occlusal or gingival walls.
- Dye penetration through the gingival or occlusal wall to one-third of the axial wall.
- Dye penetration through the gingival or occlusal wall to two-thirds of the axial wall.
- Dye penetration throughout the axial wall.
3. Statistical Analysis
4. Results
4.1. Micro-Tensile Bond Strength (μTBS) Test and Failure Mode Analysis
4.2. Microleakage Evaluation with CLSM Images
4.3. Microleakage Evaluation with Score
5. Discussion
6. Conclusions
- Giomer, RMGI, and alkasite exhibited no significant difference in the bond strength with dentin in this study. The pH 4 condition yielded a decreased bond strength for the giomer compared with that of the subgroups, which were immediately tested or stored at a neutral pH.
- RMGI and alkasite showed a lower microleakage than the giomers. There was a significant difference in the microleakage scores according to the margin location. The difference in scores varied according to the materials; a higher microleakage score was observed in the enamel margin than in the gingival margin for RMGI and alkasite, and higher microleakage scores were observed in the gingival margin than in the enamel margin for giomer.
- For RMGI and alkasite, the specimens stored at pH 4 showed significantly lower microleakage than those stored at pH 7.
- RMGI and alkasite might be adopted in generalized or localized low-pH clinical conditions.
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dionysopoulos, D.; Koliniotou-Koumpia, E.; Helvatzoglou-Antoniades, M.; Kotsanos, N. Fluoride release and recharge abilities of contemporary fluoride-containing restorative materials and dental adhesives. Dent. Mater. J. 2013, 32, 296–304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, J.-Y.; Li, Y.-Q.; Smales, R.; Yip, K.-K. Restoration of teeth with more-viscous glass ionomer cements following radiation-induced caries. Int. Dent. J. 2002, 52, 445–448. [Google Scholar] [CrossRef] [PubMed]
- Loudon, J.A. Next generational fuji IX-a proposed universal dental material—But not yet ‘set in cement’. Oral Biol. Dent. 2014, 2, 5. [Google Scholar] [CrossRef] [Green Version]
- Ngo, H.; Mount, G.; Peters, M. A study of glass-ionomer cement and its interface with enamel and dentin using a low-temperature, high-resolution scanning electron microscopic technique. J. Esthet. Resto. Dent. 1999, 11, 223. [Google Scholar]
- Sidhu, S.K.; Watson, T.F. Resin-modified glass ionomer materials. A status report for the American Journal of Dentistry. Am. J. Dent. 1995, 8, 59–67. [Google Scholar]
- Deliperi, S.; Bardwell, D.N.; Wegley, C.; Congiu, M.D. In vitro evaluation of giomers microleakage after exposure to 33% hydrogen peroxide: Self-etch vs total-etch adhesives. Oper. Dent. 2006, 31, 227–232. [Google Scholar] [CrossRef]
- Francois, P.; Fouquet, V.; Attal, J.-P.; Dursun, E. Commercially available fluoride-releasing restorative materials: A review and a proposal for classification. Materials 2020, 13, 2313. [Google Scholar] [CrossRef]
- Khalid, H.; Aleesa, N.; Grosjean, M.; Hill, R.; Wong, F. Characterisation of a bioactive SiO2-CaO-CaF2-Na2O glass used in composites. Dent. Mater. 2021, 37, 1–9. [Google Scholar] [CrossRef]
- Vallittu, P.K.; Boccaccini, A.R.; Hupa, L.; Watts, D.C. Bioactive dental materials-Do they exist and what does bioactivity mean? Dent. Mater. 2018, 34, 693–694. [Google Scholar] [CrossRef]
- Burke, F.; Ray, N.; McConnell, R. Fluoride-containing restorative materials. Int. Dent. J. 2006, 56, 33–43. [Google Scholar] [CrossRef]
- Ranjitkar, S.; Kaidonis, J.A.; Smales, R.J. Gastroesophageal reflux disease and tooth erosion. Int. J. Dent. 2012, 2012, 479850. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sreebny, L.M. Saliva in health and disease: An appraisal and update. Int. Dent. J. 2000, 50, 140–161. [Google Scholar] [CrossRef] [PubMed]
- Baliga, S.; Muglikar, S.; Kale, R. Salivary pH: A diagnostic biomarker. J. Indian Soc. Periodontol. 2013, 17, 461. [Google Scholar] [CrossRef] [PubMed]
- Moreau, J.L.; Xu, H.H. Fluoride releasing restorative materials: Effects of pH on mechanical properties and ion release. Dent. Mater. 2010, 26, e227–e235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takahashi, N.; Nyvad, B. Caries ecology revisited: Microbial dynamics and the caries process. Caries Res. 2008, 42, 409–418. [Google Scholar] [CrossRef]
- Gudmundsson, K.; Kristleifsson, G.; Theodors, A.; Holbrook, W.P. Tooth erosion, gastroesophageal reflux, and salivary buffer capacity. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 1995, 79, 185–189. [Google Scholar] [CrossRef]
- Holbrook, W.; Furuholm, J.; Gudmundsson, K.; Theodors, A.; Meurman, J.H. Gastric reflux is a significant causative factor of tooth erosion. J. Dent. Res. 2009, 88, 422–426. [Google Scholar] [CrossRef]
- Muppalla, J.K.; Harikumar, V.; Sarathchandra, P.; Reddy, S.J.; Rajani, P. Effect of modulated photoactivation of bulkfill composite on microleakage in fluorosed and nonfluorosed teeth: A confocal laser scanning microscopy study. J. Conserv. Dent. 2020, 23, 180. [Google Scholar] [CrossRef]
- Mohamed, N.I.; Safy, R.K.; Elezz, A.F.A. Microtensile bond strength, marginal leakage, and antibacterial effect of bulk fill resin composite with alkaline fillers versus incremental nanohybrid composite resin. Eur. J. Dent. 2021, 15, 425–432. [Google Scholar] [CrossRef]
- Saad, A.; Inoue, G.; Nikaido, T.; Ikeda, M.; Burrow, M.; Tagami, J. Microtensile Bond Strength of Resin-Modified Glass Ionomer Cement to Sound and Artificial Caries—Affected Root Dentin with Different Conditioning. Oper. Dent. 2017, 42, 626–635. [Google Scholar] [CrossRef]
- Bollu, I.P.; Hari, A.; Thumu, J.; Velagula, L.D.; Bolla, N.; Varri, S.; Kasaraneni, S.; Nalli, S.V.M. Comparative evaluation of microleakage between nano-ionomer, giomer and resin modified glass ionomer cement in class V cavities-CLSM study. J. Clin. Diagnostic Res. 2016, 10, ZC66. [Google Scholar] [CrossRef] [PubMed]
- Restrepo, M.; Jeremias, F.; Santos-Pinto, L.; Cordeiro, R.C.; Zuanon, A.C. Effect of fluoride varnish on enamel remineralization in anterior teeth with molar incisor hypomineralization. J. Clin. Pediatr. Dent. 2016, 40, 207–210. [Google Scholar] [CrossRef] [PubMed]
- Souza, J.F.D.; Fragelli, C.M.B.; Paschoal, M.A.B.; Campos, E.A.; Cunha, L.F.; Losso, E.M.; Cordeiro, R.D.C.L. Noninvasive and multidisciplinary approach to the functional and esthetic rehabilitation of amelogenesis imperfecta: A pediatric case report. Case Rep. Dent. 2014, 2014, 127175. [Google Scholar] [CrossRef] [PubMed]
- Carrilho, E.; Cardoso, M.; Marques Ferreira, M.; Marto, C.M.; Paula, A.; Coelho, A.S. 10-MDP based dental adhesives: Adhesive interface characterization and adhesive stability—A systematic review. Materials 2019, 12, 790. [Google Scholar] [CrossRef] [Green Version]
- Tsuchiya, S.; Nikaido, T.; Sonoda, H.; Foxton, R.M.; Tagami, J. Ultrastructure of the dentin-adhesive interface after acid-base challenge. J. Adhes. Dent. 2004, 6, 183–190. [Google Scholar]
- Guan, R.; Takagaki, T.; Matsui, N.; Sato, T.; Burrow, M.F.; Palamara, J.; Nikaido, T.; Tagami, J. Dentin bonding performance using Weibull statistics and evaluation of acid-base resistant zone formation of recently introduced adhesives. Dent. Mater. J. 2016, 35, 684–693. [Google Scholar] [CrossRef] [Green Version]
- Van Meerbeek, B.; Yoshihara, K.; Yoshida, Y.; Mine, A.; De Munck, J.; Van Landuyt, K. State of the art of self-etch adhesives. Dent. Mater. 2011, 27, 17–28. [Google Scholar] [CrossRef]
- Kasraei, S.; Haghi, S.; Valizadeh, S.; Panahandeh, N.; Nejadkarimi, S. Phosphate Ion release and alkalizing potential of three bioactive dental materials in comparison with composite resin. Int. J. Dent. 2021, 2021, 5572569. [Google Scholar] [CrossRef]
- Gonulol, N.; Ozer, S.; Sen Tunc, E. Water sorption, solubility, and color stability of giomer restoratives. J. Esthet. Restor. Dent. 2015, 27, 300–306. [Google Scholar] [CrossRef]
- Tsujimoto, A.; Barkmeier, W.W.; Takamizawa, T.; Latta, M.A.; Miyazaki, M. Depth of cure, flexural properties and volumetric shrinkage of low and high viscosity bulk-fill giomers and resin composites. Dent. Mater. J. 2017, 36, 205–213. [Google Scholar] [CrossRef] [Green Version]
- Toledano, M.; Osorio, E.; Osorio, R.; García-Godoy, F. Microleakage of Class V resin-modified glass ionomer and compomer restorations. J. Prosthet. Dent. 1999, 81, 610–615. [Google Scholar] [CrossRef]
- Afraaz, A.; Borugadda, R.; Mandava, J.; Chalasani, U.; Ravi, R.; Pamidmukkala, S.; Boddeda, M.R.; Athkuri, S. Evaluation of Marginal Adaptation and Wear Resistance of Nanohybrid and Alkasite Restorative Resins. J. Clin. Diagn. Res. 2020, 14, 16–20. [Google Scholar] [CrossRef]
- Szczesio-Wlodarczyk, A.; Polikowski, A.; Krasowski, M.; Fronczek, M.; Sokolowski, J.; Bociong, K. The Influence of Low-Molecular-Weight Monomers (TEGDMA, HDDMA, HEMA) on the Properties of Selected Matrices and Composites Based on Bis-GMA and UDMA. Materials 2022, 15, 2649. [Google Scholar] [CrossRef] [PubMed]
- Braga, R.R.; Ballester, R.Y.; Ferracane, J.L. Factors involved in the development of polymerization shrinkage stress in resin-composites: A systematic review. Dent. Mater. 2005, 21, 962–970. [Google Scholar] [CrossRef] [PubMed]
- Sunico, M.C.; Shinkai, K.; Katoh, Y. Two-year clinical performance of occlusal and cervical giomer restorations. Oper. Dent. 2005, 30, 282. [Google Scholar]
- Sofan, E.; Sofan, A.; Palaia, G.; Tenore, G.; Romeo, U.; Migliau, G. Classification review of dental adhesive systems: From the IV generation to the universal type. Ann. Stomatol. 2017, 8, 1. [Google Scholar]
- Gupta, S.K.; Gupta, J.; Saraswathi, V.; Ballal, V.; Acharya, S.R. Comparative evaluation of microleakage in Class V cavities using various glass ionomer cements: An in vitro study. J. Interdiscip. Dent. 2012, 2, 164. [Google Scholar] [CrossRef]
- Sooraparaju, S.G.; Kanumuru, P.K.; Nujella, S.K.; Konda, K.R.; Reddy, K.; Penigalapati, S. A comparative evaluation of microleakage in class v composite restorations. Int. J. Dent. 2014, 2014, 685643. [Google Scholar] [CrossRef]
- Davidson, C.L. Resisting the curing contraction with adhesive composites. J. Prosthet. Dent. 1986, 55, 446–447. [Google Scholar] [CrossRef]
- Pinto-Sinai, G.; Brewster, J.; Roberts, H. Linear coefficient of thermal expansion evaluation of glass ionomer and resin-modified glass ionomer restorative materials. Oper. Dent. 2018, 43, E266–E272. [Google Scholar] [CrossRef]
- Nie, J.; Yap, A.; Wang, X. Influence of shrinkage and viscosity of flowable composite liners on cervical microleakage of class II restorations: A micro-CT analysis. Oper. Dent. 2018, 43, 656–664. [Google Scholar] [CrossRef] [PubMed]
- Ilie, N. Comparative effect of self-or dual-curing on polymerization kinetics and mechanical properties in a novel, dental-resin-based composite with alkaline filler. Materials 2018, 11, 108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nayak, M.; Shenoy, V. Sorption and Solubility of Alkasite Restorative Material-An In Vitro Study. IOSR J. Dent. Med. Sci. 2019, 18, 69–73. [Google Scholar]
- Huang, C.; Kei, L.H.; Wei, S.H.; Cheung, G.S.; Tay, F.R.; Pashley, D.H. The influence of hygroscopic expansion of resin-based restorative materials on artificial gap reduction. J. Adhes. Dent. 2002, 4, 61–71. [Google Scholar]



| Material | Composition | Filler | Manufacturer |
|---|---|---|---|
| Beautifil Flow Plus | Bis-GMA, TEGDMA, Initiator (53%) | S-PRG filler, Aluminofluoro-borosilicate glass (47%) | Shofu, Kyoto, Japan |
| Fuji II LC | Polyacrylic acid, HEMA, UDMA, distilled water, Initiator (45%) | Fluroalumino silicate glass, poly-HEMA (55%) | GC corporation, Tokyo, Japan |
| Cention N | UDMA, Aromatic aliphatic UDMA, Initiators (Ivocerin) (42%) | Barium aluminium silicate glass, Calcium barium aluminium fluorosilicate glass, Calcium fluorosilicate glass, Ytterbium trifluoride, Isofiller (58%) | Ivoclar vivadent, Schaan, Liechtenstein |
| All-Bond Universal | 10-MDP phosphate monomer, HEMA, Bis-GMA, Ethanol, Water, Initiators | Bisco, Schaumburg, IL, USA | |
| Group G | Group R | Group A | |
|---|---|---|---|
| Immediate | 12.32 ± 5.56 Aa | 8.86 ± 4.85 Aa | 10.21 ± 5.28 Aa |
| pH 4-storage | 7.44 ± 4.58 Ba | 8.86 ± 4.07 Aab | 9.35 ± 4.02 Ab |
| pH 7-storage | 11.30 ± 4.25 Aa | 7.64 ± 3.43 Aa | 8.74 ± 3.59 Aa |
| pH 10-storage | 8.69 ± 4.62 Aba | 7.70 ± 3.40 Aa | 7.66 ± 3.47 Aa |
| Groups | Margin Location | p Value with Margin Location | pH 4 | pH 7 | pH 10 | p Value with pH |
|---|---|---|---|---|---|---|
| G a | Occlusal | 0.001 | 2.857 ± 1.069 | 2.714 ± 0.756 | 2.428 ± 0.787 | 0.796 |
| Gingival | 4.286 ± 1.380 | 3.143 ± 0.535 | 4.714 ± 1.254 | 0.120 | ||
| R b | Occlusal | 0.001 | 1.571 ± 0.535 | 2.000 ± 0.577 | 2.143 ± 0.690 | 0.212 |
| Gingival | 1.000 ± 0.000 A | 1.714 ± 0.756 B | 1.143 ± 0.378 AB | 0.032 | ||
| A b | Occlusal | 0.001 | 1.714 ± 0.756 | 1.429 ± 0.787 | 2.143 ± 0.900 | 0.212 |
| Gingival | 1.143 ± 0.378 A | 1.429 ± 0.535 B | 1.429 ± 0.535 AB | 0.032 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.-J. Comparative Evaluation of Bond Strength and Microleakage of Three Ion-Releasing Restorative Materials at Various pH Levels. Appl. Sci. 2022, 12, 6796. https://doi.org/10.3390/app12136796
Kim H-J. Comparative Evaluation of Bond Strength and Microleakage of Three Ion-Releasing Restorative Materials at Various pH Levels. Applied Sciences. 2022; 12(13):6796. https://doi.org/10.3390/app12136796
Chicago/Turabian StyleKim, Hyun-Jung. 2022. "Comparative Evaluation of Bond Strength and Microleakage of Three Ion-Releasing Restorative Materials at Various pH Levels" Applied Sciences 12, no. 13: 6796. https://doi.org/10.3390/app12136796
APA StyleKim, H.-J. (2022). Comparative Evaluation of Bond Strength and Microleakage of Three Ion-Releasing Restorative Materials at Various pH Levels. Applied Sciences, 12(13), 6796. https://doi.org/10.3390/app12136796






